Abstract
Type 2 diabetes is a highly prevalent and chronic metabolic disorder. Recent evidence suggests that formation of toxic aggregates of the islet amyloid polypeptide (IAPP) might contribute to β-cell dysfunction and disease. However, the mechanism of protein aggregation and associated toxicity is still unclear. Misfolding, aggregation and accumulation of diverse proteins in different organs is the hallmark in the group of protein misfolding disorders (PMDs), including highly prevalent illnesses affecting the central nervous system such as Alzheimer’s and Parkinson’s diseases. In this review we will discuss the current understanding of the mechanisms implicated in the formation of protein aggregates in pancreas and associated toxicity in the light of the longstanding knowledge from neurodegenerative disorders associated with protein misfolding.
Keywords: Type 2 diabetes, amyloid, protein misfolding, islet amyloid polypeptide, β-cells, prions
Protein misfolding and disease
Protein misfolding disorders (PMDs) are diseases where at least one protein or peptide has been shown to misfold, aggregate, and accumulate in tissues where the disease-specific damage occurs. There are at least 30 different PMDs, including several neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington disease (HD), transmissible spongiform encephalopathies (TSE), and amyotrophic lateral sclerosis (ALS), as well as diverse systemic disorders such as familial amyloid polyneuropathy, type 2 diabetes (T2D), secondary amyloidosis, and dialysis-related amyloidosis [1]. The first line of evidence that linked protein misfolding and aggregation with disease came from postmortem histopathological studies showing that a typical feature of each disease is the accumulation of protein deposits composed by a different protein, such as amyloid-β (Aβ) and tau in AD, α-synuclein in PD, poly-Q extended huntingtin in HD, islet amyloid polypeptide (IAPP) in T2D and prion protein (PrP) in TSEs [1]. Perhaps the most compelling evidence for the key role of misfolded proteins came from genetic studies. Mutations in the genes encoding the proteins that predominantly compose the aggregates have been genetically associated with inherited transmission of many PMDs [1,2]. Inheritance of these mutations resulted in earlier onset and increased severity of the disease than in sporadic cases, and were associated with a more extensive burden of protein aggregates [2]. Furthermore, transgenic expression of disease-specific human genes harboring the associated mutations in animal models reproduced several clinical and pathological characteristics of PMDs, supporting the key contribution of protein aggregates in these diseases [3]. In the case of TSEs the prominent role for misfolded protein aggregates is well supported by the fact that these diseases can be transmitted from individual-to-individual by the sole administration of misfolded prion protein aggregates (Box 1).
BOX 1. When misfolded protein aggregates behave as infectious agents: the Prion story.
Prion diseases represent some of the most intriguing PMDs, which can be transmitted by infection where the infectious agent is thought to be solely composed by the misfolded protein [144]. The disease is transmitted by seeding the aggregation of the normal prion protein, resulting in the accumulation of large quantities of these toxic aggregates in the brain [144,145]. Prion infectivity is dependent on the ability of misfolded prion protein aggregates to nucleate the misfolding and aggregation of the host prion protein [145]. Interestingly, the formation of misfolded aggregates in T2D, as well as in all other PMDs, follows a seeding/nucleation mechanism similar to the process of prion replication, in which pre-formed polymers seed the aggregation of the monomeric protein [145]. The similarities between the molecular mechanism of prion propagation and the process of protein misfolding and aggregation in PMDs suggest that misfolded aggregates have an inherent ability to be transmissible [36,145]. Indeed, a series of recent studies have shown that the pathological hallmarks of various PMDs, including Alzheimer’s, Parkinson’s, Huntington’s diseases, and some forms of systemic amyloidosis, can be induced under experimental conditions by administration of tissue homogenates carrying the respective misfolded protein aggregates (for reviews see [145–147]). The transmission of protein misfolding from molecule-to-molecule and cell-to-cell may play a major role in the spreading of protein aggregates during the progression of these diseases [148]. Whether IAPP misfolding and aggregation can also be transmitted using the prion principle is yet to be explored.
Even though T2D is often considered as a PMD, few studies have investigated the involvement of protein misfolding in disease pathogenesis. The subject has been mostly neglected in the diabetes field despite the fact that the evidence implicating the accumulation of misfolded protein aggregates in T2D pathogenesis are comparable to other diseases, such as AD or PD. In this article, we will review the literature and discuss the role of protein misfolding and aggregation in T2D.
The role of protein misfolding and aggregation in type 2 diabetes
T2D is a complex metabolic disease characterized by chronic insulin resistance, progressive loss of β-cell function and β-cell mass [4], which leads to impaired insulin release and hyperglycemia. In a prediabetic context, compensatory increases in insulin secretion from β-cells protects against hyperglycemia. However, genetic and environmental factors are believed to predispose some individuals (~20% of the population) to β-cell failure under conditions of chronic insulin resistance [5]. This transition to dysfunction and loss of β-cells are frequently ascribed to the consequences of glucolipotoxicity [6,7], islet cholesterol accumulation [8], and islet inflammation [9].
Accumulating evidence suggests that toxic aggregates of IAPP may contribute to β-cell dysfunction and disease [10,11]. IAPP is a 37 amino acid neuroendocrine polypeptide hormone [12] also known as amylin [13]. The phenomenon of IAPP accumulation associated with T2D, particularly in elderly individuals, was first described in 1901 as “islet hyalinization” [14]. However, the clinical significance of this observation was not appreciated as islet amyloid was not found in many diabetic patients, and some non-diabetic individuals also exhibited islet amyloid, albeit at much lower amounts [15–17]. More recently, it has been established that islet amyloid deposits are actually present in over 90% of T2D patients [16,18–21]. The fact that IAPP aggregates can be observed in non-diabetic individuals is not surprising considering that in studies of other PMDs (such as AD or PD) it is clear that aged individuals free of disease symptoms which are in the process of developing the disease show substantial amounts of these aggregates [22,23]. Several studies have linked IAPP aggregation with β-cell loss and progression of T2D. Autopsy studies suggested that IAPP aggregates are associated with loss of β-cell mass [4,18,21]. IAPP aggregation was suggested to be an important cause for declining β-cell function in clinically transplanted islets [24]. A mutation in the IAPP gene that elevates its aggregation propensity [25,26] is associated with early induction of T2D [27,28]. Longitudinal studies in animal models that spontaneously develop T2D (non-human primates and domestic cats) showed that formation of IAPP aggregates precede β-cell dysfunction and clinical signs of the disease [29–32]. Finally, transgenic mice and rats over expressing human IAPP spontaneously developed clinical and pathological hallmarks of T2D [33]. Although the link between IAPP aggregation and β-cell loss is convincing, the cause and origin of IAPP aggregation and the mechanism of toxicity is not completely understood.
The biological, biochemical and aggregation properties of IAPP
In order to conceptualize the process of IAPP aggregation, we will describe the biological and biochemical features of IAPP, its sequence propensity to aggregate, and the pathways responsible for IAPP misfolding and aggregation. The IAPP amino acid sequence, especially at the amino and carboxyl terminals, is highly conserved, suggesting an important physiological role [34]. The functions that have been proposed for IAPP include inhibition of insulin secretion, delay in gastric emptying, diminished appetite, suppression of glucagon release (reviewed in [34]), and suppression of tumorigenesis [35]. However, the exact role of IAPP function and dysfunction in diabetes is not fully understood.
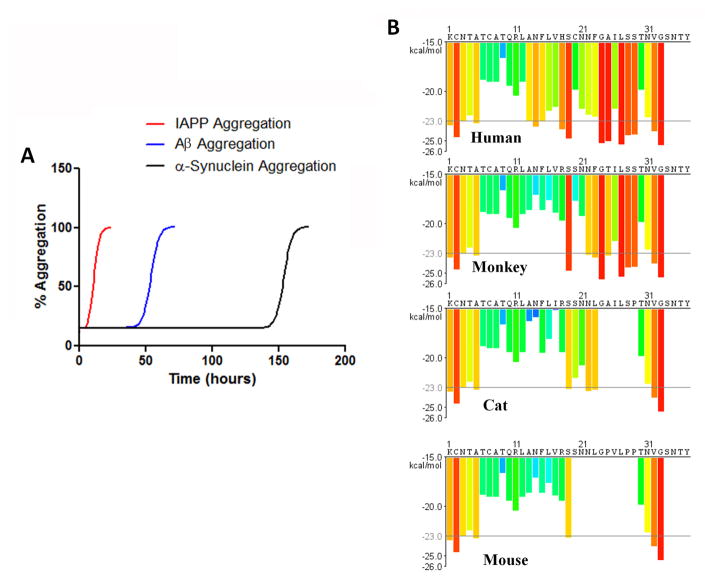
Amyloidogenic proteins/peptides form multimeric aggregates following a seeding-nucleation mechanism (Box 1). This model can be divided in two different kinetic phases [36,37]: The first phase is the lag phase, where the monomeric protein acquires a misfolded structure able to self-associate, forming small oligomers known as seeds or nuclei. Formation of the initial seeds is thermodynamically unfavorable and is a kinetically slow, rate-limiting step. Seeds formed during the lag phase act as a template for the recruitment of other monomeric proteins in the second phase, resulting in exponential growth of the oligomers, forming a continuum of larger aggregates [36,37]. The propensity of a given protein to form amyloid aggregates depends on its sequence, the stability of the folded conformation, the concentration of the protein, and the interaction with various factors including other proteins, membranes, extracellular matrix elements, and other cofactors. In the case of IAPP there is some evidence that interaction with intracellular membranes may play a role in the formation of toxic oligomers [38,39]. Protein sequences highly prone to forming amyloid exhibit a short lag phase and a large amount of the monomer will be incorporated into the aggregate. In our experience studying the kinetic of amyloid formation of many different amyloid proteins, human IAPP is one of the most amyloidogenic proteins. Compared to other PMD-associated proteins, such as Aβ or α-synuclein, IAPP aggregates much faster at similar concentrations, exhibiting a significantly shorter lag phase (Figure 1A).
Figure 1. IAPP propensity to form amyloid aggregates.
A: The figure shows a schematic representation of the typical kinetics of human IAPP aggregation compared with other PMD-associated proteins. In our experience, at low micromolar concentrations, physiological temperature and pH, IAPP forms aggregates in minutes, whereas Aβ needs hours and α-synuclein needs days to bypass the lag phase before aggregating exponentially. B: Amyloidogenicity of IAPP from human, rhesus monkey, cat and mouse was studied in silico using a structure-based algorithm originally described by Eisenberg and colleagues [157]. The amino acid sequence is plotted along the X-axis. Each histogram bar represents one hexa-peptide starting at the indicated position in the sequence and is colored according to its Rosetta energy. Orange-red segments with energy below the indicated energetic threshold of −23 kcal/mol (gray line), are predicted to form fibrils.
Although the IAPP sequence is generally conserved, there are a few critical interspecies differences, especially in residues 20–29, which is considered the domain responsible for amyloidogenicity (Figure 1B) [40]. Interestingly, species that are known to express an amyloid-prone sequence of IAPP (including humans, non-human primates, and cats) are also the species known to spontaneously develop T2D [41]. Islet amyloid has been found present in 80% of diabetic cats [31,42] and 100% of diabetic cynomolgus macaques [29,30]. The three proline substitutions (proline is a well-known beta-sheet breaking amino acid [43]) within the 20–29 amyloidogenic region in rodent IAPP substantially reduces the propensity of IAPP to misfold and aggregate compared to human IAPP [43,44]. Spontaneous T2D is not reported in rodents, and the rodent models that are often used to recapitulate T2D, such as high fat diet-induced diabetic mice, or ob/ob mice, do not produce islet amyloid [45]. Several groups have developed mice and rats expressing human IAPP (hereafter called tg-hIAPP mice or rats) [46]. Although some of these models require a prediabetic condition or a high fat diet, over-expressed human IAPP has been reported to form toxic aggregates resulting in β-cell loss leading to T2D pathology [33,47].
The process of amyloid formation produces a dynamic equilibrium of a continuum of aggregates of different sizes and properties, ranging from small oligomers (dimers, trimers, tetramers, hexamers, etc.), larger but still soluble oligomers (containing from 12–50 units), to protofibers and large amyloid fibrils. The nature of the most toxic structure of IAPP aggregates, as well as the aggregates implicated in other PMDs, is not completely clear. In this article we refer to protein aggregates as the heterogeneous mixture of polymers of different sizes which likely compose the structures present in the patients’ tissue. Initially it was thought that large aggregates were the most pathologically relevant structures, but recent reports have provided compelling evidence for smaller and soluble oligomers as the triggering agents in disease [48–50]. Some investigators have proposed that the formation and deposition of large amyloid fibrils could be a protective mechanism to sequester and isolate toxic oligomers [51]. Although this is an attractive hypothesis, it is likely that both soluble misfolded oligomers and larger aggregates, even those deposited in tissues, might be toxic, perhaps by different mechanisms [52,53]. In addition, since protein aggregates appear to be dynamic structures in equilibrium with each other, it is possible that large amyloid deposits may serve as a reservoir for more toxic smaller oligomers [54,55]. Finally, at this time we cannot rule out that the most relevant structures might be off-pathway of the amyloid formation process, such as for example the recently identified anti-parallel β-sheet oligomers known as cylindrins [56].
IAPP synthesis, processing and clearance
IAPP is predominantly expressed by β-cells as the 89 amino acid pre-pro-IAPP. The 22 amino acids signaling peptide is cleaved off in the endoplasmic reticulum (ER), producing pro-IAPP, which is further processed by the endo-proteases pro-hormone convertase 2 (PC2), PC1/3 and carboxypeptidase E in the late Golgi and secretory granules in a pH-dependent manner [57–59]. These same enzymes are responsible for processing pro-insulin. Post-translational modifications, including amidation of the COOH-terminal and disulfide bridge formation between residues 2 and 7, are pre-requisites for full biological activity [60]. Processed IAPP remains stored with insulin in secretory granules in a 1~2:50 molar ratio (IAPP:insulin). Insulin and pro-insulin inhibit IAPP aggregation, with insulin being more potent [61–64]. These inhibitory effects, together with the low pH in the secretory granules, are believed to maintain IAPP in a soluble state. IAPP is co-expressed and co-secreted with insulin by β-cells in response to glucose stimuli [21,65,66]. However, in a state of high insulin demand such as insulin resistance, IAPP expression increases compared to insulin, enhancing the probability of aggregate formation. Supporting this view, transgenic mice expressing relatively low levels of human IAPP require pharmacological induction of insulin resistance to develop diabetes and islet amyloid [67]. The evidence suggests that IAPP oligomerization begins intra-cellularly, perhaps in secretory granules [68], but large amyloid deposits accumulate extra-cellularly, as in several other amyloid diseases.
In addition to elevated expression, impaired degradation may also contribute to elevated IAPP levels, promoting aggregation (Table 1). Insulin-degrading enzyme (IDE), a Zn2+-metalloprotease involved in the clearance of insulin [69], can also degrade IAPP [70]. IDE has been identified as a T2D susceptibility gene and a 40% reduction of IDE levels in β-cells from T2D patients has been reported [71–73]. Pharmacological inhibition of IDE reduced IAPP degradation in RIN-m5F cells, leading to enhanced formation of toxic IAPP aggregates and cytotoxicity [73]. A recent report suggested that pharmacological inhibition of IDE may restore glucose tolerance and increase plasma insulin and IAPP levels in obese mice [74]. However, this mouse model does not express amyloidogenic IAPP. The effect of genetic or pharmacological reduction of IDE on IAPP aggregation in a tg-hIAPP model has not been studied. Neprilysin (NEP) is a type 2 zinc-containing metalloprotease present on the surface of various cell types, including β-cells [75]. It degrades IAPP [76] and can prevent amyloid formation [77]. Elevated neprilysin levels are reported in mice over-expressing human IAPP compared to age-matched wild type controls [75]. The interpretation of these results is that sustained elevation of neprilysin is a compensatory mechanism, aimed at degradation of extracellular IAPP and reducing its accumulation in amyloid deposits.
Table 1.
Pathways involved in aggregate formation, clearance and cell death in T2D and PMDs of the CNSa
| Protein (Pathway) | Mouse models of CNS-PMDs | Mouse models of T2D | Relation to human diseases |
|---|---|---|---|
| Insulin degrading enzyme (IDE) | CNS-specific IDE over expression leads to marked reduction of Aβ aggregates and associated pathology in AD mice model [158] | Linkage of IDE to T2D and AD by genome wide association studies [71–72, 159] | |
| Neprilysin (NEP) | CNS-specific NEP over expression prevents Aβ plaque formation [160] | Elevated mRNA level in tg-hIAPP model harboring islet amyloid [75] | NEP activity in cerebrospinal fluid is associated with dementia and amyloid-β42 levels in Lewy body disease [161] |
| UCH-L1 (Ubiquitin proteasome system) | Age dependent loss of UCH-L1 activity in the hippocampus of AD mouse model. Administered UCH-L1 restores memory deficit in AD mouse model [86] |
Hemizygous deficiency leads to significant β-cell loss and sever hyperglycemia [87] | Reduced level of UCH-L1 observed in T2D islets. Mutation in UCH-L1 linked to early onset PD and down regulation of UCHL1 associated with idiopathic PD and AD [85, 87] |
| Atg7 (Autophagy) | CNS-specific deletion of Atg7 leads to age-dependent accumulation of inclusion bodies and neuronal loss [162] | β-cell specific Atg7 deletion induces hIAPP aggregation, β-cell dysfunction and glucose impairment [96, 98] | |
| XPB1 (ER stress) | sXBP1 is upregulated in the brains of ALS, HD and TSEs patients and genetic reduction of XPB1 was neuro-protective in mouse models of ALS, PD and HD [128] | Elevated level of sXBP1 observed in mice over expressing human IAPP [126] | XBP1 polymorphism associated with prediabetic traits in a Chinese population [129] |
| NLRP3 and Caspase1 (Inflammasome) | Deficiency of NLRP3 or caspase1 prevented pathological and behavioral alterations in AD mouse models. Caspase1 deficiency decreased neuronal loss and increase survival in ALS mouse model [162, 163] | IL-1β production by islet macrophages of mice harboring islet amyloids. [138] | Enhanced expression of active caspase-1 in the brains of patients with mild cognitive impairment, AD and ALS. A combined elevation of IL-1β and IL-6 increases the risk of T2D [162] |
Abbreviations: Aβ, amyloid beta; AD, Alzheimers disease; ALS, amyotrophic lateral sclerosis; CNS, central nervous system; HD, Huntington disease; PD, Parkinson’s disease; PMD, protein misfolding disorder; T2D, type 2 diabetes.
Removing misfolded proteins
In long lived cells such as neurons and β-cells, it is crucial to have an efficient protein degradation machinery to avoid toxicity arising from sustained accumulation of damaged proteins. Cells mainly deploy three mechanisms to remove misfolded proteins: the ubiquitin proteasome system, autophagy, and aggresome formation. Defects in these mechanisms may result in the accumulation of misfolded and aggregated proteins. Table 1 summarizes current evidence implicating dysfunction of cellular degradation machinery in T2D and neurodegenerative PMDs.
The ubiquitin proteasome system (UPS)
The UPS is the predominant member of the cellular clearance machinery for the degradation of short lived, damaged, abnormal proteins. Compelling evidence has shown impaired UPS function leading to accumulation of polyubiquitinated proteins in neurodegenerative disorders associated with protein misfolding [78–81]. Proteins tagged with ubiquitin chains are targeted to the 26S proteasome. Next, deubiquinating enzymes hydrolyze ubiquitin chains, which is required for substrate entry into the proteolytic barrel of the proteasome [82]. Ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1), a member of the deubiquinating enzyme family, is abundantly expressed in β-cells [83]. Mutations in UCH-L1 have been linked to early onset PD [84], and down-regulation of UCH-L1 has been associated with idiopathic PD and AD [85]. Moreover, exogenous administration of UCH-L1 restored memory deficit in an AD model [86]. Reduced levels of UCH-L1 were also observed in islets from T2D patients in comparison with body mass index matched controls [87]. This deficiency resulted in the accumulation of ubiquitinated proteins in β-cells, leading to apoptosis. Further studies in human islets revealed that elevated levels of human IAPP downregulates the expression of UCH-L1 by an unknown mechanism [87]. UCH-L1 null mice displayed mild glucose intolerance when challenged with a high fat diet [88]. However, even hemizygous deficiency of UCH-L1 in mice expressing human IAPP, at an otherwise tolerable level, manifested a significant increase in β-cell apoptosis, loss of β-cell mass, and severe hyperglycemia on a standard diet [89]. Thus, it seems that elevated levels of IAPP, caused by conditions such as insulin resistance, may reduce efficiency of the UPS system. Inefficient UPS function could eventually promote aggregate formation leading to toxicity, which has been shown to occur in PMDs of the CNS [90,91].
Autophagy dysregulation
Autophagy is a major clearance mechanism for lysosomal degradation of damaged proteins and organelles. Macro-autophagy (hereafter referred as autophagy) involves the sequestration of damaged organelles or large protein aggregates into membrane-bound cargo vesicles known as autophagosomes that transport the contents to the lysosome for proteolysis. Although autophagy is considered an adaptive process, current studies suggest that a basal level of autophagy is always active and is involved in protein quality control [92]. Autophagy abnormalities in PMDs of the CNS lead to accumulation of protein aggregates which results in cytotoxicity [93]. This can be reversed by pharmacological activation of autophagy [94]. A significantly high volume of autophagic vacuoles and autophagosomes has been detected in islet β-cells from T2D patients compared to healthy controls, indicating alterations of the autophagy pathway [95]. Studies in β-cells from tg-hIAPP mice suggested that human IAPP aggregates impair lysosomal degradation of the autophagosome’s cargo, resulting in accumulation of autophagic vacuoles [96]. However, the mechanism of impairment remains to be determined. Moreover, genetic or pharmacological up regulation of autophagy protected the cells from IAPP mediated toxicity [96–98]. In contrast, pharmacological down regulation of autophagy increased the vulnerability of cells to IAPP aggregates, suggesting a vicious cycle whereby IAPP aggregates reduce autophagic flux, which further promotes IAPP aggregation, leading to toxicity. β-cell specific knock out of Atg7 (a crucial initiator of autophagy) induced toxic IAPP aggregate formation, leading to β-cell dysfuction and early induction of T2D in tg-hIAPP mice (Table 1) [96,98]. It is important to note that autophagy is important for the maintenance of β-cell homeostasis. Islet β-cell specific genetic reduction of autophagy (by Atg7 deficiency), even in the absence of human IAPP expression, led to mild glucose intolerance and elevated blood glucose levels [99]. However, in the presence of IAPP aggregates, this reduction of autophagy resulted in severe diabetes even under standard diet [96,98]. It was further shown that obesity-associated insulin resistance led to an enhanced autophagic flux in β-cells to meet the increasing demand for insulin. However, in the presence of IAPP aggregates, the reduction of lysosomal degradation prevented this compensatory increase in autophagic flux [98].
The aggresome
Proteins that escape canonical degradation machineries can be temporarily sequestered to diminish cytotoxicity via the formation of cytosolic inclusions at the microtubule organizing center, known as aggresomes [100]. Recently, p62/SQSTM1 was reported to function in formation of cytosolic protein inclusions and their clearance by a process of protein aggregate specific autophagy, termed aggrephagy [101]. Evidence indicates that p62 positive aggresomes can be eventually digested by UPS and lysosomal machinery [102–104]. Accumulation of p62 positive protein aggregates was noted in β-cells expressing human IAPP and enhanced formation of p62 positive inclusion bodies were associated with reduced IAPP-induced β-cell apoptosis [96,105]. Furthermore, it has been demonstrated that p62 can specifically bind to aggregation-prone human IAPP and temporarily sequester it in a less toxic form [96]. However, these p62 inclusions must be degraded by the autophagy/lysosome pathway for long-term protection of β-cells, as described for α-synuclein- or huntingtin-containing p62 inclusions in neurons [106,107].
Mechanisms of IAPP induced toxicity
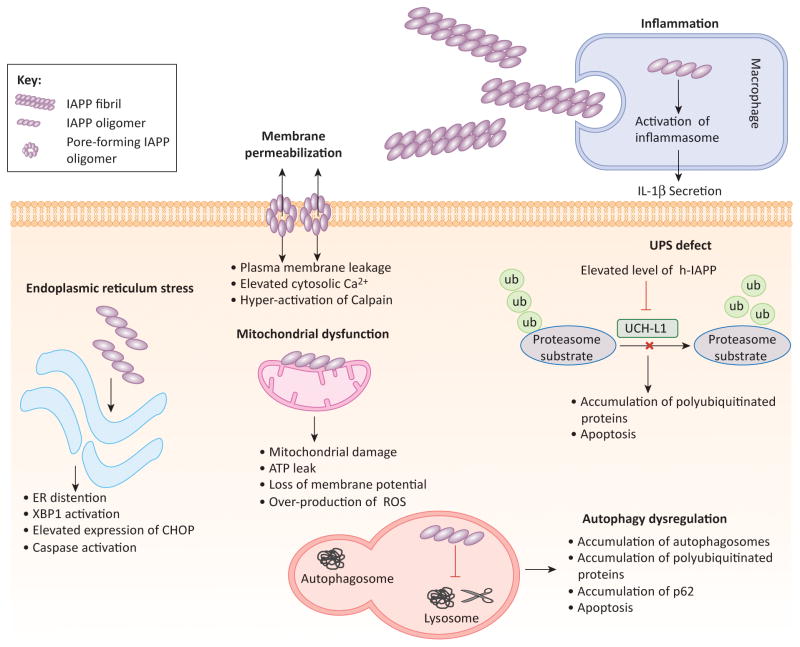
Several mechanisms have been proposed to elucidate IAPP aggregate mediated β-cell dysfunction and death in T2D. Membrane permeabilization, calpain hyperactivation, induction of endoplasmic reticulum (ER) stress, dysregulation of the clearance pathways and induction of inflammation have all been reported (Figure 2). Interestingly, the same pattern of cellular defects has also been described in other PMDs, mostly those affecting the CNS [50]. Current evidence implicating protein aggregate mediated cellular dysfunction in T2D and brain PMDs is summarized in Table 1. Neuronal dysfunction and death by disease specific protein aggregates have been studied in detail. In the following sections we will discuss our current understanding of the putative mechanisms by which IAPP aggregates may induce β-cell dysfunction and provide a comparison with other misfolded oligomeric proteins responsible for neuronal death in PMDs affecting the CNS.
Figure 2. Molecular pathways implicated in β-cell damage mediated by the formation and accumulation of IAPP aggregates.
Illustration of major cellular pathways implicated in the toxicity of IAPP aggregates against β-cells. Figure elements are not drawn to scale.
Abbreviations: ER, endoplasmic reticulum; ROS, reactive oxygen species; UPS, ubiquitin proteasome system.
Membrane permeabilization
It has been suggested that IAPP aggregates might produce cytotoxicity by forming pores that permeabilize cell membranes, notably those that compose the protein synthesis and secretory pathways (i.e. the ER, secretory vesicles and plasma membranes) [108]. It has been reported that IAPP oligomers form pore-like structures in the membrane resulting in leakage followed by Ca2+ dysregulation leading to cytotoxicity [109–111]. Similar mechanisms have been reported for Aβ, α-synuclein and PrP oligomers [112,113]. Accumulating data suggests that lipid bilayers may promote IAPP aggregation by direct interaction with negatively charged phospholipids, such as phosphatidyl serine promoting interaction of the positively charged IAPP monomer with the membrane [114]. Binding of negatively charged heparan sulfate proteoglycan, present on the cell surface, to positively charged N-terminal of IAPP and pro-IAPP, have also been shown to promote the aggregation of the peptide [115,116]. Interestingly, a high concentration of non-amyloidogenic rat IAPP has been shown to induce leakage in artificial lipid bilayers [117]. However, similar concentration of rat IAPP did not produce cellular toxicity [117], indicating that the relationship between membrane leakage and toxicity may not be direct. It should be noted that studies using lipid bilayer mimetics that likely differ in composition to the relevant intracellular and plasma membranes, while potentially providing mechanistic insights, do not mimic the complex intracellular environment (ionic concentrations, other proteins, cofactors like glycosaminoglycans, pH, chaperones, ubiquitination etc.). Moreover the itinerary of how toxic oligomers arrive at (or are formed in) various intracellular sites is poorly understood. Perhaps most important, it remains unclear why, in the setting of insulin resistance, some individuals are prone to form β-cell oligomers, β-cell failure and diabetes while others successfully express, fold and secrete (or degrade) IAPP.
Mitochondrial damage
Mitochondrial dysfunction is a crucial pathogenic event both in T2D and PMDs of the CNS (reviewed in [118,119]). Aβ and α-synuclein aggregates have previously been shown to disrupt mitochondrial function by forming pores in the mitochondrial membrane [120]. Recently, toxic IAPP oligomers have been observed within disrupted mitochondrial membranes in T2D patients and in tg-hIAPP mice [108]. Furthermore, studies in cells exposed to IAPP aggregates indicated a loss of mitochondrial membrane potential, ATP depletion, and other mitochondrial-associated damage [121]. Unstable mitochondrial membrane potential leads to over production of reactive oxygen species, which has recently been shown to be an initiator of toxicity by IAPP aggregates [122,123].
Endoplasmic reticulum stress
In general the ER is well-equipped to meet the high secretory demand of β-cells (approximately 10,000 proinsulin molecules per β-cell per minute [124]). However, genetic or environmental alterations, as well as the accumulation of protein aggregates, are known to produce chronic stress beyond the capacity of ER, leading to deleterious consequences. Several groups have reported morphological changes related to ER stress, such as distention of the ER [125], and induction of ER stress-associated death response markers, such as C/EBP homologous protein (CHOP) and activation of caspases in islet β-cells from T2D patients [126]. Neither controls nor the islets from type 1 diabetic individuals manifested these changes, suggesting that sustained ER stress could be a very specific pathological feature of T2D [126]. Using primary cell lines, islets, and rodent models (tg-hIAPP), it was shown that induction of ER-stress is specifically associated to the expression of the amyloidogenic human IAPP sequence [126]. Moreover, IAPP-mediated cytotoxicity was prevented, at least partially, by either reduction of CHOP or by increase of the endogenous ER-chaperone BIP [127]. X-Box Binding Protein-1 (XBP1) is a crucial member of the ER stress signaling cascade [128], and a polymorphism in XBP-1 has been associated with pre-diabetic traits in a Chinese population [129]. Activation of XBP1, evident by increased levels of spliced XBP1 (sXBP1), was observed in the brains of ALS, HD and TSE patients, and genetic reduction of XPB1 provided neuro-protection in mouse models of ALS, PD and HD (reviewed in [128]). Elevated sXBP-1 was observed only in mice that over-express human IAPP [126] (Table 1). Similar results were obtained from primary culture of human pancreatic cells upon exposure to IAPP aggregates. Although XBP1 deficiency in mice led to reduced insulin secretion followed by mild glucose intolerance [130], the effect of IAPP aggregate formation was not tested in this model.
Inflammation
IAPP aggregates might also induce inflammation in islets of Langerhans. IAPP aggregates were observed in macrophages present in pancreatic tissues of biopsy samples from T2D patients, as well as in diabetic monkeys, cats, and tg-hIAPP mice [131]. Although these macrophages could not efficiently remove the aggregates, internalization of IAPP aggregates led to secretion of multiple inflammatory cytokines including IL-1β in cell culture studies [132–135]. Interestingly, a three-fold increased risk of developing T2D in individuals with elevated levels of both IL-1β and IL-6, but not IL-6 alone, has been reported [136]. Using bone marrow derived dendritic cells as a model, it has been shown that phagocytized human IAPP oligomers activates the NLRP3 inflammasome, leading to IL-1β secretion [137]. Moreover, a recent study indicated that human IAPP oligomers induce a pro-inflammatory response in islet resident macrophages, including IL-1β secretions leading to islet dysfunction in tg-hIAPP mouse model [138]. Interestingly, studies in various PMDs have shown that activation of inflammasome and consequent IL-1β secretion seems to be a common mechanism by which protein aggregates produce tissue damage [139].
IAPP aggregates in non-pancreatic tissues
Severe and uncontrolled diabetes damages numerous non-pancreatic tissues, including kidney (diabetic nephropathy), sensory neurons (diabetic neuropathy) and heart (diabetic cardiomyopathy). IAPP deposits have been observed in several of these tissues in T2D patients. In a study conducted on biopsy-proven T2D nephropathy patients, 48.3% exhibited IAPP deposits in kidneys [140]. Incidence of renal lesions, such as glomerular nodular lesions, glomerulosclerosis, and tubular interstitial lesions were more severe in patients with IAPP deposition than patients without IAPP deposition, suggesting a pathological role of IAPP deposits [140]. This study also suggested that elevated plasma IAPP may induce this deposition, but it is not the only determining factor. IAPP deposition is also observed in temporal lobe gray matter of the brains from T2D patients [141]. Congophilic IAPP deposits were found in both blood vessels and perivascular spaces, suggesting influx from the peripheral circulation. Finally, significantly high levels of oligomeric IAPP were reported in the failing hearts of T2D patients [142]. Interestingly, failing hearts from obese prediabetic individuals also exhibited oligomeric IAPP, suggesting that this process of accumulation may initiate decades before onset of overt T2D. Normal hearts and failing hearts from patients without diabetes did not show IAPP aggregates [142]. Using a transgenic rat model expressing human IAPP, it was further shown that prediabetic and diabetic rats manifest cardiac hypertrophy associated with IAPP accumulation and pure synthetic IAPP aggregates induce structural and functional defects in cadiomyocytes [142]. These findings certainly warrant further research to explore a yet undiscovered role for IAPP aggregates in non-pancreatic tissue damage associated with T2D. It is also possible that IAPP aggregates may interact with other disease-causing aggregates through a process of heterologous seeding (Box 2).
BOX 2. Cross-talk between IAPP and other protein aggregates.
Multiple proteins may aggregate simultaneously in PMDs. Even multiple PMDs may coexist in a single individual [149]. Interestingly, the fact that the process, as well as the end-products and intermediates, of the seeding-nucleation mechanism are similar in all PMDs raises the possibility that seeds composed by one protein may catalyze the polymerization of other proteins [149]. This process of heterologous seeding, also known as “cross-seeding”, has been extensively described using pure preparations of proteins in vitro, especially among proteins with some degree of sequence homology [149].
Epidemiological studies have shown that one PMD may be a significant risk factor for a second PMD. It has been shown that a large percentage of AD patients are simultaneously suffering from T2D or impaired fasting glucose [150]. Furthermore, AD patients show a higher incidence of islet amyloidosis than healthy individuals. T2D patients also exhibit an increased risk of developing AD compared with age-matched nondiabetic individuals [151]. Although the mechanism responsible for the risk association between AD and T2D is unclear, several hypotheses have been proposed, including alterations in insulin signaling, hypercholesterolemia, and oxidative stress. Another interesting possibility is that misfolded proteins implicated in AD and T2D may interact between each other, promoting their heterologous seeding and accelerating the pathological onset [149]. This idea is supported by in vitro studies showing that IAPP and Aβ can cross-seed each other, stimulating amyloid formation [152,153]. In addition, IAPP has been found associated to amyloid plaques in AD patients [153]. Further, Aβ deposits and hyperphosphorylated tau, hallmark features of AD, have been reported in the islets of T2D patients [154], and the majority of Aβ colocalized with IAPP aggregates in severely affected areas. Moreover a recent study reported that that intravenous injection of preformed Aβ fibrils can trigger islet amyloid formation in the pancreas of a transgenic mouse model overexpressing human IAPP [155]. However, this group did not observe any Aβ immune-reactivity in the pancreas during an analysis of a relatively small numbers (n=4) of T2D patients. The interaction may not be restricted to AD and T2D proteins, as oligomeric α-synuclein was recently reported to be present in islets of T2D patients and was speculated to impair glucose-stimulated insulin release from β-cells [156]. However, the presence of protein aggregates composed of other proteins in T2D pancreas remains an important question for future studies.
Concluding remarks
The presence of misfolded IAPP aggregates of different sizes ranging from small soluble oligomers to large fibrillar aggregates deposited in the Islets of Langerhans of patients affected by T2D is well established. The crucial question is whether these aggregates are inert bystanders that result as a consequence of the tissue damage during the disease, or whether they play a crucial role in the pathogenesis. The diabetes field has mostly ignored the putative relevance of IAPP aggregates in T2D. This is surprising considering that the evidence for a key role of these aggregates in disease pathogenesis is compelling and very similar to those that have elevated protein aggregates as the widely-accepted cause of various neurodegenerative diseases. Considering T2D as a protein misfolding disorder will open an entire new area of research and uncover novel targets for therapeutic intervention. The mechanism and factors implicated in the transition from prediabetic conditions to β-cell failure and T2D are not clear. Convincing evidence indicates that the formation of IAPP oligomers and accumulation of islet amyloid might significantly contribute to this process. Factors promoting IAPP misfolding and aggregation in a prediabetic milieu are not well understood. However, genetic susceptibility and physiological and cellular changes during aging, such as progressive loss of proteostasis efficiency [143], may play a crucial role. In fact, all the sporadic cases of PMDs affecting the CNS are largely associated with aging, suggesting a link between protein misfolding and aging. Irrespective of the cause of aggregation, misfolded aggregates including islet amyloid, seem to share common mechanisms of formation, intermediates, and end-products, as well as pathways of cellular toxicity. Thus, inhibition of protein misfolding and aggregation might be a common therapeutic strategy to prevent some of the most prevalent, insidious and chronic diseases of our time.
HIGHLIGHTS.
Accumulation of islet amyloid is present in >90% of patients with type 2 diabetes
There is evidence for a role of misfolded IAPP aggregates in β-cell dysfunction
IAPP aggregates are similar to protein aggregates implicated in neurological diseases
Type 2 diabetes should be considered as a Protein Misfolding Disease
Acknowledgments
This study was supported in part by a grant from the National Institute of Health (R01 GM100453) to CS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998;282:1075–1079. doi: 10.1126/science.282.5391.1075. [DOI] [PubMed] [Google Scholar]
- 3.Moreno-Gonzalez I, Soto C. Natural animal models of neurodegenerative protein misfolding diseases. Curr Pharm Des. 2012;18:1148–1158. doi: 10.2174/138161212799315768. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, et al. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Assaad W, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 7.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 8.Brunham LR, et al. Cholesterol in beta-cell dysfunction: the emerging connection between HDL cholesterol and type 2 diabetes. Curr Diab Rep. 2010;10:55–60. doi: 10.1007/s11892-009-0090-x. [DOI] [PubMed] [Google Scholar]
- 9.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 10.Haataja L, et al. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull RL, et al. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 12.Westermark P, et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper GJ, et al. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988;85:7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opie EL. On the relation of chronic interstitial pancreatitis to the islans of Langerhans and to diabetes melutus. J Exp Med. 1901;5:397–428. doi: 10.1084/jem.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig G, Heitner H. On occurrence of island amyloidosis of the pancreas in diabetes mellitus. Z Gesamte Inn Med. 1967;22:814–819. [PubMed] [Google Scholar]
- 16.Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77:91–94. doi: 10.1517/03009734000000014. [DOI] [PubMed] [Google Scholar]
- 17.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- 18.Clark A, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 19.Betsholtz C, et al. Islet amyloid polypeptide (IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res. 1989;183:484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KH, et al. Islet amyloid, islet-amyloid polypeptide, and diabetes mellitus. N Engl J Med. 1989;321:513–518. doi: 10.1056/NEJM198908243210806. [DOI] [PubMed] [Google Scholar]
- 21.Jurgens CA, et al. beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–2640. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pike KE, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 23.Chetelat G, et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westermark P, et al. Is aggregated IAPP a cause of beta-cell failure in transplanted human pancreatic islets? Curr Diab Rep. 2005;5:184–188. doi: 10.1007/s11892-005-0007-2. [DOI] [PubMed] [Google Scholar]
- 25.Ma Z, et al. Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid. 2001;8:242–249. doi: 10.3109/13506120108993820. [DOI] [PubMed] [Google Scholar]
- 26.Sakagashira S, et al. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am J Pathol. 2000;157:2101–2109. doi: 10.1016/S0002-9440(10)64848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seino S. S20G mutation of the amylin gene is associated with Type II diabetes in Japanese. Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus Diabetologia. 2001;44:906–909. doi: 10.1007/s001250100531. [DOI] [PubMed] [Google Scholar]
- 28.Lee SC, et al. The islet amyloid polypeptide (amylin) gene S20G mutation in Chinese subjects: evidence for associations with type 2 diabetes and cholesterol levels. Clin Endocrinol (Oxf) 2001;54:541–546. doi: 10.1046/j.1365-2265.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 29.Howard CF., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986;29:301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- 30.de Koning EJ, et al. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta-cell population. Diabetologia. 1993;36:378–384. doi: 10.1007/BF00402271. [DOI] [PubMed] [Google Scholar]
- 31.Ma Z, et al. Quantitative immunohistochemical analysis of islet amyloid polypeptide (IAPP) in normal, impaired glucose tolerant, and diabetic cats. Amyloid. 1998;5:255–261. doi: 10.3109/13506129809007298. [DOI] [PubMed] [Google Scholar]
- 32.Guardado-Mendoza R, et al. Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–13997. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janson J, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westermark P, et al. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 35.Venkatanarayan A, et al. IAPP-driven metabolic reprogramming induces regression of p53-deficient tumours in vivo. Nature. 2015;517:626–630. doi: 10.1038/nature13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto C, et al. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien TD, et al. Human islet amyloid polypeptide expression in COS-1 cells. A model of intracellular amyloidogenesis. Am J Pathol. 1995;147:609–616. [PMC free article] [PubMed] [Google Scholar]
- 39.Gurlo T, et al. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westermark P, et al. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cefalu WT. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006;47:186–198. doi: 10.1093/ilar.47.3.186. [DOI] [PubMed] [Google Scholar]
- 42.Henson MS, O’Brien TD. Feline models of type 2 diabetes mellitus. ILAR J. 2006;47:234–242. doi: 10.1093/ilar.47.3.234. [DOI] [PubMed] [Google Scholar]
- 43.Soto C, Estrada L. Amyloid inhibitors and beta-sheet breakers. Subcell Biochem. 2005;38:351–364. doi: 10.1007/0-387-23226-5_18. [DOI] [PubMed] [Google Scholar]
- 44.Betsholtz C, et al. Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251:261–264. doi: 10.1016/0014-5793(89)81467-x. [DOI] [PubMed] [Google Scholar]
- 45.Chatzigeorgiou A, et al. The use of animal models in the study of diabetes mellitus. In Vivo. 2009;23:245–258. [PubMed] [Google Scholar]
- 46.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, et al. The pathogenic mechanism of diabetes varies with the degree of overexpression and oligomerization of human amylin in the pancreatic islet beta cells. FASEB J. 2014;28:5083–5096. doi: 10.1096/fj.14-251744. [DOI] [PubMed] [Google Scholar]
- 48.Haataja L, et al. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 50.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Ann Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 52.Zraika S, et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 2010;53:1046–1056. doi: 10.1007/s00125-010-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 54.Maji SK, et al. Amyloid as a depot for the formulation of long-acting drugs. PLoS Biol. 2008;6:e17. doi: 10.1371/journal.pbio.0060017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shahnawaz M, Soto C. Microcin amyloid fibrils are a reservoir of toxic oligomeric species. J Biol Chem. 2012;287:11665–11676. doi: 10.1074/jbc.M111.282533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laganowsky A, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335:1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marzban L, et al. Role of beta-cell prohormone convertase (PC)1/3 in processing of pro-islet amyloid polypeptide. Diabetes. 2004;53:141–148. doi: 10.2337/diabetes.53.1.141. [DOI] [PubMed] [Google Scholar]
- 58.Marzban L, et al. Role of carboxypeptidase E in processing of pro-islet amyloid polypeptide in {beta}-cells. Endocrinology. 2005;146:1808–1817. doi: 10.1210/en.2004-1175. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, et al. The prohormone convertase enzyme 2 (PC2) is essential for processing pro-islet amyloid polypeptide at the NH2-terminal cleavage site. Diabetes. 2001;50:534–539. doi: 10.2337/diabetes.50.3.534. [DOI] [PubMed] [Google Scholar]
- 60.Westermark P, et al. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 61.Westermark P, et al. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–206. doi: 10.1016/0014-5793(95)01512-4. [DOI] [PubMed] [Google Scholar]
- 62.Jaikaran ET, et al. Pancreatic beta-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem J. 2004;377:709–716. doi: 10.1042/BJ20030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janciauskiene S, et al. B cell granule peptides affect human islet amyloid polypeptide (IAPP) fibril formation in vitro. Biochem Biophys Res Commun. 1997;236:580–585. doi: 10.1006/bbrc.1997.7014. [DOI] [PubMed] [Google Scholar]
- 64.Kudva YC, et al. A novel assay in vitro of human islet amyloid polypeptide amyloidogenesis and effects of insulin secretory vesicle peptides on amyloid formation. Biochem J. 1998;331:809–813. doi: 10.1042/bj3310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahn SE, et al. Glucose stimulates and potentiates islet amyloid polypeptide secretion by the B-cell. Horm Metab Res. 1991;23:577–580. doi: 10.1055/s-2007-1003759. [DOI] [PubMed] [Google Scholar]
- 66.Mulder H, et al. Islet amyloid polypeptide and insulin gene expression are regulated in parallel by glucose in vivo in rats. Am J Physiol. 1996;271:E1008–E1014. doi: 10.1152/ajpendo.1996.271.6.E1008. [DOI] [PubMed] [Google Scholar]
- 67.Couce M, et al. Treatment with growth hormone and dexamethasone in mice transgenic for human islet amyloid polypeptide causes islet amyloidosis and beta-cell dysfunction. Diabetes. 1996;45:1094–1101. doi: 10.2337/diab.45.8.1094. [DOI] [PubMed] [Google Scholar]
- 68.Gurlo T, et al. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebrahim A, et al. Identification of the metal associated with the insulin degrading enzyme. Biochem Biophys Res Commun. 1991;181:1398–1406. doi: 10.1016/0006-291x(91)92094-z. [DOI] [PubMed] [Google Scholar]
- 70.Bennett RG, et al. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275:36621–36625. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- 71.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 72.Zeggini E, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett RG, et al. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes. 2003;52:2315–2320. doi: 10.2337/diabetes.52.9.2315. [DOI] [PubMed] [Google Scholar]
- 74.Maianti JP, et al. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature. 2014;511:94–98. doi: 10.1038/nature13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zraika S, et al. Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes. 2007;56:304–310. doi: 10.2337/db06-0430. [DOI] [PubMed] [Google Scholar]
- 76.Guan H, et al. Degradation of islet amyloid polypeptide by neprilysin. Diabetologia. 2012;55:2989–2998. doi: 10.1007/s00125-012-2678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zraika S, et al. Neprilysin impedes islet amyloid formation by inhibition of fibril formation rather than peptide degradation. J Biol Chem. 2010;285:18177–18183. doi: 10.1074/jbc.M109.082032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gregori L, et al. Amyloid beta-protein inhibits ubiquitin-dependent protein degradation in vitro. J Biol Chem. 1995;270:19702–19708. doi: 10.1074/jbc.270.34.19702. [DOI] [PubMed] [Google Scholar]
- 79.Lindersson E, et al. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–12934. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- 80.Snyder H, et al. Aggregated and monomeric alpha-synuclein bind to the S6’ proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278:11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- 81.Kristiansen M, et al. Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell. 2007;26:175–188. doi: 10.1016/j.molcel.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 83.Lopez-Avalos MD, et al. Evidence for a role of the ubiquitin-proteasome pathway in pancreatic islets. Diabetes. 2006;55:1223–1231. doi: 10.2337/db05-0450. [DOI] [PubMed] [Google Scholar]
- 84.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 85.Choi J, et al. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 86.Gong B, et al. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 87.Costes S, et al. beta-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes. 2011;60:227–238. doi: 10.2337/db10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chu KY, et al. Ubiquitin C-terminal hydrolase L1 is required for pancreatic beta cell survival and function in lipotoxic conditions. Diabetologia. 2012;55:128–140. doi: 10.1007/s00125-011-2323-1. [DOI] [PubMed] [Google Scholar]
- 89.Costes S, et al. UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in beta-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy. Autophagy. 2014;10:1004–1014. doi: 10.4161/auto.28478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma J, Lindquist S. Conversion of PrP to a self-perpetuating PrPSc-like conformation in the cytosol. Science. 2002;298:1785–1788. doi: 10.1126/science.1073619. [DOI] [PubMed] [Google Scholar]
- 91.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 92.Choi AM, et al. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 93.Ravikumar B, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 94.Berger Z, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 95.Masini M, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 96.Rivera JF, et al. Autophagy defends pancreatic beta cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014;124:3489–3500. doi: 10.1172/JCI71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morita S, et al. Autophagy protects against human islet amyloid polypeptide-associated apoptosis. J Diabetes Investig. 2011;2:48–55. doi: 10.1111/j.2040-1124.2010.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim J, et al. Amyloidogenic peptide oligomer accumulation in autophagy-deficient beta cells induces diabetes. J Clin Invest. 2014;124:3311–3324. doi: 10.1172/JCI69625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung HS, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 100.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 101.Kirkin V, et al. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 102.Wong ES, et al. Autophagy-mediated clearance of aggresomes is not a universal phenomenon. Hum Mol Genet. 2008;17:2570–2582. doi: 10.1093/hmg/ddn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirilyuk A, et al. An intrinsically disordered region of the acetyltransferase p300 with similarity to prion-like domains plays a role in aggregation. PLoS One. 2012;7:e48243. doi: 10.1371/journal.pone.0048243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnston JA, et al. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rivera JF, et al. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic beta-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death Differ. 2010;18:415–426. doi: 10.1038/cdd.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bjorkoy G, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watanabe Y, et al. p62/SQSTM1-dependent autophagy of Lewy body-like alpha-synuclein inclusions. PLoS One. 2012;7:e52868. doi: 10.1371/journal.pone.0052868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gurlo T, et al. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang CJ, et al. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J Biol Chem. 2010;285:339–348. doi: 10.1074/jbc.M109.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janson J, et al. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 111.Mirzabekov TA, et al. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 112.Hirakura Y, et al. Amyloid peptide channels: blockade by zinc and inhibition by Congo red (amyloid channel block) Amyloid. 2000;7:194–199. doi: 10.3109/13506120009146834. [DOI] [PubMed] [Google Scholar]
- 113.Quist A, et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Engel MF, et al. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad Sci U S A. 2008;105:6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abedini A, et al. Characterization of the heparin binding site in the N-terminus of human pro-islet amyloid polypeptide: implications for amyloid formation. Biochemistry. 2006;45:9228–9237. doi: 10.1021/bi0510936. [DOI] [PubMed] [Google Scholar]
- 116.Jha S, et al. Mechanism of amylin fibrillization enhancement by heparin. J Biol Chem. 2011;286:22894–22904. doi: 10.1074/jbc.M110.215814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cao P, et al. Islet amyloid polypeptide toxicity and membrane interactions. Proc Natl Acad Sci U S A. 2013;110:19279–19284. doi: 10.1073/pnas.1305517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol Cell Endocrinol. 2009;297:34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 119.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 120.Camilleri A, et al. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim Biophys Acta. 2013;1828:2532–2543. doi: 10.1016/j.bbamem.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 121.Li XL, et al. Involvement of mitochondrial dysfunction in human islet amyloid polypeptide-induced apoptosis in INS-1E pancreatic beta cells: An effect attenuated by phycocyanin. Int J Biochem Cell Biol. 2011;43:525–534. doi: 10.1016/j.biocel.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Li XL, et al. Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Int J Biochem Cell Biol. 2009;41:1526–1535. doi: 10.1016/j.biocel.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 123.Konarkowska B, et al. Thiol reducing compounds prevent human amylin-evoked cytotoxicity. FEBS J. 2005;272:4949–4959. doi: 10.1111/j.1742-4658.2005.04903.x. [DOI] [PubMed] [Google Scholar]
- 124.Costes S, et al. beta-Cell failure in type 2 diabetes: a case of asking too much of too few? Diabetes. 2013;62:327–335. doi: 10.2337/db12-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marchetti P, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–2494. doi: 10.1007/s00125-007-0816-8. [DOI] [PubMed] [Google Scholar]
- 126.Huang CJ, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 127.Geissl G. Tabes or diabetic neuropathy. MMW Munch Med Wochenschr. 1978;120:1402. [PubMed] [Google Scholar]
- 128.Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15:233–249. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- 129.Feng N, et al. Common variants in PERK, JNK, BIP and XBP1 genes are associated with the risk of prediabetes or diabetes-related phenotypes in a Chinese population. Chin Med J (Engl) 2014;127:2438–2444. [PubMed] [Google Scholar]
- 130.Lee AH, et al. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108:8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Koning EJ, et al. Macrophages and pancreatic islet amyloidosis. Amyloid. 1998;5:247–254. doi: 10.3109/13506129809007297. [DOI] [PubMed] [Google Scholar]
- 132.Westwell-Roper C, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 133.Gitter BD, et al. Human amylin stimulates inflammatory cytokine secretion from human glioma cells. Neuroimmunomodulation. 2000;7:147–152. doi: 10.1159/000026432. [DOI] [PubMed] [Google Scholar]
- 134.Yates SL, et al. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem. 2000;74:1017–1025. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- 135.Westwell-Roper CY, et al. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1beta production and beta-cell dysfunction. Diabetes. 2014;63:1698–1711. doi: 10.2337/db13-0863. [DOI] [PubMed] [Google Scholar]
- 136.Spranger J, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 137.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Westwell-Roper CY, et al. IL-1 mediates amyloid-associated islet dysfunction and inflammation in human islet amyloid polypeptide transgenic mice. Diabetologia. 2015;58:575–585. doi: 10.1007/s00125-014-3447-x. [DOI] [PubMed] [Google Scholar]
- 139.Masters SL, O’Neill LA. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med. 2011;17:276–282. doi: 10.1016/j.molmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 140.Gong W, et al. Amylin deposition in the kidney of patients with diabetic nephropathy. Kidney Int. 2007;72:213–218. doi: 10.1038/sj.ki.5002305. [DOI] [PubMed] [Google Scholar]
- 141.Jackson K, et al. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Demuro A, et al. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 143.Cuanalo-Contreras K, et al. Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol. 2013;2013:638083. doi: 10.1155/2013/638083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soto C. Transmissible Proteins: Expanding the Prion Heresy. Cell. 2012;149:968–977. doi: 10.1016/j.cell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moreno-Gonzalez I, Soto C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol. 2011;22:482–487. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morales R, et al. Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog. 2013;9:e1003537. doi: 10.1371/journal.ppat.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 151.Biessels GJ, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 152.Ono K, et al. Exogenous amyloidogenic proteins function as seeds in amyloid beta-protein aggregation. Biochim Biophys Acta. 2014;1842:646–653. doi: 10.1016/j.bbadis.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 153.Fawver JN, et al. Islet Amyloid Polypeptide (IAPP): a Second Amyloid in Alzheimer’s Disease. Curr Alzheimer Res. 2014;11:928–940. doi: 10.2174/1567205011666141107124538. [DOI] [PubMed] [Google Scholar]
- 154.Miklossy J, et al. Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol Aging. 2010;31:1503–1515. doi: 10.1016/j.neurobiolaging.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oskarsson ME, et al. In Vivo Seeding and Cross-Seeding of Localized Amyloidosis: A Molecular Link between Type 2 Diabetes and Alzheimer Disease. Am J Pathol. 2015;185:834–846. doi: 10.1016/j.ajpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 156.Steneberg P, et al. The type 2 diabetes-associated gene ide is required for insulin secretion and suppression of alpha-synuclein levels in beta-cells. Diabetes. 2013;62:2004–2014. doi: 10.2337/db12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thompson MJ, et al. The 3D profile method for identifying fibril-forming segments of proteins. Proc Natl Acad Sci U S A. 2006;103:4074–4078. doi: 10.1073/pnas.0511295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leissring MA, et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 159.Bertram L, et al. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 2000;290:2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 160.Poirier R, et al. Neuronal neprilysin overexpression is associated with attenuation of Abeta-related spatial memory deficit. Neurobiol Dis. 2006;24:475–483. doi: 10.1016/j.nbd.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 161.Maetzler W, et al. Neprilysin activity in cerebrospinal fluid is associated with dementia and amyloid-beta42 levels in Lewy body disease. J Alzheimers Dis. 2010;22:933–938. doi: 10.3233/JAD-2010-101197. [DOI] [PubMed] [Google Scholar]
- 162.Heneka MT, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meissner F, et al. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci U S A. 2010;107:13046–13050. doi: 10.1073/pnas.1002396107. [DOI] [PMC free article] [PubMed] [Google Scholar]