Abstract
Ethanol-induced neuronal loss is closely related to the pathogenesis of fetal alcohol spectrum disorders. The cerebellum is one of the brain areas that are most sensitive to ethanol. The mechanism underlying ethanol neurotoxicity remains unclear. Our previous in vitro studies have shown that the double-stranded RNA (dsRNA)-activated protein kinase (PKR) regulates neuronal apoptosis upon ethanol exposure and ethanol activates PKR through association with its intracellular activator RAX. However, the role of PKR and its interaction with RAX in vivo have not been investigated. In the current study, by utilizing N-PKR−/− mice, C57BL/6J mice with a deficient RAX-binding domain in PKR, we determined the critical role of RAX/PKR association in PKR-regulated ethanol neurotoxicity in the developing cerebellum. Our data indicate that while N-PKR−/− mice have a similar BAC profile as wild-type mice, ethanol induces less brain/body mass reduction as well as cerebellar neuronal loss. In addition, ethanol promotes interleukin-1β (IL-1β) secretion, and IL-1β is a master cytokine regulating inflammatory response. Importantly, ethanol-promoted IL-1β secretion is inhibited in the developing cerebellum of N-PKR−/− mice. Thus, RAX/PKR interaction and PKR activation regulate ethanol neurotoxicity in the developing cerebellum, which may involve ethanol-induced neuroinflammation. Further, PKR could be a possible target for pharmacological intervention to prevent or treat fetal alcohol spectrum disorder (FASD).
Keywords: Alcohol, Neuron, Cerebellum, Apoptosis, Fetal alcohol spectrum disorders, PKR, Rax
Introduction
Maternal alcohol abuse during pregnancy may cause damage to the developing fetal brain and lead to fetal alcohol spectrum disorders (FASDs). Neuronal loss is one of the most devastating effects of fetal alcohol exposure. Microcephaly, learning deficits, and behavioral disorders, which are common in FASDs, are closely related to neuronal loss in the brain. The cerebellum is one of the brain areas that are most sensitive to ethanol, especially if exposed to ethanol during the third trimester of human embryo brain development [1]. Children with FASDs show many symptoms specifically associated with cerebellar damage. In the animal models, exposure of rodents to ethanol during postnatal days (PD) 4–9, a portion of the brain growth spurt period that parallels the human third trimester, or a binge-like episode of ethanol exposure at PD4 or 5, causes a significant neuronal loss in the cerebellum [2, 3]. Therefore, the vulnerability of neurons to ethanol differs among brain regions and changes with developmental stages. Although the molecular mechanisms underlying the spatiotemporal window of ethanol susceptibility remain unclear, neuroinflammation has been suggested as one of the mechanisms for ethanol-induced neurodegeneration [4]. Reduction of proinflammatory cytokines, such as interleukin-1β (IL-1β), has been shown to ameliorate ethanol-induced motor impairments [5].
The double-stranded RNA (dsRNA)-activated protein kinase (PKR) is an important mediator in orchestrating cellular response to a variety of insults, such as viral infection, oxidative stress, and serum deprivation [6]. In the absence of dsRNA, PKR activation is mediated by direct binding of its protein activators, PACT (protein kinase, interferon-inducible double-stranded RNA-dependent activator) in humans or its murine homologue, and RAX (PKR-associated protein X), via its N-terminus-binding domain. A mutation of the N-terminal dsRNA/RAX-binding domain abolishes the binding of dsRNA or RAX with PKR and thus PKR activation. Upon activation, PKR undergoes dimerization and autophosphorylation and regulates its downstream targets. The α-subunit of eukaryotic initiation factor-2 (eIF2α), a protein critically involved in protein synthesis initiation, is the most characterized substrate of PKR. In addition to regulating translation, PKR also transcriptionally regulates the expression of various genes, some of which encode proteins involved in apoptosis. Activation of PKR has been associated with a number of neurodegenerative diseases, such as Parkinson’s disease [7], Huntington’s disease [8], Alzheimer’s disease (AD) [9], and Creutzfeldt-Jakob disease (CJD) [10]. Further, the inflammasome, a key multiprotein oligomer that regulates cellular IL-1β production and inflammatory response, has been recently shown as a downstream target of PKR [11]. It has been reported that inflammasome/ IL-1β mediates ethanol neurotoxicity [4]. Lippai et al. showed that ethanol upregulates IL-1β and induces neuroinflammation through NLRP3 inflammasome [12]. Our previous work has shown that PKR regulates ethanol-induced neuronal apoptosis in vitro through RAX/PKR association [13]. Downregulation of PKR by genetic manipulation or pharmacological inhibitors ameliorated ethanol-induced neuronal apoptosis. More importantly, overexpression of wild-type RAX enhanced ethanol-induced RAX/PKR association, PKR activation, and neuronal apoptosis, while mutation of RAX reversed the consequences. Our more recent data further indicated that ethanol regulates RAX/PKR interaction via microRNA-29b/Sp1/RAX cascade [14]. However, the functions of PKR in ethanol neurotoxicity in vivo have not been established.
In the current study, by utilizing N-PKR−/− mice, we determined the role of RAX/PKR association in PKR-regulated ethanol neurotoxicity in the developing cerebellum. The N-PKR−/− mice are C57BL/6J mice with a targeted deletion of exon 2 and 3 at the N-terminus dsRNA/RAX-binding domain [15]. They are physically normal, showing no visible differences compared to their WT counterparts with regard to appearance and behavior [15]. Although this truncated PKR is catalytically active, it fails to bind RAX or dsRNA. Therefore, this strain of mice offers an excellent system to test whether RAX-mediated PKR activation regulates ethanol-induced neurotoxicity. By comparing the blood alcohol concentration (BAC) profiles, brain/body mass alterations and neuronal loss upon ethanol exposure between N-PKR−/− mice and their wild-type (wt) counterparts, we determined the critical role of RAX/PKR interaction in mediating PKR activation and ethanol neurotoxicity. Our data indicate that while N-PKR−/− mice have a similar BAC profile as wild-type mice, ethanol induces less brain/body mass reduction as well as cerebellar neuronal apoptosis. In addition, ethanol promotes IL-1β secretion and IL-1β is a master cytokine regulating inflammatory response. Importantly, ethanol-promoted IL-1β secretion is inhibited in the developing cerebellum of N-PKR−/− mice. Thus, RAX/PKR interaction and PKR activation regulate ethanol neurotoxicity in the developing cerebellum, which may involve ethanol-induced neuroinflammation. PKR could be a possible target for pharmacological intervention to prevent or treat FASD.
Materials and Methods
Materials
Anti-actin (A5441) and anti-calbindin-D-28K (C9848) antibodies were purchased from Sigma-Aldrich. Anti-NeuN antibody (MAB377) was obtained from Millipore. Antibodies directly against PKR (sc-6282), p-Thr-451-PKR (sc-101784), and RAX (sc-377103) were purchased from Santa Cruz, Dallas, TX. Antibodies against eIF2a (#5324), p-Ser51-eIF2a (#3398), and cleaved caspase-3 (#9661) were obtained from Cell Signaling, Beverly, MA.
Animals
The N-PKR−/− mice in C57BL/6J background [15] were kindly provided by Dr. Ganes C. Sen at Cleveland Clinic Lerner Research Institute. The wild-type C57BL/6 mice were obtained from Harlan Laboratory (Indianapolis, IN). All animals were housed and bred in the Animal Facility at the University of Kentucky Medical Center. N-PKR−/− and wild-type mice were age- and gender-matched and were used at least a month after delivery. All procedures were approved by the NIH and the Animal Care and Use Committee of the University of Kentucky.
Confirmation of pkr Gene Deletion
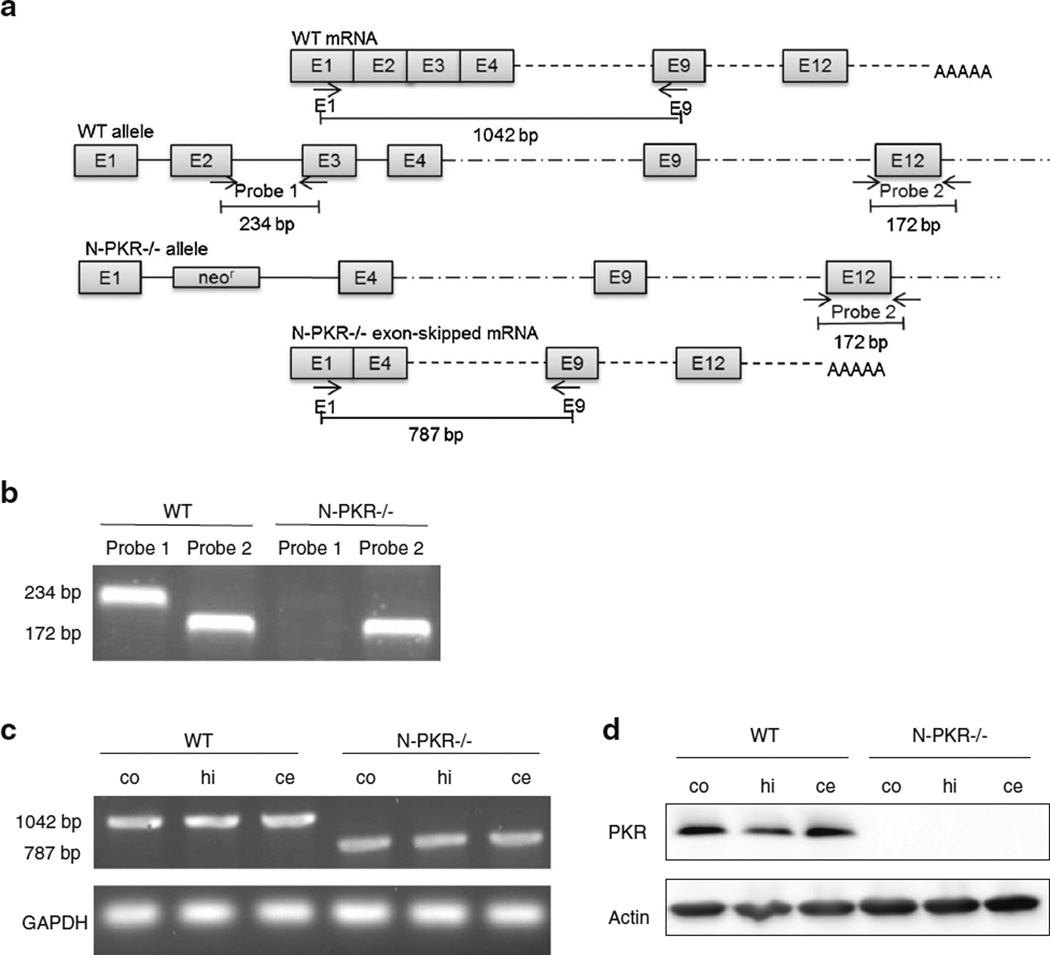
Genomic tail DNA was extracted from wt and N-PKR−/−mice using EZ Tissue/Tail DNA Isolation Kit (EZ Bioresearch, Saint Louis, MO) to verify the deletion of the N-terminal part of the pkr gene. The concentration of DNA was determined spectrophotometrically, and 100 ng of DNA was used as the template for amplification of pkr gene. According to the paper describing the generation of N-PKR −/− mice, the exon 2 and 3 of pkr gene were replaced by neomycin resistance gene [15]. Therefore, the following primers were designed to amplify a 234-bp fragment between exon 2 and 3 (probe 1) that is only present in wt but not in N-PKR−/− genomic DNA: 5′-CCTTGAGACAGACCCTGG TCACCC-3′ and 5′-GGGGGTGGGTTA GAGTATATGTTG CATGGG-3′. As a control, the following primers were used to amplify a 172-bp fragment (probe 2) in exon 12 of the pkr gene that is present in both wt and N-PKR−/− genomic DNA: 5′-TTTTGTATCCAGATACAAAACCCGGTGCCTCT-3′ and 5′-CCCTTTCGAGTGTATATACTCCACTCCGGTCA-3′. Reverse transcription PCR was also used to confirm the deletion of the N-terminal region of the pkr gene in N-PKR−/− mice. RNA was isolated from the neocortex, cerebellum, and hippocampus of adult mice using the TRIzol method (Life Technologies, Grand Island, NY). One microgram RNA from each brain region was reverse transcribed using Superscript™ II Reverse Transcriptase (Life Technologies, Grand Island, NY), and 100 ng of cDNA was used as the template for amplification of pkr gene. A set of primers were used: 5′-CAGGGAACGGAGGGCGAATAGAT-3′ and 5′-CAGGAT CATAGTCAACTCCCTCCCAAC-3′. This set of primers was designed to amplify a 1042-bp fragment from wt pkr transcript but only a 787-bp fragment from deletion mutant transcript of pkr gene. The amplified RT-PCR products were sequenced by an automated sequencer. For PCR, the initial denaturation was at 95 °C for 3 min, followed by 35 cycles of 94 °C/30 s, 60 °C/30 s, and 72 °C/60 s, and a final extension of 72 °C for 10 min. The PCR-amplified products were electrophoresed onto a 2.0 % agarose gel and visualized by ethidium bromide.
Animal Ethanol Exposure
A total of 84 pups (42N–PKR−/− and 42 wt) with seven animals in each treatment group were used in this study. An ethanol exposure paradigm, which had been shown to cause significant neurodegeration in mice, was applied [16, 17]. Briefly, on PD4, litters were culled to seven pups per litter, and the pups were randomly assigned to each of the six groups (suckle, saline on PD4, saline on PD4–9, 2.2 g/kg ETOH on PD4, 4.4 g/kg ETOH on PD4, and 4.4 g/kg ETOH daily on PD4–9). At 9 a.m. each day, the pups were weighed and a single injection of 10 or 20 % alcohol-containing solution in sterile saline was given intraperitoneally (i.p.) either on PD4 only or daily over PD4–9. The daily volume injected was 27.5 µl per gram of body weight. Immediately after the injection, the pups were returned to their mothers and allowed to nurse. The saline groups received saline either on PD4 or daily over PD4–9. The suckle controls were littermates of saline or ethanol treatment groups.
BACs
BACs were determined by measuring the tail blood samples. The blood samples were obtained 15min, 30min, 45min, 1 h, 2 h, 4 h, 6 h, and 8 h after ethanol injection on PD4. BACs were measured using an ethanol assay kit from Sigma-Aldrich, St. Louis, MO (product number MAK076), following the manufacturer’s directions.
Body/Brain Weights
The procedure for measuring the body and brain weights has been described previously [16]. Briefly, the body weights of wt and N-PKR−/− mice were determined at 9 a.m. on PD4–10. The brain weights were measured at 9 a.m. on PD10. The pups were anesthetized with ketamine and perfused intracardially with 0.9 % saline, followed by a fixative containing 4.0 % (w/v) paraformaldehyde. Each brain was removed. The weights of the total brain, the forebrain, and the cerebellum were measured by using an analytic balance (MS104S, Mettler Toledo, Columbus, OH).
Primary Culture of CGNs
Cerebellar granule neurons (CGNs) were isolated from PD7 pups and cultured as described previously [18, 19]. Briefly, neonatal mice at PD7 were decapitated, and the cerebella were carefully collected. The tissue was minced with a sterile razor blade and suspended in 10 ml of trypsin (0.025 %) solution at 37 °C. After incubation for 15 min, an equal volume of solution containing DNase (130 Knuitz U/ml) (D4527, Sigma-Aldrich, St. Louis, MO) and trypsin inhibitor (0.75 mg/ml) (T9003, Sigma-Aldrich, St. Louis, MO) was added, and the tissue was sedimented by a brief centrifugation. The tissue was dissociated by trituration, and the cell suspension was centrifuged through a 4 % bovine serum albumin solution. The cell pellet was re-suspended at a concentration of 1.5×106 cells/ml in medium consisting of Neurobasal-A medium (10888-022, Life Technologies, Grand Island, NY) containing B-27 serum-free supplement (17504-044, Life Technologies, Grand Island, NY), 1 mM l-glutamine (25030-081, Life Technologies, Grand Island, NY), 100 U/ ml penicillin, and 100 µg/ml streptomycin (15140-148, Life Technologies, Grand Island, NY). Cells were then placed in poly-D-lysine-coated cell culture plates. Cells were incubated at 37 °C in a humidified environment containing 5 % CO2 for 48 h before ethanol treatment.
In Vitro Ethanol Exposure Protocol
The method for ethanol exposure in vitro has been described previously [13]. Due to the volatility of ethanol, a sealed container was used to maintain ethanol levels in the culture medium. With this method, ethanol concentrations in the culture medium could be accurately maintained and confirmed by using an ethanol assay kit from Sigma-Aldrich, St. Louis, MO. A series of concentration of 200, 400, and 800 mg/dl was used in this study as described previously [19].
Viability of the CGNs
The procedure to determine the number of viable CGNs in culture has been described previously by using a Cell Counting Kit-8 (CCK-8, Dojindo Molecular, Rockville, MD) [14]. Briefly, CGNs were cultured in a 96-well microplate at a density of 3×105 cells/well for 24 h. The cells were then treated with ethanol at 200, 400, or 800 mg/dl for 24, 48, or 72 h. Untreated CGN cells were used as a negative control. There were three replicates for each treatment. After treatment, 10 µl of CCK-8 solution was added to each well of the plate, and the cells were incubated at 37 °C for 2 h. The optical density at a wavelength of 450 nm was measured with a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA). The results were expressed as the mean± S.E. from three independent experiments.
Tissue Preparation for Immunohistochemistry
The procedure for tissue preparation for immunohistochemistry has been described previously [20]. Briefly, the pups were weighed in the morning of postnatal day 10, anesthetized, and perfused by PBS, followed by 4% paraformaldehyde fixation. The brains were removed and stored in fixative at 4 °C for 24 h followed by 30 % sucrose solution at 4 °C for 24 h for cryoprotection. The cryoprotected brains were exhaustively cut on a freezing microtome into sagittal sections at a nominal thickness of 40 µm. The serial sections were immediately placed into a cryoprotectant anti-freezing solution in consecutive order and stored at −20 °C for further processing.
Immunohistochemical Procedures
Mouse brain sections were processed as previously described [13, 20]. Antibodies for neuron-specific markers, anti-NeuN (1:4000) for granule neurons, and anti-Calbindin-D-28K (1: 4000) for Purkinje cells were used followed by biotinylated goat anti-mouse secondary IgG (1:1000). The tissue sections were then treated with an avidin-biotinylated-peroxidase kit (ABC Elite kit, Vector Laboratories, Burlingame, CA). The specificity of each antibody on the brain sections was confirmed by the absence of staining in the control experiments which lacked either primary or secondary antibodies. The immunostained sections were mounted onto gelatin-coated glass slides, examined with light microscopy, and their images were digitized by a slide scanner (LS-2000, Nikon Inc., Melville, NY).
Stereological Cell Counting
After Purkinje cells and the granule neurons were labeled with anti-calbindin-D-28K or anti-NeuN, respectively, the numbers of both neurons in each section were counted by unbiased stereology that has been described previously [16, 20, 21]. Purkinje cells and the granule neurons were visualized using a ×60 oil immersion objective on an Olympus BX-51 microscope equipped with a motorized stage and stereology software (Bioquant Life Science, V8.40.20; Bioquant Image Analysis, Nashville, TN). Serial sagittal sections across the cerebellum were collected, and every fifth section was used. A counting frame (200×200 µm2) was optically superimposed over the granule or Purkinje cell layer within each section. Fifteen to 20 dissectors were randomly positioned by the stereology program over the defined tissue area, and dissectors of different size were used for the granule neurons (20× 20 µm2) and Purkinje cells (50×50 µm2) based on our previous experience. Data were tabulated in the Bioquant database. The total numbers (N) of both neurons were estimated using the formula: N=total cells counted×1/ssf×1/asf×1/tsf, where “ssf” is the section sampling fraction, “asf” is the area sampling fraction, and “tsf” is the thickness sampling fraction.
Immunoblotting and Immunoprecipitation
The procedure for immunoblotting has been previously described [19, 22]. Briefly, neonatal mice were anesthetized with ketamine. The cerebella were dissected and lysed with RIPA buffer [150 mM NaCl, 0.1 % sodium dodecyl sulfate (SDS), 50 mM Tris (pH 8.0), 0.5 % deoxycholic acid sodium, 1 % Nonidet P-40 (NP-40), 0.1 mg/ml phenylmethylsulfonyl fluoride, 3 % aprotinin, and 1 mM sodium orthovanadate] on ice for 30 min. Cell lysates were centrifuged at 13,000 rpm at 4 °C for 15 min. The supernatant was collected afterward, and the protein concentration was measured using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). An aliquot of 40-µg cerebellar protein was loaded into each lane of an SDS-polyacrylamide gel. The protein was electrophoretically transferred to nitrocellulose membranes followed by blocking with 5 % BSA in 0.01 MTBS (pH 7.4) and 0.05 % Tween-20 (TBST) at room temperature for 1 h. The blots were probed with primary antibodies at 4 °C overnight. After three washes with TBST, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG (Amersham, Arlington Heights, IL) for 1 h, and the bands were visualized with enhanced chemiluminescence method (Amersham, Pittsburgh, PA). Experiments were replicated three times, and results were quantified by densitometry and expressed as the mean±S.E.
Immunoprecipitation was performed as previously described [22]. Briefly, an aliquot of 200 µg of cerebellar protein was incubated with antibodies against PKR or RAX (1:50), respectively, overnight at 4 °C. Twenty microliters of protein A/G conjugated to agarose (Santa Cruz, Dallas, Texas) was added to the lysate, and the mixture was incubated for 3 h at 4 °C. Immunoprecipitates were collected by centrifugation at 10,000×g for 10 min. Thereafter, the pellets were washed and resuspended in 20 µl of 3× SDS sample buffer and analyzed for the expression of specific proteins by immunoblotting.
ELISA
The procedure for ELISA has been previously described [12]. Briefly, 8 h after ethanol treatment, tissue lysates were prepared from the cerebellum in RIPA buffer containing protease and phosphatase inhibitors (1 mM PMSF, 1 mM NaF, 2 mM Na3VO4, 20 mM Na4P2O7). The cerebella were homogenized with a sonicator and then clarified by centrifugation. The IL-1β levels in the cerebella lysate supernatants were measured by using a Mouse IL-1β ELISA kit (ELM-IL1b-CL, RayBiotech, Norcross GA). The results were expressed as the mean±S.E. from three independent experiments.
Statistical Analysis
The procedure for statistical analysis has been described previously [19]. Briefly, differences among treatment groups were analyzed by analysis of variance (ANOVA). p<0.05 was considered statistically significant. If significant differences were detected, specific post hoc comparisons between treatment groups were examined by Student–Newman–Keul tests.
Results
Confirmation of PKR Gene Disruption in N-PKR−/− Mice
PCR and RT-PCR were performed to confirm the deletion of exon 2 and 3 at the N-terminal of the pkr gene in the N-PKR−/− mice (Fig. 1a). The PCR results (Fig. 1b) confirmed the disruption of exon 2 and 3 in the tail DNA of N-PKR−/− mice but present in the strain-matched C57BL/6 wt mice, which is consistent with the previous report [15]. RT-PCR (Fig. 1c) and Western blot ( Fig. 1d) data further confirmed the disruption of exon 2 and 3 of pkr gene in the cerebral cortex, hippocampus, and cerebella of N-PKR−/− mice used in the study.
Fig. 1.
Confirmation of the disruption of the pkr gene in N-PKR−/− mice. a Schematic representation of the pkr gene in wt and N-PKR−/− as well as the primers used in the PCR and RT-PCR analysis. b Part of exon 2 and 3 was missing in the tail DNA of N-PKR−/− mice but present in the strain-matched C57BL/6 wt mice. c Total RNAs were isolated from the cerebral cortex (co), hippocampus (hi), cerebellum (ce) of wt, and N-PKR −/− mice at age of PD6. The transcrips of pkr gene of wt or N-PKR−/− mice were determined by RT-PCR. d PKR protein levels in the cerebral cortex (co), hippocampus (hi), cerebellum (ce) of wt, and N-PKR−/− mice at age of PD6 were determined by immunoblotting
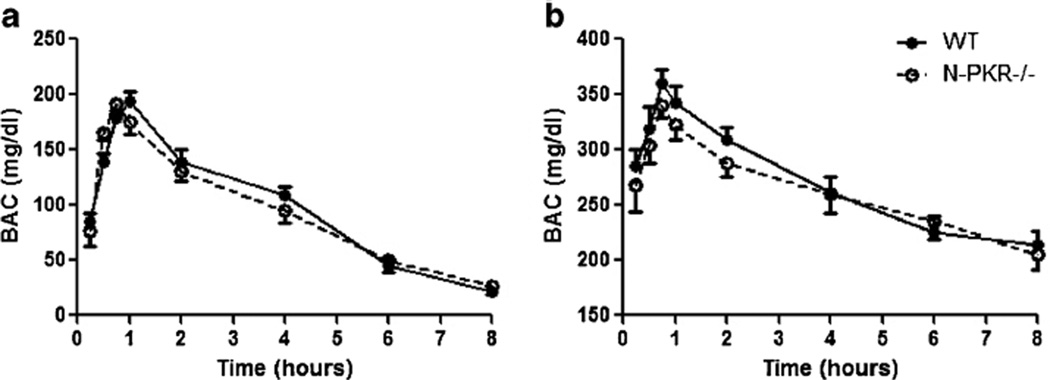
N-PKR−/− and wt Mice Had Similar BAC Profiles
To determine whether disruption of pkr gene may alter BAC profile, a time course study of BACs was applied to wt and N-PKR−/− mice following the single i.p. injection of alcohol at 2.2 g/kg or 4.4 g/kg on PD4. These ethanol exposure paradigms for BAC determination have been used previously [17]. At both doses, N-PKR−/− mice displayed a BAC curve that was similar to wt mice with a peak at 45 min after ethanol treatment (Fig. 2). In particular, the alcohol levels remained above 200 mg/dl for over 8 h following 4.4 g/kg dose that has been shown to produce neuronal apoptosis in neonatal mice [23]. The results indicate that the disruption of pkr gene in its N-terminal does not affect ethanol absorption or metabolism.
Fig. 2.
BAC profiles are similar in wt and N-PKR−/− mice. Ethanol was administered at a dose of 2.2 g/kg (a) or 4.4 g/kg (b) as a single intraperitoneal injection to wt and N-PKR−/− mice on PD4. BACs were measured at 15 min, 30 min, 45 min, 1 h, 2 h, 4 h, 6 h, and 8 h after ethanol treatment. Data are expressed as the mean±S.E. (n=7)
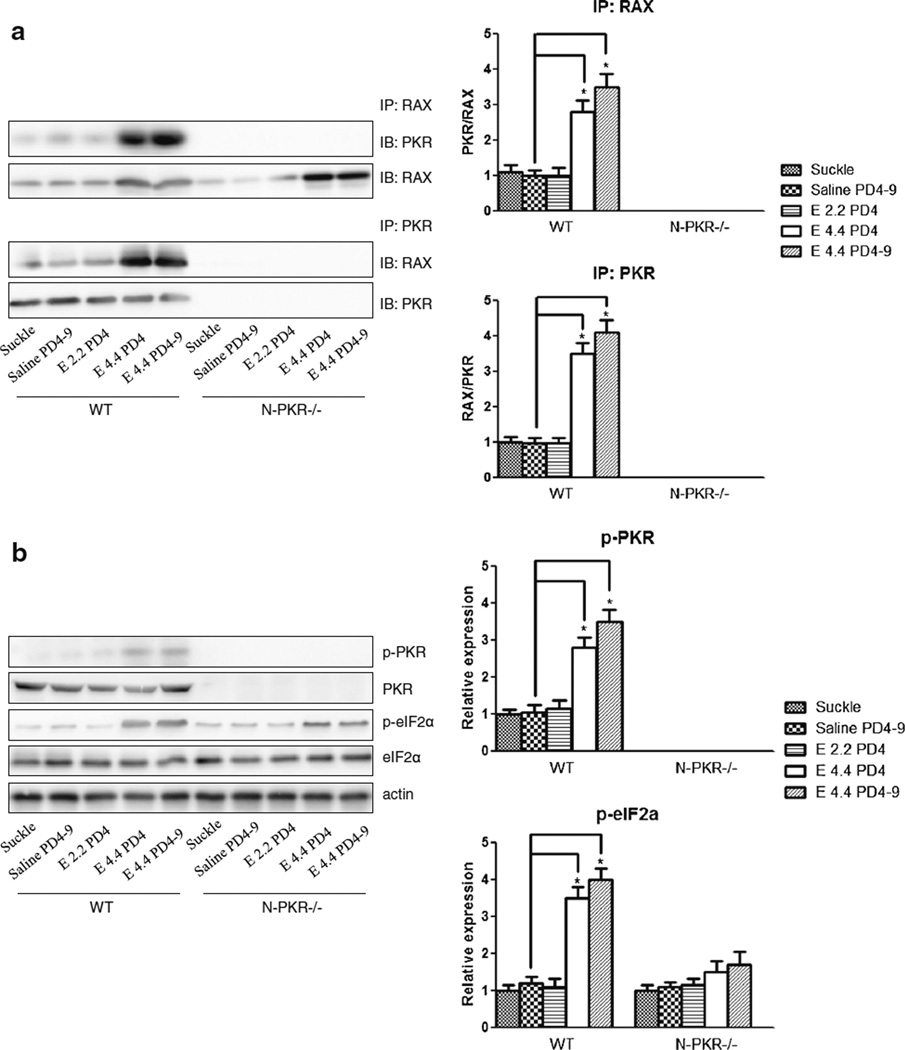
Ethanol-Induced RAX/PKR Association and PKR Activation in the Developing Cerebellum Is Inhibited in N-PKR−/− Mice
We have previously shown that ethanol-induced PKR activation is mediated by RAX/PKR association in vitro which is confirmed by the phosphorylation of its downstream target eIF2α [13]. Since the N-terminus-binding domain of PKR is the binding site for RAX, we sought to determine the binding of RAX/PKR as well as PKR activation upon ethanol exposure in the cerebellum of N-PKR−/− and wt mice. As shown in Fig. 3a, the binding between PKR and RAX was significantly enhanced by ethanol at 4.4 g/kg on PD4 or during PD4–9 inwtmice, but the enhancement was much milder in N-PKR −/− mice. No PKR/RAX interaction was observed validating the requirement of the N-terminal region for RAX association. Consequently, ethanol-induced PKR activation was inhibited in N-PKR−/− mice compared to wt mice evidenced by the phosphorylation of PKR and eIF2α in the same treatment groups (Fig. 3b). Of note, ethanol at 2.2 g/kg had minimum effects on RAX/PKR association as well as PKR activation.
Fig. 3.
Ethanol-induced RAX/PKR association and PKR activation in the developing cerebellum were inhibited in N-PKR−/− mice. a Protein samples from the cerebellum of wt and N-PKR−/− mice were collected 8 h after treatment. The mice were exposed to no ethanol (suckle), saline daily on PD4–9 (saline), 2.2 or 4.4 g/kg ethanol on PD4 (E 2.2 PD4 or E 4.4 PD4), or 4.4 g/kg during PD4–9 (E 4.4 PD4–9). The binding of RAX and PKR in the cerebellum was determined by immunoprecipitation (IP) with a RAX antibody and followed by immunoblotting (IB) with either PKR or RAX antibody; or IP with PKR antibody and IB with either a PKR or RAX antibody. The ratio of PKR/RAX or RAX/PKR in each IP experiment relative to saline controls was quantified by densitometry. b Ethanol-induced phosphorylation of PKR and eIF2α in the cerebellum of wt or N-PKR−/− mice was determined by immunoblotting, quantified by densitometry and normalized to matched actin levels. Data are expressed as the mean±S.E. of three independent experiments. *p<0.05
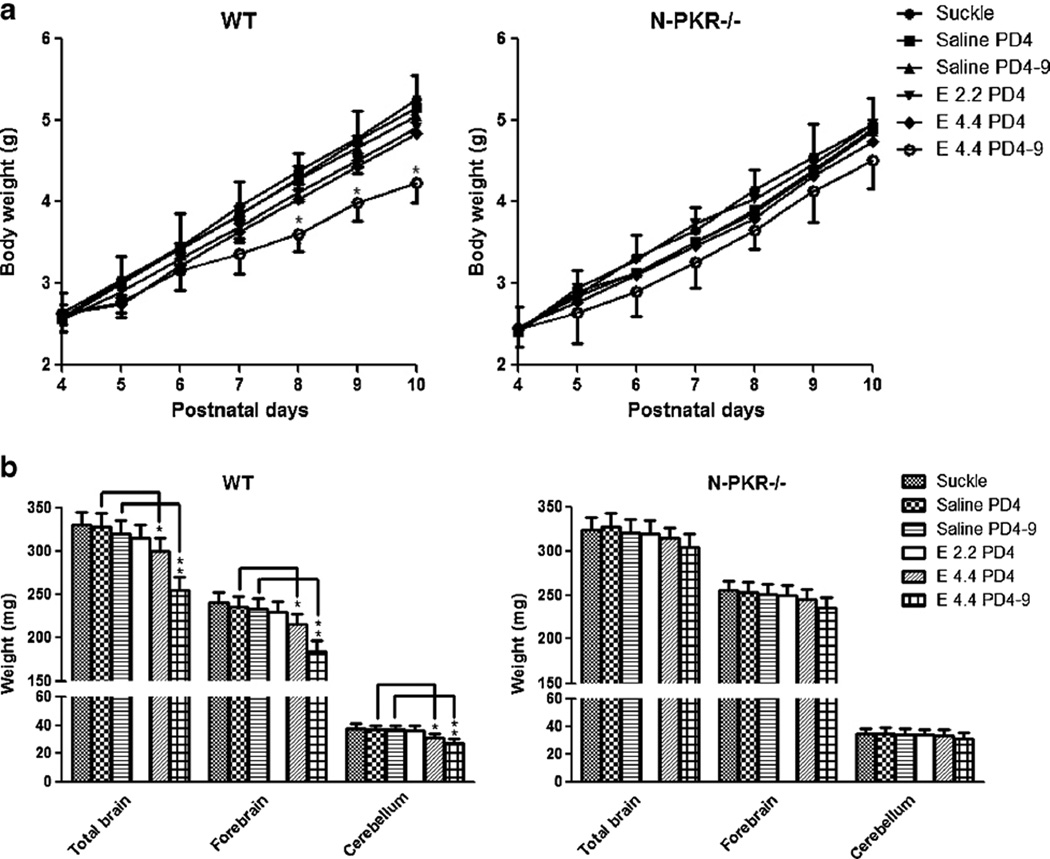
N-PKR−/− Mice Are More Resistant to Ethanol-induced Body Weight Loss and Microcephaly than wt Mice
Ethanol exposure on PD4–9 has been previously shown to reduce the body weight and brain mass [17]. To compare the effects of ethanol exposure on the weights of the body and brain in N-PKR−/− and wt mice, the body weights over PD4– 10 and brain weights on PD10 were determined. As shown in Fig. 4a, the body weights in all six groups for N-PKR−/− and wt mice progressively increased over PD4–10. However, distinct ethanol treatment appeared to have different effects on the body weight. The wt mice that received daily injection of ethanol at a dose of 4.4 g/kg over PD4–9 had significant less body weight than saline controls measured on PD8–10. These animals resumed normal weight gain after suspension of ethanol treatment on PD10 (data no shown). In contrast, the reduction of body weight by the same ethanol treatment was statistically insignificant in N-PKR−/− mice. Ethanol exposure at 2.2 or 4.4 g/kg on PD4 alone on either N-PKR−/− or wt mice did not significantly affect body mass compared with the suckle or saline controls.
Fig. 4.
N-PKR−/− mice are more resistant to ethanol-induced body weight loss and microcephaly than wt mice. a Body weight profiles of N-PKR−/− and wt mice were generated during PD4–10 with or without ethanol treatment. Data are mean±S.E. (n=7). *p<0.05 versus the saline control. b Ethanol-induced alterations in the weights of total brain, forebrain, and cerebellum were compared between the wt and N-PKR−/− mice on PD10. Data are expressed as the mean±S.E. (n=7). *p<0.05; **p<0.01
To determine the effect of N-PKR−/− on ethanol-induced microcephaly, the brain weights of wt and N-PKR−/− mice were determined on PD10 after ethanol exposure. First, as shown in Fig. 4b, the weights of total brain, forebrain, and cerebellum of N-PKR−/− mice in the control groups were not significantly different from those of wt mice, indicating disruption of pkr gene does not affect brain growth. Second, ethanol exposure reduced the weights of these brain tissues of wt mice in a dose-dependent manner. Third, ethanol at 4.4 g/kg on PD4 or 4.4 g/kg on PD4–9 significantly reduced the weights of these brain tissues in wt mice. In contrast, ethanol failed to decrease any of the weights of total brain, forebrain, or cerebellum of N-PKR−/− mice in all three paradigms.
CGNs Isolated from N-PKR−/− Mice Are More Resistant to Ethanol-Induced Apoptosis than Those from wt Mice
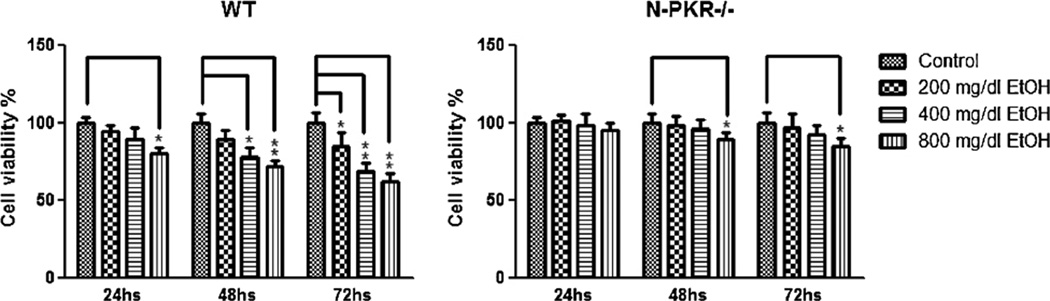
One of the most devastating consequences of ethanol exposure on the developing cerebellum is ethanol-induced neuronal loss. The primary culture of the CGNs isolated from the neonatal cerebellum has been widely used to examine ethanol neurotoxicity [18]. To determine the effect of N-PKR−/− on ethanol-induced neuronal apoptosis, primary culture of CGNs isolated from N-PKR−/− mice and wt mice at age PD7 was exposed to a series of physiologically relevant concentrations of ethanol, and the viable neuronal numbers were determined 24, 48, and 72 h thereafter. As shown in Fig. 5, ethanol at concentrations of 200, 400, and 800 mg/dl reduced the numbers of wt CGNs in a time- and dose-dependent manner, which is consistent with previous reports [19, 24]. In contrast, the CGNs from N-PKR−/− mice were more resistant to ethanol toxicity. Ethanol at 200 and 400 mg/dl did not significantly reduce the neuronal numbers at all three time-points. While ethanol at 800 mg/dl did not reduce the number at 24 h, it did reduce the numbers significantly at 48 and 72 h, which, however, was much milder than that in wt mice.
Fig. 5.
CGNs isolated from N-PKR−/− mice are more resistant to ethanol-induced apoptosis than those from wt mice. Primary cultured CGNs extracted from wt or N-PKR−/− mice were treated with 200, 400, or 800 mg/dl ethanol for 24, 48, or 72 h. Cell viabilities were determined 24, 48, or 72 h after ethanol treatment using Cell Counting Kit-8. Experiments were replicated three times, and data are expressed as the mean±S.E. *p<0.05; **p<0.01 versus matched control groups
N-PKR−/− Mice Are More Resistant to Ethanol-Induced Neuronal Loss in the Developing Cerebellum
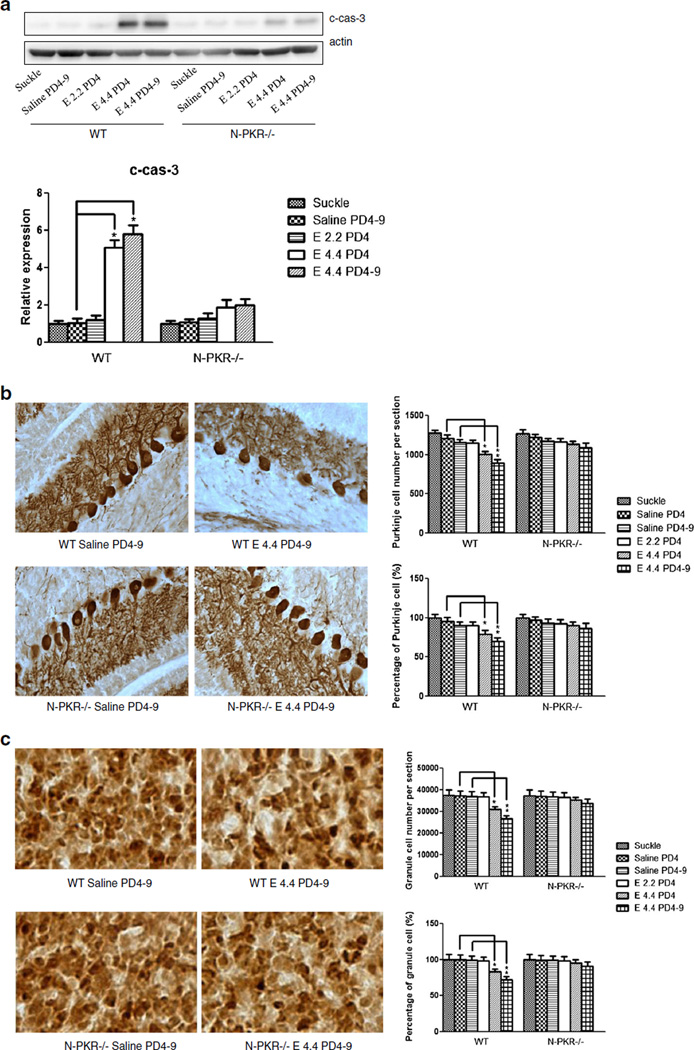
Previously, it has been shown that ethanol exposure causes neuronal apoptosis in the developing cerebellum of the mouse [16, 19, 25, 26]. To determine whether ethanol toxicity observed in cultured CGNs reflects the in vivo conditions, ethanol-induced caspase-3 activation as well as neuron number alteration was determined in the cerebellum of N-PKR−/− and wt mice. We first applied immunoblotting to examine the expression of cleaved caspase-3 in the cerebella of ethanol-exposed animals. The results showed that while ethanol at 2.2 g/kg on PD4 did not activate caspase-3 in both strains, ethanol at 4.4 g/kg on PD4 or 4.4 g/kg daily during PD4–9 increased the levels of cleaved caspase-3 in both wt and N-PKR−/− mice, but the increases were significantly milder in N-PKR−/− than in wt mice under the same paradigm (Fig. 6a). We then quantified the Purkinje neurons and granule neurons in the cerebellum by stereological cell counting after immu-nohistochemical labeling. Purkinje neurons were labeled by anti-calbindin-D28K antibody [27], and the granule neurons were stained by anti-NeuN antibody [28, 29] in the cerebellum on PD10. In the absence of ethanol treatment, the number of Purkinje cells and granule cells is similar in the N-PKR−/− and wt mice (Fig. 6b, c), suggesting that N-PKR−/− does not affect the survival of either neurons. With ethanol treatment, both Purkinje and granule neurons in wt mice showed a greater vulnerability to ethanol evidenced by the significant reductions of the neuronal numbers. However, the reductions in both types of neuron by ethanol were insignificant in N-PKR−/− mice.
Fig. 6.
N-PKR−/− mice are more resistant to ethanol-induced neuronal loss in the developing cerebellum. a Protein samples from the cerebella of wt or N-PKR−/− mice were collected 8 h after ethanol treatment. Ethanol-induced activation of caspase-3 (cleaved caspase-3, c-cas-3) was determined by immunoblotting, qualified by densitometry and normalized to matched actin levels. Data are expressed as the mean± S.E. of three independent experiments. *p<0.05. b Ethanol-induced alteration in the numbers of Purkinje cells in the cerebellum of wt or N-PKR −/− mice was determined on PD10 by stereological counting. Purkinje cells were labeled with an anti-calbindin D28K antibody. c Ethanol-induced alteration in granule neurons in the cerebellum of wt than N-PKR −/− mice was also determined on PD10. The granule neurons were labeled with anti-NeuN antibody, and intense brown staining was assessed. Seven animals were used for each group, and data are expressed as the mean±S.E. *p<0.05; **p<0.01
Ethanol-Enhanced IL-1β Production in the Developing Cerebellum in wt Mice Is Inhibited in N-PKR−/− Mice
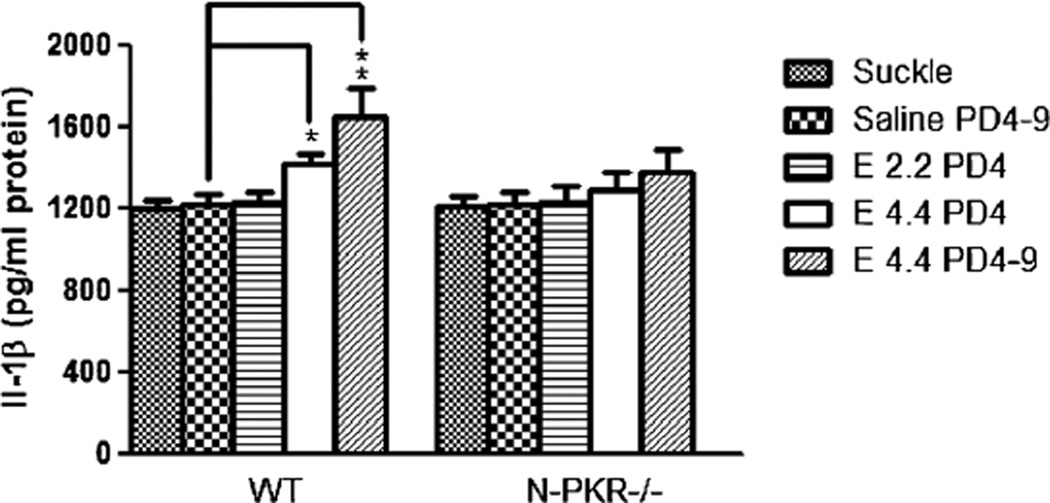
Recently, PKR has been identified as an important regulator of inflammasome which is a critical component of innate immune system [11, 30]. Inflammasome is a multiprotein oligomer that promotes the maturation and secretion of inflammatory cytokines such as IL-1β. Inflammatory cytokines have long been suggested as important mediators for ethanol neurotoxicity [31]. Crews’ group has shown that inflammasome/ IL-1β signaling mediates ethanol-induced inhibition of hip-pocampal neurogenesis [4]. Hence, we thought to determine whether N-PKR−/− alters ethanol-induced IL-1β production in the developing cerebellum. As shown in Fig. 7, ethanol exposure at 2.2 g/kg on PD4 did not significantly promote the IL-1β production in the cerebellum of both strains. However, ethanol at 4.4 g/kg on PD4 or 4.4 g/kg over PD4–9 significantly increased IL-1β production in the cerebellum of wt mice. In contrast, the increase of IL-1β production by ethanol was insignificant in N-PKR−/− mice.
Fig. 7.
Ethanol-enhanced IL-1β production in the developing cerebellum was inhibited in N-PKR−/− compared to that in wt mice. IL-1β in the cerebellar lysates of wt or N-PKR−/− mice was analyzed by ELISA assay 8 h after ethanol treatment. Data are mean±SEM of three experiments. *p<0.05; **p<0.01
Discussion
The results from this study indicate an important role of RAX/ PKR association in regulating PKR activity as well as ethanol neurotoxicity. The N-PKR−/− mice express a truncated PKR protein which is defective in dsRNA/RAX binding but remains catalytically intact. In addition, the N-PKR−/− mice are physically normal with no visible differences in appearance and behavior compared to their WT counterparts [15]. Thus, the N-PKR−/− mice are an ideal model for investigating RAX-mediated PKR activation and their roles in ethanol neurotoxicity. Our previous in vitro work has shown that the levels of PKR in the developing cerebellum are relatively even from PD3–21, but ethanol-induced PKR activation is varied among these dates, which positively correlates the levels of RAX and binding of RAX/PKR [13]. It is worth noting that, although high expression of RAX enhances cell death caused by ethanol, ceramide, and IL-3 deprivation, overexpression of RAX alone is not sufficient to cause cell death [32, 33]. Instead, it is the enhanced association of RAX/PKR that accounts for PKR activation and the neuronal loss. In the current study, we found that, despite having similar BAC profiles in wt or N-PKR−/− mice, ethanol’s effects on body/brain weights as well as neuronal numbers in the developing cerebellum are different. Ethanol-induced reductions in body/brain weights are much milder in N-PKR−/− mice. This result confirms that, in addition to regulating cell survival/ death, PKR is a key regulator of protein synthesis through eIF2α. It appears that ethanol-inhibited PKR/eIF2-regulated protein synthesis contributes to body/brain weight reduction observed in wt mice. More importantly, N-PKR−/− renders the cerebellar neuron protection against ethanol-induced neuronal loss. N-PKR−/− not only decreases ethanol-induced apoptosis in the primary cultured CGNs but also protects against ethanol-induced loss of the granule neurons and Purkinje cells in the cerebellum after ethanol exposure on PD4 or PD4–9. Collectively, our data indicate that the interaction of RAX/PKR is critical for ethanol-induced PKR activation and ethanol neurotoxicity. The developing cerebella of the mice with deficient PKR in the N-terminus RAX-binding domain are less susceptible to ethanol-induced neuronal loss and microcephaly compared to wt mice.
Neuroinflammation has been suggested as a mechanism underlying ethanol neurotoxicity, and ethanol sensitizes blood and brain inflammatory response [31]. PKR has been shown as a key regulator of the cellular inflammatory process and controls the production of IL-1β and HMGB1 in response to a variety of physiochemical insults [11]. In primary cultures containing mouse neurons, astrocytes, and microglia, inhibition of PKR prevents activation of NF-kB and leads to a strong decrease in production and release of tumor necrosis factor α (TNF-α) and IL-1β [6]. Blocking of TLR4/IL-1R1, the receptor of IL-1, abolishes ethanol-induced inflammatory processes and cell death [34–36].
In this study, we have shown that RAX/PKR interaction and PKR activation regulate ethanol neurotoxicity in the developing cerebellum. Blockade of RAX/PKR association in N-PKR−/− mice inhibits ethanol-induced PKR activation, IL-1β production, and neuronal apoptosis. Our data indicate that ethanol neurotoxicity may involve ethanol-induced neuroinflammation, which may, at least partially, account for PKR’s actions. Moreover, since pharmacological inhibitors of PKR are available [13], PKR could be a target for pharmacological intervention to prevent or treat FASD.
Acknowledgments
The authors would like to thank Dr. Ganes C. Sen at Cleveland Clinic Lerner Research Institute for providing the N-PKR−/− mice. This work was supported by NIH grant RO1AA020051 to GC.
Footnotes
Conflicts of Interest The authors declare that they have no conflict of interest.
Contributor Information
Hui Li, Department Pharmacology & Nutritional Sciences, University of Kentucky College of Medicine, MN306, UKMC, 800 Rose street, Lexington, KY 40536, USA.
Jian Chen, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian 361001, China.
Yuanlin Qi, Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Fujian Medical University, Fuzhou, Fujian 350004, China.
Lu Dai, Graduate Center for Toxicology, University of Kentucky College of Medicine, Lexington, KY 40536, USA.
Mingfang Zhang, Department of Physiology and Pathophysiology, School of Basic Medical Sciences, Fujian Medical University, Fuzhou, Fujian 350004, China.
Jacqueline A. Frank, Department Pharmacology & Nutritional Sciences, University of Kentucky College of Medicine, MN306, UKMC, 800 Rose street, Lexington, KY 40536, USA
Jonathan W. Handshoe, University of Kentucky, Lexington, KY 40536, USA
Jiajun Cui, Department Pharmacology & Nutritional Sciences, University of Kentucky College of Medicine, MN306, UKMC, 800 Rose street, Lexington, KY 40536, USA.
Wenhua Xu, Department of Neurology, Affiliated Provincial Hospital of Anhui Medical University, Hefei 230001, China.
Gang Chen, Email: gangchen6@uky.edu, Department Pharmacology & Nutritional Sciences, University of Kentucky College of Medicine, MN306, UKMC, 800 Rose street, Lexington, KY 40536, USA.
References
- 1.West JR. Fetal alcohol-induced brain damage and the problem of determining temporal vulnerability: a review. Alcohol Drug Res. 1987;7:423–441. [PubMed] [Google Scholar]
- 2.Karacay B, Li S, Bonthius DJ. Maturation-dependent alcohol resistance in the developing mouse: cerebellar neuronal loss and gene expression during alcohol-vulnerable and -resistant periods. Alcohol Clin Exp Res. 2008;32:1439–1450. doi: 10.1111/j.1530-0277.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 3.Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- 4.Zou J, Crews FT. Inflammasome-IL-1beta signaling mediates ethanol inhibition of Hippocampal neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, et al. Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun. 2011;(25 Suppl 1):S155–S164. doi: 10.1016/j.bbi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Marchal JA, Lopez GJ, Peran M, Comino A, Delgado JR, Garcia-Garcia JA, et al. The impact of PKR activation: from neurodegener-ation to cancer. FASEB J: Off Publ Fed Am Soc Exp Biol. 2014;28:1965–1974. doi: 10.1096/fj.13-248294. [DOI] [PubMed] [Google Scholar]
- 7.Bando Y, Onuki R, Katayama T, Manabe T, Kudo T, Taira K, et al. Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson’s disease and Huntington’s disease. Neurochem Int. 2005;46:11–18. doi: 10.1016/j.neuint.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Peel AL, Rao RV, Cottrell BA, Hayden MR, Ellerby LM, Bredesen DE. Double-stranded RNA-dependent protein kinase, PKR, binds preferentially to Huntington’s disease (HD) transcripts and is activated in HD tissue. Hum Mol Genet. 2001;10:1531–1538. doi: 10.1093/hmg/10.15.1531. [DOI] [PubMed] [Google Scholar]
- 9.Peel AL, Bredesen DE. Activation of the cell stress kinase PKR in Alzheimer’s disease and human amyloid precursor protein transgenic mice. Neurobiol Dis. 2003;14:52–62. doi: 10.1016/s0969-9961(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 10.Paquet C, Bose A, Polivka M, Peoc’h K, Brouland JP, Keohane C, et al. Neuronal phosphorylated RNA-dependent protein kinase in Creutzfeldt-Jakob disease. JNeuropathol Exp Neurol. 2009;68:190–198. doi: 10.1097/NEN.0b013e318196cd7c. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, et al. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94:171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Ma C, Bower KA, Ke Z, Luo J. Interaction between RAX and PKR modulates the effect of ethanol on protein synthesis and survival of neurons. J Biol Chem. 2006;281:15909–15915. doi: 10.1074/jbc.M600612200. [DOI] [PubMed] [Google Scholar]
- 14.Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, et al. MicroRNA-29b regulates ethanol-induced neuronal apoptosis in the developing cerebellum through Sp1/Rax/PKR. J Biol Chem. 2014 doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, et al. Deficient signaling in mice devoid of double-stranded RNA-depen-dent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonthius DJ, Tzouras G, Karacay B, Mahoney J, Hutton A, McKim R, et al. Deficiency of neuronal nitric oxide synthase (nNOS) worsens alcohol-induced microencephaly and neuronal loss in developing mice. Brain Res Dev Brain Res. 2002;138:45–59. doi: 10.1016/s0165-3806(02)00458-3. [DOI] [PubMed] [Google Scholar]
- 17.de Licona HK, Karacay B, Mahoney J, McDonald E, Luang T, Bonthius DJ. A single exposure to alcohol during brain development induces microencephaly and neuronal losses in genetically susceptible mice, but not in wild type mice. Neurotoxicology. 2009;30:459–470. doi: 10.1016/j.neuro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Luo J, West JR, Pantazis NJ. Nerve growth factor and basic fibroblast growth factor protect rat cerebellar granule cells in culture against ethanol-induced cell death. Alcohol Clin Exp Res. 1997;21:1108–1120. [PubMed] [Google Scholar]
- 19.Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, et al. MicroRNA-29b regulates ethanol-induced neuronal apoptosis in the developing cerebellum through SP1/RAX/PKR cascade. J Biol Chem. 2014;289:10201–10210. doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, et al. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-gamma agonists. Brain Behav Immun. 2011;(25 Suppl 1):S137–S145. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- 23.Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Kouzoukas DE, Li G, Takapoo M, Moninger T, Bhalla RC, Pantazis NJ. Intracellular calcium plays a critical role in the alcohol-mediated death of cerebellar granule neurons. J Neurochem. 2013;124:323–335. doi: 10.1111/jnc.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C, et al. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- 26.Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- 27.Light KE, Belcher SM, Pierce DR. Time course and manner of Purkinje neuron death following a single ethanol exposure on postnatal day 4 in the developing rat. Neuroscience. 2002;114:327–337. doi: 10.1016/s0306-4522(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 28.Guo W, Crossey EL, Zhang L, Zucca S, George OL, Valenzuela CF, et al. Alcohol exposure decreases CREB binding protein expression and histone acetylation in the developing cerebellum. PLoS ONE. 2011;6:e19351. doi: 10.1371/journal.pone.0019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400–409. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- 30.Kang R, Tang D. PKR-dependent inflammatory signals. Sci Signal. 2012;5:e47. doi: 10.1126/scisignal.2003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 32.Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J Biol Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 33.Bennett RL, Blalock WL, May WS. Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J Biol Chem. 2004;279:42687–42693. doi: 10.1074/jbc.M403321200. [DOI] [PubMed] [Google Scholar]
- 34.Valles SL, Blanco AM, Azorin I, Guasch R, Pascual M, Gomez-Lechon MJ, et al. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res. 2003;27:1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- 35.Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 36.Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]