Abstract
Although the inner ear has long been reported to be susceptible to middle ear disease, little is known of the inflammatory mechanisms that might cause permanent sensorineural hearing loss. Recent studies have shown inner ear tissues are capable of expressing inflammatory cytokines during otitis media. However, little quantitative information is available concerning cytokine gene expression in the inner ear and the protein products that result. Therefore, this study was conducted of mouse middle and inner ear during acute otitis media to measure the relationship between inflammatory cytokine genes and their protein products with quantitative RT-PCR and ELISA, respectively. Balb/c mice were inoculated transtympanically with heat-killed Haemophilus influenzae and middle and inner ear tissues collected for either quantitative RT-PCR microarrays or ELISA multiplex arrays. mRNA for several cytokine genes was significantly increased in both the middle and inner ear at 6 hours. In the inner ear, these included MIP-2 (448 fold), IL-6 (126 fold), IL-1β (7.8 fold), IL-10 (10.7 fold), TNFα (1.8 fold), and IL-1α (1.5 fold). The 24 hour samples showed a similar pattern of gene expression, although generally at lower levels. In parallel, the ELISA showed the related cytokines were present in the inner ear at concentrations higher by 2 to 122 fold higher at 18 hours, declining slightly from there at 24 hours. Immunohistochemistry with antibodies to a number of these cytokines demonstrated they occurred in greater amounts in the inner ear tissues. These findings demonstrate considerable inflammatory gene expression and gene products in the inner ear following acute otitis media. These higher cytokine levels suggest one potential mechanism for the permanent hearing loss seen in some cases of acute and chronic otitis media.
Keywords: cytokine, middle ear, inner ear, inflammation, gene expression, protein expression
1. Introduction
The inner ear is at risk in both acute and chronic otitis media (OM), often demonstrating considerable pathology and hearing loss (Cureoglu et al., 2005; Joglekar et al., 2010; Pearson et al., 2014). However, the mechanisms underlying the transient and permanent sensorineural hearing loss in OM are poorly defined. Previous DNA array screening studies showed cytokine genes are upregulated in the cochleas of BALB/c mice given transtympanic heat-killed bacteria and in cochleas of C3H/HeJ mice with chronic middle ear inflammation (Ghaheri et al., 2007; Ghaheri et al., 2007; MacArthur et al., 2011; Tokarz et al., 2013; MacArthur et al., 2013b). These inflammatory cytokines are predominantly distributed in the cochlear lateral wall as inoculation of middle ears with bacterial components also causes decrease in cochlear blood flow, stria vascularis damage, cytokine expression by spiral ligament fibrocytes, and decreased auditory function (Ichimiya et al., 2003; Sone et al., 2003; MacArthur and Trune, 2006; Moon et al., 2006; Moon et al., 2007; Tsuprun et al., 2008; Woo et al., 2010; Oh et al., 2012). Chronic OM leads to even more significant and permanent inner ear pathology (Cureoglu et al., 2005; MacArthur and Trune, 2006; MacArthur et al., 2008; Joglekar et al., 2010). Thus, cochlear damage from OM appears to be a combination of local inflammatory cytokine induction, as well as cytokines and bacterial components invading the inner ear from the middle ear, presumably through the round window membrane (Cureoglu et al., 2005).
These studies suggest inner ear tissues actively participate in the innate immune response by producing cytokines that might cause local damage. Characterizing these reactive mechanisms in the inner ear would provide us with a greater understanding of disease processes and potentially lead to better targeted therapies to protect the ear from hearing loss or restore hearing once it does occur. Gene expression studies above suggest cytokines might be produced within the inner ear, but their profiles are unknown. It is not clear how many are present, their concentrations, how quickly they are produced upon OM induction, or how long they persist in the ear as middle ear inflammation wanes. Therefore, the present study was conducted to evaluate inner ear cytokine gene expression during middle ear inflammation. Quantitative RT-PCR and ELISA were conducted on acute OM mice to measure inner ear inflammatory cytokine genes and their products. Insights into the extent and timing of these innate inflammatory processes could significantly advance our efforts to protect the inner ear from immune disease processes.
2. Materials and methods
2.1. Animals
Female Balb/c mice were obtained from Jackson Laboratories at 10–12 weeks of age and inoculated transtympanically with heat-killed Haemophilus influenzae (H flu). Both middle and inner ear tissues were collected for quantitative RT-PCR microarrays, multiplex ELISA arrays, or immunohistochemistry to evaluate inflammatory gene expression and gene products that are impacting the inner ear. These assays used cytokine profiles designed by our laboratory to evaluate those most relevant to middle and inner ear disease. All animal procedures in the study were approved by the OHSU Institutional Animal Care and Use Committee according to federal guidelines.
2.2. Acute OM induction
The acute middle ear disease mouse model employed has been described previously (MacArthur et al., 2006). Middle ear inflammation in Balb/c mice was created by bilateral transtympanic inoculation with heat-killed H flu in PBS. Tissues were harvested at key time points for the respective analyses below. Middle and inner ears were removed and separated. Middle ears were processed individually while left and right inner ears were combined to get adequate material. Untreated mice served as controls. A total of eight samples per treatment and time point were processed, except for VEGF (4 samples).
It should be noted that the PBS vehicle alone induces minor inflammation in the middle ear, making the H flu injections immunostimulatory from the perspective of both bacteria and vehicle. However, we have reported previously that inflammatory changes in the middle ear due to PBS alone are not as significant as those induced by bacteria (MacArthur et al., 2006; MacArthur et al., 2011). Therefore, for the present study, untreated ears are used as the control for gene and protein expression.
2.3. Quantitative RT-PCR analyses
Tissues were collected at 6, 24, and 72 hours, and 1 week after inoculation to determine the impact of bacterial induction of cytokine gene expression. Six hours was chosen as the first time point because that is the peak of gene expression following inoculation (unpublished observations). Tissues were homogenized and mRNA extracted for quantitative RT-PCR of inflammatory cytokine genes according to our standard protocol (MacArthur et al., 2011). Tissue RNA was extracted with the Qiagen (Valencia, CA) RNeasy Mini Kit by transferring to tubes with 600 µl of extraction buffer and homogenizing with a PowerGen 125. RNA was quantified using a NanoDrop and all samples were made up to a concentration of at least 25 ng/µl. Total RNA (200 ng) was reverse-transcribed using RT2 First Strand Kit (SABiosciences Corp, Frederick, MD) using the manufacturer’s instructions. Then samples were prepared for Real-time PCR using the RT2 Real-time SYBR Green/Rox PCR master mix. Real-time RT-PCR studies were conducted on an ABI Step One Plus system (Carlsbad, CA) utilizing custom PCR Arrays (SABiosciences Corp, Frederick, MD) optimized for reaction conditions, primers, and probe. These custom PCR Array plates were made by SABiosciences Corp (Frederick, MD) to measure expression of key inflammation related cytokines common to middle ear disease. These included several interleukins (IL-1α, IL-1β, IL-6, IL-10), tumor necrosis factor alpha (TNFα), vascular endothelial growth factor (VEGF), macrophage inflammatory proteins (MIP-2α or Cxcl2; MIP-1α or Ccl3), and keratinocyte-derived chemokine (KC, now called Cxcl1), a macrophage recruiter and activator that shares homology with human IL-8, as does MIP-2α. The statistical significance and fold change were calculated using the ΔΔCt method with the aid of SABiosciences PCR Array Data Analysis Web Portal. The housekeeping gene used for this method was glyceraldehyde-3-phosphate dehydrogenase.
2.4. Multiplex ELISA
Preliminary studies(MacArthur et al., 2011) showed the transient expression of cytokine genes in the middle ear is largely confined to the first 2 days following inoculation. Therefore, proteins resulting from cytokine gene expression were examined at 18, 24, 36, and 48 hours following inoculation. Middle and inner ear tissues were harvested at these time points, separated as above, and prepared for protein isolation. A total of 36 middle and inner ear samples were processed for each time point.
Tissues were washed with cold 0.1 M phosphate buffer and placed in a 1.5 ml microcentrifuge tube with 100 µl of T-PER® Tissue Protein Extraction Reagent (Thermo Scientific Pierce) containing dithiothreitol (1.0 µM) and Protease Inhibitor Cocktail (1:100). T-PER is a mild tissue cell lysis solution designed for total protein extraction. Samples are stored at −80°C until homogenized and then again at −80°C until assayed. Homogenization was carried out using the BioMasher®, composed of a disposable micro homogenizer, filter column, and collection tube. After grinding and centrifuging at 14,000 rpm at 4°C to separate the bone fragments, the supernatant was agitated at 4°C for 20 minutes in a TurboMix Vortex attachment for a Vortex-Genie 2 and spun again for 10 min at 14,000 rpm at 4°C. Protein concentration was determined using the Bradford or Pierce Protein 660 mm Protein Assay. Samples are adjusted to 2–3 µg/µl and diluted further at 1:2 or 1:3 in their respective assays to achieve approximately 30 µg of protein per well to normalize the cytokine measures.
Cytokine proteins were measured by custom services available from Aushon SearchLight Protein Array Technology (Trune et al., 2011). This is a microplate-based array in which cytokines are bound by capture antibodies in a single well and detected by binding with a secondary antibody conjugated with a chemiluminescent label. Their inflammatory cytokine profile contained most cytokines of general interest in middle ear inflammatory disease. Cytokines not available from Searchlight were analyzed by the testing service of Milliplex MAP. Milliplex™ MAP is a bead-based suspension array using the Luminex xMAP technology in which fluorescent-coded beads (microspheres) have cytokine capture antibodies on their surface to bind the proteins. Final analysis is with flow cytometry.
Together these two services included the cytokines and chemokines above, as well as IL-9, IL-10, IL-12, MIP-1β (Ccl4), granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF), intercellular adhesion molecule 1 (ICAM-1), leukemia inhibitory factor (Lif), gamma interferon induced monokine (MIG or Cxcl9), granulocyte chemotactic protein 2 (LIX or Cxcl5), monocyte chemotactic protein–1 (MCP-1 or Ccl2), eosinophil chemotactic protein (eotaxin or Ccl11), interferon-gamma inducible protein 10 (IP-10 or Cxcl10), and RANTES (Ccl5), a monocyte chemoattractant that shares the Ccr1 receptor with MIP-1α. Tissue samples for each time point were assayed in duplicate or triplicate according to the protocols of the company and averaged for final determination of fold change in cytokine production compared to untreated control ears for statistical significance.
2.5. Immunohistochemistry
Immunohistochemistry of the inner ear was conducted to ascertain which inner ear regions demonstrated the gene products in question and to localize those inner ear regions where they might have the greatest inflammatory impact. Acute OM was induced as above and mice (N=5) were killed after 24 hours. Mice were sedated and perfused intracardially with 3% paraformaldehyde in 0.1 M phosphate buffer. Ear tissues were removed, decalcified in EDTA, and embedded in paraffin. Serial 5 µm sections were mounted on glass slides, heated at 50 °C for 30 minutes, and deparaffinized. Sections were stained with primary antibodies against toll-like receptors (TLR) 2, 4 and 9 (Novus Biologicals, Littleton, CO), IL-1α, IL-1β, IL-6, MIP-2 (Abnova, Taipei City, Taiwan), and G-CSF (Abcam, Cambridge, MA). These antibodies were selected because they have been consistently reported to be involved in middle ear inflammation and they showed significant expression in both the RT-PCR and ELISA studies. Control sections were exposed to buffer only and no primary antibody. Primary antibodies were detected with secondary antibodies conjugated to AlexaFluor 488 (Invitrogen/Molecular Probes, Eugene, OR). Sections were observed with a BioRad MRC1024 laser confocal attachment on a Nikon TE300 inverted fluorescence microscope.
3. Results
3.1. Middle ear
The middle ear expressed numerous cytokine genes at 6 hours, some several hundred fold higher than normal (Table 1). The most dramatic upregulation was seen for MIP-1α, MIP-2α, IL-6, and KC, although significantly elevated expression was seen for all the others tested except VEGF, which trended to underexpression. All were still overexpressed at 24 hours, although the level of expression was subsiding. By 72 hours, expression was still elevated, albeit resolving to lower levels, with only IL-10 not significantly different from controls. MIP-2α, IL-1β, and KC were still showing significant gene expression as late as one week after inoculation (Table 1). In contrast to the other cytokines, VEGF expression tended to be reduced. These data typify the middle ear inflammatory gene response with a single inoculation of heat-killed H flu and established the gene expression basis for cytokine and chemokine protein synthesis in the ELISA analyses.
Table 1.
Middle ear gene expression (fold change)
| qRT-PCR |
||||
|---|---|---|---|---|
| Cytokine | 6 hours | 24 hours | 72 Hours | 1 Week |
| MIP-1α (Ccl3) | 52.9 | 63.4 | 14.0 | 1.4 |
| MIP-2α (Cxcl2) | 1083.6 | 404.2 | 72.1 | 2.9 |
| IL-1α | 5.7 | 5.6 | 3.0 | 0.8 |
| IL-1β | 36.1 | 20.3 | 4.5 | 1.6 |
| IL-6 | 557.6 | 30.2 | 9.5 | 1.7 |
| IL-10 | 34.9 | 4.6 | 1.5 | 1.4 |
| TNFα | 4.4 | 7.8 | 1.7 | 1.0 |
| KC (Cxcl1) | 638.5 | 50.1 | 11.8 | 3.6 |
| VEGF | 0.2 | 0.7 | 0.6 | - |
Bold, t-test probability ≤ 0.05
Middle ear ELISA measurements (Table 2) reflected a significant elevation of cytokine and chemokine production in parallel with the gene upregulation above (Table 1). At 18 hours, numerous proteins were measured at a greater than 2 fold increase from control tissues. These included MIP-1α, MIP-2α, IL-1β, IL-6, and KC. In fact, the same cytokine genes showing increased expression (Table 1) also showed elevated levels of their respective proteins. Additional cytokine proteins overexpressed in this assay included ICAM-1, G-CSF, LIF, RANTES, IL-12, LIX, M-CSF, and IL-5. Numerous other cytokines were measured at fold changes greater than one, but were not statistically significant. Thus, overall the ELISA results at 18 hours reflected a comprehensive and significant production of inflammatory factors within the middle ear. Other cytokines and chemokines were not present at sufficient levels to be detected (ND, Table 2), even in the inflamed middle ear.
Table 2.
Middle ear inflammatory protein levels (fold change)
| Multiplex ELISA |
|||||
|---|---|---|---|---|---|
| Cytokine | 18 Hours | 24 Hours | 36 Hours | 48 Hours | Service |
| MIP-1α (Ccl3) | 19.0 | 24.9 | 10.5 | 9.2 | Milliplex |
| MIP-2α (Cxcl2) | 34.0 | 131.2 | 35.2 | 12.9 | Searchlight |
| IL-1α | 2.4 | 4.6 | 1.3 | 1.5 | Searchlight |
| IL-1β | 27.1 | 23.3 | 5.3 | 3.4 | Searchlight |
| IL-6 | 75.3 | 124.7 | 11.5 | 5.6 | Searchlight |
| IL-10 | 1.5 | 1.8 | 1.2 | 1.6 | Searchlight |
| TNFα | 2.4 | 3.7 | 1.6 | 1.2 | Searchlight |
| KC (Cxcl1) | 10.9 | 7.6 | 5.6 | 4.4 | Milliplex |
| GM-CSF | 3.3 | 3.8 | 3.2 | 1.4 | Searchlight |
| VEGF | 2.3 | 4.2 | 4.9 | 2.6 | Searchlight |
| ICAM-1 | 2.4 | 3.4 | 2.0 | 1.7 | Searchlight |
| G-CSF | 30.9 | 29.8 | 22.1 | 14.7 | Milliplex |
| LIF | 17.2 | 18.9 | 15.8 | 10.9 | Milliplex |
| RANTES | 5.4 | 3.9 | 2.1 | 1.1 | Milliplex |
| IL-12 | 6.0 | 5.6 | 2.5 | 2.7 | Milliplex |
| IL-9 | 0.9 | 1.5 | 1.6 | 1.3 | Milliplex |
| LIX (Cxcl5) | 1.9 | 1.5 | 1.3 | 1.1 | Milliplex |
| M-CSF | 1.8 | 1.7 | 1.4 | 1.4 | Milliplex |
| IL-2 | 0.7 | 0.8 | 1.2 | 0.6 | Milliplex |
| IL-5 | 1.5 | 1.5 | 0.9 | ND | Milliplex |
| IL-7 | 1.3 | 0.9 | 1.1 | 1.2 | Milliplex |
| IL-15 | 1.4 | 1.0 | 0.7 | 0.9 | Milliplex |
| IL-17 | 1.2 | 1.3 | 1.0 | 0.9 | Milliplex |
| MIG (Cxcl9) | 1.4 | 1.1 | 1.1 | 0.5 | Milliplex |
| Eotaxin (Ccl11) | 0.7 | 0.9 | 0.9 | 0.6 | Milliplex |
| ND (Milliplex): IL-3, IL-4, IL-13, IFN-γ, MIP-1β (Ccl4), IP-10 (Cxcl10), MCP-1 (Ccl2) | |||||
Bold, t-test probability ≤ 0.05
ND, not determinable, below detection limits
A number of the inflammatory cytokine proteins were still elevated at 24 hours, some reaching higher levels than at 18 hours (Table 2). This reflected a continuous production by their respective genes and suggested the inflammatory response was still robust. By 36 hours, the production of these proteins was beginning to subside (Table 2) and by 48 hours few were statistically significant. At this stage, most were present at less than 2 fold of normal levels. However, even at 48 hours, MIP-1α, MIP-2α, IL-1β, IL-6, KC, G-CSF, and LIF were still present in elevated amounts. These protein measures reflect the prolonged production and presence of cytokines in the middle ear following a single inoculation of H flu and thus establish the potential inflammatory influence on the inner ear.
3.2. Inner ear
In parallel with the middle ear inflammation, the same pattern of cytokine genes was expressed by the inner ear tissues at 6 hours (Table 3). The RT-PCR results showed all genes (except VEGF) to be significantly elevated at this time point. This included MIP-1α (9 fold), MIP-2α (448 fold), IL-1α (1.5 fold), IL-1β (7.8 fold), IL-6 (126 fold), IL-10 (11 fold), TNFα (1.8 fold), and KC (495 fold). This reflected a short time interval between gene expression in the middle ear to elevation of the same genes in the inner ear tissues. The 24 hour samples showed a similar pattern of gene expression, although at dramatically declining levels. Nevertheless, several cytokines were still significantly upregulated in inner ear tissues at this time. Even at 72 hours and one week postinoculation, several cytokine genes were still functionally elevated (MIP-2α, IL-1β, and KC). These results demonstrate a rapid and prolonged expression of inflammatory genes in the inner ear tissues with a single inoculation of heat-killed bacteria into the middle ear.
Table 3.
Inner ear gene expression (fold change)
| qRT-PCR |
||||
|---|---|---|---|---|
| Cytokine | 6 Hours | 24 Hours | 72 Hours | 1 week |
| MIP-1α (Ccl3) | 9.1 | 6.9 | 1.0 | 1.2 |
| MIP-2α (Cxcl2) | 448.1 | 76.5 | 7.9 | 5.4 |
| IL-1α | 1.5 | 1.9 | 1.0 | 1.1 |
| IL-1β | 7.8 | 7.4 | 2.2 | 2.2 |
| IL-6 | 126.0 | 5.9 | 0.9 | 1.3 |
| IL-10 | 10.7 | 1.6 | 1.8 | 0.7 |
| TNFα | 1.8 | 2.6 | 1.0 | 1.1 |
| KC (Cxcl1) | 495.3 | 49.5 | 7.9 | 5.5 |
| VEGF | 0.4 | 0.9 | 0.8 | - |
Bold, t-test probability ≤ 0.05
As in the middle ear, the most active inner ear genes paralled the highest levels of cytokine proteins (Table 4). The 18 hour ELISA showed many of the cytokines were present in the inner ear at concentrations higher by 2 fold or greater. The most significant cytokines produced in the inner ear were MIP-1α, MIP-2α, IL-1β, IL-6, IL-10, KC, GM-CSF, G-CSF, LIF, and RANTES. This same pattern of elevated inner ear cytokine and chemokine proteins was seen at 24 hours, as well. By 36 hours, most had declined, although several were still over twice normal levels, including MIP-1α, MIP-2α, IL-1β, IL-6, IL-10, KC, G-CSF, LIF, and RANTES. Most had declined further at 48 hours, although some were still elevated above normal levels (MIP-1α, MIP-2α, IL-6, IL-10, and G-CSF). These analyses reflect the significant and prolonged cytokine production by inner ear tissues following a single inoculation of the middle ear with heat-killed bacteria.
Table 4.
Inner ear inflammatory protein synthesis (fold change)
| Multiplex ELISA |
|||||
|---|---|---|---|---|---|
| Cytokine | 18 Hours | 24 Hours | 36 Hours | 48 Hours | Service |
| MIP-1α (Ccl3) | 3.8 | 5.1 | 9.6 | 3.3 | Milliplex |
| MIP-2α (Cxcl2) | 16.6 | 6.6 | 2.7 | 3.4 | Searchlight |
| IL-1α | 7.3 | 4.4 | 1.3 | 0.8 | Searchlight |
| IL-1β | 10.3 | 5.5 | 1.5 | 3.0 | Searchlight |
| IL-6 | 122.1 | 145.4 | 3.1 | 12.9 | Searchlight |
| IL-10 | 2.7 | 2.2 | 2.5 | 3.2 | Searchlight |
| TNFα | 2.2 | 1.6 | 0.8 | 1.2 | Searchlight |
| KC (Cxcl1) | 5.5 | 3.3 | 3.3 | 1.3 | Milliplex |
| GM-CSF | 4.0 | 4.5 | 1.0 | 1.0 | Searchlight |
| VEGF | 0.9 | 2.0 | 1.2 | 1.5 | Searchlight |
| ICAM-1 | 1.8 | 2.2 | 1.2 | 0.7 | Searchlight |
| G-CSF | 62.7 | 78.2 | 55.6 | 27.1 | Milliplex |
| LIF | 4.5 | 6.0 | 11.6 | 5.0 | Milliplex |
| RANTES | 3.0 | 3.3 | 5.4 | 1.4 | Milliplex |
| IL-9 | 1.1 | 1.1 | 1.0 | 1.2 | Milliplex |
| LIX (Cxcl5) | 1.3 | 1.2 | 1.4 | 1.0 | Milliplex |
| M-CSF | 1.0 | 0.9 | 1.5 | 0.8 | Milliplex |
| IL-2 | 0.9 | 0.7 | 0.8 | 0.8 | Milliplex |
| IL-17 | 1.1 | 1.1 | 1.3 | 0.9 | Milliplex |
| MIG (Cxcl9) | 1.4 | 1.3 | 1.8 | 1.3 | Milliplex |
| Eotaxin (Ccl11) | 0.8 | 1.0 | 1.3 | 1.0 | Milliplex |
| IL-7 | ND | 1.0 | 1.3 | 1.1 | Milliplex |
| ND (Milliplex): IL-3, IL-4, IL-5, IL-12, IL-13, IL-15, IFN-γ, MIP-1β (Ccl4), IP-10 (Cxcl10), MCP-1 (Ccl2) | |||||
Bold, t-test probability ≤ 0.05
ND, not determinable, below detection limits
Because our particular interest was inner ear inflammatory processes during middle ear disease, the most significantly affected protein levels were quantified at the time points of greatest impact. Inner ear concentrations of several cytokines from 18 and 24 hours were measured and compared to normal inner ears levels (Table 5). All cytokines were significantly elevated and some reached concentrations in the ear of hundreds of pg/ml. For example, MIP-2α was present at levels as high as 697 pg/ml at 18 hours, while IL-1α reached levels of 1747 pg/ml. IL-6 and G-CSF were the most dramatically elevated, reaching concentrations in the inner ear of 10,497 and 3,649 pg/ml, respectively. Both of these cytokines were still at similar concentrations at 24 hours, as well. These significant levels of cytokines reflect the extent to which inner ear tissues are exposed to inflammatory factors during a single inoculation of heat killed H flu.
Table 5.
Inner ear inflammatory cytokine concentrations (pg/ml)
| ELISA (pg/ml) |
||||
|---|---|---|---|---|
| Cytokine | Control | 18 Hours | 24 Hours | |
| MIP-1α (Ccl3) | 21.0 | 79.9 | 107.8 | |
| Range | 0–21 | 64–109 | 100–115 | |
| Probability | 0.010 | 0.0002 | ||
| Fold change | 3.8 | 5.1 | ||
| MIP-2α (Cxcl2) | 28.5 | 472.3 | 188.4 | |
| Range | 25–31 | 248–697 | 136–241 | |
| Probability | 0.018 | 0.012 | ||
| Fold change | 16.6 | 6.6 | ||
| IL-1α | 144.2 | 1058.0 | 640.2 | |
| Range | 137–151 | 368–1747 | 315–965 | |
| Probability | 0.002 | 0.039 | ||
| Fold change | 7.3 | 4.4 | ||
| IL-1β | 85.2 | 881.3 | 467.1 | |
| Range | 81–90 | 567–1195 | 416–518 | |
| Probability | 0.010 | 0.017 | ||
| Fold change | 10.3 | 5.5 | ||
| IL-6 | 60.6 | 7395.8 | 8808.2 | |
| Range | 51–70 | 4295–10497 | 4971–12645 | |
| Probability | 0.036 | 0.045 | ||
| Fold change | 122.1 | 145.4 | ||
| KC (Cxcl1) | 125.3 | 693.5 | 411.8 | |
| Range | 78–202 | 634–755 | 304–574 | |
| Probability | 0.0004 | 0.035 | ||
| Fold change | 5.5 | 3.3 | ||
| G-CSF | 40.1 | 2518.2 | 3138.8 | |
| Range | 4–79 | 1675–3649 | 2604–3783 | |
| Probability | 0.014 | 0.0009 | ||
| Fold change | 62.7 | 78.2 | ||
| LIF | 10.0 | 42.7 | 57.0 | |
| Range | 0–16 | 29–64 | 48–68 | |
| Probability | 0.038 | 0.002 | ||
| Fold change | 4.5 | 6.0 | ||
| RANTES | 6.1 | 18.5 | 20.3 | |
| Range | 4–7 | 12–26 | 10–35 | |
| Probability | 0.036 | 0.14 | ||
| Fold change | 3.0 | 3.3 | ||
In both the middle ear and inner ear there were multiple cytokines that were not measurable (ND in Tables 2 and 4). These cytokines either do not occur in these tissues, or occur at such low levels that they were not detectable with the level of sensitivity provided by the capture and detection antibody pairs available in the testing services. Because the testing services have optimized their assays to measure normal levels of all cytokines, the fact that they are not detected indicates they occur at very low levels compared to the other cytokines and are not relevant to middle and inner ear inflammation.
3.3. Correlation between RT-PCR and ELISA
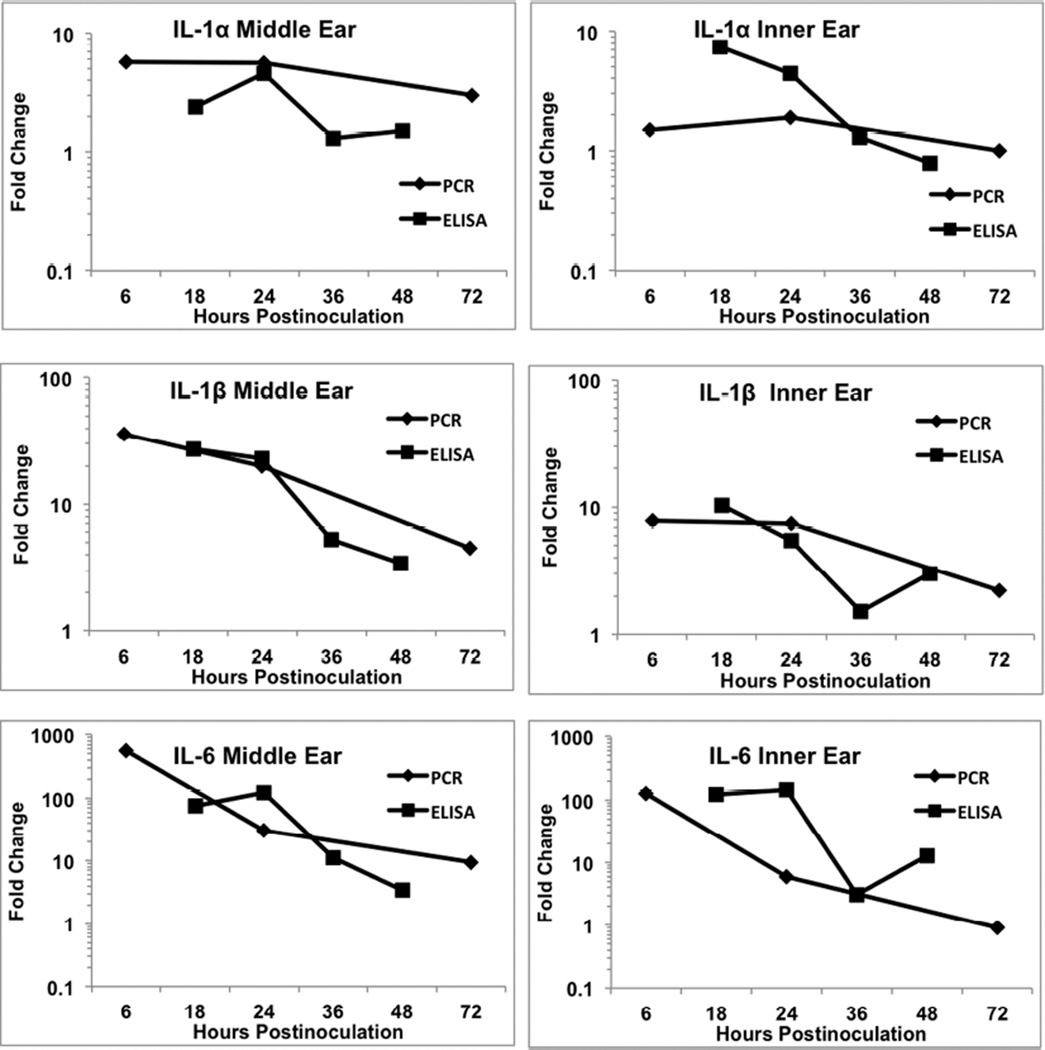
A major interest in studies of gene expression is how well does qRT-PCR results correlate with ELISA measures of actual gene product. Therefore, a comparison was made of fold change in the most active cytokines for middle ear and inner ear to assess this relationship (Figs. 1–2). IL-1α showed the least amount of gene upregulation among the cytokines and this was reflected in comparable small changes in actual protein produced both for middle ear and inner ear (Fig. 1). The increase in ELISA lagged that of PCR with its peak at 24 hours in the middle ear. IL-1β also had overlapping plot lines that indicated its gene expression and gene product increases were very similar (Fig. 1). Much stronger reaction to inflammation was seen for the IL-6 gene and its protein, but these also showed comparable fold changes during the inflammatory reaction (Fig. 1). Like IL-1α, IL-6 demonstrated a peak in gene expression at 6 hours with its peak of protein production at 24 hours.
Figure 1.
Comparison of fold change in cytokine gene expression (RT-PCR) and protein levels (ELISA) for IL-α, IL-1β, and IL-6. All three cytokines showed comparable levels of increase between the two methods. In some cases, the increase in protein levels lagged that of gene activity, such as IL-6. The levels of activity were generally comparable between the middle ear and inner ear.
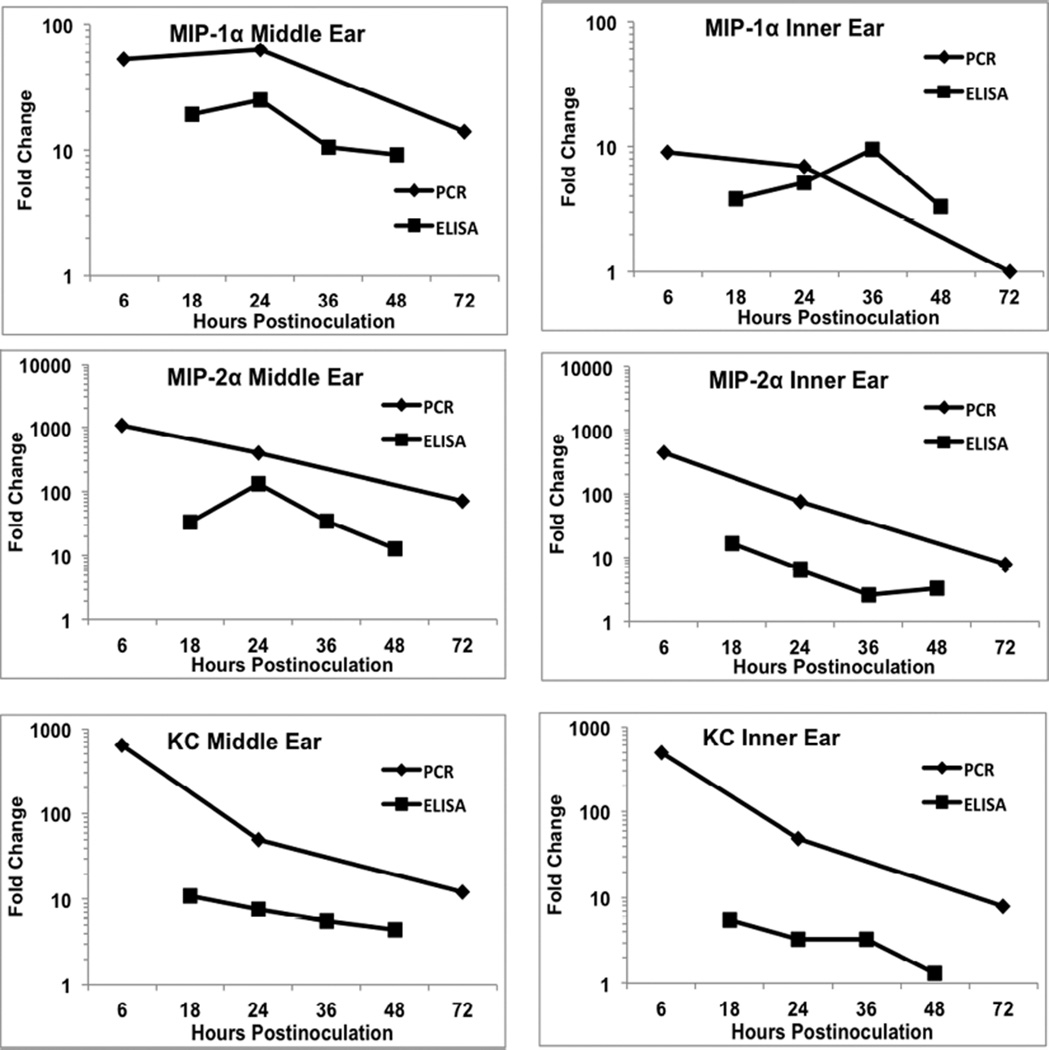
Figure 2.
Comparison of fold change in cytokine gene expression (RT-PCR) and protein levels (ELISA) for Mip-1α, Mip-2α, and KC. In general, these cytokines showed much greater levels of gene upregulation relative to actual protein measures. The fold change in gene expression sometimes was 2 orders of magnitude greater than the change in protein levels, such as KC. As with the cytokines in Fig. 1, the levels of activity were generally comparable between the middle ear and inner ear.
Slightly different correlative patterns of gene expression and protein production were seen for MIP-1α, MIP-2α, and KC (Fig. 2). In the case of these cytokines, the gene expression fold change was higher than the fold change in ELISA. This suggested that the magnitude of gene activation did not result in a significant elevation in gene product. This was particularly notable in activity of KC where gene expression was increased several hundred fold while actual measureable protein was increased less than 10 fold.
A number of observations can be made regarding these comparisons between PCR and ELISA. Generally, the magnitude of changes was comparable between the middle ear and inner ear, both peaking early and declining rapidly after that. This demonstrated little or no lag in activation of inner ear genes following inflammation induction in the middle ear. Often the peak in ELISA change lagged that of gene upregulation, with many proteins having their highest level measured at 24 hours. The fold change in ELISA seldom was greater than gene expression fold change, and in many cases showed considerably less increase.
3.4. Immunohistochemistry of inner ear
Overall the immunohistochemistry changes in the inner ear were much less dramatic than the ELISA results would suggest. This was quite surprising given the significant increase in protein levels due to the middle ear inflammation. In general, the middle ear immunohistochemistry showed prominent staining along the inflamed mucosa lining the cochlear wall, but this was seldom paralleled by comparable inner ear levels.
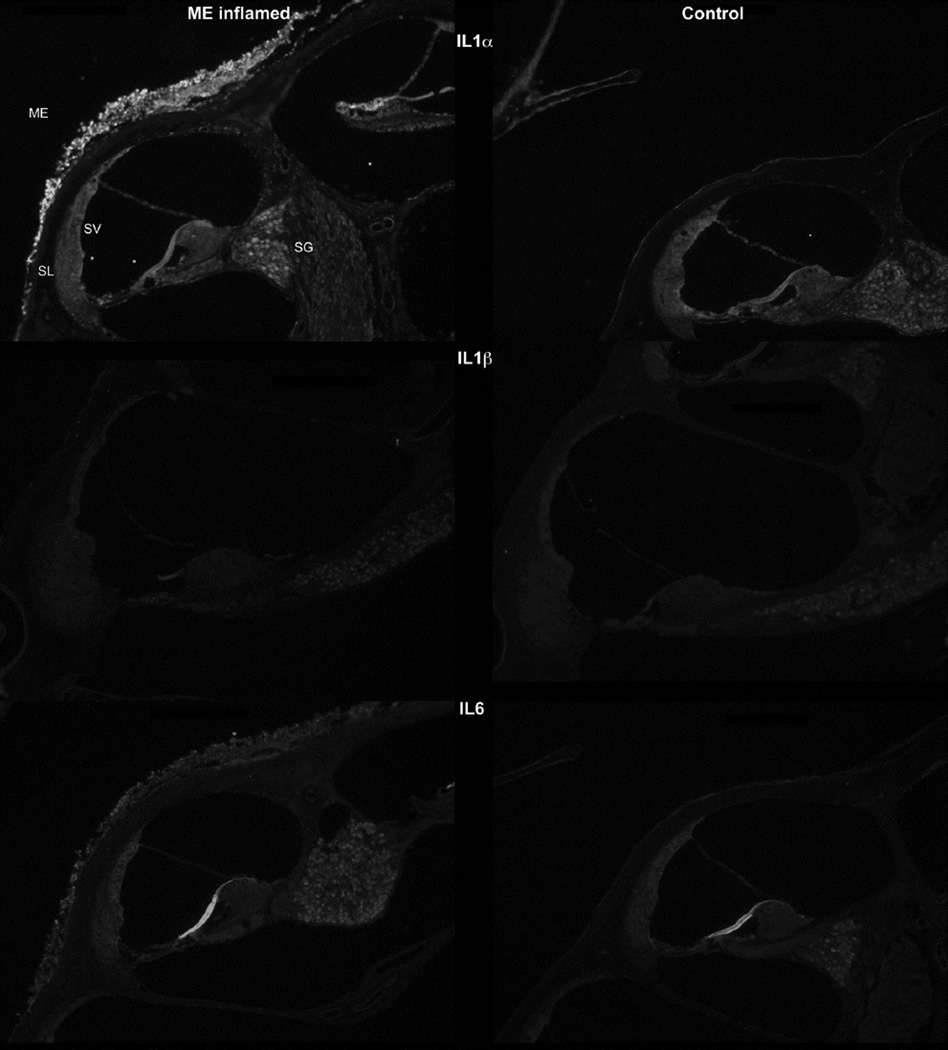
IL-1β
This cytokine stained prominently along the inflamed middle ear mucosa (Fig. 3), demonstrating the increased thickness of this epithelium that occurs within hours of inoculation with bacteria. Within the inner ear itself, only moderately increased staining was seen. The spiral ligament and lateral wall appeared slightly brighter than the control ears, suggesting only a little accumulation of the cytokine there. The spiral ganglion was brighter, indicating potential buildup around the nerve cell bodies. Another prominent accumulation of reaction product was that lining the blood vessels of the bony capsule of the cochlea. This appeared to be within the endothelial cells of the vessels since perfusion would have removed any immune cell debris from the blood that potentially would be stained.
Figure 3.
IL-α: IL-1α immunohistochemistry (top row) showed greater staining after bacterial inoculation. Heavier staining was particularly seen in the middle ear (ME) mucosa lining the cochlear bone. Inner ear staining was slightly greater in the spiral ganglion (SG) and the bone lining the perilymphatic spaces. (SL, spiral ligament; SV, stria vascularis)
IL-1β: There was very little difference in IL-1β staining between the normal and inoculated ears (middle row).
IL-6: Immunostaining showed IL-6 was present in small amounts in the normal middle and inner ears, but increased significantly in the infected mice. Following inoculation, the inflamed middle ear mucosa lining the cochlear wall was stained. The inner ear showed slightly greater staining of the lateral wall and spiral ganglion.
IL-1β
Little or no staining for this cytokine was observed in the ears, suggesting it did not accumulate to any extent during the inflammatory response (Fig. 3).
IL-6
Staining for this cytokine showed considerable accumulation of it in the thickened middle ear mucosa lining the bony capsule of the inner ear (Fig. 3). The cytokine also appeared to be slightly more pronounced in the stria vascularis following inoculation. The spiral ganglion stained brighter, suggesting a higher quantity around the cell bodies.
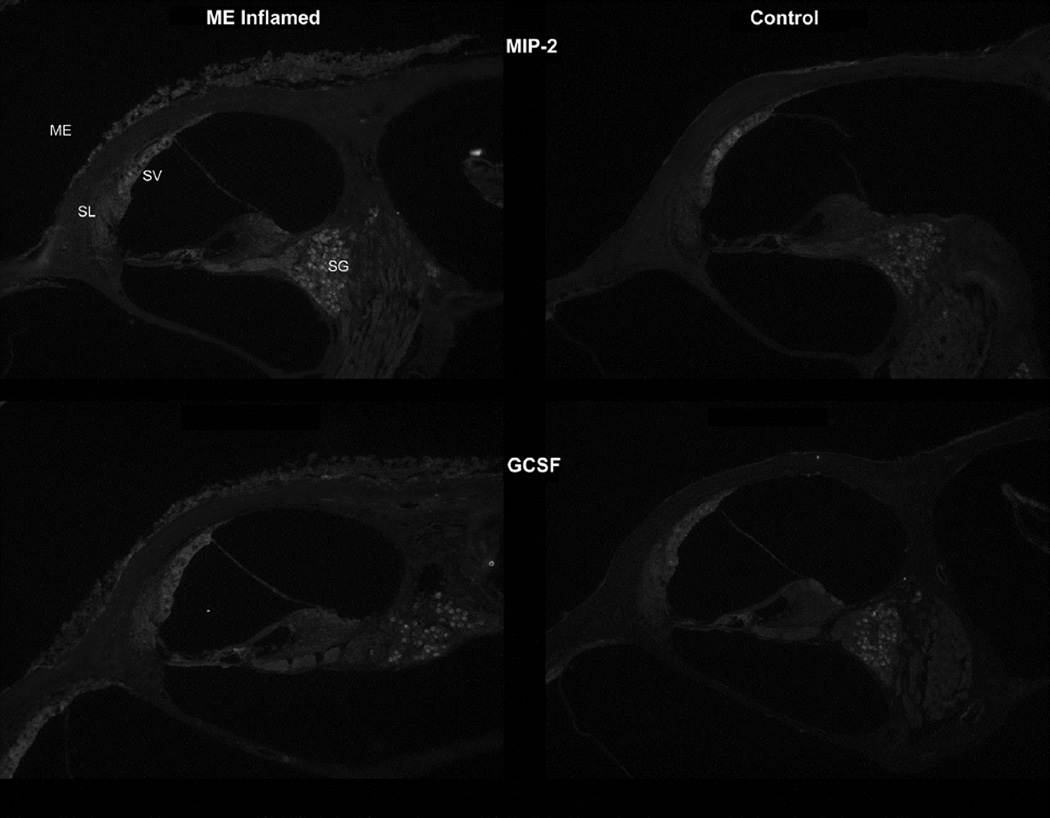
MIP-2α
Mip-2α (Cxcl2) also stained in the middle and inner ears following inoculation (Fig. 4). The inflamed and thickened mucosa lining the middle ear space was moderately stained, demonstrating this cytokine was involved in the local inflammatory response. The bony cochlear capsule under the middle ear space showed blood vessels with greater staining as well. The spiral ligament also showed slightly more stain in the inflamed mice, as did the spiral ganglion neuron bodies.
Figure 4.
MIP-2: Staining for MIP-2 (top row) was moderately stronger in the inflamed middle and inner ears. Increased staining was seen in the middle ear (ME) mucosa lining the tympanic cavity. Strongest cochlear staining due to inflammation was seen in the spiral ligament (SL) and spiral ganglion (SG).
G-CSF: Staining for granulocyte colony-stimulating factor (GCSF, bottom row) was present in light amounts in the normal, uninflamed ear and showed moderately increased staining after inoculation with the bacteria, particularly the mucosal lining of the middle ear. There also was possibly increased staining for the cytokine in the inflamed inner ear, particularly in the lateral wall.
G-CSF
The granulocyte colony stimulating factor (Fig. 4) antibody showed the typical middle ear muscosal staining in the inflamed ears. Inner ear lateral wall staining was slightly more pronounced as well. The blood vessels of the stria vascularis appeared enlarged and its entire depth was more brightly stained. Also, the intensity of staining in the spiral ganglion neurons was slightly brighter, although it was not as pronounced as with other cytokines.
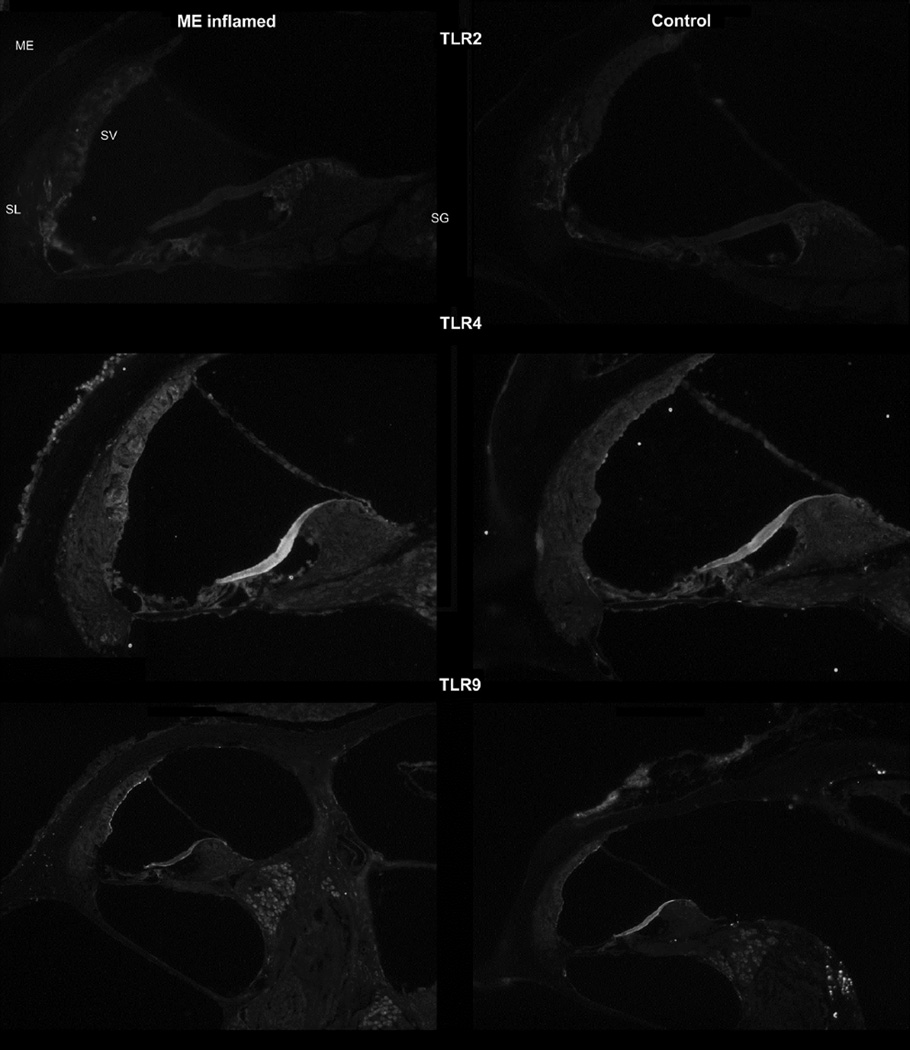
To assess the role of toll-like receptors (TLR) in cochlear inflammation, an assessment was made of their presence with immunohistochemistry. Not only was it of interest to determine their locations in the normal ear, but also how they might be upregulated during a local inflammatory response to potentially increase the expression of downstream cytokines. Therefore, evaluations were made of TLR’s 2, 4, and 9.
TLR2
Toll-like receptor 2 (Fig. 5) showed slightly increased staining in the inflamed ear. The middle ear mucosa did not show any appreciable stain for this TLR. The spiral ligament of the cochlear lateral wall showed significant staining in the control mice, and this appeared more intense in the inflamed mice. In addition, the stria vascularis demonstrated the presence of TLR2 in the inflamed ear, but none in the control. Thus the lateral wall appeared to be the major location for this receptor.
Figure 5.
TLR-2: Immunostaining for Toll-like receptor 2 (top row) showed slightly stronger staining in the lateral wall of the cochlea, both in the spiral ligament (SL) and stria vascularis (SV). The mucosal lining of the middle ear (ME) space was not significantly stained with TLR2 antibody.
TLR-4: Immunostaining for Toll-like receptor 4 (middle row) in the inflamed mice showed much stronger staining in the lateral wall of the cochlea, particularly stria vascularis (SV), as well as the spiral ganglion (SG). The mucosal lining of the middle ear (ME) space was significantly stained with TLR4 antibody.
TLR-9: Staining for TLR-9 (bottom row) showed minimal changes within the inner ear after inoculation. More prominent staining was only seen along the marginal cells of the stria vascularis and potentially higher intensity label in the spiral ganglion.
TLR4
This receptor showed significantly increased presence in the inflamed middle and inner ears (Fig. 5). The inflamed middle ear mucosa was stained heavily, predominantly the inflammatory cells lining the middle ear mucosa during the inflammation process. The distinct outline of these cells could be seen with this antibody, suggesting they were covered with these receptors, and producing more, in their inflammatory response. The lateral wall also stained more intensely with this antibody, showing particularly stronger staining of the stria vascularis during inflammation. Cells of the spiral ganglion also appeared to stain more intensely during the inflammatory response.
TLR9
Antibodies for this receptor showed greater staining intensity in the middle ear mucosa (Fig. 5), though not as strong as those for TLR4. There also was some staining of the cochlear bone under the middle ear mucosa. It appeared to be limited to cells within the spaces of the bony capsule. The stria vascularis also appeared to stain slightly greater in the inflamed condition. The marginal cells of the stria were the brightest staining. The spiral ganglion cell bodies also appeared to stain slightly greater in the inflamed ears.
The immunohistochemistry showed significant staining for a number of the cytokines and TLRs in the inflamed ear. These results demonstrate that the considerable upregulation of cytokine genes demonstrated in the RT-PCR and ELISA studies did result in greater levels of the cytokines being present in the inner ear tissues. These overall results reflect the considerable expression of inflammatory factors in the inner ear during middle ear inflammation.
4. Discussion
4.1. Inner ear inflammatory response
The key finding of this study is that tissues within the cochlea are capable of expressing cytokine mRNA, leading to significant local cytokine production. This means the cochlea is not simply secondarily susceptible to migrating inflammatory factors from the middle ear, but rather actively participates in a pronounced local immune response. The local production of inflammatory factors and cytokines explains the extensive cochlear inflammation and remodeling often seen with prolonged middle ear disease. This also provides a molecular basis for the transient and permanent sensorineural hearing loss often reported with acute OM. This parallels findings from other laboratories showing the fibrocytes of the cochlear lateral wall are capable of expressing inflammatory cytokines following bacterial stimulation of the middle ear (Woo et al., 2010; Oh et al., 2012).
This study also demonstrated that a broad range of cytokines are produced by the cochlear tissues. While many other studies have concentrated on single cytokines in detail, the use of more comprehensive RT-PCR and ELISA panels here showed several inflammatory factors are operating simultaneously. Thus, the inflammatory cascade within the cochlea is not a simple production of, and reaction to, a single cytokine in isolation, but rather a complex array of immune factors impacting cochlear homeostasis. Also noteworthy was the consistent results between the PCR and ELISA methods, as demonstrated by comparable fold changes within the cochlea. This parallel of gene activity and protein synthesis (transcription and translation) is taken as evidence the immune response processes are significant and real. Furthermore, identification of these cochlear inflammatory processes may be beneficial in the design of therapies to protect the inner ear during both acute and chronic middle ear inflammation.
4.2. Parallel middle and inner ear inflammation
Results also showed that the upregulated cytokines were similar between the middle ear and inner ear. In fact, the fold change analysis showed the degree to which they are activated is relatively similar in the two locations of the ear. These cytokines expressed are involved in a number of functions. Many cause immune cell proliferation (IL-1α,β, IL-6, KC) and prolongation of the inflammatory response. Other cytokines upregulated in the middle and inner ear induce tissue remodeling (e.g., TNFα). This close parallel in the inflammatory cytokine response between the middle and inner ear has not been demonstrated previously.
Another demonstrated parallel between the two ear regions is the transient nature of the response. Previous studies of the middle ear response to heat-killed bacteria showed inflammation was largely resolved by 7 days (MacArthur et al., 2006). This transient response has now been demonstrated for the inner ear as well following middle ear inflammation induction.
4.3. Impact on hearing
While it has been demonstrated by this study and others that inflammation occurs in the inner ear during otitis media, little is known of exactly what cochlear homeostatic processes are compromised in order to cause the associated hearing loss. One cochlear function demonstrated to be affected is ion homeostasis. Because inner ear function is largely dependent on proper ion and fluid balances, any compromise of these functions would lead to hearing loss. It was discussed above that the fibrocytes of the lateral wall are significantly affected by middle ear inflammation (Woo et al., 2010; Oh et al., 2012). This would have an impact on K+ ion transport and its recycling into endolymph. It also is now known that numerous genes related to tight junctions, gap junctions, aquaporins, and Cl−, K+ and Na+ transport are also compromised in the inner ear following middle ear inflammation (MacArthur et al., 2013a; MacArthur et al., 2013b). This parallels findings in other tissues that ion homeostasis is compromised by inflammation (Eisenhut, 2006; Al-Sadi and Ma, 2007; Choi et al., 2007; Peng et al., 2012; Petecchia et al., 2012) Therefore, not only is endolymph production and maintenance at risk, but also the endothelial cell tight junctions that are necessary for preservation of the blood labyrinth barrier (Danese et al., 2007; Lemichez et al., 2010; Trune and Canlon, 2012). Steroid treatment partially resolves the hearing loss in these mice (MacArthur et al., 2008), and it is known junctional proteins, vessel integrity, and ion homeostasis genes are controlled by steroids as much as those related to inflammation (Felinski and Antonetti, 2005; Trune and Canlon, 2012).
Another process compromised by middle ear disease is tissue remodeling. Numerous genes involving growth factors, bone morphogenetic proteins, and matrix metalloproteinases are affected in both the middle ear and inner ear during middle ear disease (MacArthur et al., 2006; Sautter et al., 2011; MacArthur et al., 2013a; MacArthur et al., 2013b). Thus, significant connective tissue and bone reorganization in the inner ear follows acute and chronic middle ear disease. These processes also can affect structural homeostasis and healing in the inner ear to cause both transient and permanent hearing loss.
4.4. Conclusions
The results of the present study demonstrate a comprehensive and prolonged inflammation of the inner ear by a single inoculation of the middle ear with heat killed bacteria. This transient inflammation in the middle ear is sufficient to induce a parallel response by inner ear tissues involving the production of the same cytokine profile. Thus, even moderate or mild middle ear inflammation can potentially have a detrimental effect on the sensorineural components of the inner ear. This expands our understanding of the widespread impact of otitis media and the susceptibility of the inner ear. This establishes the breadth of potential processes in the cochlea that may be at risk in cases of middle ear disease. Further research into the control or mitigation of such inflammation will be critical to provide the level of protection needed for children and adults with prolonged otitis media and other middle ear inflammatory conditions.
Highlights.
Middle ear inflammation causes significant inflammation in the inner ear
Both occur within hours of the initial inflammatory insult in the middle ear
The same inflammatory cytokines are expressed in the middle ear and inner ear
The predominant inner ear tissues affected are the lateral wall and spiral ganglion
These processes demonstrate a potential mechanism for hearing loss in otitis media
Acknowledgements
This research was supported by research grant R01 DC009455 and core grant P30 DC005983 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- G-CSF
granulocyte colony stimulating factor
- H flu
Haemophilus influenza
- IL
interleukin
- KC
keratinocyte-derived chemokine
- MIP
macrophage inflammatory protein
- OM
otitis media
- PBS
phosphate buffered saline
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- TLR
toll-like receptor
- TNFα
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Experimental Work: DRT, JBK, FAH, BEL, CJM
Article Preparation: DRT, JBK, FAH, BEL, CJM
All authors have approved submission of this article
Conflict of interest
The authors declare they have no conflicts of interest.
References
- Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Choi YS, Kim SJ, Son EJ, Choi HS, Yoon JH. Interleukin-1beta suppresses epithelial sodium channel beta-subunit expression and ENaC-dependent fluid absorption in human middle ear epithelial cells. Eur. J. Pharmacol. 2007;567:19–25. doi: 10.1016/j.ejphar.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Cureoglu S, Schachern PA, Rinaldo A, Tsuprun V, Ferlito A, Paparella MM. Round window membrane and labyrinthine pathological changes: an overview. Acta Otolaryngol. 2005;125:9–15. doi: 10.1080/00016480410022534. [DOI] [PubMed] [Google Scholar]
- Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J. Immunol. 2007;178:6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- Eisenhut M. Changes in ion transport in inflammatory disease. J Inflamm (Lond) 2006;3:5. doi: 10.1186/1476-9255-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr. Eye Res. 2005;30:949–957. doi: 10.1080/02713680500263598. [DOI] [PubMed] [Google Scholar]
- Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine acute otitis media. Laryngoscope. 2007;117:22–29. doi: 10.1097/01.mlg.0000240170.48584.73. [DOI] [PubMed] [Google Scholar]
- Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol. Head Neck Surg. 2007;137:332–337. doi: 10.1016/j.otohns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Ichimiya I, Yoshida K, Suzuki M, Mogi G. Expression of adhesion molecules by cultured spiral ligament fibrocytes stimulated with proinflammatory cytokines. Ann. Otol. Rhinol. Laryngol. 2003;112:722–728. doi: 10.1177/000348940311200813. [DOI] [PubMed] [Google Scholar]
- Joglekar S, Morita N, Cureoglu S, Schachern PA, Deroee AF, Tsuprun V, Paparella MM, Juhn SK. Cochlear pathology in human temporal bones with otitis media. Acta Otolaryngol. 2010;130:472–476. doi: 10.3109/00016480903311252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez E, Lecuit M, Nassif X, Bourdoulous S. Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat. Rev. Microbiol. 2010;8:93–104. doi: 10.1038/nrmicro2269. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Hausman F, Kempton JB, Choi D, Trune DR. Otitis media impacts hundreds of mouse middle and inner ear genes. PLoS One. 2013a;8:e75213. doi: 10.1371/journal.pone.0075213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CJ, Hausman F, Kempton JB, Sautter N, Trune DR. Inner ear tissue remodeling and ion homeostasis gene alteration in murine chronic otitis media. Otol. Neurotol. 2013b;34:338–346. doi: 10.1097/MAO.0b013e31827b4d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CJ, Hausman F, Kempton JB, Trune DR. Murine middle ear inflammation and ion homeostasis gene expression. Otol. Neurotol. 2011;32:508–515. doi: 10.1097/MAO.0b013e31820e6de4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear. Res. 2006;219:12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Kempton JB, DeGagne J, Trune DR. Control of chronic otitis media and sensorineural hearing loss in C3H/HeJ mice: glucocorticoids vs mineralocorticoids. Otolaryngol. Head Neck Surg. 2008;139:646–653. doi: 10.1016/j.otohns.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CJ, Trune DR. Mouse models of otitis media. Curr. Opin. Otolaryngol. Head Neck Surg. 2006;14:341–346. doi: 10.1097/01.moo.0000244193.97301.d7. [DOI] [PubMed] [Google Scholar]
- Moon SK, Park R, Lee HY, Nam GJ, Cha K, Andalibi A, Lim DJ. Spiral ligament fibrocytes release chemokines in response to otitis media pathogens. Acta Otolaryngol. 2006;126:564–569. doi: 10.1080/00016480500452525. [DOI] [PubMed] [Google Scholar]
- Moon SK, Woo JI, Lee HY, Park R, Shimada J, Pan H, Gellibolian R, Lim DJ. Toll-like receptor 2-dependent NF-kappaB activation is involved in nontypeable Haemophilus influenzae-induced monocyte chemotactic protein 1 up-regulation in the spiral ligament fibrocytes of the inner ear. Infect. Immun. 2007;75:3361–3372. doi: 10.1128/IAI.01886-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Woo JI, Lim DJ, Moon SK. ERK2-dependent activation of c-Jun is required for nontypeable Haemophilus influenzae-induced CXCL2 upregulation in inner ear fibrocytes. J. Immunol. 2012;188:3496–3505. doi: 10.4049/jimmunol.1103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson F, Mann KD, Rees A, Davis A, Pearce MS. The Effect of Childhood Infection on Hearing Function at Age 61 to 63 Years in the Newcastle Thousand Families Study. Ear Hear. 2014 doi: 10.1097/AUD.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ. Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2012;53:5016–5028. doi: 10.1167/iovs.11-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab. Invest. 2012;92:1140–1148. doi: 10.1038/labinvest.2012.67. [DOI] [PubMed] [Google Scholar]
- Sautter NB, Delaney KL, Hausman FA, Trune DR. Tissue remodeling in the acute otitis media mouse model. Int. J. Pediatr. Otorhinolaryngol. 2011;75:1368–1371. doi: 10.1016/j.ijporl.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Hayashi H, Yamamoto H, Tominaga M, Nakashima T. A comparative study of intratympanic steroid and NO synthase inhibitor for treatment of cochlear lateral wall damage due to acute otitis media. Eur. J. Pharmacol. 2003;482:313–318. doi: 10.1016/j.ejphar.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Tokarz SA, Pang J, Grosz A, Kempton JB, Trune DR, Pillers DA. Age-related cochlear cytokine gene expression in the BALB/cJ mouse with systemic versus intratympanic dosing of steroid drugs. Acta Otolaryngol. 2013;133:685–691. doi: 10.3109/00016489.2013.771407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Canlon B. Corticosteroid therapy for hearing and balance disorders. Anat Rec (Hoboken) 2012;295:1928–1943. doi: 10.1002/ar.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Larrain BE, Hausman FA, Kempton JB, MacArthur CJ. Simultaneous measurement of multiple ear proteins with multiplex ELISA assays. Hear. Res. 2011;275:1–7. doi: 10.1016/j.heares.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuprun V, Cureoglu S, Schachern PA, Ferrieri P, Briles DE, Paparella MM, Juhn SK. Role of pneumococcal proteins in sensorineural hearing loss due to otitis media. Otol. Neurotol. 2008;29:1056–1060. doi: 10.1097/MAO.0b013e31818af3ad. [DOI] [PubMed] [Google Scholar]
- Woo JI, Pan H, Oh S, Lim DJ, Moon SK. Spiral ligament fibrocyte-derived MCP-1/CCL2 contributes to inner ear inflammation secondary to nontypeable H. influenzae-induced otitis media. BMC Infect. Dis. 2010;10:314. doi: 10.1186/1471-2334-10-314. [DOI] [PMC free article] [PubMed] [Google Scholar]