Abstract
Objective
To use a large-scale multi-institutional dataset to quantify the prevalence of packed red blood cell transfusions and examine the associations between transfusion and perioperative outcomes in gynecologic cancer surgery.
Methods
The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) participant use file was queried for all gynecologic cancer cases between 2010 and 2012. Demographic, preoperative and intraoperative variables were compared between transfusion and non-transfusion groups using chi-squared, Fisher’s exact and Wilcoxon rank-sum test. The primary endpoint was 30-day composite morbidity. Secondary endpoints included composite surgical site infections, mortality and length of stay.
Results
A total of 8,519 patients were analyzed, and 13.8% received a packed red blood cell transfusion. In the multivariate analysis, after adjusting for key clinical and perioperative factors, including preoperative anemia and case magnitude, transfusion was associated with higher composite morbidity (OR = 1.85, 95% CI 1.5 – 2.24), surgical site infections (OR 1.80, 95% CI 1.39 – 2.35), mortality (OR 3.38, 95% CI 1.80 – 6.36) and length of hospital stay (3.02 days v. 7.17 days, p <0.001).
Conclusions
Blood transfusions are associated with increased surgical wound infections, composite morbidity and mortality. Based on our analysis of the NSQIP database, transfusion practices in gynecologic cancer should be scrutinized. Examination of institutional practices and creation of transfusion guidelines for gynecologic malignancies could potentially result in better utilization of blood bank resources and clinical outcomes among patients.
INTRODUCTION
Blood is a precious, costly resource that is often over utilized and transfused with great variation in clinical practice. According to the US Department of Health and Human Services there were more than 13.5 million units of blood transfused in 2011 at an average cost of $225.42/unit [1]. Perioperative surgical transfusion rates in gynecologic oncology patients fluctuate greatly with some studies reporting rates as low as 3% [2] and others as high as 77% [3–5]. This wide variation in practice patterns may be attributed to vague clinical practice guidelines combined with conflicting data in cancer patients.
Several large randomized controlled trials have suggested that a more restrictive transfusion protocol in surgical and critically ill patients is associated with improved clinical outcomes [6–10]. Although there have been no randomized controlled trials in oncology patients, there is ample evidence in the colorectal cancer surgery literature to suggest that blood transfusions themselves are immunosuppressive and associated with increased rates of infection, perioperative morbidity, disease progression and mortality [11, 12].
There is compelling evidence that questions the liberal use of blood transfusion in colorectal surgery and critically ill patients; however, uncertainties remain about the application of these data to gynecologic cancer patients. There are limited data examining the effects of blood transfusions on perioperative outcomes after gynecologic cancer surgery. Furthermore, to date, most of the studies in gynecologic cancer have been single-institution studies evaluating outcomes in a single disease site such as cervix or ovary. Awareness of national blood transfusion practices in gynecologic oncology could potentially result in better utilization of blood bank resources and both short- and long-term clinical outcomes among patients. We hypothesized that blood transfusions are associated with increased morbidity in gynecologic oncology surgical patients. We used a large-scale multi-institutional dataset, the National Surgical Quality Improvement Program database, to quantify the prevalence of perioperative blood transfusion and examine the effect of transfusion on perioperative outcomes.
MATERIALS AND METHODS
The American College of Surgeons National Surgical Quality Improvement Program (ASC-NSQIP) is a multi-institutional comprehensive database containing perioperative information on surgical patients. Trained risk-assessment nurses in participating hospitals collect preoperative patient characteristics, intraoperative data and 30-day morbidity and mortality. The specific methodology has been reported previously [13]. De-identified patient information is available to all participating institutions through the ASC-NSQIP participant use file (PUF).
The ASC-NSQIP PUF was queried for all gynecologic cases between 2010 and 2012 and limited to cases with ICD-9 codes associated with malignant gynecologic neoplasms (vulva, vagina, cervix, uterus, and ovary). CPT codes for which the transfusion rate was zero were excluded based on the findings by Bernard et al.[14] Extreme outliers were excluded from the analysis which included patients with preoperative transfusion greater than 4 units, those undergoing emergency procedures, pelvic exenteration, or procedures with operative time less than 30 minutes.
A total of 8,519 cases were included for analysis. The demographic data assessed included: age, body mass index (BMI), ethnicity (Hispanic and non-Hispanic) and race (white, black, other). Medical comorbidities and risk factors analyzed included: American Society of Anesthesiologists (ASA) class, presence of disseminated cancer, presence of ascites, receipt of neoadjuvant chemotherapy within 30 days of surgery, smoking, steroid use, hypertension requiring medication management, dyspnea, COPD, disease site (uterus, ovary, vagina/vulva, or cervix), preoperative bleeding disorders and more than 10% body weight loss in last six months. Perioperative factors evaluated included: preoperative labs (including hematocrit, INR, platelets and albumin), operating time, anesthesia time, procedure complexity, wound classification and procedure type. Procedure complexity was assessed by using total work relative value scales (WRVU), which has been previously shown to be an appropriate surrogate marker for surgical complexity [13]. Procedure type was defined as minimally invasive surgery (MIS) or open. Perioperative variables with less than 1% incidence were excluded.
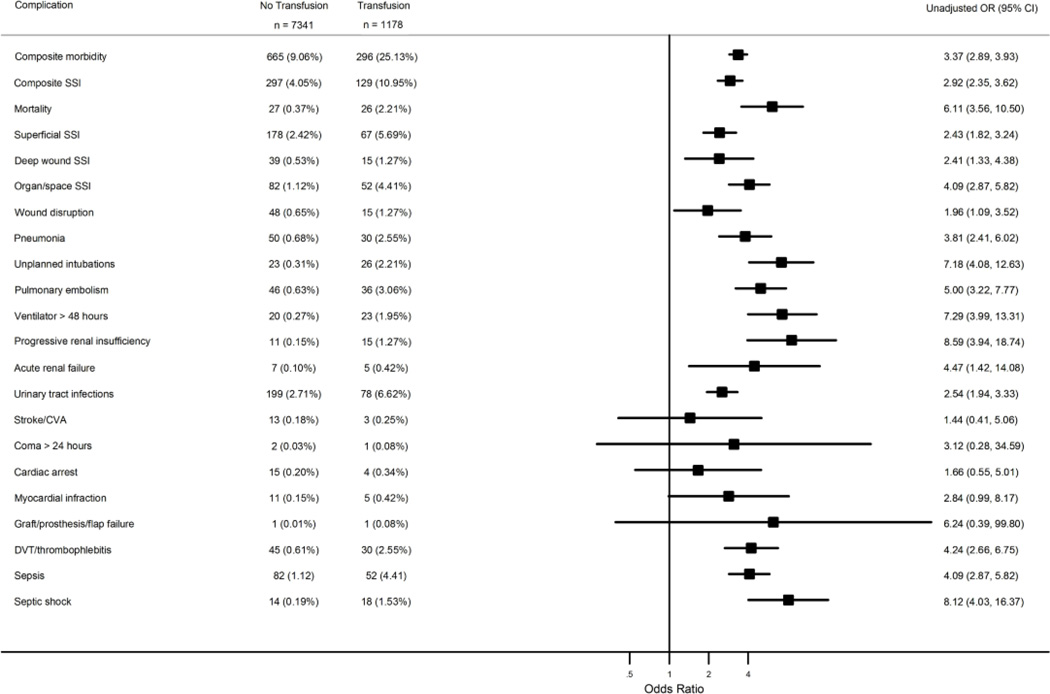
Patients were divided into two groups: those who received a blood transfusion and those who did not receive a blood transfusion. The variable for transfusion includes those patients who received a transfusion in the operating room until up to 72 hours post-operatively. The primary endpoint for the study was 30-day composite morbidity (based on the occurrence of 1 or more of the 20 adverse events defined by NSQIP, excluding transfusion, which are listed in Figure 1). Secondary endpoints examined were: 30-day composite infectious morbidity (superficial, deep or organ/space surgical site infections), the 20 adverse events defined by NSQIP, mortality, and length of stay.
Figure 1.
Forest plot of risk of 30-day postoperative outcomes by transfusion status. Odds ratios and 95% confidence intervals from univariate analysis of 30-day postoperative outcomes as a function of risk group (transfusion status), with number (%) of events by risk group
Summary statistics were used to describe demographic, preoperative and intraoperative variables. Chi-squared and Fisher’s exact tests were used to test for differences between those who received a blood transfusion and those who did not receive a blood transfusion for categorical variables, and the Wilcoxon rank-sum test was used to compare medians between groups for continuous variables.
Univariate logistic regression was used to model the logit of the probability of composite morbidity as a function of whether or not a patient received a transfusion and several other potential prognostic factors. A saturated model including all factors with a P < 0.20 was built and backward elimination was used in a multivariate analysis to construct a parsimonious model, removing factors one at a time until all remaining factors remained statistically significant. Preoperative hematocrit was retained as a continuous variable in all models. Adjusted odds ratios and corresponding 95% confidence intervals for each factor remaining in the model are reported. P < 0.05 was considered statistically significant. This modeling strategy was repeated for composite surgical site infections (SSI). However, since there were only 53 events for mortality, a forward selection strategy was used to build a multivariate model. The model began with transfusion (yes/no), and then the factor with the smallest P was added and the model was refit. All factors with P < 0.05 were retained and this process was repeated until no remaining factors could enter the model. This strategy avoided overfitting the model. All analyses were performed using STATA™ 13.0 for Macintosh (StatCorp LP, College Station, Texas). The study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board.
RESULTS
We identified 8,906 patients with the diagnosis of gynecologic malignancy in the NSQIP database. Three hundred eighty-seven patients were excluded for the following reasons: emergency case (n=61), exenterative procedure (n=88), preoperative transfusion more than 4 units (n=79), operative time less than 30 min (n=74) and CPT codes with transfusion rate of zero (n=85). Of the 8,519 patients who met our inclusion criteria, 1178 or 13.8% (95% CI 13.1% – 14.6%) received a blood transfusion within 72 hours of surgery.
Procedures were grouped according to primary CPT code and organ system (Table 1). Laparoscopy was the most common procedure performed (n=3,916), followed by open abdominal hysterectomy (n=2,483) then laparotomy for tumor reductive surgery (n=1,773). Laparotomy associated with a tumor reductive surgery had the highest propensity for blood transfusion with 35.08% of patients receiving at least one transfusion, followed by vaginectomy (23.53%) and laparotomy for adnexal surgery (18.18%). Laparoscopy was associated with the lowest likelihood of having a transfusion (2.32%).
Table 1.
Procedure groups included in analysis by CPT code
| Organ/system | CPT | N | Patients Transfused, N (%) |
|---|---|---|---|
| Vulvectomy | 56630 – 56637 | 100 | 8 (8.00%) |
| Vaginectomy | 57106 – 57111 | 17 | 4 (23.53%) |
| Trachelectomy | 57530 – 57531 | 15 | 2 (13.33%) |
| Laparotomy, Hysterectomy | 58150 – 58210 | 2483 | 426 (17.16%) |
| Vaginal Surgery, Hysterectomy | 58260 – 58285 | 131 | 5 (3.82%) |
| Laparoscopy | 58542 – 58573 | 3916 | 91 (2.32%) |
| Laparotomy, adnexal surgery | 58720 | 33 | 6 (18.18%) |
| Laparotomy, Tumor Reductive Surgery | 58950 – 58960 | 1773 | 622 (35.08%) |
Comparison of the demographics and preoperative characteristics of patients who received a blood transfusion and those who did not are displayed in Table 2. Compared to those patients who did not receive a transfusion, patients who were transfused were more likely to be older, thinner, non-white, have a higher ASA class, have disseminated cancer, dyspnea, ovarian cancer, a bleeding disorder. Comparison of preoperative laboratory variables between patients who received a blood transfusion and those who did not are displayed in Table 3. Compared to those patients who did not receive a transfusion, patients who were transfused were more likely to have a lower preoperative hematocrit, and a preoperative albumin less than 3 (P < 0.001 for all). While our primary interest was to evaluate the association of morbidity with transfusion use, we considered the above factors in our multivariate analysis of morbidity in an effort to account for potential bias in differences between those patients who did and did not receive transfusions. Importantly, we accounted for presence of disseminated cancer and preoperative anemia in our multivariate analysis. Table 4 displays the comparison of intraoperative variables in patients who were transfused and those who were not. Compared to those patients who did not receive a transfusion, patients who were transfused were more likely to have longer operating time, longer anesthesia time, increased surgical complexity, contaminated or dirty wounds and undergone laparotomy (P < 0.001 for all).
Table 2.
Demographic and preoperative characteristics in patients who were transfused and those who were not transfused
| No Transfusion group (n = 7341) |
Transfusion group (n = 1178) |
p-value* | |
|---|---|---|---|
| Age, years | <0.001 | ||
| Mean ± sd | 60.77 ± 12.24 | 62.50 ±12.64 | |
| Median (range) | 61 (18 – 89) | 63 (19 – 89) | |
| Missing | 43 | 8 | |
| BMI, kg/m2 | <0.001 | ||
| Mean ± sd | 32.80 ± 9.53 | 29.72 ± 8.56 | |
| Median (range) | 31 (10.97–102.50) | 27.63 (15.79–76.89) | |
| Missing | 52 | 11 | |
| Race | |||
| White | 5,749 (88.38%) | 845 (82.36%) | <0.001 |
| Black | 510 (7.84%) | 115 (11.21%) | <0.001 |
| Other | 246 (3.78%) | 66 (6.43%) | <0.001 |
| Missing | 836 | 333 | |
| Ethnicity | |||
| Hispanic | 490 (6.67%) | 72 (6.11%) | 0.470 |
| ASA class | <0.001 | ||
| I | 261 (3.56%) | 25 (2.12%) | |
| II | 3631 (49.46%) | 454 (38.54%) | |
| III | 3248 (44.24%) | 645 (54.75%) | |
| IV | 192 (2.62%) | 52 (4.41%) | |
| V | 1 (0.01%) | 1 (0.08%) | |
| None assigned | 8 (0.11%) | 1 (0.08%) | |
| Disseminated cancer | 894 (11.38%) | 284 (42.84%) | <0.001 |
| Presence of ascites | 179 (2.44) | 38 (3.23%) | 0.111 |
| Chemotherapy < 30 days | 69 (0.94%) | 60 (5.09%) | 0.421 |
| Tobacco Use | 902 (12.29) | 131 (11.12%) | 0.255 |
| Diabetes | 1201(16.37) | 207 (17.57%) | 0.304 |
| Steroid use | 124 (1.69) | 28 (2.38%) | 0.098 |
| Hypertension | 3662 (49.88%) | 568 (48.22%) | 0.288 |
| Dyspnea | 511 (6.96%) | 130 (11.04%) | <0.001 |
| COPD | 179 (2.44%) | 38 (3.23%) | 0.111 |
| Cancer | <0.001 | ||
| Uterus | 5239 (71.37%) | 431 (36.59%) | |
| Ovary | 1369 (18.65%) | 658 (55.86%) | |
| Vagina/Vulva | 118 (1.61%) | 14 (1.19%) | |
| Cervix | 615 (8.38%) | 75 (6.37%) | |
| Bleeding disorder | 140 (1.91%) | 52 (4.41%) | <0.001 |
| >10% weight loss in last 6 months | 87 (1.19%) | 64 (5.43%) | <0.001 |
BMI = body mass index, ASA = American Society of Anesthesiologists class; COPD = chronic obstructive pulmonary disease
Chi-squared and Fisher’s-exact test for categorical variables and the Wilcoxon rank-sum test to compare medians between groups for continuous variables
Table 3.
Comparison of preoperative laboratory variables in patients who were transfused and those who were not transfused
| No Transfusion group (n = 7341) |
Transfusion group (n = 1178) |
p-value* | |
|---|---|---|---|
| Hematocrit, g/dL | <0.001 | ||
| ≥ 36 | 5698 (80.87%) | 484 (42.53%) | |
| ≥ 30 < 36 | 1179 (16.73%) | 463 (40.69%) | |
| > 21 – < 30 | 157 (2.23%) | 186 (16.34%) | |
| ≤ 21 | 12 (0.17%) | 5 (0.44%) | |
| Missing | 295 | 40 | |
| INR | 0.040 | ||
| ≤ 1.5 | 3277 (98.70%) | 669 (97.66%) | |
| >1.5 | 43 (1.30%) | 16 (2.34%) | |
| Missing | 4021 | 493 | |
| Albumin | <0.001 | ||
| ≤ 3 | 129 (3.01%) | 118 (14.55%) | |
| > 3 | 4160 (96.99%) | 693 (85.45%) | |
| Missing | 3052 | 367 |
BMI = body mass index, ASA = American Society of Anesthesiologists class; COPD = chronic obstructive pulmonary disease
Chi-squared and Fisher’s-exact test for categorical variables and the Wilcoxon rank-sum test to compare medians between groups for continuous variables
Table 4.
Intraoperative variables in patients who were transfused and those who were not transfused
| No Transfusion group (n = 7341) |
Transfusion group (n = 1178) |
p-value* | |
|---|---|---|---|
| Operation time, minutes | <0.001 | ||
| Mean ± sd | 172.94 ± 78.81 | 220.04 ± 102.96 | |
| Median (range) | 160 (30 – 1028) | 203 (31 – 919) | |
| Missing | 4 | 0 | |
| Anesthesia Time, minutes | <0.001 | ||
| Mean ± sd | 228.76 ± 93.72 | 286.99 ± 111.34 | |
| Median (range) | 212 (52 – 858) | 273.50 (71 – 910) | |
| Missing | 4426 | 686 | |
| Procedure complexity | <0.001 | ||
| Mean ± sd | 32.31 ± 14.14 | 47.97 ± 25.28 | |
| Median (range) | 31.63 (5.27 – 199.55) | 40.05 (12.16 – 187.34) | |
| Wound Classification | |||
| Clean | 200 (2.72%) | 55 (4.67%) | 0.001 |
| Clean/Contaminated | 7010 (95.49%) | 1059 (89.90%) | <0.001 |
| Contaminated | 113 (1.54%) | 51 (4.33%) | <0.001 |
| Dirty/Infected | 18 (0.25%) | 13 (1.10%) | <0.001 |
| Procedure type | <0.001 | ||
| MIS | 3951 (53.82%) | 96 (8.15%) | |
| Open | 3390 (46.18%) | 1082 (91.85%) |
Chi-squared and Fisher’s-exact test for categorical variables and the Wilcoxon rank-sum test to compare medians between groups for continuous variables
In the univariate analysis, transfusion was associated with higher composite morbidity (9.06% v. 25.13%, OR 3.37; 95% CI 2.89 – 3.93), increased composite surgical site infections (4.05% v. 10.95%, OR 2.92; 95% CI 2.35 – 3.62), and increased mortality (0.37% v. 2.21%, OR 6.11; 95% CI 3.56 – 10.50). Transfusion was also associated with increased rates of wound disruption, pneumonia, unplanned intubations, pulmonary embolism, ventilator use for longer than 48 hours, progressive and acute renal failure, urinary tract infections, deep vein thrombosis or thrombophlebitis, sepsis and septic shock (P < 0.01 for all). A forest plot of risk of 30-day postoperative outcomes by transfusion status is presented in Figure 1. The ORs and 95% CIs for the NSQIP 30-day adverse events are displayed in this figure. Hospital length of stay was increased by 4.15 days for those receiving transfusions (3.02 days v. 7.17 days, P <0.001).
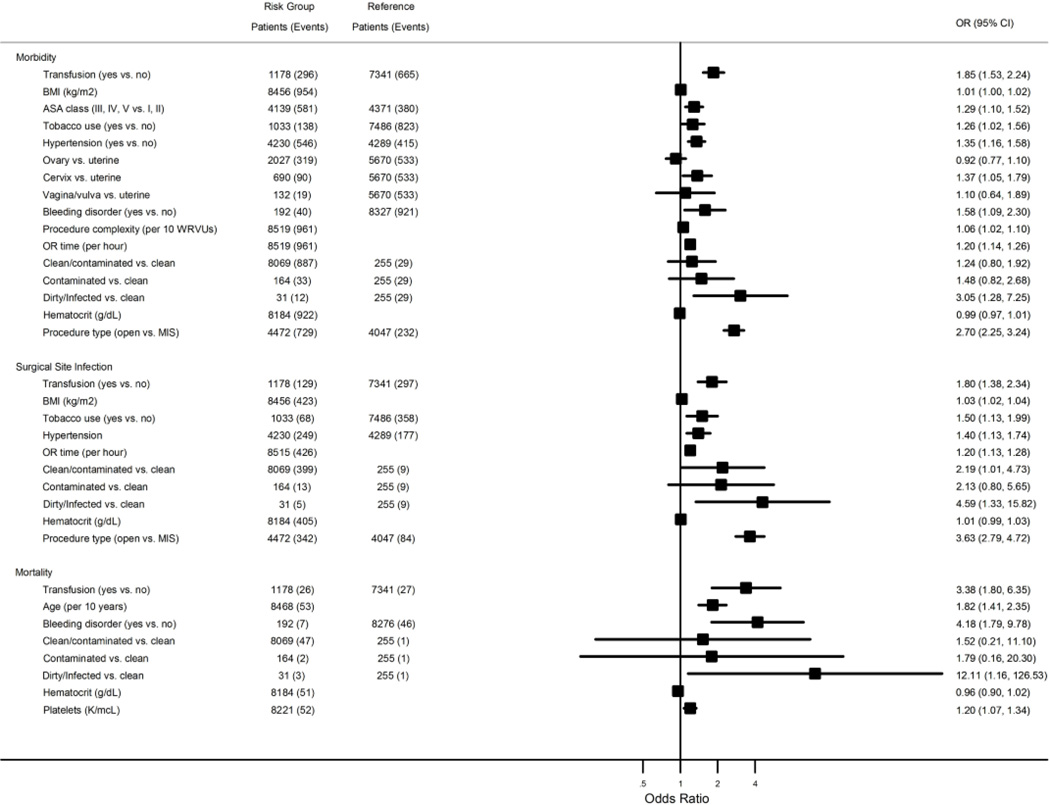
All perioperative characteristics with a P < 0.20 were taken into consideration in the multivariate analysis and included: BMI, race, ASA classification, presence of disseminated cancer, ascites, tobacco use, steroid use, hypertension, dyspnea, cancer type, bleeding disorder, weight loss, procedure complexity, wound class, preoperative hematocrit, preoperative platelet count and procedure type. Preoperative hematocrit was modeled as both a categorical and continuous variable in the multivariate analysis for morbidity and surgical site infection without a significant difference in magnitude of odds ratios; therefore, preoperative hematocrit was considered as a continuous variable in all models. Forest plots displaying multivariate logistic regression analysis for composite morbidity, composite surgical site infection and mortality are presented in Figure 2. After adjusting for the key clinical and perioperative factors which included: BMI, ASA class, tobacco use, hypertension, disease type, bleeding disorder, procedure complexity, OR time, wound classification, preoperative hematocrit and procedure type, as shown in a forest plot in Figure 2, transfusion was associated with 1.85 times increased odds of composite morbidity (OR 1.85; 95% CI 1.53 – 2.24). In the risk-adjusted analysis we also found that blood transfusions increased the odds of composite SSI (OR 1.80; 95% CI 1.38 – 2.34) and mortality (OR 3.38; 95% CI 1.80 – 6.35).
Figure 2.
Forest plots displaying multivariate logistic regression analysis for composite morbidity, composite surgical site infection and mortality. Adjusted odds ratios and 95% confidence intervals from multivariate analysis of morbidity, composite surgical site infection, and mortality as a function of various risks, with number of patients and events in each risk group
We performed several subgroup analyses to evaluate the effect of blood transfusion on different cancer types and procedure classifications. The directionality and statistical significance of the association of transfusion with increased morbidity were the same regardless of the disease type or procedure classification. In the subset of patients with ovarian cancer, transfusion was associated with a 2.68 times increased odds of composite morbidity (OR 2.68; 95% CI 2.10 – 3.42) after adjusting for BMI, presence of ascites, hypertension, bleeding disorder, procedure complexity, OR time, preoperative hematocrit and procedure type. In those patients who had undergone a tumor reductive surgery, transfusion was associated with a 1.77 times increased odds of composite morbidity (OR 1.77; 95% CI 1.33 – 2.35) after adjusting for the presence of ascites, hypertension, procedure complexity, operating time and preoperative hematocrit.
DISCUSSION
Our results suggest that blood transfusions are associated with increased morbidity, surgical site infections and mortality in gynecologic oncology patients. Perioperative blood transfusions occurred in 13.8% of patients in the NSQIP database. This rate is on the lower end of reported transfusion rates in the gynecologic oncology literature and is likely attributed to the high percentage of minimally invasive cases in this database. Minimally invasive cases accounted for 47.51% of cases in our database, but only 2.37% of transfusions. Given that postoperative transfusion is increasingly being used as a quality metric, it is critical that risk-adjusted models account for surgical approach and other important risk factors that we identified including disseminated cancer, preoperative anemia and disease site.
Anemia in gynecologic cancer patients is common and its etiology multifactorial. Animal and human studies have shown that anemia is an indicator of poor prognosis [15–18]. In 1965, Evans was one of the first researchers to show the association between anemia and decreased survival in cervix cancer [15]. Since then, there have been numerous experimental studies demonstrating worse oncologic outcomes in anemic patients [15, 19]. Knowing that anemic patients have worse outcomes, there has traditionally been a trend towards liberal use of blood products to correct anemia in gynecologic oncology patients. In two recent studies, more than half of ovarian cancer patients received at least one perioperative transfusion [3, 20]. However, there is a dearth of data in the gynecologic cancer population supporting the concept that preemptive or perioperative transfusion actually improves clinical outcomes.
Several large randomized controlled trials have suggested that a more restrictive transfusion protocol in surgical and critically ill patients is associated with improvement in short- and long-term clinical outcomes. The Transfusion Requirements in Critical Care (TRICC) [7] study showed a lower mortality rate in ICU patients who were randomized to the restrictive transfusion group (transfused for Hb < 7 g/dL) compared to patients in the liberal group (transfused for Hb < 10 g/dL). Similarly, the Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) [21] trial showed no difference in mortality in a cardiovascular high-risk cohort undergoing hip surgery randomized to restrictive (transfused for Hb < 8 g/dL) or liberal (transfused for Hb < 10 g/dL) approach. A third randomized controlled trial of patients with acute upper gastrointestinal bleeding revealed that a restrictive strategy (transfused for Hb <7 g/dL) was associated with decreased mortality and adverse events compared to rates in the liberal group (transfused for Hb < 9 g/dL) [22]. Expert opinion supports a restrictive transfusion protocol utilizing hemoglobin triggers of 7 – 8g/dL[23–25].
Our data are consistent with previous studies in colorectal surgery using the NSQIP database. Bernard and colleagues demonstrated that one unit of packed red blood cells significantly increased the risk of morbidity, mortality, pneumonia and sepsis [14]. Halabi et al. confirmed these findings in a more recent analysis and demonstrated that blood transfusions were independently associated with increased morbidity, mortality, length of stay and SSI [11].
Although the data in colorectal surgery are quite compelling, there is currently minimal evidence available to guide practitioners regarding the effect of transfusion on perioperative outcomes in gynecologic oncology. In 2005, Abu-Rustum and colleagues at Memorial Sloan-Kettering Cancer Center demonstrated that transfusion was associated with increased venous thromboembolism risk [26]. Boone et al. reported that, after the adoption of a restrictive transfusion protocol at the University of Alabama, there was no significant difference in postoperative infections, thrombotic events or mortality between those patients who received an appropriate transfusion and those who were inappropriately transfused [27]. Bakkum-Gamez et al. demonstrated that receipt of an intraoperative PRBT was associated with an increased rate of surgical site infections [28].
While the pathogenesis of increased morbidity with transfusion cannot be answered by our study, there have been several theories that have proposed physiologic mechanisms for this effect. One proposed mechanism relates to the immunomodulatory effects of blood transfusions. Alloantigens present on donor blood cells elicit cytokine mediators and cellular responses that influence the inflammatory response system [29]. Blumberg et al. demonstrated that transfused patients had significant changes in immunologic laboratory tests such as decreased IL-2 production, decreased CD4 and natural killer cells as well as increased macrophage prostaglandin E2 production [30, 31]. Thus, it has been suggested that this alteration in immune function may predispose individuals who receive perioperative blood transfusion to postoperative complications and disease progression. Furthermore, evidence from the surgical oncology transfusion-related immunomodulation literature suggests a dose-dependent increase in perioperative adverse outcomes. So although the adverse impact of transfusion occurs with even one unit, increasing units amplifies this effect [14].
Our study is limited by several factors. First, the study design is retrospective and perioperative differences exist between the two study groups. Second, we are limited to the data collected by NSQIP and therefore are inherently missing important clinical variables that have been shown to impact outcomes, such as time from diagnosis until surgery and cancer stage. While we do not have time from diagnosis until surgery, the presence of metastatic disease has been shown to as a surrogate for advanced cancer stage and more important for predicting 30-day morbidity and mortality in NSQIP studies [32, 33]. NSQIP also provides limited data on important clinical variables that may influence the receipt of blood transfusion such as estimated blood loss, hematocrit nadir and postoperative hematocrit. The database is incomplete as several fields are not mandatory; this results in a high percentage of missing values, especially for laboratory values. NSQIP has also changed its definitions for transfusion variables. Starting in 2010, NSQIP combined intraoperative and postoperative transfusion into one category and stopped collecting number of units transfused. Thus, we have only considered data from 2010–2012, but unfortunately have access to neither total units of blood transfused nor the breakdown of intraoperative and postoperative transfusion. Furthermore, NSQIP only collects data on blood transfusions up to 72 hours post-operatively; therefore, there may be individuals in the non-transfusion group who actually received a blood transfusion later in their hospital course.
To our knowledge, this study is the first evaluation of perioperative outcomes with respect to blood transfusions in gynecologic oncology patients using a national dataset. Our study found that, similar to the colorectal literature, transfusions are associated with increased surgical morbidity, infections and perioperative mortality in gynecologic cancer patients even after controlling for the presence of disseminated cancer and preoperative anemia. The American Medical Association’s Physician Consortium for Performance Improvement and the Joint Commission have both identified blood transfusions as one of the five treatments that are overused. Our study contributes to the compelling evidence from other surgical specialties that transfusions are associated with increased length of hospital stay and perioperative complications. Based on our analysis of the NSQIP database, transfusion practices in gynecologic cancer should be scrutinized. Our data suggest that individuals and institutions who have not previously modified their practice to a restrictive blood transfusion program should do so to minimize unnecessary transfusions. In addition, the creation and adoption of restrictive transfusion guidelines for gynecologic cancer surgery will likely yield better utilization of blood bank resources and improve clinical outcomes among patients.
Research Highlights.
13.8% of gynecologic surgical patients received a perioperative blood transfusion.
Transfusion is independently associated with increased perioperative morbidity.
Transfusion increases risk of perioperative mortality and surgical site infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
Disclaimer for Participant Use File Research: The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ASC NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
REFERENCES
- 1.Whitacker BI, Henry RA. The 2011 National Blood Collection and Utilization Survey. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. [Accessed 2014]. Report of the US Department of Health and Human Services; pp. 1–98. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. [Google Scholar]
- 2.Backes FJ, Brudie LA, Farrell MR, Ahmad S, Finkler NJ, Bigsby GE, et al. Short- and long-term morbidity and outcomes after robotic surgery for comprehensive endometrial cancer staging. Gynecol Oncol. 2012;125:546–551. doi: 10.1016/j.ygyno.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Warner LL, Dowdy SC, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. The impact of perioperative packed red blood cell transfusion on survival in epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2013;23:1612–1619. doi: 10.1097/01.IGC.0000436089.03581.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spirtos NM, Westby CM, Averette HE, Soper JT. Blood transfusion and the risk of recurrence in squamous cell carcinoma of the cervix: a gynecologic oncology group study. Am J Clin Oncol. 2002;25:398–403. doi: 10.1097/00000421-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Azuma C, Koyama M, Inagaki M, Ito S, Sawada M, Saji F, et al. The influence of peri-operative blood transfusion during radical hysterectomy on the prognosis of uterine cervical cancer. Transfus Sci. 1997;18:55–62. doi: 10.1016/s0955-3886(96)00077-x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study) Am J Cardiol. 2011;108:1108–1111. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishan A, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Crit Care Med. 2013;41:2354–2363. doi: 10.1097/CCM.0b013e318291cce4. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen K, Meyhoff CS, Johansson PI, Jorgensen LN, Rasmussen LS. Transfusion practice and complications after laparotomy--an observational analysis of a randomized clinical trial. Vox Sang. 2012;103:294–300. doi: 10.1111/j.1423-0410.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- 11.Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, et al. Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg. 2013;206:1024–1032. doi: 10.1016/j.amjsurg.2013.10.001. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 12.Acheson AG, Brookes MJ, Spahn DR. Effects of Allogeneic Red Blood Cell Transfusions on Clinical Outcomes in Patients Undergoing Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 13.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198:S19–S27. doi: 10.1016/j.amjsurg.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–937. 7 e1–7 e2. doi: 10.1016/j.jamcollsurg.2008.11.019. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 15.Evans JC, Bergsjo P. The influence of anemia on the results of radiotherapy in carcinoma of the cervix. Radiology. 1965;84:709–717. doi: 10.1148/84.4.709. [DOI] [PubMed] [Google Scholar]
- 16.Dunst J. Management of anemia in patients undergoing curative radiotherapy. Erythropoietin, transfusions, or better nothing? Strahlenther Onkol. 2004;180:671–681. doi: 10.1007/s00066-004-9191-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim BD, Ver Halen JP, Mlodinow AS, Kim JY. Intraoperative transfusion of packed red blood cells in microvascular free tissue transfer patients: Assessment of 30-day morbidity using the NSQIP dataset. J Reconstr Microsurg. 2013;30(2):103–114. doi: 10.1055/s-0033-1357275. [DOI] [PubMed] [Google Scholar]
- 18.Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000;57:13–19. doi: 10.1016/s0167-8140(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 19.Dunst J, Kuhnt T, Strauss HG, Krause U, Pelz T, Koelbl H, et al. Anemia in cervical cancers: impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys. 2003;56:778–787. doi: 10.1016/s0360-3016(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 20.De Oliveira GS, Jr, Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfus Med. 2012;22:97–103. doi: 10.1111/j.1365-3148.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- 21.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jairath V, Kahan BC, Gray A, Dore CJ, Mora A, Dyer C, et al. Restrictive vs liberal blood transfusion for acute upper gastrointestinal bleeding: Rationale and protocol for a cluster randomized feasibility trial. Transfus Med Rev. 2013;27(3):146–153. doi: 10.1016/j.tmrv.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers G, Gilreath J, Alwan L, Ali Bhat S, Blinder M, Cella D, et al. NCCN Guidelines Version 2.2015 Cancer- and Chemotherapy-Induced Anemia. 2015 [Google Scholar]
- 25.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 Update to The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Rustum NR, Richard S, Wilton A, Lev G, Sonoda Y, Hensley ML, et al. Transfusion utilization during adnexal or peritoneal cancer surgery: effects on symptomatic venous thromboembolism and survival. Gynecol Oncol. 2005;99:320–326. doi: 10.1016/j.ygyno.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Boone JD, Kim KH, Marques M, Straughn JM. Compliance rates and outcomes associated with a restrictive transfusion policy in gynecologic oncology patients. Gynecol Oncol. 2014;132:227–230. doi: 10.1016/j.ygyno.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Bakkum-Gamez JN, Dowdy SC, Borah BJ, Haas LR, Mariani A, Martin JR, et al. Predictors and costs of surgical site infections in patients with endometrial cancer. Gynecol Oncol. 2013;130:100–106. doi: 10.1016/j.ygyno.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao KJ. Mechanisms and new approaches for the allogeneic blood transfusion-induced immunomodulatory effects. Transfus Med Rev. 2000;14:12–22. doi: 10.1016/s0887-7963(00)80112-1. [DOI] [PubMed] [Google Scholar]
- 30.Porembka DT. Immunomodulatory effects of transfusion. Seminars in Anesthesia, Perioperative Medicine and Pain. 2001;20:36–43. [Google Scholar]
- 31.Blumberg N, Heal JM. Transfusion and recipient immune function. Arch Pathol Lab Med. 1989;113:246–253. [PubMed] [Google Scholar]
- 32.Merkow RP, Bentrem DJ, Cohen ME, Paruch JL, Weber SM, Ko CY, et al. Effect of cancer surgery complexity on short-term outcomes, risk predictions, and hospital comparisons. J Am Coll Surg. 2013;217:685–693. doi: 10.1016/j.jamcollsurg.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Merkow RP, Kmiecik TE, Bentrem DJ, Winchester DP, Stewart AK, Ko CY, et al. Effect of including cancer-specific variables on models examining short-term outcomes. Cancer. 2013;119:1412–1419. doi: 10.1002/cncr.27891. [DOI] [PubMed] [Google Scholar]