Abstract
Objective and design
Though combination antiretroviral therapy reduces the concentration of HIV-1 RNA in both plasma and cerebrospinal fluid (CSF) below the detection limit of clinical assays, low levels of HIV-1 RNA are frequently detectable in plasma using more sensitive assays. We examined the frequency and magnitude of persistent low-level HIV-1 RNA in CSF and its relation to the central nervous system (CNS) immune activation.
Methods
CSF and plasma HIV-1 RNA were measured using the single-copy assay with a detection limit of 0.3 copies/ml in 70 CSF and 68 plasma samples from 45 treated HIV-1-infected patients with less than 40 copies/ml of HIV-1 RNA in both fluids by standard clinical assays. We also measured CSF neopterin to assess intrathecal immune activation. Theoretical drug exposure was estimated using the CNS penetration-efficacy score of treatment regimens.
Results
CSF HIV-1 RNA was detected in 12 of the 70 CSF samples (17%) taken after up to 10 years of suppressive therapy, compared to 39 of the 68 plasma samples (57%) with a median concentration of less than 0.3 copies/ml in CSF compared to 0.3 copies/ml in plasma (P <0.0001). CSF samples with detectable HIV-1 RNA had higher CSF neopterin levels (mean 8.2 compared to 5.7 nmol/l; P =0.0085). Patients with detectable HIV-1 RNA in CSF did not differ in pretreatment plasma HIV-1 RNA levels, nadir CD4+ cell count or CNS penetration-efficacy score.
Conclusion
Low-level CSF HIV-1 RNA and its association with elevated CSF neopterin highlight the potential for the CNS to serve as a viral reservoir and for persistent infection to cause subclinical CNS injury.
Keywords: central nervous system, cerebrospinal fluid, HIV, immune, inflammation, neurological, reservoir, therapy
Introduction
HIV-1 enters the central nervous system (CNS) early during systemic infection and can then be detected in the cerebrospinal fluid (CSF) throughout the subsequent course of untreated infection in most individuals [1–3]. The concentration of HIV-1 RNA in the CSF averages about one-tenth of that in plasma during chronic infection, though the CSF : plasma virus ratio varies among individuals and also may change over time within individual patients [1,2,4,5] related to both the stage of systemic infection and the origins of the CSF virus [2,6].
Through the course of infection, HIV-1 is accompanied by local inflammation and immune activation that is reflected in CSF lymphocytic pleocytosis and elevated concentrations of a number of CSF inflammatory biomarkers, including neopterin (a marker for macrophage activation), and chemokines CCL2 and CXCL10 [5,7]. Later in its course, infection may also lead to brain injury reflected in changes in CSF biomarkers of neuronal injury including neurofilament light (NFL) chain, tau and amyloid proteins [8–10]. In the era before effective treatment, 20–30% of untreated patients with advanced immunosuppression developed overt HIV-associated dementia (HAD) [11,12].
Combination antiretroviral therapy (cART) effectively reduces HIV-1 RNA concentrations in both CSF and plasma to levels below the lower level of detection of assays used in clinical settings (<20–50 copies/ml) [2,4,13,14]. However, using very sensitive assays such as single-copy assay (SCA) that allows HIV-1 RNA quantification down to less than one copy of viral RNA/ ml, it is possible to detect HIV-1 RNA regularly in plasma and less commonly in CSF during suppressive therapy [15,16].
In addition to reducing the HIV-1 RNA concentrations, cART also reduces intrathecal inflammation and ongoing neural injury [14,17–19]. When it is widely available, cART has greatly reduced the incidence of HAD and opportunistic CNS infections [20–23]. However, recent studies indicate that less severe cognitive impairment is common in patients on cART despite plasma virus suppression, related either to earlier injury before treatment was initiated or to persistent low-level CNS infection accompanied by local immune activation [22,24–26].
In this study, we examined paired CSF and plasma samples from 45 patients on long-term suppressive therapy who volunteered for lumbar puncture. We used SCA to determine the frequency and magnitude of HIV-1 RNA detection in the CSF compared to plasma during suppressive cART, and also measured CSF neopterin to assess whether low concentrations of HIV-1 in CSF was associated with CNS immune activation.
Methods
Study design and participants
Paired plasma and CSF samples were derived from observational cohort studies in Gothenburg, Sweden and San Francisco, California, USA. Selection criteria included HIV-1 infection with confirmed or epidemiologically presumed clade B, treatment with cART containing at least three antiretroviral drugs (not counting ritonavir), and both plasma and CSF HIV-1 RNA below 40 copies/ml at screening by standard clinical assays. Additional criteria included the following: above 18 years of age, absence of neurological symptoms suggestive of new or progressing CNS disease, presumed medication adherence by history, no contraindications to lumbar puncture, and no active opportunistic infections or neurological disease. The study was conducted in accordance with the Declaration of Helsinki approved by the Regional Ethical Review Boards in Stockholm and Gothenburg, Sweden and the University of California San Francisco Committee on Human Research. Written informed consent was obtained from all patients.
Cerebrospinal fluid and plasma HIV-1 RNA concentrations
HIV-1 RNA concentrations in CSF and plasma were measured using the sensitive SCA following the same methods of sample preparation and assay as detailed in the earlier publication [27]. In brief, 7 ml of CSF or plasma, with a known amount of Replication-competent avian sarcoma-leukosis virus long terminal repeat with a splice acceptor (RCAS) (an avian retrovirus) added as an internal standard, was ultra-centrifuged and the pellet was extracted and subjected to complementary DNA synthesis followed by real-time PCR amplification of a 79-base pair region of HIV-1 Gag and a portion of the RCAS genome. HIV-1 RNA levels were determined using a standard curve constructed with HIV-1 of known RNA copy number. To ensure that the extraction process was successful, the level of RCAS was measured using a separate standard curve constructed with RCAS of known RNA copy number. Samples where we measured less than half of the expected amount of added RCAS internal standard were considered to have failed and were excluded from the analysis. As described earlier [16], we assigned negative results (below <0.3 copies/ml) a default ‘floor’ value of 0.2 copies/ml for further analysis including group statistics.
Biomarkers of immune suppression and activation
Cerebrospinal fluid white blood cell (WBC) counts and blood CD4+ T-cell analysis were performed in the Sahlgrenska University Hospital in Gothenburg or the San Francisco General Hospital Clinical Laboratories using standard clinical methods. Neopterin was measured in cell-free CSF by enzyme-linked immunoassay according to the manufacturer’s instructions (BRAHMS Aktienge-sellschaft, Henningsdorf, Germany) in Innsbruck, Austria. Normal reference values were below 5.8 nmol/l in CSF [28].
Central nervous system antiviral drug penetration
As an index of the theoretical CNS exposure to cART, we used the revised form of the CNS penetration-effectiveness (CPE) score developed by Letendreet al. [29,30] to calculate a rating for each patient’s regimen at the time of sampling.
Statistics
Means and SD were calculated for variables assumed to have normal distribution, and median and intraquartile range (IQR) were calculated for the remaining variables. The variables were compared independently by Mann–Whitney’s test, whereas proportions were compared using Fisher’s exact test. Spearman’s rho was calculated to test for correlation. All P values were two-sided with values less than 0.05 considered significant. Statistical analyses were performed and graphs constructed using GraphPad Prism 6 software (GraphPad, La Jolla, California, USA).
Results
Samples and patients
We analyzed a total of 76 paired CSF and plasma samples from 45 patients (Table 1). All samples had a volume of 7 ml. The extraction control failed for 14 of the 152 samples (six CSF and eight plasma); these measurements were excluded from further analysis, leaving 70 CSF and 68 plasma from 45 patients for analysis. This included 18 patients with multiple samplings: nine sampled twice, five sampled three times, three sampled four times and one sampled five times. Patients were predominantly men and middle-aged, had been diagnosed with HIV-1 for nearly a decade, were on suppressive therapy for a median of 3 years, had not changed therapy during the previous 6 months before the first study sample, and were on regimens with a median CPE score of 7. None had symptoms or signs of active CNS disease. Their blood CD4+ T cells at sampling were relatively high, but with a nadir of about 200 cells/μl. Pretherapy plasma HIV-1 RNA concentrations were available for 73% with a mean level of 4.8 log10 copies/ml, whereas pretherapy CSF HIV-1 RNA concentrations were only available for 44% with a mean concentration of 3.4 log10 copies/ml.
Table 1.
Background characteristics.
| Number of patients | 45 |
| Patients per site (San Francisco/Gothenburg) | 30/15 |
| Number of samples (total, with valid SCA results) | |
| CSF | 76 (70) |
| Plasma | 76 (68) |
| Patients with multiple samples | 18 |
| Sex (M/F) | 40/5 |
| Age (mean years, SD) | 49 (11) |
| Time of infection (median years, IQR) | 8 (4–13) |
| Time of suppressive therapy (median years, IQR) | 3 (1–5) |
| CPE (median, IQR) | 7 (7–8) |
| Blood T-cells (mean cells/μl, SD) | |
| CD4+ at first time point in study | 598 (246) |
| Nadir CD4+ | 198 (162) |
| Pretherapy HIV-1 RNA concentration (log 10 mean HIV-1 RNA/ml, SD) | |
| CSFa | 3.4 (1.0) |
| Plasmab | 4.8 (0.8) |
| CSF WBC (median per μl, IQR) | 2 (1–3) |
CPE, central nervous system penetration-efficacy; CSF, cerebrospinal fluid; IQR, interquartile range; SCA, single-copy assay; WBC, white blood cell.
n =20 samples.
n =33 samples.
Single-copy assay HIV-1 RNA measurements
Twelve of the 70 (17%) CSF samples were positive for HIV-1 RNA, which was significantly less than the 39 of 68 (57%) positive plasma samples (P <0.0001) (Table 2). The median concentration HIV-1 RNA in CSF was below the limit of detection. Using the defined ‘floor’ value of 0.2 copies/ml, the median was at this value of 0.2 copies/ml (range 0.2–3.9, IQR 0.2–0.2) compared to 0.3 copies/ml (range 0.2–15, IQR 0.2–0.9 in plasma; P <0.0001) (Table 2). Time points with detectable CSF HIV-1 RNA occurred after a median of 2.8 years of suppressive therapy (range 1.8–10) compared to 2.0 years (range 0.4–11) for time points with detectable plasma HIV-1 RNA (P =0.0917).
Table 2.
Single-copy assay HIV-1 RNA measurements.
| CSF | Plasma | P value | |
|---|---|---|---|
| Proportion of positive samples | 12/70 | 39/68 | <0.0001 |
| HIV-1 RNA concentrations during suppressive therapy (mean HIV-1 RNA copies/ml, range) | 0.3 (0.2–3.9) | 1.1 (0.2–15) | <0.0001 |
| Time of suppressive therapy to time point with detectable HIV-1 RNA (median years, range) | 2.8 (1.8–10) | 2.0 (0.4–11) | 0.0917 |
CSF, cerebrospinal fluid.
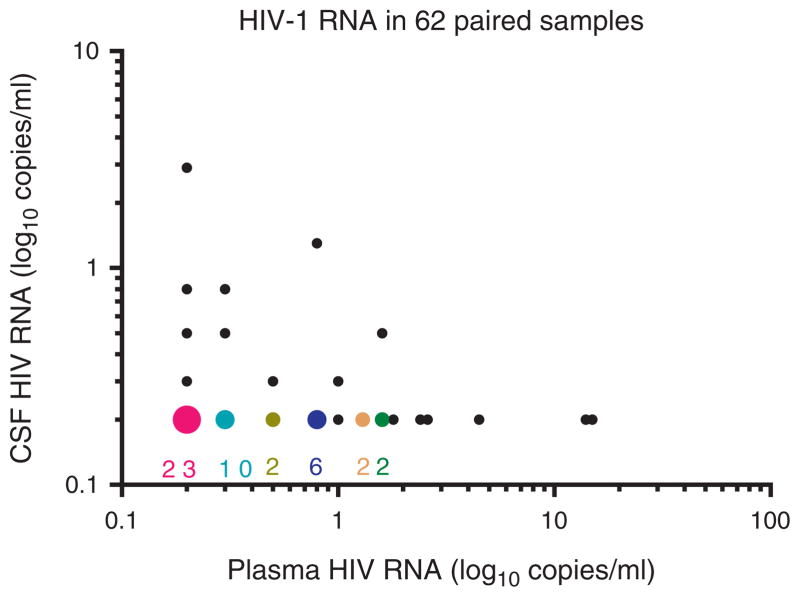
After excluding SCA measurements where the extraction had failed, 62 sample pairs with valid measurements of HIV-1 RNA in both CSF and plasma remained (Fig. 1). In these sample pairs, the proportion of positive samples was 16% in CSF and 56% in plasma. The median level was below the limit of detection in CSF (range 0.2–2.9, IQR 0.2–0.8) compared to 0.3 copies/ml (range 0.2–15, IQR 0.2–0.9 in plasma; P <0.0001). Overall, there was no correlation between CSF and plasma HIV-1 RNA levels in the sample pairs (r =−0.0071, P =0.9563).
Fig. 1. HIV-1 RNA concentrations in analyzed 62 pairs.
The figure shows SCA results in the 56 paired samples with valid results on both CSF and plasma. The larger-colored symbols indicate overlapping results in the samples with undetectable CSF HIV RNA with the number of overlaps given below each. The smaller black circles represent results of single sample pairs. CSF, cerebrospinal fluid; SCA, single-copy assay.
To avoid bias from repeated samples in individual patients, we also conducted similar calculations for measurements from the first visit for all patients. In this subset of 45 pairs, the proportion of positive samples was 14% in CSF and 61% in plasma. In this subset, the median level was again below the limit of detection in CSF (range 0.2–3.9, IQR 0.2–0.1) compared to 0.3 copies/ml (range 0.2–14, IQR 0.2–0.9 in plasma; P <0.0001).
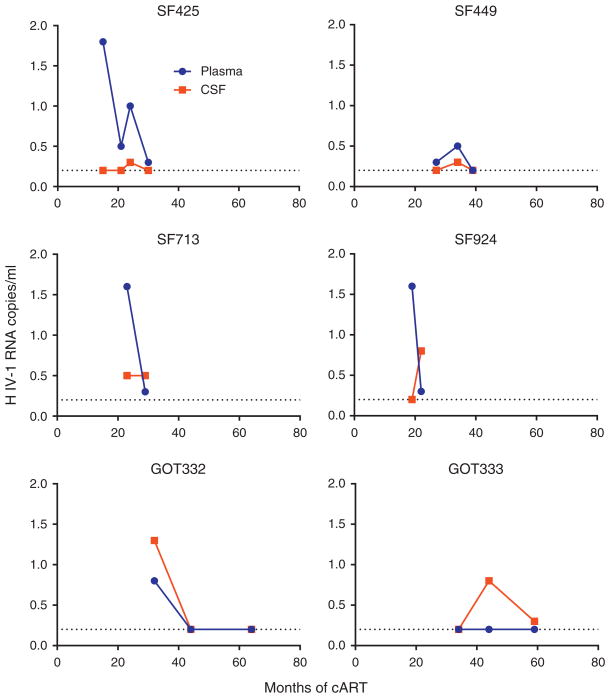
We analyzed samples from multiple time points from 18 patients. Six of these 18 patients had detectable CSF HIV-1 RNA in at least one sample. Two of these six patients in whom CSF HIV-1 RNA could be detected at least once had a detectable CSF HIV-1 RNA at more than one time point (Fig. 2). By contrast, 17 of the 18 patients with samples from multiple time points had detectable plasma HIV-1 RNA from at least one time point.
Fig. 2. HIV-1 RNA concentrations in cerebrospinal fluid and plasma.
The figure shows SCA results in the six patients where we had multiple samples from CSF and at least one CSF sample was positive. Plasma values are shown in blue circles and CSF values are shown in red squares. CSF, cerebrospinal fluid; SCA, single-copy assay.
Detectable HIV-1 RNA in cerebrospinal fluid and plasma in relation to pretreatment HIV-1 RNA concentrations, nadir CD4+ cell count and central nervous system penetration-effectiveness score
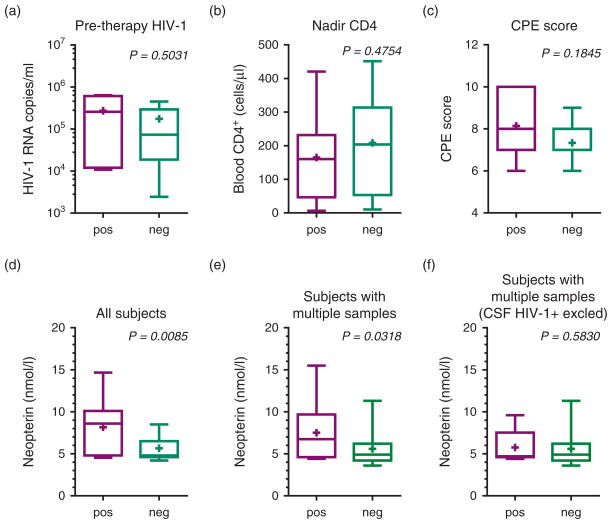
Of the 10 patients with detectable HIV-1 RNA in the CSF from at least one time point during effective therapy, we had data on pretherapy HIV-1 RNA concentration in plasma from 7. They had a mean pretherapy plasma HIV-1 RNA concentration of 4.8 (SD 0.8) log10 copies/ml compared to the 34 patients with no detectable HIV-1 RNA in CSF who had a mean concentration of plasma of 5.0 (SD, 0.7) log10 copies/ml (P = 0.5031) (Fig. 3a). Likewise, there was no significant difference in the mean nadir blood CD4+ T-cell counts in those where HIV-1 RNA could be detected at one time point or more to those where it never could be detected (165 vs. 208 cells/ μl; P =0.4754) (Fig. 3b). The median CPE of the treatment regimen was eight in the patients in whom CSF HIV-1 RNA could be detected and seven in those in whom it could not (P =0.1845) (Fig. 3c).
Fig. 3. Comparison of patients or samples with detectable and undetectable cerebrospinal fluid HIV-1 RNA by single-copy assay.
Graphs in top row compare patients with CSF HIV-1 detected at one or more time points (pos) to those without detected CSF HIV-1 RNA (neg) with respect to: (a) pretherapy plasma HIV-1 RNA concentrations; (b) blood nadir CD4+ cell count; and (c) CPE scores. Graphs in the bottom row compare the CSF neopterin concentrations in different patient and sample groupings: (d) includes all time points from all patients, comparing samples with detectable CSF HIV-1 RNA (pos) to those in which it was undetectable (neg); (e) includes only samples from patients with multiple samples and compares all time points from patients where HIV-1 RNA could be detected in at least one CSF sample (pos) to all time points from patients in which HIV-1 RNA could not be detected in CSF at any time point (neg); and (f) includes the same groups as in graph (e), but the measurements from time points where CSF HIV-1 RNA could be detected have been removed in order to compare these patients at intervals where CSF HIV-1 RNA was below the SCA detection limit. Box-plots include 10–90 percentile whiskers and mean values (‘+’). CPE, central nervous system penetration-efficacy; CSF, cerebrospinal fluid; SCA, single-copy assay.
Detectable HIV-1 RNA in relation to neopterin
We compared the levels of CSF neopterin in all samples with detectable and undetectable CSF HIV-1 RNA and found higher levels in the former (mean 8.2 compared to 5.7 nmol/l; P =0.0085) (Fig. 3d). In contrast, higher CSF neopterin was not found in patients with detectable compared to undetectable plasma HIV-1 RNA (5.9 vs. 6.2 nmol/l; P =0.1323) (not shown). For the patients with multiple samples, the mean CSF neopterin level was 7.5 nmol/l for those in whom CSF HIV-1 RNA could be detected at one time point or more compared to 5.6 nmol/l where CSF HIV-1 RNA could not be detected at any time point (P =0.0318) (Fig. 3e). When excluding the time points with detectable HIV-1 RNA in the CSF, there was, however, no significant difference in the mean values between these groups (mean 5.8 vs. 5.6 nmol/l; P =0.5830) (Fig. 3f), suggesting that the patients in whom HIV-1 RNA was detected CSF at one or more time points did not have chronically elevated levels of CSF neopterin compared to those without detectable CSF HIV RNA at any time point.
Cerebrospinal fluid neopterin in relation to blood–brain barrier integrity
We examined if CSF neopterin was related to the blood–brain barrier integrity by comparing the albumin ratio to CSF neopterin levels, but found no significant correlation (r =−0.21, P =0.0878) (not shown).
Discussion
These results show that low-level HIV-1 RNA could be measured by SCA in CSF in a substantial proportion of treated individuals with ‘undetectable’ viral loads in both CSF and plasma by conventional clinical assays. Additionally, detection of CSF HIV-1 RNA was associated with higher CSF neopterin concentrations, suggesting that the presence of CSF virus may either be a cause or a response to local immune activation.
This is the first study using SCA to measure the low HIV-1 RNA concentrations in CSF in a substantial number of patients and samples. We found that HIV-1 RNA could be detected in 17% (12 of 70 CSF samples) during suppressive therapy. This was significantly less than the 57% (39/68) plasma samples that were positive. Both the proportion of positive samples and the measured concentrations were consistent with our previous study using the same method for a smaller group of participants enrolled in a treatment intensification study [15]. The lower concentration of HIV-1 RNA in CSF than in plasma is consistent with the relationship observed in untreated HIV-1 infection where the levels of HIV-1 RNA in CSF are usually 10-fold lower than in plasma albeit with considerable variation [31]. We found CSF HIV-1 RNA after up to 10 years of suppressive therapy.
This is in agreement with smaller previous intensification studies where we have found CSF HIV-1 RNA after years of therapy using SCA and other methods [15,32]. By comparison, 57% of the plasma samples were positive with a median of 0.3 copies of HIV-1 RNA copies/ml and plasma HIV-1 RNA could be detected after up to 11 years of therapy which is comparable to what has previously been described [16].
We examined several factors that might have influenced CNS infection, but none appeared to favor the capacity for detecting CSF HIV-1 RNA in this group. CSF HIV-1 RNA did not correlate with that of plasma. This could suggest two separate reservoirs, one in the CNS and one in the periphery, which release HIV-1 virus particles independently. In favor of this explanation is that HIV-1 variants found in the CSF and the plasma during suppressive therapy can be genetically different variants [33]. Less likely, HIV-1 RNA found in the CSF is actually ‘spill over’ from the periphery, but because of temporal dispersion of viral peaks and very low levels in CSF, the associations were not seen and it would require analysis of more samples from more patients to discern this association. Whereas concentrations of plasma HIV-1 RNA found during suppressive therapy have previously been linked to pretherapy plasma HIV-1 concentrations [34], we did not find any association between the occurrence of on-therapy CSF HIV-1 RNA and pretherapy plasma HIV-1 concentrations. Likewise, because nadir CD4+ T-cell counts have been linked to neurocognitive impairment in patients on cART [35], we examined the relationship of cell count nadir to detection of CSF HIV-1 RNA, but found none. Finally, since reduced drug exposure might also predispose a person to CNS infection, we examined whether there was an association with aggregate drug penetration estimates using the CPE score, but again we found no association. Hence, we were unable to identify patient background features that predisposed to appearance of detectable CSF HIV-1 RNA in these patients with plasma and CSF HIV-1 concentrations below the conventional laboratory limits.
In the light of the lack of these associations, it is of interest that detection of CSF HIV-1 RNA was associated with CSF neopterin levels. CSF neopterin has proved to be a useful biomarker of local, intrathecal immune activation [28]. It is produced predominantly by cells of the monocyte–macrophage lineage and likely also by astrocytes in response to interferon gamma and other signals [36]. CSF neopterin is elevated in untreated viremic patients with particularly high levels in HAD patients. Likewise, suppressive cART reduces these levels rapidly, though not fully to normal, and a previous study showed that immune activation in the CNS as measured by CSF neopterin continued even after years of suppressive therapy [37]. It has also been demonstrated that asymptomatic CSF ‘virological escape’ (measurable HIV-1 RNA in CSF by standard assays during cART) was associated with elevated levels of CSF neopterin above that of patients without escape [38]. Additionally, another study using a different assay for HIV-1 RNA quantification showed that patients with CSF HIV-1 RNA concentrations above 2 copies/ml had higher levels of CSF neopterin than those with undetectable CSF HIV-1 RNA by this assay [39]. We also used CSF neopterin as our outcome biomarker for CSF immune activation. As in the previously mentioned studies, we found that the presence of HIV-1 RNA at very low levels was linked to higher levels of CSF neopterin, indicating that measuring these low levels of CSF virus is associated with an independent biological measurement. We also found that the patients who had detectable CSF HIV-1 RNA at one time point did not have elevated levels of CSF neopterin at other time points. This suggests that these patients may not have had ‘chronic’ immune activation in the CSF, but rather that release of these very low levels of HIV-1 in the CNS may lead to macrophage activation and neopterin production even during suppressive therapy. Alternatively, the sequence of events may be reversed macrophage activation in the CNS, provoked by some other factor, which could lead to the production of virions. This additionally raises the question of whether these ‘blips’ of detectable CSF HIV-1 RNA, either by standard assays or by SCA, might also have neuropathological consequences through this increase in the local immune activation. The association between elevated levels of CSF neopterin and detectable CSF HIV-1 RNA also suggests that CSF neopterin might be used as a surrogate marker for low CNS-grade infection in evaluating treatment regimens and various strategies for CNS viral eradication. Whether the low, but clearly abnormal, levels of neopterin detected in these patients indeed reflect a level of local immune activation sufficient to chronically impair neurological function and damage CNS integrity is uncertain. Addressing this issue will require longitudinal study of the predictive value of low levels of CSF neopterin on neurological function.
The study highlights the CNS as a potential reservoir for HIV-1 during suppressive therapy. It has been suggested that the CNS is a ‘sanctuary site’ where HIV-1 replication can persist during suppressive therapy by replicating at a low level since some drugs penetrate less well across the blood–brain barrier. In this study, we found that there was no association between the estimated CPE and the occurrence of CSF HIV-1 RNA. We have previously examined this question and found that treatment intensification did not affect CSF HIV-1 RNA or CSF immune activation, suggesting that there is no, or very little, ongoing replication in the CNS during suppressive therapy with undetectable HIV-1 RNA concentrations in both CSF and plasma by standard assays [15,32]. In addition, when we sequenced CSF HIV-1 RNA, we found little evidence of viral evolution in the CSF during therapy [33]. These findings taken together do not entirely rule out ongoing replication in the CSF during suppressive therapy. Perhaps, additional drugs are not enough to inhibit the ongoing replication in the CNS or the methods used were not sensitive or precise enough to measure these small changes. The range of CPE values in our study was narrow, and the patients were selected on the basis of both plasma and CSF viral suppression below 40 copies/ml. In this setting, the CPE score may not provide a sufficiently precise measure of CNS drug effectiveness. Nevertheless, there is no evidence in support of ongoing replication in the CNS during suppressive therapy. Hence, whether the presence of small amounts of HIV-1 RNA in the CSF during suppressive therapy is the result of production of HIV-1 virions within the CNS by cells that were infected before the initiation of therapy or relates to transport of virions across the blood–brain barrier or migration of infected cells into the CNS at the time of sampling needs to be further studied.
One limitation of our study was that the SCA only amplifies HIV-1 RNA from approximately 80% of HIV-1 B strains due to primer and probe mismatch [27]. We did not have access to pretherapy samples to test for primer and probe match. Our HIV-1 RNA concentration determinations are therefore likely to be an underestimate, though with a similar effect on CSF and plasma samples.
In conclusion, in this study, we show that HIV-1 RNA can be detected in 17% CSF samples during suppressive therapy, which is significantly less than the 57% plasma samples that were positive. The concentration of HIV-1 RNA in CSF was also lower than in plasma. We found that HIV-1 RNA could be detected in the CSF up to after 10 years of suppressive therapy and is associated with elevated neopterin levels. This suggests that HIV-1 virions continue to drive immune activation and possibly neurological injury during suppressive therapy. The findings of HIV-1 RNA in the CSF during suppressive therapy also indicate that the CNS could be a possible reservoir for HIV-1 during suppressive therapy.
Acknowledgments
Source of funding: This work was supported by: National Institutes of Health (NIH): NIMH R21MH096619, UCSF CTSI from NCATS UL1 TR000004, and Delaney AIDS Research Enterprise U19 AI096109; The Foundation for AIDS Research (amfAR) (108021–49-RFRL); Sahlgrenska Academy at the University of Gothenburg (ALFGBG-11067); and the Swedish Research Council (2007–7092).
Footnotes
R.W.P., M.G. and S.P. designed the study. R.W.P. and M.G. oversaw and characterized the study patient cohort. R.W.P., M.G. and J.P. collected samples and interviewed patients. V.D. and D.F. analyzed samples. V.D., R.W.P. and J.P. analyzed data. All authors participated in preparation of the manuscript.
Conflicts of interest
R.W.P. has been a consultant to Merck and Co and received honoraria and travel expenses from AbbVie for a meeting presentations.
The other authors have no conflict of interest to declare.
References
- 1.Gisslen M, Fuchs D, Svennerholm B, Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999;21:271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Spudich SS, Nilsson AC, Lollo ND, Liegler TJ, Petropoulos CJ, Deeks SG, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- 5.Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanstrom R, Coffin J. HIV-1 pathogenesis: the virus. Cold Spring Harb Perspect Med. 2012;2:a007443. doi: 10.1101/cshperspect.a007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinque P, Brew BJ, Gisslen M, Hagberg L, Price RW. Cerebrospinal fluid markers in central nervous system HIV infection and AIDS dementia complex. Handb Clin Neurol. 2007;85:261–300. doi: 10.1016/S0072-9752(07)85017-2. [DOI] [PubMed] [Google Scholar]
- 8.Hagberg L, Fuchs D, Rosengren L, Gisslen M. Intrathecal immune activation is associated with cerebrospinal fluid markers of neuronal destruction in AIDS patients. J Neuroimmunol. 2000;102:51–55. doi: 10.1016/s0165-5728(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 9.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 10.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 12.Wong MH, Robertson K, Nakasujja N, Skolasky R, Musisi S, Katabira E, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68:350–355. doi: 10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]
- 13.Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology. 2003;60:1388–1390. doi: 10.1212/01.wnl.0000058768.73358.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 15.Dahl V, Lee E, Peterson J, Spudich SS, Leppla I, Sinclair E, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204:1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz A, Stahle L, Hagberg L, Svennerholm B, Fuchs D, Gisslen M. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36:823–828. doi: 10.1080/00365540410025320. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair E, Ronquillo R, Lollo N, Deeks SG, Hunt P, Yiannoutsos CT, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69:1536–1541. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- 20.d’Arminio Monforte A, Cinque P, Mocroft A, Goebel FD, Antunes F, Katlama C, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 21.Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 22.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lescure FX, Omland LH, Engsig FN, Roed C, Gerstoft J, Pialoux G, et al. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: a Danish nationwide cohort study. Clin Infect Dis. 2011;52:235–243. doi: 10.1093/cid/ciq041. [DOI] [PubMed] [Google Scholar]
- 24.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 25.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18 (Suppl 1):S75–78. [PubMed] [Google Scholar]
- 26.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 27.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letendre S, FitzSimons C, Ellis R, Clifford D, Collier AC, Gelman BB, et al. Correlates of CSF viral loads in 1221 volunteers of the CHARTHER cohort. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- 31.Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197 (Suppl 3):S294–306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz A, Verhofstede C, D’Avolio A, Watson V, Hagberg L, Fuchs D, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–596. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 33.Dahl V, Gisslen M, Hagberg L, Peterson J, Shao W, Spudich S, et al. An example of genetically distinct HIV-1 variants in cerebrospinal fluid and plasma during suppressive therapy. J Infect Dis. 2013;209:1618–1622. doi: 10.1093/infdis/jit805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldarelli F, Palmer S, King MS, Wiegand A, Polis MA, Mican J, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cano OD, Neurauter G, Fuchs D, Shearer GM, Boasso A. Differential effect of type I and type II interferons on neopterin production and amino acid metabolism in human astrocyte-derived cells. Neurosci Lett. 2008;438:22–25. doi: 10.1016/j.neulet.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 38.Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]