Abstract
Introduction
Calpain is a family of cysteine proteases that has an important role in the initiation, regulation and execution of cell death. Our recent studies using a hypercholesterolemic swine model demonstrated that in the setting of metabolic syndrome calpain inhibition improved collateral dependent perfusion, and increased expression of proteins implicated in angiogenesis and vasodilation. In this study, we hypothesized that calpain inhibition (CI) would decrease myocardial apoptosis in the same model.
Methods
Yorkshire swine, fed a high cholesterol diet for four weeks, then underwent placement of an ameroid constrictor on the left circumflex artery. Three weeks later animals received either: no drug, high cholesterol control group (HCC; n= 8); low dose CI (0.12 mg/kg; LCI, n= 9); or high dose CI (0.25 mg/kg; HCI, n= 8). The high cholesterol diet and CI were continued for five weeks, after which the pig was euthanized and the left ventricular myocardium was harvested and analyzed via TUNEL staining, oxyblot analysis and western blots. Data was analyzed via the Kruskal-Wallis test.
Results
The percentage of apoptotic cells to total cells in ischemic myocardial territory was significantly decreased in the LCI and HCI groups compared to the HCC group as shown by TUNEL staining (p= 0.018). There was a significant decrease in pro-apoptotic proteins including cleaved caspase 3 (p =0.001), caspase 9 (p = 0.003), cleaved caspase 9 (p=0.004), Bax (p=0.0262), BAD (p= 0.049), p-BAD (p= 0.007) , Erk 1/2 (p= 0.016) and a statistically insignificant decrease in caspase 3 (p= 0.737). There was a significant increase in anti-apoptotic proteins including BCL-2 (p= 0.025) and p-BCL2 (p= 0.004). In the ischemic myocardium, there was a significant increase in several pro-angiogenic proteins in the LCI and HCI groups compared to the HCC group including p-AKT (p=0.0001), p-eNOS (p= 0.003) and eNOS (p=0.006) with a statistically insignificant increase seen in AKT (p=0.311). CI significantly decreased tissue oxidative stress in both the LCI and HCI groups as compared to the HCC group as shown by Oxyblot analysis (p= 0.021).
Conclusions
In the setting of hypercholesterolemia, CI decreases apoptosis and the expression of proteins in pro apoptotic signaling pathways. CI also increased expression of proteins implicated anti apoptotic pathways and improves oxidative stress in ischemic myocardial tissue.
Introduction
Metabolic syndrome is associated with an increased incidence of coronary artery disease and coronary artery disease mortality. 1 In patients who are not candidates for traditional revascularization strategies such as coronary bypass grafting or percutaneous coronary intervention, regenerative therapies are an attractive theoretical option. However, nearly all attempts have met with little success. 2,3 Patients with end-stage coronary artery disease have been shown to have advanced myocardial and endothelial dysfunction and abnormal myocardial and vascular signaling. 4,5 Similarly patients with metabolic syndrome have diminished angiogenic responses to chronic ischemia and alterations in a variety of mechanisms contributing to vascular bed dysfunction including the formation of collateral vessels. 6,7 We and other laboratories have reported that a major mechanism leading to vascular dysfunction in metabolic syndrome is increased oxidative stress leading to over production of reactive oxygen species (ROS) and cellular apoptosis. 7,8,9 [Figure 1]
Figure 1. Apoptotic Pathways.
Multiple signaling pathways involved in cellular apoptosis.
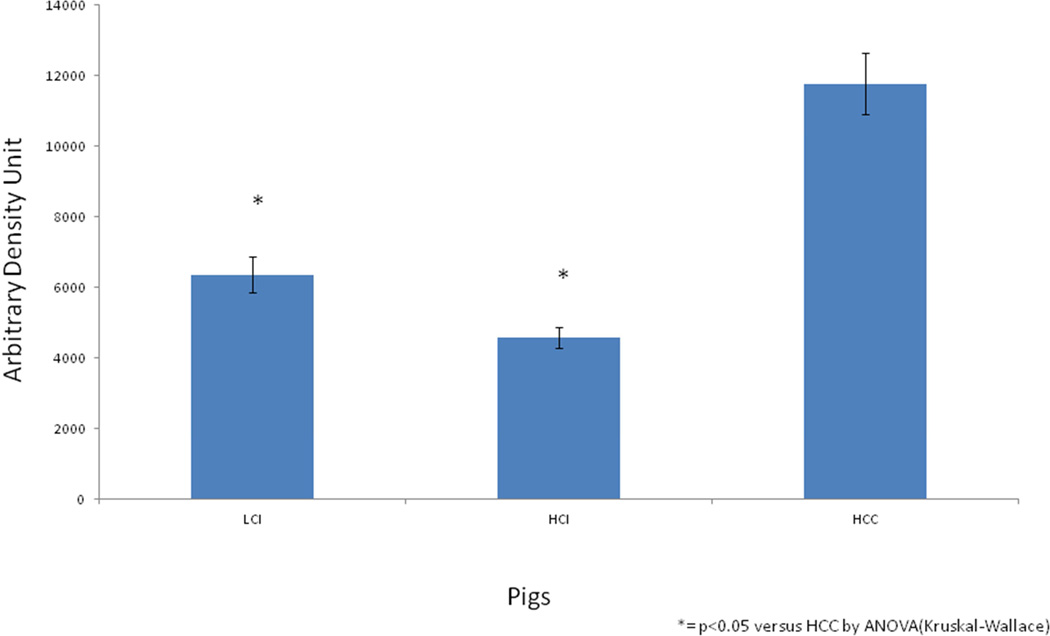
Results of Calpain Inhibition on Oxidative Stress in Ischemic Myocardial hypermetabolic Pig Tissue. CI significantly decreased tissue oxidative stress in both the LCI and HCI groups as compared to the HCC group as shown by oxiblot analysis (p= 0.021). (*) = p<0.05 versus HCC by ANOVA (Kruskal-Wallace)
Recent studies have suggested calpains control fundamental cellular functions such as cytoskeleton remodeling, cell cycle regulation, gene expression and cell death in all tissue. 10 Myocardial calpain plays an essential role in the ubiquitin/proteasome protein degradation pathway that removes proteins whose abnormal accumulation causes cardiomyocyte apoptosis and heart failure. 11, 12 In ischemic heart disease, calpain activation has been found to promote left ventricular remodeling after myocardial infarction by disassembling cell-cell adhesion via degradation of N-cadherin. 10 However, uncontrolled activation of calpain has been proven to be involved in the pathogenesis of myocardial ischemia and dysfunction. 13 Similarly, in metabolic syndrome, hyper activation of calpain has been linked to myocardial and vascular inflammation and impaired collateral formation. 14 Conversely, complete knockout of calpain has been proven to abolish neovessel integration and lumen formation. Taken together, these findings suggest that a regulated level of calpain is necessary for basic physiological function and that over-activation of calpain leads to tissue dysfunction. 15 In support of this notion, previous research has shown that moderate inhibition of calpain can improve neovasculature intervention and lumen formation during pathological angiogenesis and improve global hemodynamics and left ventricular contractility in myocardial ischemia. 13,16 The mechanism by which uncontrolled activation of calpain leads to cardiac dysfunction is still under investigation. Therefore, calpain represents an important target for rectifying key vascular defects associated with pathological angiogenesis. 16
We recently found that calpain activity was significantly increased in the ischemic myocardium of pigs with metabolic syndrome and that calpain inhibition improved collateral dependent perfusion, and increased expression of proteins implicated in angiogenesis and vasodilatation. 17 Since collateral vessel formation is in part regulated by apoptosis, the purpose of this study is to examine the role of calpain on myocardial apoptosis in the setting of chronic ischemia and MS conditions. In the current study, we hypothesized that calpain inhibition could be affecting the heart not only via angiogenic pathways but also by helping to inhibit ischemia induced cell death. The findings reported here implicate a role of calpain on myocardial apoptosis is essentially aimed at identifying the molecular mechanisms by which calpain inhibition may exert a beneficial effect on chronic myocardial ischemia.
In this study, we hypothesized that dose dependent calpain inhibition (CI) would have a protective effect on the heart against chronic ischemia-induced myocardial apoptosis in a clinically relevant model of pig with metabolic syndrome.
Methods
Animal Model
Twenty- Five juvenile (7weeks old) Yorkshire swine (E.M. Parsons and Sons, Hadley MA) were fed a high fat/ high cholesterol diet for four weeks. The swine then underwent placement of an ameroid constrictor on the left circumflex artery to induce chronic myocardial ischemia. Three weeks later, animals received either: no drug, (high cholesterol control group, HCC; n= 8); a low dose of the of Calpain inhibitor MDL28170 (CI) drug (0.12 mg/kg daily; LCI, n= 9); or a high dose of Calpain inhibitor MDL28170 (CI) drug (0.25 mg/kg daily; HCI, n= 8). The high cholesterol diet and calpain inhibitors were continued for five weeks, after which the pig was euthanized and the left ventricular myocardium was harvested. All animals were observed to ensure: complete consumption of food and supplemental, unlimited access to water and a warm non-stressful housing environment for the duration of the experiment. Endocardial tissue was analyzed via TUNEL staining, oxiblot analysis and western blots. Data was analyzed via the Kruskal-Wallis test. The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments.
Surgical Procedures
Anesthesia
Anesthesia was induced by intramuscular injection of telazol (4.4mg/kg). General anesthesia was maintained with a gas mixture of oxygen at 1.5–2L/min and isoflurane at 0.75–3.0%concentration. Animals were endotracheally intubated and mechanically ventilated at 12–20 breaths/min.
Ameroid Constrictor Placement
One dose of enrofloxacin 5mg/kg was administered to each pig prior to the induction of general anesthesia. Animals were then prepped and draped in the usual sterile fashion. A left mini-thoracotomy was performed to expose the heart. The left atrial appendage was retracted and the proximal left circumflex artery was dissected free at the take off from the left main artery. The ameroid constrictor was placed around the left circumflex artery (Research Instruments NW, Excondido, CA). Next the pericardium was re-approximated and the surgical incision was closed in layers. Intramuscular bupenorphone (0.03mg/kg) was administered and a 72h fentanyl patch (4ug/kg) was placed near the incision site to control post operative pain. All animals received 325mg of aspirin for thrombo-embolic prophylaxis and enrofloxacin 68mg daily for a total of 5 days.
Cardiac Harvest
General anesthesia was induced and the pigs were prepped and draped in the usual sterile fashion. A median sternotomy was performed to expose the heart. Myocardial perfusion was measured by injecting isotope-labeled microspheres at rest and with demand pacing at 150 beats/min was performed. Then euthanasia was before by exsaguination and the hearts were harvested.
The area of the chronically ischemic left ventricle immediately adjacent to the left circumflex artery (distal to the ameroid constrictor) was sectioned out into full thickness pieces. The normally perfused left ventricle immediately adjacent to the left anterior descending artery was sectioned out into full thickness pieces as well. (A previous study reporting on myocardial perfusion analysis confirmed that the ischemic territory has the lowest microsphere counts and the normally perfused normal ventricle had the highest microsphere counts). 18
The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments. Animals were cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals”.
ApopTag Peroxidanse InSitu Apoptosis Detection Kit (Millipore, Billerica, MA)
Frozen myocardium was sectioned (10-um thickness) and fixed in 1% paraformaldehyde in PBS, pH 7.4. for 10minutes at room temperature. Samples were washing in PBS for 2X5 minutes. Samples were then post-fixed in precooled ethanol:acetic acid 2:1 for 5 minutes at −20 degrees C in a coplin jar and washed in PBS for 2X5minutes. Samples were quenched in 3.0% hydrogen peroxide in PSB for 5 minutes at room temperature and rinsed in PBS for 2X5minutes. 75 uL/5cm2 of Equilibration Buffer was directly added to the specimen and incubated for 10 seconds at room temperature. Excess liquid was tapped off and 55ul/5cm2 of Working Strength TdT Enzyme was added and samples were incubated in a humidified chamber at 37 degrees for 1 hour. Working Strength Stop/Wash Buffer was then added for 10 minutes at room temperature. The samples were washed in PBS for 3×1minute and Anti-Digoxigenin Peroxidase Conjugate was applied at 65 uL/5cm2 of surface covered and incubated in a humidified chamber for 30minutes at room temperature. Samples were then washed in PBS for 4×2minutes. Working Strength Peroxidase Substrate was then applied with enough to cover the entire specimen (75uL/5cm2) and was set to stain for 3–6 minutes at room temperature. The color was monitored for development by looking at the slide under a microscope. The specimen was washed in dH2O for 3×1minute and incubated for 5 minutes in dH2O. Counter stain in 0.5% (w:V) methyl green was applied for 10 minutes at room temperature. The specimen was washing in 3 changes of dH2O and then 3 changes of 100% N-Butanol. The specimen was dehydrated by moving the slide through 3 jars of xylene and a glass coverslip was applied in mounting medium. Images were captured at x20 magnification with a Nikon E800 Eclipse microscope (Nikon, Toyko, Japan) in five random fields.
Protein Expression
Forty micrograms of radio-immuno-precipitation assay (Boston BioProducts, Ashland, MA) soluble fraction of myocardial lysates were fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis 3%–8% Tris-Acetate gel (NuPage Novex Mini Gel). The protein was then transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA) and incubated overnight at 4 degrees C with primary antibodies at dilutions recommended by the manufacturer against cleaved caspase 3, caspase 9, cleaved caspase 9, Bax, BAD, p-BAD, caspase 3, BCL-2, p-BCL2, p-AKT , ERK ½, p-ENOS, ENOS, and AKT (all from Cell Signaling, Danvers, MA). Membranes were incubated with the appropriate horseradish peroxidase-linked secondary antibody for 1h at room temperature (Jackson ImmunoResearch, West Grove, PA). Immune complexes were visualized with enhanced chemiluminescence and images were captured with a digital camera system (G-Box, Syngene, Cambride, England). Band densitometry was quantified as arbitrary light units using Image J Software. All membranes were probed with GAPDH (Cell Signaling) to correct for loading error.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Frozen myocardium from the ischemic territory was sectioned (10-um thickness). Apoptotic cells were identified using the commercially available ApopTag detection kit (Millipore) according to the manufacturer’s instructions. Images were captured at 20X magnification using Nikon E800 Eclipse microscope (Nikon, Toyko, Japan) in multiple random fields from each animal. The percentage of Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)- positive cardiomyocytes was measured using Image J software in a blinded fashion.
Data Analysis
All results are reported as a mean +/− standard deviation. One way analysis of variance was used to compare the means among groups followed by a Kruskal-Wallis post-hoc test to compare the means among groups using GraphPad Prism 5.0 Software (GraphPad Software Inc, Sand Diego, Ca). Protein expression is normalized to GAPDH in all pigs and is reported as fold change compared with the hyper metabolic control pigs (HCC).
Results
TUNEL STAINING
The percentage of apoptotic cells to total cells in ischemic myocardial territory was significantly decreased in the LCI ( 0.106%) and HCI (0.223%) groups compared to the HCC (0.402%) group as shown by TUNEL Staining (p= 0.018). [Table 1]
Table 1. Results of Calpain Inhibition on Ischemic Myocardial Pig Tissue.
The percentage of apoptotic cells to total cells in ischemic myocardial territory was significantly decreased in the LCI and HCI groups compared to the HCC group as shown by TUNEL Staining. There was a significant decrease in pro-apoptotic proteins including cleaved caspase 3, caspase 9, cleaved caspase 9, Bax, BAD, p-BAD, and an insignificant decrease in caspase 3. There was a significant increase in anti-apoptotic proteins including p-AKT, p-eNOS, eNOS, BCL-2 and p-BCL2 with an insignificant increase seen in AKT. CI significantly decreased tissue oxidative stress in both the LCI and HCI groups as compared to the HCC group as shown by Oxyblot Analysis.
| LCI | HCI | HCC | p-Value | |
|---|---|---|---|---|
| TUNEL Staining | 0.106%* | 0.223%* | .402%* | 0.018 |
| Cleavedcaspase 3 | 0.511+/−0.178 | 0.678+/−0.240 | 1.0 | 0.001 |
| Caspase 3 | 0.973+/−0.261 | 0.981+/−0.290 | 1.0 | 0.737 |
| Caspase 9 | 0.672+/−0.529 | 0.653+/−0.758 | 1.0 | 0.00.3 |
| Cleaved caspase 9 | 0.648+/−0.119 | 0.717+/−0.136 | 1.0 | 0.004 |
| Bax | 0.734+/−0.483 | 0.808+/−0.279 | 1.0 | 0.0262 |
| BAD | 0.301+/−0.488 | 0.707+/−0.991 | 1.0 | 0.049 |
| p-BAD | 0.469+/−0.359 | 0.656+/−0.499 | 1.0 | 0.014 |
| ERK1/2 | 0.660+/−0.222 | 0.900+/−0.282 | 1.0 | 0.016 |
| BCL-2 | 2.395+/−0.954 | 1.103+/−0.423 | 1.0 | 0.025 |
| P-BCL2 | 4.378+/−2.851 | 2.481+/−2.546 | 1.0 | 0.004 |
| P-AKT | 1.423+/−0.567 | 0.731+/−0.251 | 1.0 | 0.0001 |
| AKT | 1.263+/−0.613 | 1.180+/−0.636 | 1.0 | 0.311 |
| pENOS | 8.518+/−6.433 | 3.452+/−1.879 | 1.0 | 0.003 |
| ENOS | 1.260+/−0.665 | 1.870+/−0.761 | 1.0 | 0.006 |
| P-Bad/Bad | 0.993+/−.453 | 0.814+/−0.698 | 1.0 | 0.0861 |
| P-BLC-2/BCL2 | 1.813+/−1.089 | 1.477+/−1.183 | 1.0 | 0.200 |
| PENOS/ENOS | 3.313+/−1.372 | 2.280+/−1.689 | 1.0 | 0.0226 |
| pAKT/AKT | 1.327+/−0.743 | 0.801+/−0.546 | 1.0 | 0.176 |
| Oxyblot | 0.540 | 0.390 | 1.0 | 0.021 |
All numbers are load controlled to GAPDH and normalized to HCC except where (*).
P Values determined via ANOVA (Krustal-Wallace)
Legend:
LCI – High Cholesterol, Low Dose Calpain Inhibitor Pigs
HCI- High Cholesterol, High Dose Calpain Inhibitor Pigs
HCC- High Cholesterol Control, No Drug Pigs
OXIBLOT ANALYSIS
CI significantly decreased tissue oxidative stress in both the LCI and HCI groups as compared to the HCC group as shown by Oxiblot analysis (p= 0.021). [Figure 1]
PRO-APOPOTIC SIGNALING
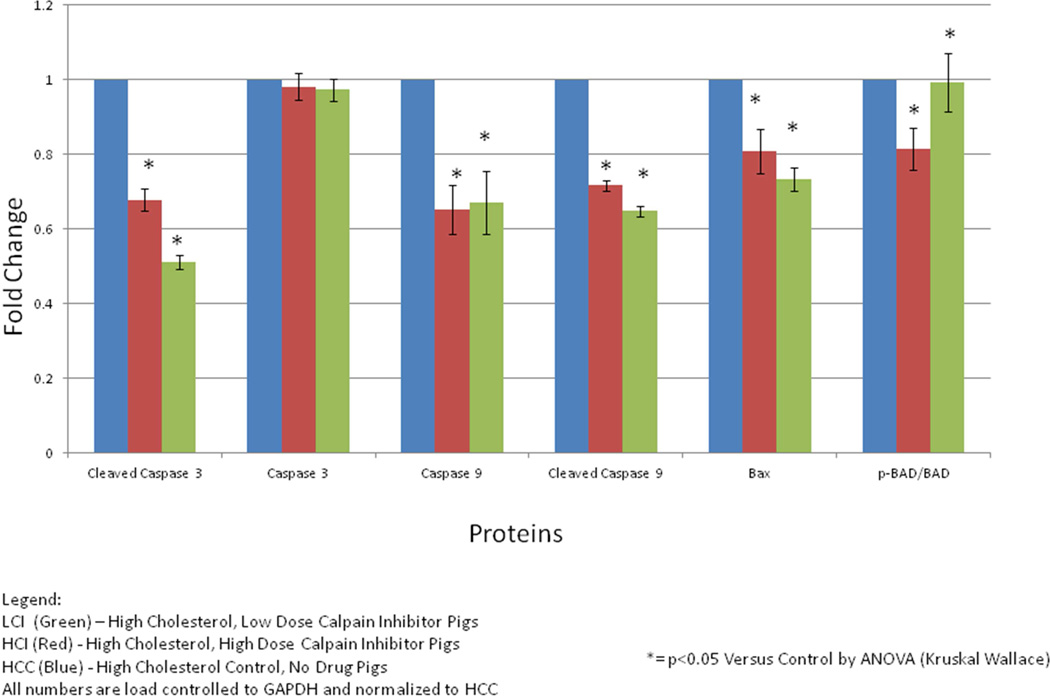
There was a significant decrease in pro-apoptotic proteins in the CI pigs compared to the HCC pigs including cleaved caspase 3 (p =0.001), caspase 9 (LCI p = 0.003), cleaved caspase 9 (p=0.004), Bax (p=0.0262), BAD (p= 0.049), p-BAD (p= 0.007), ERK 1/2 (p= 0.0157) and a statistically insignificant decrease in caspase 3 (p= 0.737). [Figure 2]
Figure 2. Results of Calpain Inhibition on Pro-Apopotic Protein Signaling in Ischemic Myocardial hypermetabolic Pig Tissue.
There were significant decreases in pro-apoptotic proteins including cleaved caspase 3 (p =0.001), caspase 9 (p = 0.003), cleaved caspase 9 (p=0.004), Bax (p=0.0262), BAD (p= 0.049), p-BAD (p= 0.007), ERK 1/2(p= 0.0157) and an insignificant decrease in caspase 3 (p= 0.737). Results figured as ratios of active form of protein (phosphorylated) to inactive form of protein where appropriate. All numbers are load controlled to GAPDH and normalized to HCC. (*)= p<0.05 versus HCC by ANOVA (Kruskal-Wallace)
ANTI-APOPTOTIC SIGNALING
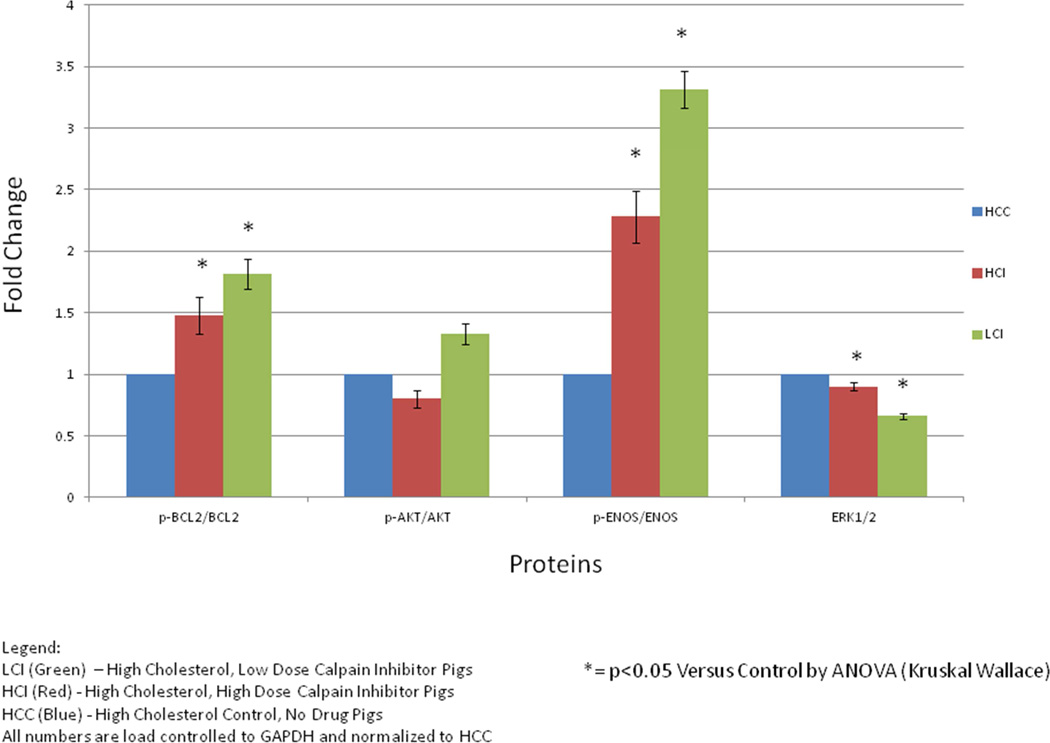
There was a significant increase in anti-apoptotic proteins in the CI pigs compared to the HCC pigs including p-AKT (p=0.0001), p-eNOS (p= 0.003), eNOS (p=0.006), BCL-2 (p= 0.025) and p-BCL2 (p= 0.004) with an insignificant increase seen in AKT (p=0.311). [Figure 3]
Figure 3. Results of Calpain Inhibition on Anti-Apoptotic Protein Signaling in Ischemic Myocardial hypermetabolic Pig Tissue.
There was a significant increase in anti-apoptotic proteins including p-AKT (p=0.0001), p-eNOS (p= 0.003) and eNOS (p=0.006), BCL-2 (p= 0.025) and p-BCL2 (p= 0.004) with an insignificant increase seen in AKT (p=0.311). Results figured as ratios of active form of protein (phosphorylated) to inactive form of protein where appropriate. All numbers are load controlled to GAPDH and normalized to HCC. (*)= p<0.05 versus HCC by ANOVA (Kruskal-Wallace)
Discussion
Up to 20–37% of patients with coronary artery disease cannot undergo coronary artery bypass grafting or percutaneous coronary intervention secondary to their high risk for surgical procedures. 7 Most of these patients suffer from multiple co-morbid conditions including: tobacco use, hypertension, hyperlipidemia, diabetes, and endothelial dysfunction. Clinical trials for angiogenic and cell therapy to treat these patients have been, so far, unsuccessful for severe coronary artery disease. This indicates that we do not currently understand the basic mechanisms of collateral vessel formation in chronic myocardial ischemia. Most of this research was performed in animal models for myocardial ischemia that did not take into account the patients co-morbidities. This is especially true in the setting of metabolic syndrome conditions which are associated with vascular dysfunction and increased oxidative stress. 7
The most novel aspect of our work is our animal model which can answer the question of how co-morbidities affect coronary artery disease in high surgical risk patients. Our group previously reported that a high fat diet leads to most components of metabolic syndrome including weight gain, glucose intolerance, dyslipidemia and hypertension. As a result, using our already proven method of developing chronic myocardial ischemia via the use of an ameroid constrictor around the left circumflex artery, we were able to create a pig model of coronary artery disease in the setting of metabolic syndrome that we feel will better represent patients with CAD and their associated co-morbidities. 19
Calpain activation is up-regulated under cell stress and hypoxic conditions. High glucose levels are known to induce apoptosis in cardiomyocytes via oxidative stress and elevation of calcium ions. Kumar et al showed that high glucose induced ROS and mitosolic calcium overload plays a role in proteolytic cleavage of calpain-1 in rat cardiomyocytes and activates its downstream targets promoting apoptosis. 20 In conjunction with previously sited research, it therefore follows that calpain would be a good therapeutic target for both ischemia and high glucose induced myocardial disease. We recently reported that calpain inhibition may improve collateral dependent myocardial perfusion in this pig model of diet induced metabolic syndrome. 17 The findings reported in this study implicate a role of calpain on myocardial apoptosis and show that moderate calpain inhibition exerts a beneficial effect on chronic myocardial ischemia in the setting of metabolic syndrome.
In this study, inhibition of oxidative stress was confirmed by the decreased tissue oxidative stress in both the LCI and HCI groups as compared to the HCC group. The benefit was seen to a greater extent in the HCI group as compared to the LCI group. This is consistent with previous research that suggests that both glucose and ischemia can induce calpain induced apoptosis in mycocytes and that calpain inhibition can protect cardiac cells. 20
We also found that calpain inhibition significantly improves cell survival in the ischemic myocardial territory. CI was found to significantly decrease the percentage of apoptotic cells in the ischemic myocardial tissue compared to the HCC pigs. To further determine the mechanism by which this occurs we did protein analysis to study different apoptotic pathways.
Youn et al established a critical role for calpin signaling in modulating endothelial cell function via VEGF dependent signaling to eNos via AMPK and AKT. 21 AKT is also known to inhibit apoptosis via the mitochondrial and Ca2+ induced death pathways by inhibiting the caspases, Bad and Bax. This is consistent with our results showing that calpain inhibition decreased cleaved caspase 3, Bax, BAD, p-BAD, and increased p-AKT, eNOS and p-eNOS. It should be noted that the p-AKT/AKT ratio and the change in AKT values were both not found to be significantly different between the groups however p-AKT, the active form of the protein, was found to be significantly increased in the calpain inhibited pigs. This suggests that calpain inhibition still had an effect on the functioning form of this protein. Likewise, caspase 3 was not found to be significantly different amongst the three groups but its active form, cleaved caspase 3, was found to be significantly lower in the calpain inhibited groups.
BCL-2 inhibits the mitochondrial apoptotic pathway inhibiting Cyc-C and Apaf-1 activation of Caspase 9 and inhibiting p53 induction of cell death. This is consistent with our research that showed an increase in BCL-2 and significant decrease in caspase 9 and cleaved caspase 9 suggesting another pathway by which calpain works to induce cell death.
The MAPK/ERK pathway is a chain of proteins involved in cellular apoptosis that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. Our findings suggest that calpain inhibition down regulated this pathway in ischemic myocardial tissue suggesting a third means by which calpain inhibition could lead to decreased cellular apoptosis.
Importantly, our research shows that both the high dose calpain inhibitor and the low dose calpain inhibitor improved apoptotic signaling, oxidative stress and percentage of apoptotic cells in ischemic myocardial tissue in the setting of metabolic syndrome. In addition, it identifies three different apoptotic pathways through which calpain works. This is consistent with previous research suggesting that moderate inhibition of calpain can improve neovasculature and lumen formation during pathological angiogenesis and thus improve global hemodynamic and left ventricular contractility in myocardial ischemia. 13,15,16 The question that still needs to be answered is exactly where calpain is having this effect.
In our study, it is interesting to note that our data suggests oxidative stress is improved to a greater extent by the high dose calpain inhibitor than by the low dose calpain inhibitor (Fig.1).In fact, high dose calpain inhibition reduced oxidative stress by almost 67% (from ~12,000 units to ~4,000 units), low dose (LCI) reduced oxidative stress by around 50%, suggesting that calpain inhibition-mediated improvement of myocardial apoptosis is in fact dose-dependent. Future work will be needed to address the precise dose-dependent effects. Interestingly, the dose-dependent effects of calpain inhibition are not obvious from the Western blot data involving modulation of apoptotic cell signaling molecules. However, it is clear that some amount of moderate calpain inhibition has a statistically significant benefit when compared to the no drug control pigs. This suggests that calpain inhibition may be working via an oxidative pathway to improve downstream signaling affecting myocardial apoptosis in chronic ischemia. Ongoing work in our lab is aimed at examining the oxidative stress pathways’ roles in mediating the effects of high dose and low dose calpain inhibition.
Limitations
The currently available calpain inhibitors are not selective for the different calpain members so it was impossible for us to tell which calpain members the inhibitors were working on. Autolysis could have a role in calpain function in response to metabolic syndrome. In the Western blot experiments, we could observe proteolytically derived calpain fragments. The nature and role of possible autolysis, however, are not known. While it has been shown that complete inhibition of calpain is detrimental to tissue, our study does not consistently show that the low dose or high dose is the preferred amount. While we can confidently predict that some form of calpain inhibition is beneficial, we have not identified the adequate dose in this study.
In summary, our findings demonstrated that CI treatment promotes survival protein expression, inhibits the apoptosis pathway and decreases oxidative stress in pigs with metabolic syndrome and chronic myocardial ischemia. Future studies will provide critical insight for the mechanism by which calpain inhibition may improves oxidative stress.
Acknowledgement
This research project was supported in part by the National Institute of Health R01 grants-HL-46716(F.W.S); NIGMS/NIH grant 1P20GM103652 01A1 (Project# 3) and American Heart Association Grant-in-Aid 14GRNT20460291 (M.R.A.); Rhode Island Foundation-RIF-20123834 and NIH-5P20 GM1P20GM103652 (J.F.), NIH Training grant 5T32-HL094300-03 (Drs Elmadhun and Sabe). National Heart, Lung and Blood Institute, National Institutes of Health- 5R25HL088992-06 (Hunter Mitchell).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galassi A, Reynolds K, He J. Metabolic Syndrome and Risk of Cardiovascular Disease: A Meta-Analysis. The Americal Journal of Medicine. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 2.Lahan RJ, Rezaee M, Post M, Sellke FW, Braeckman RA, Hung D, Simons M. Intracoronary and Intravenous Administration of Basic Fibroblast Growth Factor: Mycocardial and Tissue Distribution. Drug Metabolism & Disposition. 1999;27:821–826. [PubMed] [Google Scholar]
- 3.Baumgartner WA, Burrows S, del Nido PJ, Gardner TJ, Goldberg S, Gorman RC, Letsou GV, Mascette A, Michler RE, Puskas JD, Rose EA, Rosengart TK, Sellke FW, Shumway SJ, Wilke N. Recommendations of the National Heart, Lung and Blood Institute Working Group on Future Direction in Cardiac Surgery. Circulation. 2005;111:3007–3013. doi: 10.1161/CIRCULATIONAHA.104.530154. [DOI] [PubMed] [Google Scholar]
- 4.El-Tamimi H, Mansour M, Wargovich TJ, Hill JA, Kerensky RA, Conti CR, Pepine CJ. Constrictor and dilator responses to intracoronary acetylcholine in adjacent segments of the same coronary artery in patients with coronary artery disease. Endothelial function revisited. Circulation. 1994;89:45–51. doi: 10.1161/01.cir.89.1.45. [DOI] [PubMed] [Google Scholar]
- 5.Werns SW, Walton JA, Hsia HH, Nabel EG, Sanz ML, Pitt B. Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation. 1989;79:287–291. doi: 10.1161/01.cir.79.2.287. [DOI] [PubMed] [Google Scholar]
- 6.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of Diabetes Mellitus on Formation of Coronary Collateral Vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 7.Boodhwani M, Sellke FW. Therapeutic Angiogenesis in Diabetes and Hypercholesterolemia: Influence of Oxidative Stress. Antioxidants & Redox Signaling. 2009;11:1945–1959. doi: 10.1089/ars.2009.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calver A, Collier J, Vallance P. Inhibition and Stimulation of Nitric Oxide Synthesis in the Human Forearm Arterial Bed of Patients with Insulin-dependent Diabetes. J. Clin.Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 10.Kudo-Sakamoto Y, Akazawa H, Ito K, Takano J, Yano M, Yabumoto C, Naito AT, Oka T, Lee J-K, Sakata Y, Suzuki J-I, Saido T-C, Komuro I. Calpain-dependent Cleavage of N- cadherin Is Involved in the Progression of Post-myocardial Infarction Remodeling. The Journal of Biological Chemistry. 2014;289:19408–19419. doi: 10.1074/jbc.M114.567206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagishi S-I, Edelstein D, Du X-L, Brownlee M. Hyperglycemia Potentiates Collagen-Induced Platelet Activation Through Mitochondrial Superoxide Overproduction. Diabetes. 2001;50:1491–1494. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 12.Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Meledez JG, Ronnins J, Lynch RA, Marreez Y, Dorn GW. Cardiomyocyte Degeneration with Calpain Deficiency Reveals a Critical Role in Protein Homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 13.Khalil PN, Neuhof C, Hiss R, Pollhammer M, Khalil MN, Neuhob H, Fritz H, Siebeck M. Calpain Inhibition Reduces Infarct Size and Improves Global Hemodynamics and Left Ventricular Contractility in a Porcine Myocardial Ischemia/Reperfusion Model. European Journal of Pharmacology. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Stalker TJ, Gong Y, Scalia R. The Calcium- Dependent Protease Calpain Causes Endothelial Dysfunction in Type 2 Diabetes. Diabetes. 2005;54:1132–1140. doi: 10.2337/diabetes.54.4.1132. [DOI] [PubMed] [Google Scholar]
- 15.Hoang MV, Nagy JA, Fox JEB, Senger DR. Moderation of Calpain Activity Promotes Neovascular Integration and Lumen Formation during VEGF-Induced Pathological Angiogenesis. PLoS One. 2010;5:e13612. doi: 10.1371/journal.pone.0013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang MV, Smith LEH, Senger DR. Calpain Inhibitors Reduce Retinal Hypoxia In Ischemic Retinopathy By Improving Neovascular Architecture and Functional Perfusion. Biochemica et Biophysica Acta 1812. 2010:549–557. doi: 10.1016/j.bbadis.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabe AA, Elmadhun NY, Feng J, Liu YH, Potz BA, Abid R, Abbott D, Sellke FW. Calpain inhibition improves myocardial perfusion in a swine model of chronic myocardial ischemia. Circulation. 2014;130(Suppl 2):A19666. [Google Scholar]
- 18.Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–S149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmadhun NY, Sabe AA, Robich MP, Chu LM, Lassaletta AD, Sellke FW. The Pig As a Valuable Model for Testing The Effect Resveratrol to Prevent Cardiovascular Disease. Annals Of The New York Academy of Sciences. 2013:130–135. doi: 10.1111/nyas.12216. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Kain V, Sitasawad SL. High Glucose Induces Ca 2+ Overload and Oxidative Stress Contribute to Apoptosis of Cardiac Cells Through Mitochondrial Dependent and Independent Pathways. Biochemica et Biophysica Acta. 2012:907–920. doi: 10.1016/j.bbagen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Youn JY, Want T, Cai H. An Exrin/Calpain/PI3K/AMPK/eNOSs1179 Signaling Cascade Mediating VEGF-Dependent Endothelial Nitric Oxide Production. Circulation Research. 2009;104:50–59. doi: 10.1161/CIRCRESAHA.108.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]