Abstract
Long acting nanoformulated antiretroviral therapy (nanoART) can sustain plasma drug levels and improve its biodistribution. Cell targeted-nanoART can achieve this and bring drug efficiently to viral reservoirs. However, if such improvements affect antiretroviral responses remains unknown. To these ends, we tested folic acid (FA)-linked poloxamer407 coated-ritonavir boosted atazanavir (FA-nanoATV/r) nanoparticles for their ability to affect chronic HIV-1 infection in humanized mice. Following three every other week 100 mg/kg FA-nanoATV/r intramuscular injection administered to infected animals viral RNA was at or below the detection limit, cell-associated HIV-1p24 reduced and CD4+ T cell counts protected. The dosing regimen improved treatment outcomes more than two fold from what was reported for untargeted nanoATV/r. We posit that these nanoformulations have potential for translation to human use.
Keywords: folic acid receptor, long-acting nanoformulated antiretroviral therapy, human immunodeficiency virus type one, pharmacokinetics, pharmacodynamics, non-obese diabetic severe combined immunodeficient mice
Combination antiretroviral therapy can reduce, but not eliminate, human immunodeficiency virus (HIV) replication. Therapeutic limitations, including adherence to therapeutic regimens and inadequate drug penetration to viral reservoirs can lead to treatment failures. To this end, our laboratories developed long-acting antiretroviral nanoformulations (nanoART). These were demonstrated to improve antiviral activities (Balkundi et al., 2010). Weekly parenteral administration of poloxamer188 formulated ritonavir-boosted atazanavir (P188-ATV/r) for 6 weeks provided up to a 3-log viral load reduction in humanized HIV-1 infected NOD/scid-IL-2Rγcnull (NSG) mice (Dash et al., 2012). Despite these pharmacodynamics (PD) advantages, high dose, volume of injection and dosing frequency precluded nanoART translation to human use (Gautam et al., 2013; Nowacek et al., 2010; Roy et al., 2012). Such limitations were compounded by injection site irritations and high dose volume required to achieve plasma ATV/r levels sufficient for viral inhibition (Gautam et al., 2013). In order to reduce dose and injection volume we developed a folic acid (FA) modification approach to target the folate receptor on macrophages (Puligujja et al., 2013). Advantage in antiviral activity of FA-nanoATV/r was demonstrated in NSG mice following pre-exposure prophylaxis (PrEP) regimens. The present study on FA-nanoATV/r treated NSG mice builds on prior PK and PD studies. The promising results lay a foundation to further develop nanoformulations for clinical use(Puligujja et al., 2015).
Physicochemical characterization
FA-nanoATV/r nanoformulations (FA-P407-ATV/r) were prepared by high-pressure homogenization(Puligujja et al., 2013). Physicochemical characteristics including particle size, charge, polydispersity (PDI) and shape were determined. Particle size, polydispersity and zeta potential ranged from 257 to 433 nm, 0.17 to 0.33 and −8.9 to −12.1 mV for FA-nanoATV and FA-nanoRTV.
Infection and nanoART treatments
The University of Nebraska Medical Center Institutional Review Board approved human fetal tissue usage. CD34+- hematopoietic stem cells (HSC) were isolated from human fetal liver by immune selection (Miltenyl Biotec Inc, Auburn, CA) then transplanted into NSG mice at birth (Gorantla et al., 2007). At 22 weeks of age mice were infected with a 104 tissue culture infective dose50 (TCID50)/mouse of HIV-1ADA by intraperitoneal injection. Ten weeks later mice were administered 100 mg/kg FA-nanoATV/r intramuscularly with booster doses at 2 and 4 weeks. Replicate animals were untreated. All mice were sacrificed at week 6. Mice were maintained on a folate deficient diet from 2 weeks before and throughout the study. This enabled serum folate levels of < 25 nM that are comparable to humans (Figure 1A).
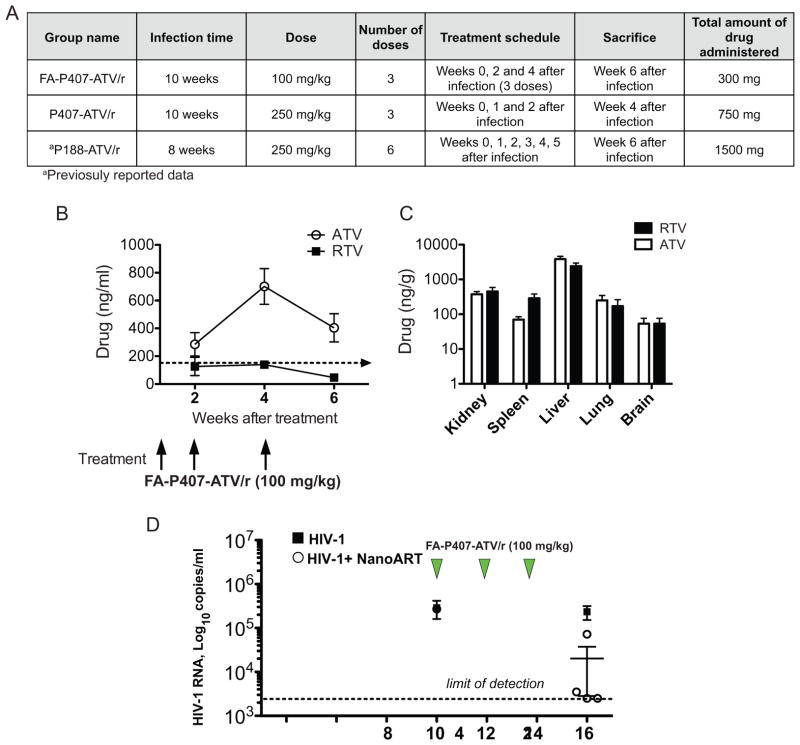
Figure 1.
Comparison of pharmacokinetics and viral loads between targeted and untargeted nanoformulations in CD34+ hematopoietic stem cell transplanted humanized NOD/scid-IL-2Rγcnull mice (A) Table describing the infection and treatment scheme in FA-P407-ATV/r and untargeted groups. Pharmacokinetics of FA-P407-ATV/r. FA-P407-ATV/r (100 mg/kg) was administered after the mice were infected for 10 weeks. A boosting dose of 100 mg/kg was again administered 2 and 4 weeks following the initial dose. Plasma was collected at indicated time points. (B) ATV (open circle) and RTV (closed box) concentrations in plasma were determined by UPLC-MS/MS. Tissues were collected 6 weeks following the initial dose. Data are expressed, as mean ± SEM. (n=5) (C) Tissue ATV and RTV concentrations were determined by UPLC–MS/MS. All data are expressed as mean ± SEM. (n≥3) (D) Plasma viral load in CD34+-HSC-NSG mice during and following FA-P407-ATV/r treatment. Humanized NSG mice were either treated with 3 doses of 100 mg/kg FA-P407-ATV/r at 10, 12 and 14 weeks following HIV-1 infection or untreated. Plasma viral loads from FA-P407-ATV/r (open circle) and untreated (Closed boxes) groups were determined before and after treatment as illustrated. Data are expressed, as mean ± SEM. Data are statistically significant at P <0.05 with Mann-Whitney test (n≥4).
Plasma and tissue drug distribution
Plasma samples were collected at weeks 2, 4 and 6 after drug administration. Mouse tissues were collected after sacrifice. Drug concentrations were determined by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)(Huang et al., 2011). Following treatment with FA-nanoATV/r, plasma ATV concentrations were maintained above the minimum effective concentration (MEC) of 150 ng/ml(Porte, 2006) throughout the study. At 2, 4 and 6 weeks ATV levels were 285±84, 701±128 and 404±101 ng/ml respectively. Corresponding plasma RTV concentrations were 126±65, 139±27 and 46±13 ng/ml respectively (Figure 1B). Tissue drug levels in liver, lung, spleen and kidney were 3835±792, 250±95, 69±14 and 374±72 ng/g for ATV and 2407±554, 171±90, 289±93 and 452±136 ng/g for RTV respectively (Figure 1C).
Viral load determinations
To determine antiviral efficacy of FA-nanoATV/r, pre- and post-treatment viral loads were determined in blood from HIV-1 infected-CD34+-HSC-NSG mice. Viral load (RNA copies/ml) was determined in plasma using COBAS Amplicor System v1.5 kit (Roche Molecular Diagnostics, Switzerland) (Dash et al., 2012). Individual mouse viral loads before treatment with FA-nanoATV/r were 0.7 x 105, 5.0 x 105, 2.0 x 105, 3.2 x 105, and 0.7 x 105 viral RNA copies/ml. Treatment with FA-P407-ATV/r produced an up to 3 log decrease in the viral load and was statistically significant at P<0.05 with Mann-Whitney test (Figure 1D); with 2 mice showing undetectable viral RNA, one with a 2-log decrease (3 x 103 viral RNA copies/ml) and the fourth exhibiting a log decrease (7 x 104 viral RNA copies/ml). The variance in viral levels is likely attributable to humanization, mouse-human cell biology and drug biodistribution differences and irradiation-associated toxicities. The average post-infection and post-treatment viral loads for the untreated group were 2.9 x 105 and 2.2 x 105 viral RNA copies/ml.
Flow cytometric analyses
To assess whether FA-nanoATV/r treatment provided immune protection, we determined the percent of human CD45, CD3, CD4 and CD8 positive cells in blood, spleen and bone marrow. The number of human CD45+, CD3+, CD4+ and CD8+ cells (BD Pharmingen, San Diego, CA) were determined by fluorescence-activated cell sorting (FACS) using a BD FACSDiva® (BD Immunocytometry Systems, Mountain View, CA) system. CD4+ lymphocyte percentages were determined from the CD3+ gated cells. After sacrifice, CD4+ and CD8+ T cell percentages were calculated from CD45+ cells; total CD3+ cell number was considered to be sum of CD4+ and CD8+ cell numbers.
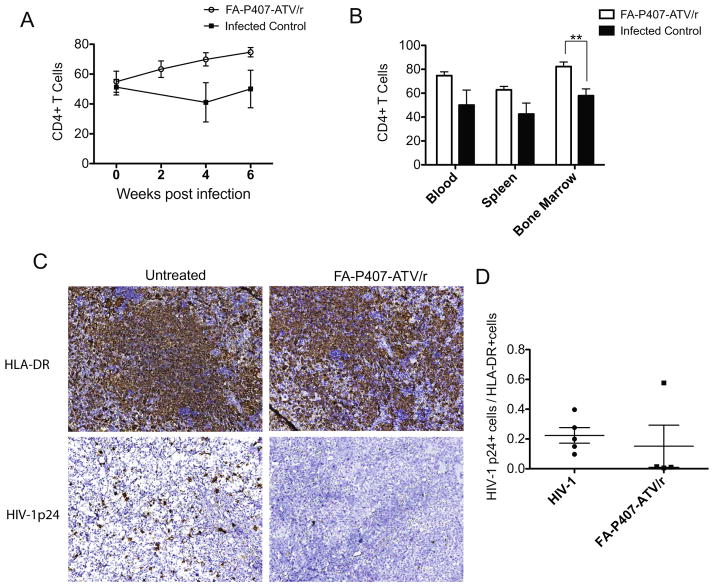
The average pre-infection CD4+ T cell percentages among the untreated and FA-nanoATV/r treated groups in blood were 54.8±7.1% and 51.2±5.2% of the CD3+ T cell population, respectively. After the initial 100 mg/kg dose the average CD4+ T cell percentage in blood was 63.0±5.5% at 2 weeks post treatment. A second dose was administered at week two and CD4+ T cell percentages in blood increased to 69.8±4.4% at week 4. After the third dose CD4+ T cell percentages increased further to 74.7±3.1% at week 6. In contrast, the CD4+ T cell percentages in infected and untreated mice dropped to 50.0±12.5% at week 6 (Figure 2A). The uninfected and untreated mice had CD4+ T cell percentages of 73.5±3.9%. The CD4+ T cell percentages in spleen and bone marrow were 62.7±2.9 and 82.3±3.8% for the FA-nanoATV/r group and 42.5±9.1 and 57.8±5.7% for the infected and untreated mice (Figure 2B). Moreover, the CD4+ T cell percentages in uninfected and untreated mice in spleen and bone marrow were 62.4±2.9 and 90.2±1.7%. The corresponding CD4:CD8 T cell ratios in blood, spleen and bone marrow are shown in supplementary figures 1A and 1B.
Figure 2. Pharmacodynamics of FA-P407-ATV/r treatment in CD34+ hematopoietic stem cell transplanted NSG mice.
The percent of CD4+ T cells among CD3+ T cells and the CD4+ to CD8+ cell ratios were determined using fluorescence-activated cell sorting between treated (open box) and untreated infected control (closed box) groups. (A) CD4+ T cells in blood during the study and (B) CD4+ T cells in blood, spleen and bone marrow at the end of the study. Data are expressed as mean ± SEM. ** Statistically different at P<0.01 using unpaired Student’s t-test (n=5). Spleen immunohistochemistry was performed for HIV-1p24 antigens following FA-P407-ATV/r treatment. Humanized NSG mice were infected with HIV-1ADA for 10 weeks and then treated with 100 mg/kg FA-P407-ATV/r at 0, 2 and 4 weeks. (C) Spleens were collected from treated and untreated groups and immunostained with antibodies for either HLA-DR or HIV-1p24, then visualized with DAB staining. (D) Antiretroviral efficacy was determined by counting the number of HIV-1p24+ cells and HLA-DR+ cells from five random microscopic fields per mouse and then calculating the HIV-1p24+ to HLA-DR+ cell ratios. Data are expressed, as mean ± SEM (n≥4).
Immunohistochemistry
Spleens collected at sacrifice were fixed with 10% neutral buffered formalin (Fisher scientific, Kalamazoo, MI) and paraffin embedded. Five μm thick serial sections were collected and immunostained (Figure 2C) with human leukocyte antigen; HLA-DR (clone CR3/43; 1:100) and mouse monoclonal antibody against HIV-1p24 (clone Kal-1; 1:10)(Puligujja et al., 2015). Antiretroviral activity was determined by dividing the total HIV-1p24+ cells by total HLA-DR+ cells in five random microscopic fields/mouse. The ratio of HIV-1p24+ cells among HLA-DR+ cells in spleen was 0.15±0.14 for the FA-nanoATV/r group compared to 0.22±0.05 in the untreated mice (Figure 2D).
There is a significant interest for the development of long-acting ART nanoformulations (Dolgin, 2014). Although these show improved drug PK and tissue distribution, the relatively high dose and volume of injection remain a limitation. FA-nanoATV/r was improved over non-targeted formulations in both these parameters (Puligujja et al., 2013). For the latter, the PD efficacy of FA-nanoATV/r in a PrEP regimen protected humanized mice against acute HIV infection (Puligujja et al., 2015). However the efficacy of FA-nanoATV/r in treating chronic HIV-1 infection was not investigated. Thus, we evaluated PD outcomes of FA-nanoATV/r in chronically HIV-1 infected CD34+-HSC-NSG mice.
Targeted FA-P407-ATV/r improved the drug-dosing regimens (Dash et al., 2012). FA-P407-ATV/r provided a significant decrease in viral loads compared to untreated infected mice (P<0.05, Mann-Whitney test). It is known that higher plasma drug concentrations are needed for efficient antiviral responses (Winston et al., 2005). The plasma ATV levels following FA-P407-ATV/r were above the MEC. Additionally, in comparison with untargeted P407-ATV/r (Supplementary Figure 2), FA-P407-ATV/r treatments showed enhanced viral load suppression. This can be attributed again to the enhanced plasma drug concentrations with FA targeting. Although the dosing regimen was not identical between the studies, the suppression in viral load seen supports the potential of FA-P407-ATV/r to reduce dosing frequencies.
We reported previously on the antiviral activity of nanoART in CD34+- NSG mice(Dash et al., 2012). In that study CD34+-HSC-NSG were infected for 8 weeks and treated weekly for 6 weeks with 250 mg/kg P188-ATV/r. The mice were sacrificed 7 weeks after the first dose. We compared the viral loads from this previously published study with the viral loads after FA-nanoATV/r treatment (Figure 1D). In comparison to the P188-ATV/r study, the dose of 100 mg/kg FA-nanoATV/r was 2.5 times less than what was administered with P188-ATV/r. Also, the frequency of FA-nanoATV/r treatment was half the frequency of the P188-ATV/r treatment. Taken together, FA-nanoATV/r treatment with 5 times less total drug than P188-ATV/r was sufficient to reduce the viral load to undetectable levels. The effectiveness of FA targeting enables FA-nanoATV/r to be administered once every 2 weeks.
Flow cytometry analysis in blood, spleen and bone marrow demonstrated CD4+ T cell count drops after 10 weeks of HIV-1 infection. These then returned gradually to uninfected levels after treatment. Although not statistically significant, immunohistochemical staining for HIV-1p24 antigen in spleen after FA-nanoATV/r treatment decreased over time. These results demonstrate the potential of FA-nanoATV/r to treat a chronic viral infection. Recent phase II studies underway for monthly or every other month ARV injections will permit future development of folic acid targeting of longer acting antiretrovirals.
Supplementary Material
The CD4+ to CD8+ cell ratios were determined using fluorescence-activated cell sorting between treated (open box) and untreated infected control (closed box) groups. (A) CD4+ to CD8+ ratios in blood over time are shown along with (B) CD4+ to CD8+ ratios in blood, spleen and bone marrow at the end of the study. Data are expressed as mean ± SEM. ** Statistically different at P<0.01 using unpaired Student’s t-test (n=5).
Humanized NSG mice were treated with 3 doses of 250 mg/kg poloxamer 407-ATV/r every week from week 10 to 12 after HIV-1 infection and viral RNA copies/ml were determined.
Highlights.
Improved pharmacodynamics of FA-nanoATV/r were shown during chronic HIV-1 infection of CD34+-HSC-NSG mice
In mice treated every other week with 100 mg/kg FA-nanoATV/r showed viral RNA copies/ml below limit of detection
FA-nanoATV/r enabled dosing reductions of 2.5 times over nondecorated nanoATV/r to achieve viral suppression
Acknowledgments
This work was supported by the University of Nebraska Foundation which includes individual donations from Carol Swarts and Frances and Louie Blumkin, the Vice Chancellor’s office of the University of Nebraska Medical Center, ViiV Healthcare and National Institutes of Health grants P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, P30 MH062261 and R01 AG043540. The authors thank Jaclyn Knibbe, Selena Dickinson and Edward Makarov for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balkundi S, Nowacek AS, Roy U, Martinez-Skinner A, McMillan J, Gendelman HE. Methods development for blood borne macrophage carriage of nanoformulated antiretroviral drugs. Journal of visualized experiments: JoVE. 2010 doi: 10.3791/2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, Gelbard HA, McMillan J, Gorantla S, Poluektova LY. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. Aids. 2012;26:2135–2144. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. Long-acting HIV drugs advanced to overcome adherence challenge. Nature medicine. 2014;20:323–324. doi: 10.1038/nm0414-323. [DOI] [PubMed] [Google Scholar]
- Gautam N, Roy U, Balkundi S, Puligujja P, Guo D, Smith N, Liu XM, Lamberty B, Morsey B, Fox HS, McMillan J, Gendelman HE, Alnouti Y. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrobial agents and chemotherapy. 2013;57:3110–3120. doi: 10.1128/AAC.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, Wood C, Dewhurst S, Gendelman HE, Poluektova L. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2−/−gammac−/− mice. Journal of virology. 2007;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gautam N, Bathena SP, Roy U, McMillan J, Gendelman HE, Alnouti Y. UPLC-MS/MS quantification of nanoformulated ritonavir, indinavir, atazanavir, and efavirenz in mouse serum and tissues. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2011;879:2332–2338. doi: 10.1016/j.jchromb.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacek AS, McMillan J, Miller R, Anderson A, Rabinow B, Gendelman HE. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2010;5:592–601. doi: 10.1007/s11481-010-9198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte CJLlBD, Blaschke T, Boucher CAB, Fletcher CV, Flexner C, Gerber J, Kashuba ADM, Schapiro JM, Burger DM. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Reviews in Antiviral Therapy. 2006;2006:4–14. [Google Scholar]
- Puligujja P, Balkundi SS, Kendrick LM, Baldridge HM, Hilaire JR, Bade AN, Dash PK, Zhang G, Poluektova LY, Gorantla S, Liu XM, Ying T, Feng Y, Wang Y, Dimitrov DS, McMillan JM, Gendelman HE. Pharmacodynamics of long-acting folic acid-receptor targeted ritonavir-boosted atazanavir nanoformulations. Biomaterials. 2015;41:141–150. doi: 10.1016/j.biomaterials.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu XM. Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections. Nanomedicine: nanotechnology, biology, and medicine. 2013;9:1263–1273. doi: 10.1016/j.nano.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy U, McMillan J, Alnouti Y, Gautum N, Smith N, Balkundi S, Dash P, Gorantla S, Martinez-Skinner A, Meza J, Kanmogne G, Swindells S, Cohen SM, Mosley RL, Poluektova L, Gendelman HE. Pharmacodynamic and antiretroviral activities of combination nanoformulated antiretrovirals in HIV-1-infected human peripheral blood lymphocyte-reconstituted mice. The Journal of infectious diseases. 2012;206:1577–1588. doi: 10.1093/infdis/jis395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston A, Bloch M, Carr A, Amin J, Mallon PW, Ray J, Marriott D, Cooper DA, Emery S. Atazanavir trough plasma concentration monitoring in a cohort of HIV-1-positive individuals receiving highly active antiretroviral therapy. The Journal of antimicrobial chemotherapy. 2005;56:380–387. doi: 10.1093/jac/dki235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The CD4+ to CD8+ cell ratios were determined using fluorescence-activated cell sorting between treated (open box) and untreated infected control (closed box) groups. (A) CD4+ to CD8+ ratios in blood over time are shown along with (B) CD4+ to CD8+ ratios in blood, spleen and bone marrow at the end of the study. Data are expressed as mean ± SEM. ** Statistically different at P<0.01 using unpaired Student’s t-test (n=5).
Humanized NSG mice were treated with 3 doses of 250 mg/kg poloxamer 407-ATV/r every week from week 10 to 12 after HIV-1 infection and viral RNA copies/ml were determined.