Abstract
Few studies have examined anxiety recurrence after symptom remission in the primary care setting. We examined anxiety recurrence in the Coordinated Anxiety Learning and Management (CALM) trial. From 2006-2009, CALM randomized adults with anxiety disorders (generalized anxiety disorder, panic disorder, social anxiety disorder, and post-traumatic stress disorder) in primary care clinics to usual care (UC) or a collaborative care (CC) intervention of pharmacotherapy and/or cognitive behavioral therapy. We examined 274 patients who met criteria for anxiety remission (Brief Symptom Inventory for anxiety and somatization (BSI-12) < 6) after 6 months of randomized treatment and completed a follow-up of 18 months. Logistic regression and receiver operating characteristics (ROC) were used to identify predictors of anxiety recurrence (BSI-12 ≥ 6 and 50% increase from 6-month ratings) during the year following remission. Recurrence was lower in CC (29%) compared to UC (41%) (p = 0.04). Patients with comorbid depression or lower self-perceived socioeconomic status particularly benefited (in terms of reduced recurrence) if assigned to CC instead of UC. In the multivariable logistic regression model, smoking, being single, Anxiety Sensitivity Index score, functional impairment at month 6 due to residual anxiety (measured with the Sheehan Disability Scale), and treatment with benzodiazepines were associated with subsequent anxiety recurrence. ROC identified prognostic subgroups based on the risk of recurrence. Our study was exploratory, and our findings require replication. Future studies should also examine the effectiveness of relapse prevention programs in patients at highest risk for recurrence.
Keywords: anxiety disorders, relapse, risk factors, primary care, collaborative care, integrated health care
Introduction
Although many trials have examined the efficacy of short-term treatments in anxiety disorders, few trials have examined how those treatment gains are maintained (Batelaan et al., 2010; Bruce et al., 2005; Calkins et al., 2009; Rodriguez et al., 2005; Scholten et al., 2013). Anxiety recurrence is the emergence of symptoms after remission has been achieved. A recent study found that 20% of patients with remitted anxiety will experience anxiety recurrence (Scholten et al., 2013). Identifying which patients have the highest risk for symptom recurrence is important so that relapse prevention resources can be directed to patients with the greatest need. Furthermore, identifying patient and treatment characteristics associated with increased recurrence risk may help in the development of new relapse prevention programs.
Previous research has found that baseline anxiety severity (Bruce et al., 2005; Scholten et al., 2013), anxiety sensitivity (fear that anxiety symptoms will be noticed by others or that the symptoms indicate serious illness) (Calkins et al., 2009; Mitchell et al., 2014; Scholten et al., 2013), and depression (Batelaan et al., 2010; Bruce et al., 2005; Rodriguez et al., 2005) are associated with anxiety recurrence. Higher rates of anxiety recurrence have been identified in patients with generalized anxiety disorder (GAD) and panic disorder (PD) compared to other anxiety disorders (Rodriguez et al., 2005). Still, little is known about predictors of anxiety recurrence in the primary care setting (Rodriguez et al., 2006), where most patients with anxiety receive treatment (Young et al., 2001). Moreover, prior studies examining risk factors for anxiety recurrence have 1) relied on lifetime anxiety diagnoses to retrospectively determine anxiety remission and recurrence making them vulnerable to recall bias (Batelaan et al., 2010; Calkins et al., 2009; Scholten et al., 2013) or 2) used observational designs instead of monitoring for recurrence after a course of treatment (Batelaan et al., 2010; Bruce et al., 2005; Calkins et al., 2009; Rodriguez et al., 2005; Scholten et al., 2013).
The Coordinated Anxiety Learning and Management (CALM) trial addressed the need for studies examining anxiety treatment in the primary care setting (Sullivan et al., 2007). In CALM, primary care patients with GAD, PD, generalized social anxiety disorder (SAD), and/or post-traumatic stress disorder (PTSD) were randomized to usual care (UC) or collaborative care (CC), which employed evidence-based pharmacotherapies and/or cognitive behavioral therapy (CBT) (Craske et al., 2011; Roy-Byrne et al., 2010). In contrast to prior anxiety recurrence studies, in CALM patients were assessed for symptom remission after a course of randomized treatment, and remitted patients were then prospectively monitored for recurrence.
We studied patients in the CALM trial whose anxiety and somatization symptoms had remitted after 6 months of randomized treatment, and we determined the recurrence rate of their symptoms over the subsequent year. We also examined several possible predictors of symptom recurrence. By identifying which patients are likely (and unlikely) to maintain treatment gains, this paper complements prior CALM data analyses which identified patient populations likely to improve with treatment (Kelly et al., 2014). To our knowledge, our study is the first to examine anxiety recurrence in the collaborative care model and the largest prospective study to examine recurrence in the primary care setting.
Methods
The CALM methodology and anxiety remission outcomes have been detailed previously (Roy-Byrne et al., 2010; Sullivan et al., 2007). Data analyses were done using the NIMH-supported CALM public access database, Version 1. CALM was conducted in primary care settings in four U.S. cities (Little Rock, Seattle, Los Angeles, and San Diego) from 2006-2009. The research was approved by Institutional Review Boards at each site and the RAND Survey Research Group (Santa Monica, California). Written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki.
Participants
Descriptions of CALM participants have been published previously (Roy-Byrne et al., 2010). All patients were fluent in English or Spanish, 18-75 years old, and met criteria for GAD, PD, SAD, or PTSD on the DSM-IV Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). CALM trial exclusion criteria included 1) treatment by a mental health professional at intake, 2) psychosis, 3) cognitive impairment, and 4) recent (prior three months) alcohol dependence or illicit drug use with the exception of marijuana.
CALM randomized 1,004 patients to CC (n = 503) or UC (n = 501), and 872 (87%) patients (CC n = 444, UC n = 428) completed a follow-up of 6 months. Of the 872 patients, 314 (36%) (CC n = 195; UC n = 119) met criteria for remission at month 6. We defined symptom remission as a 12-item Brief Symptom Inventory for anxiety and somatization (BSI-12) score < 6, consistent with prior studies (Roy-Byrne et al., 2010; Schat et al., 2013). The BSI-12 is a reliable and valid self-report measure of global anxiety and somatization symptoms in the past week (Derogatis and Melisaratos, 1983; Morlan and Tan, 1998) and has been used to measure anxiety in studies examining long-term outcomes (Andreescu et al., 2007; Lang et al., 2006; Schat et al., 2013). The BSI-12 sums the scores from 12 questions that are each scored 0-4 with higher scores indicating more severe symptoms.
Of the 314 patients with remitted symptoms at month 6, we examined the 274 (87%) (CC n = 171; UC n = 103) who completed a follow-up of 18 months. The 40 (13%) patients (CC n = 24; UC n = 16) with remission at month 6 but who were excluded due to incomplete follow-up were significantly younger (35 vs 45 years), more likely to feel they had less than enough money (45% vs 29%), more anxious at baseline (BSI-12 15 vs 12), and prescribed more psychotropics (2 vs 1) compared to the patients included in analyses. Table 1 describes characteristics of patients included in the study. Table 1 also identifies the characteristics that were more common in CC remitters compared to UC remitters (i.e. female sex, comorbid major depressive disorder (MDD), higher baseline depression and anxiety scores, treatment with CBT, and higher patient satisfaction). This is consistent with prior CALM analyses that found CC especially outperformed UC in achieving remission if patients were female, had comorbid MDD, or had higher baseline depression and anxiety scores (Kelly et al., 2014) and also consistent with prior CALM analyses that found higher patient satisfaction and more frequent CBT use in CC compared to UC (Roy-Byrne et al., 2010).
Table 1.
Characteristics of patients with anxiety remission at month 6.
| Overall | Collaborative care | Usual care | P value | |
|---|---|---|---|---|
| N=274 | N = 171 | N = 103 | ||
| Baseline (pretreatment) characteristics | ||||
| Age, mean (range)a | 45 | 45 (21-75) | 45 (18-74) | 0.94 |
| Female, n (%) | 187 (68) | 126 (74) | 61 (59) | 0.02 |
| Hispanic, n (%) | 45 (16) | 26 (15) | 19 (18) | 0.5 |
| White (non-Hispanic), n (%) | 163 (59) | 100 (58) | 63 (61) | 0.7 |
| Black (non-Hispanic), n (%) | 29 (11) | 19 (11) | 10 (10) | 0.84 |
| Multiple races (non-Hispanic), n (%) | 28 (10) | 19 (11) | 9 (9) | 0.68 |
| US born, n (%) | 231 (84) | 145 (85) | 86 (84) | 0.86 |
| Some post-high school education, n (%) | 216 (79) | 133 (78) | 83 (81) | 0.65 |
| Patient's income (in thousands), mean (range)a | 47 (0-400) | 46 (0-400) | 48 (0-260) | 0.23 |
| Number of dependent children, mean (range)a | 1 (0-5) | 1 (0-5) | 1 (0-4) | 0.47 |
| Baseline BSI-12 (0-48 scale), mean (range)a | 12 (0-44) | 13 (0-44) | 11 (1-36) | 0.03 |
| Baseline ASI (0-90 scale), mean (range)a | 41 (17-75) | 42 (17-75) | 39 (18-68) | 0.12 |
| Baseline SDS (0-30 scale), mean (range)a | 15 (0-30) | 15 (0-30) | 15 (0-30) | 0.95 |
| Baseline PHQ-8 (0-24 scale), mean (range)a | 10 (0-24) | 11 (0-24) | 9 (0-23) | 0.02 |
| GAD diagnosis, n (%) | 203 (74) | 128 (75) | 75 (73) | 0.78 |
| PD diagnosis, n (%) | 107 (39) | 69 (40) | 38 (37) | 0.61 |
| SAD diagnosis, n (%) | 97 (35) | 58 (34) | 39 (38) | 0.52 |
| PTSD diagnosis, n (%) | 28 (10) | 22 (13) | 6 (6) | 0.07 |
| Number of anxiety disorders, n (%) | ||||
| 1 | 139 (51) | 84 (49) | 55 (53) | 0.53 |
| 2 | 111 (41) | 70 (41) | 41 (40) | 0.9 |
| 3 or 4 | 24 (9) | 17 (10) | 7 (7) | 0.51 |
| OCD diagnosis, n (%) | 13 (5) | 10 (6) | 3 (3) | 0.38 |
| MDD diagnosis, n (%) | 141 (51) | 97 (57) | 44 (43) | 0.03 |
| Suicidal ideation in past month, n (%) | 24 (9) | 17 (10) | 7 (7) | 0.51 |
| Lifetime suicide attempt, n (%) | 36 (13) | 24 (14) | 12 (12) | 0.71 |
| Alcohol abuse (past 3 months), n (%) | 9 (3) | 5 (3) | 4 (4) | 0.73 |
| Alcohol abuse (past 12 months), n (%) | 12 (4) | 8 (5) | 4 (4) | 1 |
| Alcohol dependence (past 12 months), n (%) | 7 (3) | 4 (2) | 3 (3) | 1 |
| Number of chronic medical conditions, n (%) | ||||
| 0 | 80 (29) | 50 (29) | 30 (29) | 1 |
| 1 | 68 (25) | 38 (22) | 30 (29) | 0.25 |
| 2 or more | 126 (46) | 83 (49) | 43 (42) | 0.32 |
| Self-perceived financial resources, n (%) | ||||
| More than enough money | 42 (15) | 26 (15) | 16 (16) | 1 |
| Just enough | 153 (56) | 91 (53) | 62 (60) | 0.32 |
| Less than enough | 79 (29) | 54 (32) | 25 (24) | 0.22 |
| Self-perceived SES overall (1-10 scale), mean (range)a | 6 (1-10) | 6 (1-10) | 6 (3-9) | 0.85 |
| Self-perceived SES in community (1-10 scale), mean (range)a | 6 (1-10) | 6 (1-10) | 6 (1-9) | 0.64 |
| Self-efficacy expectancy (0-8 scale), mean (range)a | 7 (2-8) | 6 (2-8) | 7 (2-8) | 0.34 |
| Outcome expectancy (0-8 scale), mean (range)a | 6 (0-8) | 6 (0-8) | 6 (2-8) | 0.88 |
| Comfort with mental health help (3-12 scale), mean (range)a | 6 (3-10) | 6 (3-10) | 6 (3-10) | 0.54 |
| 6-month characteristics | ||||
| Married or cohabitating, n (%) | 158 (58) | 91 (53) | 67 (65) | 0.06 |
| Employed, n (%) | 221 (81) | 140 (82) | 81 (79) | 0.53 |
| Any health insurance, n (%) | 238 (87) | 148 (87) | 90 (87) | 1 |
| Medicaid, n (%) | 12 (4) | 6 (4) | 6 (6) | 0.38 |
| Medicare, n (%) | 28 (10) | 13 (8) | 15 (15) | 0.1 |
| Other government insurance, n (%) | 8 (3) | 5 (3) | 3 (3) | 1 |
| Private insurance, n (%) | 217 (79) | 136 (80) | 81 (79) | 0.88 |
| Social support (SSS, 1-5 scale)a, mean (range) | 4 (1-5) | 4 (1-5) | 4 (1-5) | 0.91 |
| 6-month BSI-12 (0-48 scale)a, mean (range) | 3 (0-5) | 3 (0-5) | 3 (0-5) | 0.09 |
| 6-month ASI (0-90 scale)a, mean (range) | 28 (16-63) | 27 (16-63) | 29 (16-59) | 0.09 |
| 6-month SDS (0-30 scale)a, mean (range) | 5 (0-28) | 4 (0-22) | 5 (0-28) | 0.16 |
| 6-month PHQ-8 (0-24 scale)a, mean (range) | 4 (0-19) | 4 (0-19) | 4 (0-15) | 0.85 |
| Number of psychotropics in past 6 mo.a, mean (range) | 1.1 (0-9) | 1 (0-6) | 1 (0-9) | 0.52 |
| >2 mo. of any anxiolytic in past 6 mo., n (%) | 166 (61) | 104 (61) | 62 (61) | 1 |
| >2 mo. of antidepressant in past 6 mo., n (%) | 147 (54) | 96 (56) | 51 (50) | 0.32 |
| >2 mo. of bz in past 6 mo., n (%) | 43 (16) | 27 (16) | 16 (16) | 1 |
| >2 mo. of other anxiolytic in past 6 mo., n (%) | 13 (5) | 7 (4) | 6 (6) | 0.56 |
| Any counseling with CBT elements, n (%) | 204 (74) | 155 (91) | 49 (48) | <0.0001 |
| Counseling with ≥ 3 CBT elements sometimes provided at office visits, n (%) | 175 (64) | 143 (84) | 32 (31) | <0.0001 |
| Counseling with ≥ 3 CBT elements usually provided at office visits, n (%) | 112 (41) | 103 (60) | 9 (9) | <0.0001 |
| Smoking (cigarettes/day)a, mean (range) | 1 (0-24) | 1 (0-24) | 1 (0-20) | 0.52 |
| Patient satisfaction (2-10 scale)a, mean (range) | 8 (4-10) | 9 (4-10) | 8 (4-10) | <0.0001 |
Variables are continuous (all variables without notation are categorical).
Note: Boldface type indicates significant (p < 0.05) differences between collaborative care (CC) and usual care (UC) patients in remission after 6 months of treatment. P values were determined using Fisher's exact test for categorical variables and the Wilcoxon Rank Sum test for continuous variables.
Abbreviations: BSI-12 = 12-item Brief Symptom Inventory for anxiety and somatization; PHQ-8 = 8-item Patient Health Questionnaire (depression scale); ASI = Anxiety Sensitivity Index; SDS = Sheehan Disability Scale (functional impairment due to anxiety); GAD = generalized anxiety disorder; PD = panic disorder; SAD = generalized social anxiety disorder; PTSD = post-traumatic stress disorder; OCD = obsessive compulsive disorder; MDD = major depressive disorder; SES = socioeconomic status (1-10 scale, 10 is highest); SSS = Social Support Survey from the Medical Outcomes Study; mo. = months; bz = benzodiazepine; CBT = cognitive behavioral therapy.
Intervention
Details of the treatment strategy have been described previously (Sullivan et al., 2007). Patients in the CC and UC groups received 12 months of randomized treatment. Both CC and UC patients received care and all prescriptions from their PCP. In UC, PCPs could refer patients to outside mental health services (referrals were not permitted in CC unless for substance abuse). In CC, the PCP received one year of support from a psychiatric team, i.e. the “collaborative care model.” In CC, patients chose computer-assisted CBT (generally six 45-minute sessions) and/or pharmacotherapy. Each CC patient had an “anxiety clinical specialist” (ACS), who was tasked with identifying community resources needed to overcome treatment barriers like transportation and child care. If CC patients chose CBT, it was done by the ACS. If CC patients chose pharmacotherapy, one or two trials of a selective serotonin reuptake inhibitor (SSRI) were first-line. In the case of SSRI failure, either CBT or second-line agents (e.g. serotonin-norepinephrine reuptake inhibitors (SNRIs), mirtazapine, and benzodiazepines) were trialed. In CC, if patients achieved remission after 6 months, the remaining 6 months of randomized treatment focused on relapse prevention. Relapse prevention included monthly phone calls from their ACS which emphasized healthy lifestyle choices, continued medication adherence for one year (if they were treated with medications), and continued monthly CBT telephone booster sessions (if patients had received CBT).
Assessments
The RAND Survey Research Group raters were blind to treatment assignment and performed assessments via telephone at baseline, 6 months, 12 months, and 18 months for all patients. The baseline assessments were approximately 60 minutes, and the follow-up assessments were approximately 45 minutes because many questions were only asked at baseline. We evaluated the random treatment group assignment (CC or UC) and 55 independent variables from the RAND assessments (of more than 600 candidate variables) as potential predictors of anxiety recurrence. Putative predictors (Batelaan et al., 2010; Bruce et al., 2005; Calkins et al., 2009; Rodriguez et al., 2005; Scholten et al., 2013) and variables with reasonable clinical relevance were included as independent variables with the aim of being as expansive as possible so that potentially novel predictors were not overlooked.
Independent variables
Random assignment to CC or UC treatment group.
- Baseline (i.e. pretreatment) patient characteristics:
-
○Demographic variables: age, gender, race, Hispanic ethnicity, born in the US (Pennell et al., 2004), education, patient's income, and number of dependent children (Schumm et al., 1986).
- ○ Mental and physical health variables: baseline BSI-12, baseline Anxiety Sensitivity Index (ASI) (Peterson and Heilbronner, 1987), baseline Sheehan Disability Scale (SDS, measures functional impairment due to a psychiatric condition - anxiety in CALM) (Sheehan et al., 1996), baseline 8-item Patient Health Questionnaire (PHQ-8, measures depression) (Kroenke et al., 2009), suicidal ideation (thoughts of being “better off dead” in the past month) (Kroenke et al., 2001), number of chronic medical conditions (based on the Partners in Care Checklist) (Wells, 1999), and baseline measures on the MINI including specific anxiety disorder diagnosis (GAD, PD, SAD, PTSD), number of anxiety disorders, obsessive compulsive disorder (OCD) diagnosis, major depressive disorder (MDD) diagnosis, lifetime suicide attempts, alcohol abuse (past 3 months and past 12 months), and alcohol dependence (past 12 months).
- ○ Patient perception and beliefs variables: self-perceived ability to meet family's financial needs (Pennell et al., 2004), self-perceived socioeconomic status (SES) overall and self-perceived SES relative to those in the patient's community (Pennell et al., 2004), self-efficacy expectancy (patient's belief in his or her ability to adhere to anxiety treatment plan) and outcome expectancy (patient's belief that anxiety can be treated successfully) (Sheehan et al., 1998), and comfort in seeking help from mental health professionals (Pennell et al., 2004).
-
○
- Patient characteristics after 6 months of treatment:
- ○ Demographic variables: married or co-habiting with partner, employment status, health insurance status and type (Medicaid, Medicare, other government insurance (e.g. Tricare), or private insurance), and Medical Outcomes Study social support survey (SSS; having someone to relax with, get help from when ill, get advice from, and having someone who makes the patient feel loved – each scored 1-5 and averaged) (Sherbourne and Stewart, 1991).
- ○ Mental and physical health variables: 6-month BSI-12 (i.e. post-treatment residual anxiety with the BSI-12 < 6), 6-month ASI, 6-month SDS, 6-month PHQ-8, number of psychotropic medications prescribed for any duration in the past 6 months (Wells, 1999), > 2-month trial of an antidepressant (e.g. SSRI, SNRI, tricyclics (TCAs), bupropion) in the past 6 months, > 2-month trial of a benzodiazepine in the past 6 months, > 2-month trial of any other anxiolytic (e.g. buspirone, hydroxyzine, excluding antipsychotics) in the past 6 months (Sullivan et al., 2007; Wells, 1999), any counseling with CBT elements in the past 6 months, counseling with ≥ 3 CBT elements sometimes provided at office visits in the past 6 months, counseling from any provider with ≥ 3 CBT elements usually provided at office visits in the past 6 months (Sullivan et al., 2007), and current smoking (cigarettes per day).
- ○ Patient perception and beliefs variables: patient satisfaction (Sturm et al., 1999).
Outcome variable
Prior studies defined symptom remission in CALM as a BSI-12 < 6 at month 6 (Roy-Byrne et al., 2010). We defined subsequent anxiety recurrence as having a 12-month or 18-month BSI-12 score that was both ≥ 6 and ≥ 50% greater than the 6-month BSI-12 score. We required a 50% increase on the BSI-12 for recurrence because prior studies examining CALM defined treatment response as a 50% decrease on the BSI-12 (Roy-Byrne et al., 2010).
Statistical analysis
We used SAS version 9.3 (SAS Institute Inc., Cary, North Carolina) for statistical analyses. All p values reported are two-tailed. We examined differences between patients included in our analysis and patients excluded from analysis due to incomplete follow-up using Fisher's exact test (p < 0.05) for categorical variables and the non-parametric Wilcoxon rank-sum (WRS) test with continuity correction (p < 0.05) for continuous variables. We also used Fisher's and WRS tests to examine differences at month 6 between the CC and UC participants included in analyses. The WRS test was used for continuous variables because the data were not normally distributed as demonstrated by the Shapiro-Wilk test (p < 0.05).
We calculated anxiety recurrence rates with 95% confidence intervals (CI) using the binomial distribution. CC and UC samples were analyzed separately to determine recurrence rates and predictors of recurrence in each model of care. We also examined how interactions (pinteraction < 0.05) between treatment group assignment and patient characteristics affected recurrence in logistic regression models. Simple logistic regression was used to assess the association of each independent variable with symptom recurrence, and odds ratios with 95% confidence intervals and p values were calculated using the Wald chi-square test. All independent variables significantly associated with recurrence (p's < 0.05) were included in the backward stepwise logistic regression model. Backward stepwise logistic regression methodically eliminated variables that did not improve the model's accuracy at the Wald chi-square p < 0.05 level. The resulting multivariable models had sufficiently low multicollinearity (maximum condition index of 13.3). Due to inadequate sample size, marijuana abuse was excluded from analysis.
Free signal detection software available online (http://www.stanford.edu/~yesavage/ROC.html) was used to conduct receiver operating characteristic (ROC) analyses identifying empirically-derived prognostic subgroups based on likelihood of symptom recurrence. ROC analyses are non-parametric. The signal detection software evaluated all variables at every value (“cut-off point”) and determined the variable's optimal cut-off point (maximal sensitivity and specificity) when discriminating between patients who did and did not experience symptom recurrence (Andreescu et al., 2008; Kiernan et al., 2001). Sensitivity and specificity were weighted as equally important in our analyses. If the variable's optimal cut-off point predicted recurrence at the chi-square p < 0.01 level, the software divided the patient group into two subgroups. The ROC subgrouping stopped if no cut-off point discriminated between recurrence outcomes at the p < 0.01 level, if the resulting subgroup had less than ten patients, or after the third-order interaction level was reached. The ROC stopping criteria were determined a priori (Andreescu et al., 2008; Jakubovski and Bloch, 2014; Kiernan et al., 2001). The ROC hierarchical decision trees with the recurrence rates of prognostic subgroups are presented.
Results
Anxiety recurrence rates
In the year following 6 months of randomized CC or UC treatment, recurrence was lower in CC (29%) compared to UC (41%) (number needed to treat = 9, p = 0.04). Recurrence rates were similar across anxiety disorders (Table 2). PTSD had the highest recurrence rate (39%, 95% CI 22%-59%), while GAD had the lowest recurrence rate (33%, 26%-39%).
Table 2.
Anxiety recurrence rates in collaborative care and usual care.
| Overall | Collaborative care | Usual care | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Recurrence | Total | Recurrence | Total | Recurrence | ||||
| n | n | % (95% CI) | n | n | % (95% CI) | n | n | % (95% CI) | |
| GAD | 203 | 66 | 33 (26-39) | 128 | 37 | 29 (21-38) | 75 | 29 | 39 (28-51) |
| PD | 107 | 39 | 36 (27-46) | 69 | 22 | 32 (21-44) | 38 | 17 | 45 (29-62) |
| SAD | 97 | 34 | 35 (25-45) | 58 | 16 | 28 (17-41) | 39 | 18 | 46 (30-63) |
| PTSD | 28 | 11 | 39 (22-59) | 22 | 8 | 36 (17-59) | 6 | 3 | 50 (12-88) |
| Any disordera | 274 | 91 | 33 (28-39) | 171 | 49 | 29 (22-36) | 103 | 42 | 41 (31-51) |
Any disorder n is less than the sum of each anxiety disorder n due to comorbidity.
Abbreviations: GAD = generalized anxiety disorder; PD = panic disorder; SAD = generalized social anxiety disorder; PTSD = post-traumatic stress disorder.
Interactions between patient characteristics and treatment group
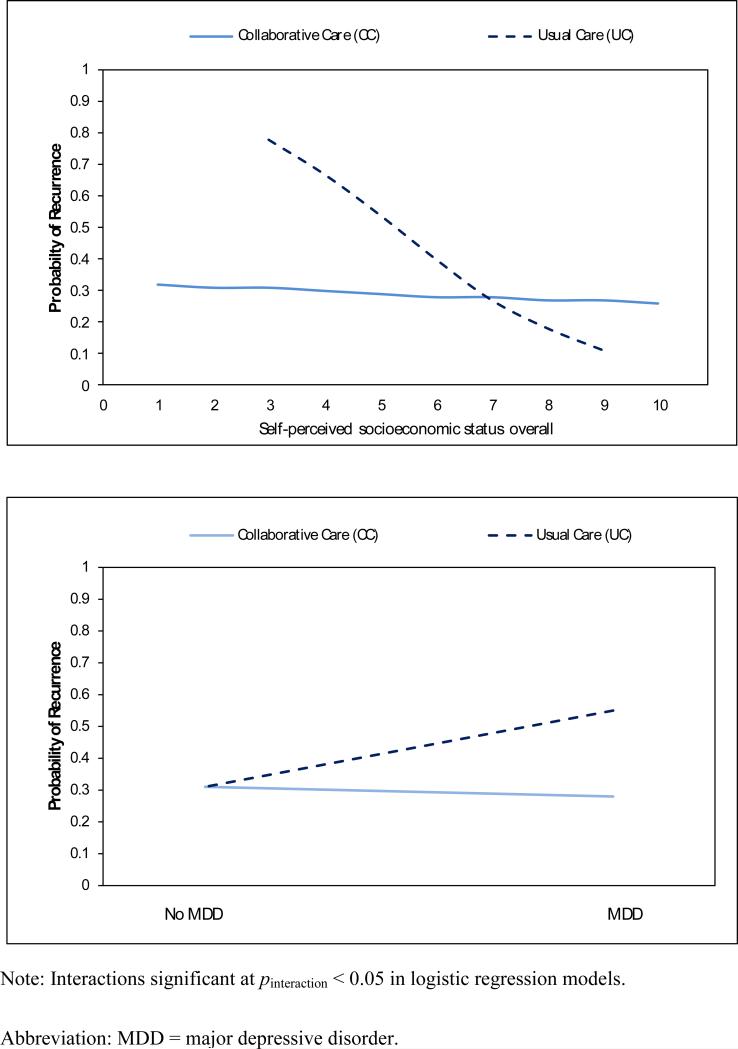
Fig. 1 displays the significant interactions between patient characteristics and treatment modality in logistic regression models estimating recurrence. Examination of significant interactions identified characteristics of patients particularly likely to benefit (in terms of lower recurrence) if assigned to CC instead of UC. Patients with low self-perceived SES had lower recurrence if they received CC (compared to UC), while patients with high self-perceived SES had lower recurrence if they received UC (compared to CC) (pinteraction = 0.01). Patients with comorbid MDD had lower recurrence if they received CC (compared to UC), while patients without MDD had the same recurrence rate whether they received CC or UC (pinteraction = 0.04).
Fig. 1. Effects of interactions between patient characteristics and random treatment assignment to collaborative care (N = 171) or usual care (N = 103) on anxiety recurrence over 1 year.
Predictors of recurrence in logistic regression models
In simple logistic regression models, 17 variables predicted symptom recurrence for the overall sample (combined CC and UC samples) (Table 3a). After backward stepwise logistic regression, smoking, being single, treatment with benzodiazepines, anxiety sensitivity (6-month ASI), and functional impairment due to anxiety at month 6 (i.e. impairment due to post-treatment residual anxiety symptoms that are below the threshold for symptom remission) (6-month SDS) remained strong predictors of recurrence in the multivariable logistic regression model (Table 3b). When CC and UC samples were analyzed separately, predictors unique to each treatment modality were identified (Table 3a and Table 3b).
Table 3.
Effects of patient and treatment characteristics on anxiety recurrence in simple (a) and multivariable (b) logistic regression models.
| a. Simple logistic regression models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment assignment |
|||||||||
| Overall | Collaborative care | Usual care | |||||||
| Independent variables | N = 274 | N = 171 | N = 103 | ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Random Treatment | |||||||||
| Collaborative Care | 0.58 | 0.35–0.98 | 0.04 | - | - | - | - | - | - |
| Baseline (pretreatment) characteristics | |||||||||
| Agea | 0.99 | 0.97-1.01 | 0.28 | 1.00 | 0.97–1.02 | 0.51 | 0.99 | 0.96–1.02 | 0.36 |
| Female | 0.86 | 0.5–1.48 | 0.58 | 0.85 | 0.41–1.79 | 0.67 | 0.87 | 0.39–1.92 | 0.72 |
| Hispanic | 1.78 | 0.93-3.42 | 0.08 | 1.70 | 0.71–4.06 | 0.23 | 1.81 | 0.66–4.92 | 0.25 |
| White (non-Hispanic) | 0.62 | 0.37-1.03 | 0.06 | 0.73 | 0.38–1.43 | 0.36 | 0.45 | 0.2–1.02 | 0.06 |
| Black (non-Hispanic) | 0.74 | 0.32–1.75 | 0.50 | 0.43 | 0.12–1.56 | 0.20 | 1.51 | 0.41–5.59 | 0.53 |
| Multiple races (non-Hispanic) | 1.87 | 0.85-4.11 | 0.12 | 1.53 | 0.56–4.14 | 0.40 | 3.22 | 0.76–13.7 | 0.11 |
| US born | 1.04 | 0.52-2.07 | 0.92 | 1.41 | 0.53–3.74 | 0.50 | 0.74 | 0.26–2.09 | 0.57 |
| Some post-high school education | 0.53 | 0.29–0.96 | 0.04 | 0.34 | 0.16–0.72 | 0.005 | 1.04 | 0.38–2.82 | 0.94 |
| Patient's income (in thousands)a | 1.00 | 0.99–1.00 | 0.29 | 1.00 | 0.99–1.01 | 0.59 | 0.99 | 0.98–1 | 0.22 |
| Number of dependent childrena | 1.17 | 0.92-1.49 | 0.21 | 1.25 | 0.92–1.71 | 0.16 | 1.03 | 0.7–1.52 | 0.86 |
| Baseline BSI-12a | 1.06 | 1.03–1.10 | 0.0003 | 1.07 | 1.03–1.12 | 0.002 | 1.06 | 1.01–1.12 | 0.02 |
| Baseline ASIa | 1.03 | 1.01-1.05 | 0.004 | 1.02 | 0.99-1.05 | 0.08 | 1.06 | 1.02-1.10 | 0.004 |
| Baseline SDSa | 1.06 | 1.02-1.10 | 0.001 | 1.06 | 1.01-1.11 | 0.01 | 1.06 | 1.01-1.12 | 0.03 |
| Baseline PHQ-8a | 1.06 | 1.02-1.11 | 0.006 | 1.07 | 1.01-1.13 | 0.02 | 1.08 | 1.01-1.17 | 0.03 |
| GAD diagnosis | 0.89 | 0.50-1.57 | 0.68 | 1.05 | 0.49–2.26 | 0.90 | 0.73 | 0.3–1.75 | 0.48 |
| PD diagnosis | 1.27 | 0.76-2.12 | 0.36 | 1.30 | 0.67–2.54 | 0.44 | 1.30 | 0.58–2.92 | 0.53 |
| SAD diagnosis | 1.14 | 0.67–1.92 | 0.63 | 0.92 | 0.46–1.87 | 0.82 | 1.43 | 0.64–3.2 | 0.39 |
| PTSD diagnosis | 1.34 | 0.60-3.00 | 0.47 | 1.51 | 0.59–3.85 | 0.39 | 1.49 | 0.29–7.75 | 0.64 |
| Number of anxiety disorders | - | - | 0.38 | - | - | 0.56 | - | - | 0.61 |
| 1 | reference | reference | reference | ||||||
| 2 | 1.20 | 0.48-3.01 | - | 1.47 | 0.73-2.96 | - | 1.51 | 0.66–3.45 | - |
| 3 or 4 | 1.46 | 0.86-2.47 | - | 1.25 | 0.39-3.97 | - | 1.31 | 0.27–6.47 | - |
| OCD diagnosis | 1.27 | 0.40-4.00 | 0.68 | 1.07 | 0.27–4.32 | 0.92 | 3.00 | 0.26–34.2 | 0.38 |
| MDD diagnosis | 1.32 | 0.80-2.18 | 0.28 | 0.91 | 0.47–1.78 | 0.79 | 2.73 | 1.21–6.16 | 0.02 |
| Suicidal ideation in past month | 1.00 | 0.41-2.45 | 0.99 | 1.04 | 0.35–3.13 | 0.94 | 1.10 | 0.23–5.17 | 0.91 |
| Lifetime suicide attempt | 1.33 | 0.65-2.74 | 0.44 | 1.29 | 0.51–3.25 | 0.59 | 1.53 | 0.46–5.11 | 0.49 |
| Alcohol abuse (past 3 months) | 1.63 | 0.43-6.25 | 0.47 | 3.91 | 0.63–24.18 | 0.14 | 0.47 | 0.05–4.7 | 0.52 |
| Alcohol abuse (past 12 months) | 1.46 | 0.45-4.74 | 0.53 | 2.62 | 0.63–10.93 | 0.19 | 0.47 | 0.05–4.7 | 0.52 |
| Alcohol dependence (past 12 months) | 1.53 | 0.33-6.97 | 0.59 | 0.83 | 0.08–8.14 | 0.87 | 3.00 | 0.26–34.2 | 0.38 |
| Number of chronic medical conditions | - | - | 0.21 | - | - | 0.36 | - | - | 0.35 |
| 0 | reference | reference | reference | ||||||
| 1 | 1.34 | 0.66-2.74 | - | 0.98 | 0.37–2.65 | - | 1.78 | 0.62–5.17 | - |
| 2 or more | 1.73 | 0.94-3.20 | - | 1.61 | 0.73–3.56 | - | 2.03 | 0.76–5.43 | - |
| Self-perceived financial resources | - | - | 0.053 | - | - | 0.11 | - | - | 0.39 |
| More than enough money | reference | reference | reference | ||||||
| Just enough | 2.73 | 1.13–6.6 | - | 3.23 | 0.9–11.68 | - | 2.31 | 0.67–7.98 | - |
| Less than enough | 3.06 | 1.21-7.76 | - | 4.16 | 1.1–15.67 | - | 2.36 | 0.59–9.37 | - |
| Self-perceived SES overalla | 0.85 | 0.72-0.99 | 0.04 | 0.97 | 0.8–1.18 | 0.74 | 0.57 | 0.4–0.8 | 0.001 |
| Self-perceived SES in communitya | 0.89 | 0.77–1.02 | 0.09 | 0.92 | 0.77–1.09 | 0.35 | 0.84 | 0.66–1.06 | 0.14 |
| Self-efficacy expectancya | 0.0.94 | 0.78-1.12 | 0.48 | 0.92 | 0.73–1.16 | 0.47 | 0.93 | 0.68–1.27 | 0.64 |
| Outcome expectancya | 1.06 | 0.91–1.24 | 0.46 | 1.01 | 0.82–1.25 | 0.91 | 1.14 | 0.89–1.45 | 0.31 |
| Comfort with mental health helpa | 1.06 | 0.93–1.21 | 0.41 | 1.07 | 0.9–1.27 | 0.42 | 1.05 | 0.84–1.33 | 0.66 |
| 6-month characteristics | |||||||||
| Married or cohabitating | 0.46 | 0.28-0.77 | 0.003 | 0.35 | 0.17–0.69 | 0.003 | 0.56 | 0.25–1.27 | 0.16 |
| Employed | 0.71 | 0.38-1.31 | 0.27 | 0.57 | 0.25–1.28 | 0.17 | 0.99 | 0.38–2.59 | 0.99 |
| Any health insurance | 0.58 | 0.28–1.17 | 0.13 | 0.47 | 0.19–1.15 | 0.10 | 0.78 | 0.24–2.5 | 0.67 |
| Medicaid | 4.29 | 1.26-14.65 | 0.02 | 2.56 | 0.5–13.17 | 0.26 | 8.11 | 0.91–72.13 | 0.06 |
| Medicare | 1.87 | 0.85-4.11 | 0.12 | 2.29 | 0.73–7.21 | 0.16 | 1.33 | 0.44–3.98 | 0.62 |
| Other government insurance (e.g. Tricare) | 0.66 | 0.13–3.35 | 0.62 | 0.62 | 0.07–5.64 | 0.67 | 0.72 | 0.06–8.2 | 0.79 |
| Private insurance | 0.51 | 0.28–0.93 | 0.03 | 0.44 | 0.21–0.96 | 0.04 | 0.62 | 0.24–1.6 | 0.32 |
| Social support (SSS)a | 0.81 | 0.63-1.04 | 0.10 | 0.97 | 0.7–1.34 | 0.84 | 0.60 | 0.39–0.91 | 0.02 |
| 6-month BSI-12a | 1.22 | 1.05-1.42 | 0.01 | 1.11 | 0.91–1.34 | 0.31 | 1.38 | 1.08–1.78 | 0.01 |
| 6-month ASIa | 1.05 | 1.02-1.09 | 0.0004 | 1.05 | 1.01-1.09 | 0.02 | 1.06 | 1.01-1.11 | 0.01 |
| 6-month SDSa | 1.11 | 1.05-1.16 | 0.0002 | 1.08 | 1.01-1.16 | 0.02 | 1.13 | 1.04-1.23 | 0.006 |
| 6-month PHQ-8a | 1.12 | 1.04-1.20 | 0.002 | 1.09 | 1–1.2 | 0.049 | 1.18 | 1.04–1.33 | 0.009 |
| Number of psychotropics in past 6 mo.a | 1.08 | 0.88–1.33 | 0.48 | 1.19 | 0.89–1.57 | 0.24 | 0.99 | 0.72–1.35 | 0.94 |
| >2 mo. of any anxiolytic in past 6 mo. | 0.90 | 0.54–1.50 | 0.69 | 1.28 | 0.64–2.56 | 0.48 | 0.55 | 0.25–1.24 | 0.15 |
| >2 mo. of antidepressant in past 6 mo. | 0.81 | 0.49-1.34 | 0.41 | 1.04 | 0.53–2.03 | 0.91 | 0.61 | 0.28–1.36 | 0.23 |
| >2 mo. of bz in past 6 mo. | 2.76 | 1.42-5.35 | 0.003 | 4.04 | 1.73–9.47 | 0.001 | 1.56 | 0.53–4.55 | 0.42 |
| >2 mo. of other anxiolytic in past 6 mo. | 1.30 | 0.41-4.10 | 0.65 | 1.02 | 0.19–5.43 | 0.98 | 1.53 | 0.29–7.96 | 0.62 |
| Any counseling with CBT elements | 0.94 | 0.53-1.66 | 0.83 | 6.73 | 0.86-52.41 | 0.07 | 0.85 | 0.39-1.88 | 0.69 |
| Counseling with ≥ 3 CBT elements sometimes provided at office visits | 0.80 | 0.48-1.35 | 0.41 | 1.58 | 0.60-4.16 | 0.36 | 0.82 | 0.35-1.93 | 0.65 |
| Counseling with ≥ 3 CBT elements usually provided at office visits | 0.75 | 0.45-1.26 | 0.27 | 1.35 | 0.68-2.69 | 0.39 | 0.16 | 0.02-1.34 | 0.09 |
| Smoking (cigarettes/day)a | 1.11 | 1.04-1.18 | 0.003 | 1.12 | 1.03–1.23 | 0.01 | 1.08 | 0.98–1.2 | 0.11 |
| Patient satisfactiona | 0.84 | 0.71-0.99 | 0.04 | 0.97 | 0.77–1.22 | 0.77 | 0.75 | 0.57–1 | 0.047 |
| b. Multivariable logistic regression models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment assignment |
|||||||||
| Overall | Collaborative Care | Usual care | |||||||
| N = 274 | N = 171 | N = 103 | |||||||
| Independent predictors | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| 6-month SDSa | 1.07 | 1.01-1.13 | 0.02 | - | - | - | - | - | - |
| Smoking (cigarettes/day)a | 1.10 | 1.02-1.18 | 0.01 | - | - | - | - | - | - |
| Married or cohabitating | 0.46 | 0.26-0.82 | 0.008 | 0.27 | 0.12-0.59 | 0.001 | - | - | - |
| 6-month ASIa | 1.05 | 1.02-1.08 | 0.004 | 1.06 | 1.01-1.1 | 0.01 | - | - | - |
| >2 mo. of benzodiazepine in past 6 mo. | 2.88 | 1.36-6.12 | 0.006 | 5.05 | 1.92-13.30 | 0.001 | - | - | - |
| Some post-high school education | - | - | - | 0.34 | 0.15–0.82 | 0.02 | - | - | - |
| Baseline ASIa | - | - | - | - | - | - | 1.06 | 1.02-1.11 | 0.003 |
| Self-perceived SES overall (1-10 scale)a | - | - | - | - | - | - | 0.55 | 0.38-0.81 | 0.002 |
| Logistic regression adjusted r2 | 0.22 | 0.26 | 0.26 | ||||||
Variables are continuous (all variables without notation are categorical).
Note: Boldface type indicates independent variable significant at Wald chi-square p < 0.05 in simple logistic regression analysis. All significant variables were included in backward stepwise logistic regression to yield final multivariable models.
Abbreviations: BSI-12 = 12-item Brief Symptom Inventory for anxiety and somatization; ASI = Anxiety Sensitivity Index; SDS = Sheehan Disability Scale; PHQ-8 = 8-item Patient Health Questionnaire (depression scale); GAD = generalized anxiety disorder; PD = panic disorder; SAD = generalized social anxiety disorder; PTSD = post-traumatic stress disorder; OCD = obsessive compulsive disorder; MDD = major depressive disorder; SES = socioeconomic status (1-10 scale, 10 is highest); SSS = Social Support Survey from the Medical Outcomes Study; mo. = months; bz = benzodiazepine; CBT = cognitive behavioral therapy.
Prognostic subgroups for recurrence in ROC analyses
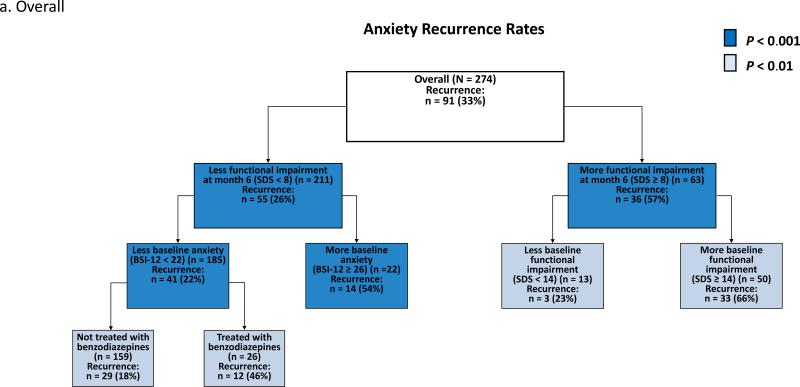
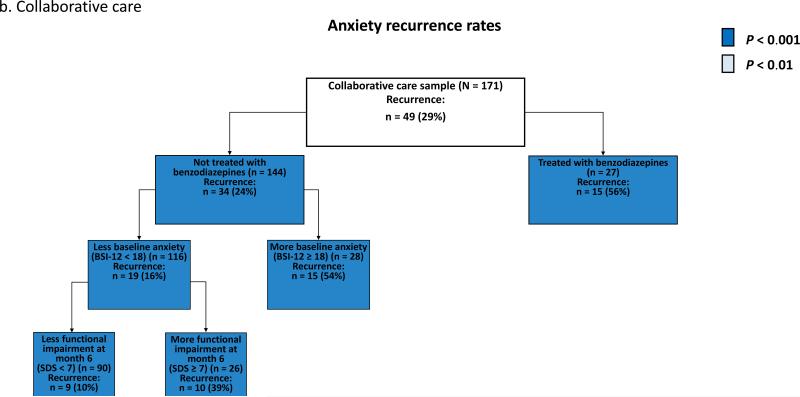
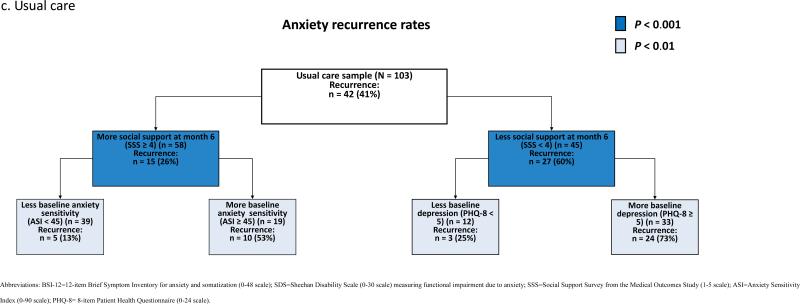
ROC analysis of the overall sample (CC and UC samples combined) identified functional impairment due to anxiety (SDS) at month 6 as the most discriminative predictor of recurrence [χ2 (df = 1, n =274) = 21.12, p < 0.00001] (Fig. 2a). ROC analysis separated patients with a relatively high risk of recurrence (66% in patients with greater functional impairment at baseline (SDS ≥ 14) and month 6 (SDS ≥ 8)) from patients a with relatively low risk of recurrence (18% in patients who were not treated with benzodiazepines and had less anxiety at baseline (BSI-12 < 22) and less functional impairment at month 6 (SDS < 8)) (Fig. 2a). Within the CC sample, ROC identified treatment with a benzodiazepine [χ2 (df = 1, n = 171) = 11.35, p = 0.0008] as the most discriminative predictor of recurrence (Fig. 2b). Within the UC sample, ROC identified the amount of social support the patient had at month 6 [χ2 (df = 1, n = 103) = 12.23, p = 0.0005] as the most discriminative predictor of recurrence (Fig. 2c).
Fig. 2. Receiver operating characteristic analysis identifying prognostic subgroups based on anxiety recurrence overall (a), in collaborative care (b), and in usual care (c).
Discussion
In the CALM study, 36% of patients with anxiety achieved remission after 6 months of treatment in the primary care setting (Roy-Byrne et al., 2010). However, we found that symptom recurrence during the year after remission was common (33% overall). Recurrence was lower in CC (29%) than in UC (41%) (number needed to treat = 9). Recurrence rates were similar across anxiety disorders (GAD, PD, SAD, or PTSD). Patients with lower self-perceived SES or comorbid MDD particularly benefited (in terms of reduced recurrence) if they received CC instead of UC. For the overall sample (CC and UC combined), multivariable logistic regression found that smoking, being single, anxiety sensitivity, functional impairment at month 6 due to residual sub-threshold anxiety, and treatment with benzodiazepines predicted anxiety recurrence. When the CC and UC samples were analyzed separately, predictors unique to each treatment group were identified. Still, in both CC and UC multivariable models, measures of anxiety severity and indicators of lower SES predicted recurrence.
The Harvard/Brown Anxiety Research Project (HARP) examined anxiety recurrence in a mental health outpatient setting, and like our study, HARP found that greater baseline depression and anxiety increased the likelihood of anxiety recurrence (Bruce et al., 2005). Our study also found that post-treatment residual anxiety symptoms (even when minimal) are important when assessing the risk of symptom recurrence. Our study did not find an association between recurrence and alcohol abuse, in contrast to HARP. Of note, CALM excluded patients with recent alcohol dependence, and all enrolled patients abusing alcohol were referred for additional treatment, unlike HARP. These differences may have muted the effects of alcohol abuse on outcomes in the CALM study. Also in contrast to HARP, but in line with the Netherlands Study of Depression and Anxiety, we found that having multiple anxiety disorders did not increase recurrence (Scholten et al., 2013).
Our study also found strong associations between recurrence and multiple low SES indices (less education, lacking private insurance, having Medicaid, smoking, being single, having limited social supports, and lower self-perceived SES) (Adler and Snibbe, 2003). A prior study examining the CALM cohort similarly found that being unemployed was associated with inconsistent partial symptom improvement (Joesch et al., 2013). Furthermore, our results fit well with numerous studies identifying higher rates of stress and psychiatric disorders in low SES patients (Abravanel and Sinha, 2014; McLeod and Kessler, 1990; Muntaner et al., 2004) .
In terms of reducing recurrence, CC was particularly beneficial for patients with lower self-perceived SES and patients with comorbid MDD. Several studies have found that collaborative care is effective in MDD (Gilbody et al., 2006). Studies have suggested that self-perceived SES is a more accurate indicator of social standing than objective measures because self-perceived SES can account for several nuances like quality of education, prospects for the future, and cultural factors (Singh-Manoux et al., 2005). CC may be particularly helpful for low SES patients because in CC each patient's ACS identified community resources and social supports to overcome many putative contributors to socioeconomic health disparities including logistic barriers to care (e.g. transportation and child care), limited social supports, insufficient health literacy, and unhealthy habits like smoking, physical inactivity, and poor diet (Adler and Snibbe, 2003). Our finding compliments a prior analysis of the CALM cohort, which found that for depressed patients and low-income patients, CC was more effective than UC at reducing anxiety (Kelly et al., 2014). In short, for anxiety patients with depression or low SES, CC appears to be particularly effective at helping patients get better and stay better.
A study finding that requires further investigation is that treatment with benzodiazepines was associated with increased symptom recurrence. A possible explanation for the increased recurrence is that benzodiazepines are second-line treatments, and thus treatment with benzodiazepines may have been a marker of treatment-refractory anxiety. Prior studies have identified reduced CBT efficacy with concurrent benzodiazepine treatment (Fava et al., 2001), the development of tolerance, and prescription abuse as significant limitations to treating anxiety disorders with benzodiazepines (Michelini et al., 1996).
A limitation (and strength) of our study is that it was not hypothesis driven. Our exploratory design allowed us to evaluate putative and potentially novel predictors of anxiety recurrence. However, our findings need to be replicated. The use of ROC provided an additional analytic method to identify patients at high risk for recurrence, and all characteristics identified with ROC were significant in simple logistic regression models. Still, given the 56 independent variables evaluated, the Bonferroni correction for the significance level of p = 0.05 would require a p value < 0.0009, which only three variables met in simple logistic regression models (baseline BSI-12 (p = 0.0003), 6-month ASI (p = 0.0004), and 6-month SDS (p = 0.0002)). Another caveat is that our findings are not specific to any single anxiety disorder. Instead, we examined symptoms across multiple anxiety disorders because primary care patients often present with comorbid anxiety disorders and a range of anxiety and somatization symptoms (Kroenke et al., 2007; Schatzberg, 2003). Another limitation is that the BSI-12 only measures symptoms during the previous week, but the BSI-12 was only assessed every 6 months in CALM. If the BSI-12 had been measured more often during the 18 months of follow-up, recurrence rates would have likely been higher and different predictors of anxiety recurrence may have been identified. However, other studies have similarly used the BSI to measure anxiety in long-term outcomes studies (Andreescu et al., 2007; Lang et al., 2006; Roy-Byrne et al., 2010; Schat et al., 2013). Finally, the differences between our analyzed sample and those excluded due to incomplete follow-up suggest our results may be more representative of patients who are older, more financially secure, less anxious, and prescribed fewer psychotropics.
Our study identified which anxiety disorder patients with symptom remission were most likely to experience symptom recurrence in the primary care setting. Our findings are meant to be hypothesis-generating and require replication. CC is effective at treating MDD (Gilbody et al., 2006), and CC has several attributes that address concerns of low SES patients; thus, it was not surprising that patients with comorbid MDD or low self-perceived SES had lower recurrence in CC compared to UC. Similarly, researchers and clinicians developing relapse prevention programs should consider addressing factors associated with increased recurrence like benzodiazepine prescription, anxiety sensitivity, smoking, being single, and post-treatment residual symptoms (even if minimal). Finally, directing relapse prevention resources to patients with the highest risk of recurrence may improve resource utilization efficiency, and future research should examine outcomes of relapse prevention programs when used in prognostic subgroups with high rates of recurrence.
Highlights.
- After anxiety remission, 33% of patients had symptoms recur within a year.
- Collaborative care reduced anxiety recurrence in the primary care setting.
- Collaborative care benefited depressed and low socioeconomic status patients most.
- Anxiety sensitivity and post-treatment residual symptoms predicted recurrence.
- Benzodiazepine prescription, smoking, and being single predicted recurrence.
Acknowledgements
The authors acknowledge the support of the National Institutes of Health 1K23MH091240 (MHB), NARSAD (MHB), the Rembrandt Foundation (MHB), Patterson Trust (MHB and JHT), UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB), 5T32MH18268 NIMH Sponsored Institutional Research Training Grant in Childhood Neuropsychiatric Disorders (JHT), American Psychiatric Association/Substance Abuse and Mental Health Services Administration (JHT). The authors wish to thank the original investigators in the CALM trial and the NIH for making the data used in this report publically available. Data used in the preparation of this article were obtained from the limited access datasets distributed from the NIH-supported “Coordinated Anxiety Learning and Management” (CALM) trial. This manuscript reflects the views of the authors and may not reflect the opinions or views of the CALM Study Investigators or the NIH.
Role of the funding source: Funders did not participate in study design, data analysis, or writing of the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Information: ClinicalTrials.gov Identifier: NCT00347269. The CALM database is available from the NIMH upon request. Information on available limited access datasets can be found at http://www.nimh.nih.gov/funding/clinical-trials-for-researchers/datasets/nimh-procedures-for-requesting-data-sets.shtml.
Contributors: All authors contributed to data interpretation and writing the report. Dr. Bloch came up with the idea for the paper. Dr. Taylor and Mr. Jakubovski analyzed the data. All authors approved the final manuscript.
Conflict of interest: None.
References
- Abravanel BT, Sinha R. Emotion dysregulation mediates the relationship between lifetime cumulative adversity and depressive symptomatology. J Psychiatr Res. 2014 doi: 10.1016/j.jpsychires.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir Psychol Sci. 2003;12:119–23. [Google Scholar]
- Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, et al. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. The British Journal of Psychiatry. 2007;190:344–9. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Mulsant BH, Houck PR, Whyte EM, Mazumdar S, Dombrovski AY, et al. Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry. 2008;165:855–62. doi: 10.1176/appi.ajp.2008.07081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batelaan N, Smit F, De Graaf R, Van Balkom A, Vollebergh W, Beekman A. Identifying target groups for the prevention of anxiety disorders in the general population. Acta Psychiatr Scand. 2010;122:56–65. doi: 10.1111/j.1600-0447.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, et al. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 2005;162:1179–87. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins AW, Otto MW, Cohen LS, Soares CN, Vitonis AF, Hearon BA, et al. Psychosocial predictors of the onset of anxiety disorders in women: Results from a prospective 3-year longitudinal study. J Anxiety Disord. 2009;23:1165–9. doi: 10.1016/j.janxdis.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Sullivan G, Sherbourne C, Bystritsky A, Rose RD, et al. Disorder-specific impact of coordinated anxiety learning and management treatment for anxiety disorders in primary care. Arch Gen Psychiatry. 2011;68:378–88. doi: 10.1001/archgenpsychiatry.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Fava G, Rafanelli C, Grandi S, Conti S, Ruini C, Mangelli L, et al. Long-term outcome of panic disorder with agoraphobia treated by exposure. Psychol Med. 2001;31:891–8. doi: 10.1017/s0033291701003592. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- Jakubovski E, Bloch M. Prognostic subgroups for citalopram response in the STAR* D trial. The Journal of clinical psychiatry. 2014;75:738–47. doi: 10.4088/JCP.13m08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch JM, Golinelli D, Sherbourne CD, Sullivan G, Stein MB, Craske MG, et al. Trajectories of change in anxiety severity and impairment during and after treatment with evidence-based treatment for multiple anxiety disorders in primary care. Depress Anxiety. 2013;30:1099–106. doi: 10.1002/da.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Jakubovski E, Bloch M. Prognostic subgroups for remission and response in the Coordinated Anxiety Learning and Management (CALM) trial. The Journal of clinical psychiatry. 2014 doi: 10.4088/JCP.13m08922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan M, Kraemer HC, Winkleby MA, King AC, Taylor CB. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods. 2001;6:35–48. doi: 10.1037/1082-989x.6.1.35. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The Phq-9. J Gen Intern Med 2001. 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, Monahan P, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–25. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Norman GJ, Casmar PV. A randomized trial of a brief mental health intervention for primary care patients. J Consult Clin Psychol. 2006;74:1173–9. doi: 10.1037/0022-006X.74.6.1173. [DOI] [PubMed] [Google Scholar]
- McLeod JD, Kessler RC. Socioeconomic status differences in vulnerability to undesirable life events. J Health Soc Behav. 1990:162–72. [PubMed] [Google Scholar]
- Michelini S, Cassano G, Frare F, Perugi G. Long-term use of benzodiazepines: tolerance, dependence and clinical problems in anxiety and mood disorders. Pharmacopsychiatry. 1996;29:127–34. doi: 10.1055/s-2007-979558. [DOI] [PubMed] [Google Scholar]
- Mitchell MA, Capron DW, Raines AM, Schmidt NB. Reduction of cognitive concerns of anxiety sensitivity is uniquely associated with reduction of PTSD and depressive symptoms: A comparison of civilians and veterans. J Psychiatr Res. 2014;48:25–31. doi: 10.1016/j.jpsychires.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Morlan KK, Tan SY. Comparison of the brief psychiatric rating scale and the brief symptom inventory. J Clin Psychol. 1998;54:885–94. doi: 10.1002/(sici)1097-4679(199811)54:7<885::aid-jclp3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Muntaner C, Eaton WW, Miech R, O'Campo P. Socioeconomic position and major mental disorders. Epidemiol Rev. 2004;26:53–62. doi: 10.1093/epirev/mxh001. [DOI] [PubMed] [Google Scholar]
- Pennell BE, Bowers A, Carr D, Chardoul S, Cheung Gq, Dinkelmann K, et al. The development and implementation of the national comorbidity survey replication, the national survey of American life, and the national Latino and Asian American survey. Int J Methods Psychiatr Res. 2004;13:241–69. doi: 10.1002/mpr.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Heilbronner RL. The anxiety sensitivity index: Construct validity and factor analytic structure. J Anxiety Disord. 1987;1:117–21. [Google Scholar]
- Rodriguez BF, Bruce SE, Pagano ME, Keller MB. Relationships among psychosocial functioning, diagnostic comorbidity, and the recurrence of generalized anxiety disorder, panic disorder, and major depression. J Anxiety Disord. 2005;19:752–66. doi: 10.1016/j.janxdis.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez BF, Weisberg RB, Pagano ME, Bruce SE, Spencer MA, Culpepper L, et al. Characteristics and predictors of full and partial recovery from generalized anxiety disorder in primary care patients. J Nerv Ment Dis. 2006;194:91–7. doi: 10.1097/01.nmd.0000198140.02154.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, et al. Delivery of evidence-based treatment for multiple anxiety disorders in primary care. JAMA. 2010;303:1921–8. doi: 10.1001/jama.2010.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schat A, van Noorden M, Noom M, Giltay E, van der Wee N, Vermeiren R, et al. Predictors of outcome in outpatients with anxiety disorders: The Leiden routine outcome monitoring study. J Psychiatr Res. 2013;47:1876–85. doi: 10.1016/j.jpsychires.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF. Treating Depression and Anxiety to Remission. J Clin Psychiatry. 2003;64:3–4. [PubMed] [Google Scholar]
- Scholten WD, Batelaan NM, van Balkom AJ, WJH Penninx B, Smit JH, van Oppen P. Recurrence of anxiety disorders and its predictors. J Affect Disord. 2013;147:180–5. doi: 10.1016/j.jad.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Schumm WR, McCollum EE, Bugaighis MA, Jurich AP, Bollman SR. Characteristics of the Kansas Family Life Satisfaction Scale in a regional sample. Psychol Rep. 1986;58:975–80. [Google Scholar]
- Sheehan D, Harnett-Sheehan K, Raj B. The measurement of disability. Int Clin Psychopharmacol. 1996;11:89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67:855–61. doi: 10.1097/01.psy.0000188434.52941.a0. [DOI] [PubMed] [Google Scholar]
- Sturm R, Gresenz C, Sherbourne C, Minnium K, Klap R, Bhattacharya J, et al. The Design of“Healthcare for Communities”: A Study of Health Care Delivery for Alcohol, Drug Abuse, and Mental Health Conditions. Inquiry. 1999:221–33. [PubMed] [Google Scholar]
- Sullivan G, Craske MG, Sherbourne C, Edlund MJ, Rose RD, Golinelli D, et al. Design of the Coordinated Anxiety Learning and Management (CALM) study: innovations in collaborative care for anxiety disorders. Gen Hosp Psychiatry. 2007;29:379–87. doi: 10.1016/j.genhosppsych.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KB. The design of Partners in Care: evaluating the cost-effectiveness of improving care for depression in primary care. Soc Psychiatry Psychiatr Epidemiol. 1999;34:20–9. doi: 10.1007/s001270050107. [DOI] [PubMed] [Google Scholar]
- Young AS, Klap R, Sherbourne CD, Wells KB. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 2001;58:55–61. doi: 10.1001/archpsyc.58.1.55. [DOI] [PubMed] [Google Scholar]