Abstract
The anterior oblique ligament (AOL) and the dorsoradial ligament (DRL) are both regarded as mechanical stabilizers of the thumb carpometacarpal (CMC) joint, which in older women is often affected by osteoarthritis. Inferences on the potential relationship of these ligaments to joint pathomechanics are based on clinical experience and studies of cadaveric tissue, but their function has been studied sparsely in vivo. The purpose of this study was to gain insight into the functions of the AOL and DRL using in vivo joint kinematic data. The thumbs of 44 healthy subjects were imaged with a clinical computed tomography scanner in functional-task and thumb range-of-motion positions. The origins and insertion sites of the AOL and the DRL were identified on the 3D bone models and each ligament was modeled as a set of three fibers whose lengths were the minimum distances between insertion sites. Ligament recruitment, which represented ligament length as a percentage of the maximum length across the scanned positions, was computed for each position and related to joint posture. Mean AOL recruitment was lower than 91% across the CMC range of motion, whereas mean DRL recruitment was generally higher than 91% in abduction and flexion. Under the assumption that ligaments do not strain by more than 10% physiologically, our findings of mean ligament recruitments across the CMC range of motion indicate that the AOL is likely slack during most physiological positions, whereas the DRL may be taut and therefore support the joint in positions of CMC joint abduction and flexion.
1. INTRODUCTION
Ligaments are thought to be important stabilizers of the versatile thumb carpometacarpal (CMC) joint. The saddle-shaped CMC articulation, which is responsible for the wide range of motion and oppositional capability of the human thumb, is characterized by little bony support, necessitating muscular and ligamentous support for stability (Leversedge, 2008). Ligament laxity is believed to destabilize the joint, leading to incongruent loading and subsequent damage of the articular cartilage that is typical of CMC osteoarthritis (OA) (Pellegrini, 2005). In response, several ligament reconstruction procedures that aim to reduce laxity and restore the center of pressure in the joint have been developed (Birman et al., 2014; Burton and Pellegrini, 1986; Eaton and Littler, 1973). The anterior oblique ligament (AOL) and the dorsoradial ligament (DRL) have received particular attention in regards to their role on joint stability.
The AOL is located on the ulnar-volar side of the CMC joint and has been classically considered the primary stabilizer of the CMC joint (Bettinger et al., 1999; Doerschuk et al., 1999; Imaeda et al., 1994, 1993; Pellegrini, 2005; Pellegrini et al., 1993; Pieron, 1973). It originates on the volar-ulnar edge of the trapezium and inserts on the volar-ulnar side of the metacarpal beak. It is widely believed that degeneration of the AOL precedes CMC OA because a direct correlation between the stage of OA and the integrity of the AOL has been documented in cadaveric specimens (Doerschuk et al., 1999; Eaton and Littler, 1973; Pellegrini, 2005, 1991; Pellegrini et al., 1993). The first CMC ligament reconstruction technique (Eaton and Littler, 1973) and the subsequent ligament reconstruction tendon interposition (LRTI) (Eaton et al., 1984; Freedman et al., 2000; Tomaino et al., 1995) were partly based on the theory that the CMC joint is kept intact primarily by the AOL. Additionally, the presence of the hormone relaxin, which is associated with ligament loosening, has been found in this ligament and has been correlated with joint laxity (Lubahn et al., 2006; Wolf et al., 2013a, 2013b). Recent studies, however, have reported structural limitations of the AOL that challenge the importance of its role on joint stability (Colman et al., 2007; Hagert et al., 2012; Ladd et al., 2012; Lin et al., 2013; Strauch et al., 1999).
In tandem with the recent skepticism on the role of the AOL, new evidence has emerged on the sturdiness and superior stabilizing potential of the DRL (Bettinger et al., 2000, 1999; Colman et al., 2007; D’Agostino et al., 2014; Hagert et al., 2012; Ladd et al., 2012; Strauch et al., 1999, 1994; Tan et al., 2011; Van Brenk et al., 1998; Zhang et al., 2013). The DRL is located on the dorsal-radial side of the CMC joint, opposite the AOL, and is described by these studies as the most robust ligamentous structure in the joint. It originates on the radial side of the dorsal trapezial tubercle and inserts on the radial-dorsal side of the metacarpal, fanning out like a deltoid. Dissection studies have reported that it is the widest, thickest, and shortest ligament (Bettinger et al., 2000, 1999; Colman et al., 2007; Strauch et al., 1994; Tan et al., 2011; Van Brenk et al., 1998; Zhang et al., 2013). Moreover, histological studies have determined that the DRL contains greater cellularity and sensory innervations when compared to the AOL, thus concluding that the DRL may have a neuromuscular function, in addition to being a more robust stabilizer (Hagert et al., 2012; Ladd et al., 2012).
Current knowledge on the roles of the AOL and DRL on joint stability and their association with OA pathogenesis is inferred from morphometric, histological, and mechanical evaluations of cadaveric ligament tissue. In vivo studies should provide further insight into the role of each of these two ligaments on the stability of the active joint. Several studies have identified the major CMC joint ligaments with MRI and ultrasound (Cardoso et al., 2009; D’Agostino et al., 2014; Gondim Teixeira et al., 2011), without providing a quantitative analysis of their function. There is a limited number of published in vivo studies (Tan et al., 2011) that have explored the roles of these ligaments on joint stability, mainly because assessing ligament function in vivo remains challenging.
One approach, which has been utilized more extensively in other joints, is to model ligaments from bone models (acquired with different imaging modalities) and joint kinematics, by representing ligament fiber bundles as the shortest paths between ligament insertion sites, optimized to bypass bony constraints (Blankevoort et al., 1991; Marai et al., 2004). Ligament recruitment, which is defined as the probability that a ligament is in tension and is computed by expressing the modeled ligament length as a percentage of the maximum length across all the tested positions (Blankevoort et al., 1991), can provide valuable information on ligament function. The rationale behind modeling ligament recruitment is that if a ligament is functioning as a joint stabilizer in a given position, then the recruitment value for that position should be high, i.e., the difference between the maximum length reached by the ligament across all positions and the computed length in that position should be very small. This method does not make any inferences about ligament properties, but simply evaluates their function based on joint motion. A recruitment value can be low in a given position either because the ligament is slack in that position or because it is strained in the maximum length position, since the outcome that it assesses is how much the ligament is allowing the joint to move. The objective of the current study was to estimate the in vivo function of the AOL and DRL by computing their maximum lengths and recruitments during three functional tasks and four thumb range-of-motion positions captured in vivo.
2. METHODS
Data Acquisition
Following approval from the Institutional Review Board and completion of informed consents, 44 healthy subjects (10 young men, age = 22.5 ± 2.5 yrs.; 11 young women, age = 23.5 ± 1.4 yrs.; 10 older men, age = 56.7 ± 9.4 yrs.; 13 older women, age = 55.4 ± 7.6 yrs.) were enrolled as part of a broader study on CMC joint biomechanics (Halilaj et al., 2014a, 2014b, 2014c, 2013). The dominant hand and wrist of each subject were imaged in a neutral wrist position, during three functional task positions—key pinch, jar grasp, jar twist—and during four maximum active range of motion (ROM) positions: thumb adduction, abduction, flexion, and extension. A thumb spica splint was used to support the wrists and thumbs during the neutral scans and custom-designed polycarbonate mechanical fixtures were used to standardize the other positions. For the functional task positions, subjects were scanned while they applied and maintained 80% of their maximum load for each specific task. Each scan lasted approximately 30–45 seconds and subjects were asked to maintain the load during the whole duration of the scan. A compression load cell (0–50lb, Model D, Honeywell International Inc., Morristown, NJ) was integrated into the fixtures and connected to a custom-written Labview (National Instruments, Austin, Texas) program that assisted the subjects in maintaining the standardized load during the entire duration of the scan. Computed tomography (CT) volume images were generated with a 16-slice clinical CT scanner (General Electric, Milwaukee, WI) at tube settings of 80 kVp and 80 mA, slice thickness of 0.625 mm, and in-plane resolution of approximately 0.3 mm × 0.3 mm, resulting in a total effective dose of radiation of less than 1.6 mSv.
CMC Joint Kinematics
Three-dimensional (3-D) models of the trapezium and first metacarpal were generated by segmenting the neutral CT volume with commercial software (Mimics, Materialise, Leuven, Belgium) and 3-D kinematics of the bones from the neutral position to each of the other positions were obtained with an established markerless bone registration algorithm (Marai et al., 2006). The posture of the first metacarpal with respect to the trapezium was computed at each position and expressed in terms of a CMC joint coordinate system (Halilaj et al., 2013). Since internal/external rotation is considered a secondary motion in the CMC joint, flexion/extension and ab/adduction were used to define CMC joint posture. In the CMC joint coordinate system, flexion/extension of the metacarpal with respect to the trapezium is defined as rotation about an axis oriented in the ulnar-to-radial direction, or the concavity of the trapezium, whereas abduction/adduction is defined as rotation about an axis oriented in the dorsal-to-volar direction, or the concavity of the metacarpal.
Ligament Modeling
The origins and insertion sites of the AOL and the DRL were manually selected on the bone models in Geomagic Studio (Geomagic®, Research Triangle Park, NC) by a trained research student, using information from cadaver studies of AOL and DRL anatomy (Bettinger et al., 1999; Ladd et al., 2012; Nanno et al., 2006) and working knowledge from a hand surgeon (Amy L. Ladd, co-author) who has studied the anatomy of the CMC joint extensively. Given that the width of these ligaments is approximately 5 mm (Ladd et al., 2012), each ligament was modeled as a set of three fibers (Fu–ulnar; Fc–central, Fr–radial) whose lengths were the shortest distances between three points equally distributed across the insertion sites (Fig. 1). The three fibers were chosen to represent the radial, central, and ulnar parts of the ligaments since the ulnar and radial regions were expected to be affected by abduction-adduction and the central regions were expected to be affected more by extension-flexion. The fibers were constrained to avoid bone penetrations (Marai et al., 2004; Rainbow et al., 2012). The bone rigid body transformations from the neutral position to the other positions were applied accordingly to determine the location of the insertion sites in the non-neutral positions. The length of each fiber at each of the scanned positions, λp, and the maximum length of each fiber across all of the positions, λmax, were computed for each subject. Individual fiber recruitment, Rp, defined as percentage of the maximum length, was computed at each position as follows:
| (1) |
Figure 1.
a) The anterior oblique ligament, which has been described as a “curtain-like” (Pieron, 1973) structure that attaches proximally on the ulnar-volar edge of the trapezium and distally on the ulnar-volar edge of the base of the metacarpal (Bettinger et al., 1999; Ladd et al., 2012; Nanno et al., 2006), was represented by three fibers that connected 3 equidistant points on the manually selected insertion sites; b) the dorsoradial ligament, which has been described as a “fan-shaped” sturdy structure that attaches proximally on the dorsoradial tubercle of the trapezium and distally on the dorsoradial edge of the metacarpal (Bettinger et al., 1999; Ladd et al., 2012; Nanno et al., 2006), was represented similarly
Rp was then related to CMC joint posture (flexion/extension and abduction/adduction). Although it is not possible to differentiate strained ligaments from slack ligaments with these methods, recruitment values can be interpreted as a probability that the ligament is strained (Blankevoort et al., 1991). Ligament fibers with recruitment values near 100% may be strained. Based on the assumption that ligaments are not strained by more than 10% of their true length, λtrue, in physiological positions (Beynnon and Fleming, 1998), there is a high probability that ligament fibers with recruitment values of less than the critical recruitment value of 91% are slack.
| (2) |
If 91% ≤ Rp ≤ λmax, the ligament may be in tension (taut), although it cannot be determined if it is.
If Rp ≤ 91%, the ligament is most likely not in tension (slack).
Therefore, the range of motion regions where Rp was greater than 91% are reported here as potential regions of ligament tension, where the ligament may be playing a stabilizing role. It is important to note that the intention of the analysis is not to identify when and if the AOL and DRL are in tension, or when and if they are not. Rather, the purpose is to determine the likelihood that the ligaments are playing a stabilizing role, based on how much the ligaments allow the joint to move and without making assumptions on the mechanical behavior of ligament tissue.
Statistical Analysis
Repeated measures analyses of variance (ANOVA) were used to compare the λmax of the three different fibers, Fu, Fc, and Fr, in each ligament. Two-way ANOVAs were used to determine the effects of sex and age on the λmax of the AOL and DRL. Pair-wise multiple comparisons between groups were carried out using the Holm-Sidak method, with the significance level set at .05. Multiple regression analyses were used to model the relationship of Rp to joint posture (AA—ab/adduction and FE—flexion/extension) across all of the positions, for each fiber:
| (3) |
where I is recruitment at a theoretical neutral position of 0° of ab/adduction and 0° of flexion/extension, whereas α and β are the rates of recruitment per degree of AA and FE, respectively.
3. RESULTS
Maximum AOL and DRL Fiber Lengths
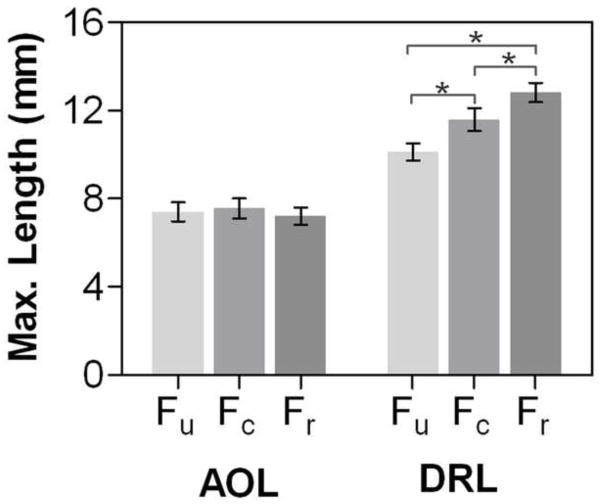
The mean (± SD) maximum lengths of the AOL Fu, Fc, and Fr fibers were 7.4 ± 1.4 mm, 7.6 ± 1.5 mm, and 7.2 ± 1.3 mm, respectively (Fig. 2). This maximum length was generally reached during the jar grasp task, where the mean joint posture was 22.5 ± 7.2° of abduction and 8.5 ± 8.1° of flexion (Fig 3). There was no significant difference among the lengths of the three AOL fibers (p = .118). Neither sex, nor age had a significant effect on the maximum AOL fiber lengths.
Figure 2.
The maximum computed lengths across all the scanned positions (mean and 95% CI shown here) were not different among fibers in the AOL, but they were significantly different in the DRL (p < .001). Cadaver studies report that both the AOL and DRL are approximately 9 to 11 mm in length, therefore our findings that the computed lengths (the minimum distances between ligament insertion sites) for the AOL are below this range indicate that the AOL may be slack, and therefore may not be providing stability, even in the maximum-length position scanned here.
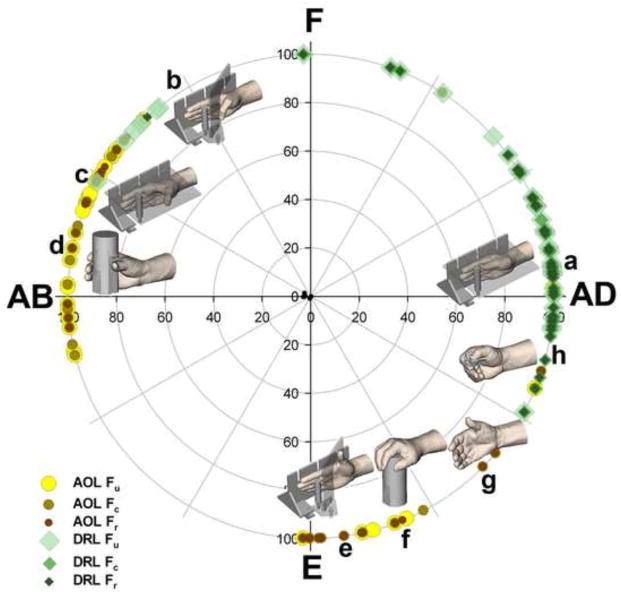
Figure 3.
The maximum lengths (100% recruitment) for the AOL fibers were generally reached during the jar grasp task, whereas the maximum lengths for the DRL fibers were reached during the adduction task
The mean (± SD) maximum lengths of the DRL fibers were 10.1 ± 1.3 mm, 11.6 ± 1.7 mm, and 12.8 ± 1.4 mm (Fig. 2). The maximum length for the DRL was generally reached during adduction, where the mean joint posture was 22.6 ± 8.3° of adduction and 3.3 ± 6.0° of flexion (Fig. 3). There was a significant difference among the lengths of the three fibers (p < .001), with the radial fiber being the longest and the ulnar fiber the shortest (Fig. 2). Additionally, the male Fu, Fc, and Fr DRL fibers were longer than female DRL fibers by a mean of 14% (p < .001), 14% (p = .004), and 10% (p =.005), respectively. There were no statistically significant differences with age group.
AOL and DRL Fiber Recruitments across the CMC Joint Range-of-Motion
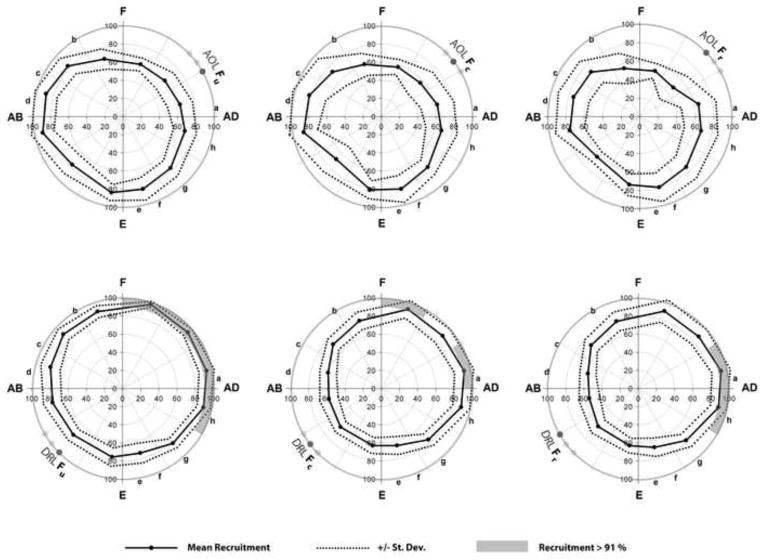
The AOL fibers had the highest recruitment values during CMC postures that involved abduction, whereas the DRL fibers had the highest mean recruitment values during CMC postures that involved adduction and flexion. Across the CMC range-of-motion, the mean recruitment values for the AOL fibers were lower than 91% (Fig. 4). The mean recruitment values for the DRL fibers ranged between 91% and 98% in adduction and flexion (Fig. 4). The radial DRL fiber had the highest recruitment in postures of adduction (mean of approximately 93%), whereas the central and ulnar DRL fibers had high recruitments during both abduction and flexion (Fig. 4). Overall, the variability of recruitment values was higher in the AOL (r =0.58, r = 0.42, r = 0.33 for the Fu, Fc, Fr regression models respectively, p < .0001 for all) than the DRL (r = 0.68, r = 0.86, r = 0.86, p < 0.0001 for all).
Figure 4.
Mean (± SD) ligament recruitment for thumb range of motion postures, as captured with the scanned positions of: (a) adduction, (b) flexion, (c) abduction, (d) jar grasp, (e) extension, (f) jar twist, (g) neutral, (h) key pinch. Adduction (AD) and flexion (F) resulted in DRL recruitment values that were higher than 91%, whereas abduction (AB) resulted in AOL recruitment values higher than 85.0%, but lower than 91%
4. DISCUSSION
Thumb carpometacarpal joint osteoarthritis is a debilitating disease of unknown pathoetiology. Joint mechanics, and ligamentous instability in particular, is often cited as a potential predisposing factor, but our understanding of ligament function in the active joint is currently limited. The aim of this study was to gain insight into the function of the healthy anterior oblique and dorsoradial ligaments by modeling their lengths and recruitments across the CMC range of motion using in vivo kinematic data. Our findings indicate that the AOL is slack across most of the range of CMC joint motion, whereas the DRL may be in tension in positions that involve joint flexion and adduction.
Our interpretation of AOL and DRL function is supported by findings reported in literature in the last two decades. Studies of cadaveric tissue have reported lengths of 9 to 12 mm for both the AOL and DRL (Bettinger et al., 1999; Ladd et al., 2012; Nanno et al., 2006; Tan et al., 2011), but our average maximum fiber lengths were approximately 7 mm and 11 mm, for the AOL and DRL, respectively, indicating that the AOL may be slack even in the maximal length position scanned here. Cadaver studies of CMC joint anatomy agree on the fact that the AOL experiences tension only at extremes of extension, abduction, and external rotation (Bettinger et al., 1999; Edmunds, 2011; Imaeda et al., 1997) and that sectioning of the AOL does not cause the joint to dislocate (Strauch et al., 1994; Van Brenk et al., 1998), but that the DRL is taut and provides support in most positions (Bettinger et al., 1999; Edmunds, 2011; Imaeda et al., 1997). It is possible that, unlike what is seen during in vitro manually aided passive range-of-motion experiments, the AOL may not be placed in tension during the active positions scanned here due to muscular constraints, for example, from the adductor pollicis. At the same time, while the DRL may be a robust stabilizer in some positions, it is not taut across all of the thumb range of motion, as reported in cadaveric studies. It is unlikely that any of these ligaments strain by more than 10% during in vivo physiological positions (Beynnon and Fleming, 1998), therefore ligament recruitment below 91% is indicative of slackness. The only positions where the AOL or the DRL may be in tension are those where recruitment falls between 91% and 100%. The DRL may be taut across a wide span of the thumb range of motion, but given mean recruitment values of less than 91%, the same cannot be said about the AOL.
The observed patterns of sex-related and fiber-related differences in the DRL maximum ligament length are consistent with the conjecture that the DRL was in tension in the maximal length position scanned in this study. Since men have larger joints than women, a longer DRL in men reflects joint size differences, which may not be captured if a ligament is slack, as in the case of the AOL. When a ligament is slack, it is also more difficult to capture differences in the length of different fibers within the same ligament, because the variability in lengths is greater. Our finding that with different motions, different parts of the DRL may be strained differently is not surprising given the width of this ligament (approximately 5 mm) (Ladd et al., 2012), the irregular shape of the mating bones, and the complex motions that the CMC joint undergoes. These finding suggests that different parts of the DRL may be responsible for keeping the joint from subluxing during different types of end positions. For example, the ulnar part of the DRL seems to support the joint during both flexion and adduction, whereas the radial part seems to support it mainly during adduction.
A few aspects of our study must be considered when interpreting the results. The analysis presented here is limited by the inability to measure true ligament resting length, which precludes the computation of true ligament strain. The purpose of this analysis, however, was to determine the probabilities (Blankevoort et al., 1991) that the AOL and DRL were in tension in the positions that we studied, without making any unsubstantiated claims on the mechanical properties of the ligaments. Interpretation of the results presented here can be further informed by previous cadaver studies that have reported mean resting lengths of approximately 9 to 12 mm for both the AOL and the DRL and by reports that the physiological strain of ligaments generally does not surpass 10% (Beynnon and Fleming, 1998). However, the study does not rely exclusively on these previous findings. Even if the maximum lengths were not known and a 10% threshold was not considered in the interpretation of the results, the fact that recruitment values are lower for the AOL than they are for the DRL across the CMC joint range of motion does not change. This finding alone informs us that the AOL allows more subluxation in the CMC joint than the DRL. A limitation of the modeling approach taken here is the reliability of ligament insertion sites on previously published descriptions, rather than on directly acquired information. The three fibers that were used to model the ligaments spanned across a width of approximately 5 mm, yet major findings on patterns of recruitment were vastly similar across them. In itself, this finding serves as a validation of the ability of the utilized method to detect differences in patterns of recruitment between ligaments, in spite of the accuracy of the selection of insertion sites. Last, we find it important to clarify that the task positions ah in Figures 3 and 4 represent the mean postures across subjects—the data that we collected covered the entire range of motion, including the abduction-extension region, although some regions, e.g. extension-adduction, were denser. This is due to the fact that there is variability in how different subjects perform a given task. Additionally, there might be a concern that the averaged recruitment values across subjects (Fig. 4) could have led to low AOL recruitment values due to a high variability in where the AOL reached its maximum length across subjects. However, this variability is similar between the AOL and DRL (Fig. 3) and low AOL recruitment values result from low recruitment values in non-maximal length positions.
In summary, using 3-D in vivo kinematic data, this study presented an analogous analysis of AOL and DRL function across the CMC joint range of motion. The findings reported here add new evidence to the existing body of literature on the AOL (Doerschuk et al., 1999; Eaton and Littler, 1973; Imaeda et al., 1997, 1994; Pellegrini, 2005) and DRL (Bettinger et al., 2000; Colman et al., 2007; Edmunds, 2011; Ladd et al., 2012; Najima et al., 1997; Strauch et al., 1999; Van Brenk et al., 1998) by reinforcing the role of the DRL as a potential stabilizer in the healthy CMC joint. Insight on the roles of AOL and DRL function on CMC joint stability should help to elucidate the link between these ligaments and CMC joint pathomechanics, motivating, in turn, improved treatment procedures for the arthritic CMC joint.
Acknowledgments
The project described was supported by Grant Number AR059185 from NIAMS/NIH. Its contents are the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH. The authors would like to thank Hayley McClintock, Arlene Garcia, Debbie Kenny, Robert Chang, James C Tarrant, Joel B Schwartz, and Bethany J Wilcox for their assistance.
Footnotes
Conflicts of Interest Statement:
The authors have no financial or personal relationships that could bias this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bettinger PC, Linscheid RL, Berger RA, Cooney WP, An KN. An anatomic study of the stabilizing ligaments of the trapezium and trapeziometacarpal joint. J Hand Surg [Am] 1999;24:786–798. doi: 10.1053/jhsu.1999.0786. [DOI] [PubMed] [Google Scholar]
- Bettinger PC, Smutz WP, Linscheid RL, Cooney WP, 3rd, An KN. Material properties of the trapezial and trapeziometacarpal ligaments. J Hand Surg Am. 2000;25:1085–1095. doi: 10.1053/jhsu.2000.18487. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: A review of previous work. Journal of Biomechanics. 1998;31:519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Birman MV, Danoff JR, Yemul KS, Lin JD, Rosenwasser MP. Dorsoradial ligament imbrication for thumb carpometacarpal joint instability. Tech Hand Up Extrem Surg. 2014;18:66–71. doi: 10.1097/BTH.0000000000000035. [DOI] [PubMed] [Google Scholar]
- Blankevoort L, Huiskes R, de Lange A. Recruitment of knee joint ligaments. J Biomech Eng. 1991;113:94–103. doi: 10.1115/1.2894090. [DOI] [PubMed] [Google Scholar]
- Burton RI, Pellegrini VD. Surgical management of basal joint arthritis of the thumb. Part II. Ligament reconstruction with tendon interposition arthroplasty. J Hand Surg [Am] 1986;11:324–332. doi: 10.1016/s0363-5023(86)80137-x. [DOI] [PubMed] [Google Scholar]
- Cardoso FN, Kim HJ, Albertotti F, Botte MJ, Resnick D, Chung CB. Imaging the ligaments of the trapeziometacarpal joint: MRI compared with MR arthrography in cadaveric specimens. AJR Am J Roentgenol. 2009;192:W13–19. doi: 10.2214/AJR.07.4010. [DOI] [PubMed] [Google Scholar]
- Colman M, Mass DP, Draganich LF. Effects of the deep anterior oblique and dorsoradial ligaments on trapeziometacarpal joint stability. J Hand Surg [Am] 2007;32:310–307. doi: 10.1016/j.jhsa.2006.12.002. [DOI] [PubMed] [Google Scholar]
- D’Agostino P, Kerkhof FD, Shahabpour M, Moermans JP, Stockmans F, Vereecke EE. Comparison of the anatomical dimensions and mechanical properties of the dorsoradial and anterior oblique ligaments of the trapeziometacarpal joint. J Hand Surg Am. 2014;39:1098–1107. doi: 10.1016/j.jhsa.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Doerschuk SH, Hicks DG, Chinchilli VM, Pellegrini VD. Histopathology of the palmar beak ligament in trapeziometacarpal osteoarthritis. J Hand Surg [Am] 1999;24:496–504. doi: 10.1053/jhsu.1999.0496. [DOI] [PubMed] [Google Scholar]
- Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg Am. 1984;9:692–699. doi: 10.1016/s0363-5023(84)80015-5. [DOI] [PubMed] [Google Scholar]
- Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55:1655–1666. [PubMed] [Google Scholar]
- Edmunds JO. Current concepts of the anatomy of the thumb trapeziometacarpal joint. J Hand Surg Am. 2011;36:170–182. doi: 10.1016/j.jhsa.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Eaton RG, Glickel SZ. Long-term results of volar ligament reconstruction for symptomatic basal joint laxity. J Hand Surg [Am] 2000;25:297–304. doi: 10.1053/jhsu.2000.jhsu25a0297. [DOI] [PubMed] [Google Scholar]
- Gondim Teixeira PA, Omoumi P, Trudell DJ, Ward SR, Blum A, Resnick DL. High-resolution ultrasound evaluation of the trapeziometacarpal joint with emphasis on the anterior oblique ligament (beak ligament) Skeletal Radiol. 2011;40:897–904. doi: 10.1007/s00256-010-1068-0. [DOI] [PubMed] [Google Scholar]
- Hagert E, Lee J, Ladd AL. Innervation patterns of thumb trapeziometacarpal joint ligaments. J Hand Surg Am. 2012;37:706–714. e1. doi: 10.1016/j.jhsa.2011.12.038. [DOI] [PubMed] [Google Scholar]
- Halilaj E, Laidlaw DH, Moore DC, Crisco JJ. Polar histograms of curvature for quantifying skeletal joint shape and congruence. J Biomech Eng. 2014a;136:094503. doi: 10.1115/1.4027938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilaj E, Moore DC, Laidlaw DH, Got CJ, Weiss APC, Ladd AL, Crisco JJ. The morphology of the thumb carpometacarpal joint does not differ between men and women, but changes with aging and early osteoarthritis. J Biomech. 2014b;47:2709–2714. doi: 10.1016/j.jbiomech.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilaj E, Rainbow MJ, Got CJ, Moore DC, Crisco JJ. A thumb carpometacarpal joint coordinate system based on articular surface geometry. J Biomech. 2013;46:1031–1034. doi: 10.1016/j.jbiomech.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilaj E, Rainbow MJ, Got C, Schwartz JB, Moore DC, Weiss A-PC, Ladd AL, Crisco JJ. In vivo kinematics of the thumb carpometacarpal joint during three isometric functional tasks. Clin Orthop Relat Res. 2014c;472:1114–1122. doi: 10.1007/s11999-013-3063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T, An KN, Cooney WP, Linscheid R. Anatomy of trapeziometacarpal ligaments. J Hand Surg [Am] 1993;18:226–231. doi: 10.1016/0363-5023(93)90352-4. [DOI] [PubMed] [Google Scholar]
- Imaeda T, Niebur G, An KN, Cooney WP. Kinematics of the trapeziometacarpal joint after sectioning of ligaments. J Orthop Res. 1994;12:205–210. doi: 10.1002/jor.1100120209. [DOI] [PubMed] [Google Scholar]
- Imaeda T, Niebur G, Cooney WP, Linscheid RL, An KN. Ligament length during circumduction of the trapeziometacarpal joint. Journal of Orthopaedic Science. 1997;2:319–327. [Google Scholar]
- Ladd AL, Lee J, Hagert E. Macroscopic and Microscopic Analysis of the Thumb Carpometacarpal Ligaments: A Cadaveric Study of Ligament Anatomy and Histology. The Journal of Bone & Joint Surgery. 2012;94:1468–1477. doi: 10.2106/JBJS.K.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leversedge FJ. Anatomy and Pathomechanics of the Thumb. Hand Clinics. 2008;24:219–229. doi: 10.1016/j.hcl.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Lin JD, Karl JW, Strauch RJ. Trapeziometacarpal Joint Stability: The Evolving Importance of the Dorsal Ligaments. Clin Orthop Relat Res. 2013 doi: 10.1007/s11999-013-2879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn J, Ivance D, Konieczko E, Cooney T. Immunohistochemical detection of relaxin binding to the volar oblique ligament. J Hand Surg [Am] 2006;31:80–84. doi: 10.1016/j.jhsa.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Marai GE, Laidlaw DH, Crisco JJ. Super-resolution registration using tissue-classified distance fields. IEEE Trans Med Imaging. 2006;25:177–187. doi: 10.1109/TMI.2005.862151. [DOI] [PubMed] [Google Scholar]
- Marai GE, Laidlaw DH, Demiralp C, Andrews S, Grimm CM, Crisco JJ. Estimating joint contact areas and ligament lengths from bone kinematics and surfaces. IEEE Trans Biomed Eng. 2004;51:790–799. doi: 10.1109/TBME.2004.826606. [DOI] [PubMed] [Google Scholar]
- Najima H, Oberlin C, Alnot JY, Cadot B. Anatomical and biomechanical studies of the pathogenesis of trapeziometacarpal degenerative arthritis. J Hand Surg Br. 1997;22:183–188. doi: 10.1016/s0266-7681(97)80058-7. [DOI] [PubMed] [Google Scholar]
- Nanno M, Buford WL, Patterson RM, Andersen CR, Viegas SF. Three-dimensional analysis of the ligamentous attachments of the first carpometacarpal joint. J Hand Surg Am. 2006;31:1160–1170. doi: 10.1016/j.jhsa.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Pellegrini VD. The ABJS 2005 Nicolas Andry Award: osteoarthritis and injury at the base of the human thumb: survival of the fittest? Clin Orthop Relat Res. 2005;438:266–276. doi: 10.1097/01.blo.0000176968.28247.5c. [DOI] [PubMed] [Google Scholar]
- Pellegrini VD., Jr Osteoarthritis of the trapeziometacarpal joint: the pathophysiology of articular cartilage degeneration. I. Anatomy and pathology of the aging joint. J Hand Surg Am. 1991;16:967–974. doi: 10.1016/s0363-5023(10)80054-1. [DOI] [PubMed] [Google Scholar]
- Pellegrini VD, Jr, Olcott CW, Hollenberg G. Contact patterns in the trapeziometacarpal joint: the role of the palmar beak ligament. J Hand Surg Am. 1993;18:238–244. doi: 10.1016/0363-5023(93)90354-6. [DOI] [PubMed] [Google Scholar]
- Pieron AP. The mechanism of the first carpometacarpal (CMC) joint. An anatomical and mechanical analysis. Acta Orthop Scand Suppl. 1973;148:1–104. doi: 10.3109/ort.1973.44.suppl-148.01. [DOI] [PubMed] [Google Scholar]
- Rainbow MJ, Crisco JJ, Moore DC, Kamal RN, Laidlaw DH, Akelman E, Wolfe SW. Elongation of the dorsal carpal ligaments: a computational study of in vivo carpal kinematics. J Hand Surg Am. 2012;37:1393–1399. doi: 10.1016/j.jhsa.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch RJ, Behrman MJ, Rosenwasser MP. Acute dislocation of the carpometacarpal joint of the thumb: an anatomic and cadaver study. J Hand Surg Am. 1994;19:93–98. doi: 10.1016/0363-5023(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Strauch RJ, Rosenwasser MP, Behrman MJ. A biomechanical assessment of ligaments preventing dorsoradial subluxation of the trapeziometacarpal joint. J Hand Surg Am. 1999;24:198–199. doi: 10.1053/jhsu.1999.jjhsu9924a1le03. [DOI] [PubMed] [Google Scholar]
- Tan J, Xu J, Xie RG, Deng AD, Tang JB. In vivo length and changes of ligaments stabilizing the thumb carpometacarpal joint. J Hand Surg Am. 2011;36:420–427. doi: 10.1016/j.jhsa.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Tomaino MM, Pellegrini VD, Jr, Burton RI. Arthroplasty of the basal joint of the thumb. Long-term follow-up after ligament reconstruction with tendon interposition. J Bone Joint Surg Am. 1995;77:346–355. doi: 10.2106/00004623-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Van Brenk B, Richards RR, Mackay MB, Boynton EL. A biomechanical assessment of ligaments preventing dorsoradial subluxation of the trapeziometacarpal joint. J Hand Surg [Am] 1998;23:607–611. doi: 10.1016/s0363-5023(98)80045-2. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Scher DL, Etchill EW, Scott F, Williams AE, Delaronde S, King KB. Relationship of Relaxin Hormone and Thumb Carpometacarpal Joint Arthritis. Clin Orthop Relat Res. 2013a doi: 10.1007/s11999-013-2960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JM, Williams AE, Delaronde S, Leger R, Clifton KB, King KB. Relationship of serum relaxin to generalized and trapezial-metacarpal joint laxity. J Hand Surg Am. 2013b;38:721–728. doi: 10.1016/j.jhsa.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Zhang A, Van Nortwick S, Hagert E, Ladd A. Thumb Carpometacarpal Ligaments Inside and Out: A Comparative Study of Arthroscopic and Gross Anatomy from the Robert A. Chase Hand and Upper Limb Center at Stanford University. Journal of Wrist Surgery. 2013;02:055–062. doi: 10.1055/s-0033-1333683. [DOI] [PMC free article] [PubMed] [Google Scholar]