Abstract
Background
Exposure to persistent organic pollutants (POPs) is associated with increased diabetes risk, although the mechanism of action is not well delineated.
Methods
We investigated established diabetes biomarkers that could implicate potential mechanistic pathways, including C-reactive protein (CRP), a marker of systemic inflammation; gamma glutamyl transferase (GGT), a liver enzyme associated with oxidative stress; and adiponectin, an adipokine modulating glucose regulation and fatty acid oxidation. These biomarkers as well as hemoglobin A1c (HA1c), and POPs [polychlorinated biphenyls (PCBs), p,p-dichlorodiphenyldichloroethylene (DDE) and polybrominated diphenyl ethers (PBDEs)] were measured in a cohort of Great Lakes sport caught fish (GLSCF) consumers. We examined associations of POPs and fish consumption with HA1c and incident diabetes, and evaluated mediation and moderation by diabetes biomarkers.
Results
Odds of incident diabetes were elevated with exposure to DDE and PCBs. DDE and PCB 118 were positively, and fish meals were inversely, associated with HA1c. CRP was inversely associated with saltwater and total fish meals, particularly in persons with higher adiposity, but did not mediate the associations of fish meals with HA1c. There were few associations of adiponectin, CRP and GGT with POPs, with the exception of positive associations of GGT with PCB 118 and with PBDEs in older persons, and a positive association of adiponectin with PBDEs. Adiponectin, CRP and GGT did not mediate associations of DDE and PCBs with HA1c or incident diabetes. However, the association of DDE with HA1c was stronger in persons with higher CRP, GGT and BMI, and lower adiponectin, while the association of PCB 118 with HA1c was stronger in persons with higher GGT.
Conclusions
These findings suggest that adiponectin, CRP and GGT did not mediate effects of POPs on diabetes or HA1c. However, POPs may have stronger effects on blood glucose in persons at higher risk for diabetes.
Keywords: Persistent organic pollutants, PCBs, DDE, diabetes, fish consumption, adiponectin, C-reactive protein, gamma glutamyl transferase
1. Introduction
There is accumulating evidence that environmental contaminants are associated with increased diabetes risk. Many studies have investigated the risk of diabetes with exposure to one or more persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs), dibenzo-p-dioxins and chemicals with related toxicological properties, and persistent pesticides such as DDT and its metabolite p,p-dichlorodiphenyldichloroethylene (DDE) (reviewed by(Kuo et al. 2013;Taylor et al. 2013). While associations have been noted in many studies, chemicals with multiple modes of action have been implicated.
Possible biologic pathways through which POPs could affect diabetes incidence have been hypothesized, including insulin resistance (Kern et al. 2004), pancreatic beta cell destruction (De Tata 2014), mitochondrial dysfunction (Lee 2011), alterations in steroid metabolism (Persky et al. 2011;Persky et al. 2012), antagonism of PPARγ expression (Remillard and Bunce 2002), and induction of low grade chronic inflammation (Fujiyoshi et al. 2006), oxidative stress (Lee et al. 2008), and autoimmunity (Langer et al. 2002). This study explored some of these potential pathways using biomarkers of diabetes risk. Selected biomarkers were C reactive protein (CRP), adiponectin, and gamma glutamyl transferase (GGT). C reactive protein is a general marker of systemic inflammation, which is increased in obese persons and associated with increased risk of type 2 diabetes, independent of obesity (Dehghan et al. 2007;Duncan and Schmidt 2006). Adiponectin, an adipokine, is positively associated with insulin sensitivity, is decreased in obesity and in patients with type 2 diabetes, and has been related to decreased risk of incident diabetes independent of adiposity (Li et al. 2009). Adiponectin has anti-inflammatory properties, including inhibition of tumor necrosis factor α (TNFα) and IL-6 production and induction of anti-inflammatory cytokines (Fantuzzi 2005). GGT is a liver enzyme associated with oxidative stress that is related to increased type 2 diabetes risk, with stronger effects in persons with higher BMI (Nakanishi et al. 2003).
A significant route of exposure to POPs is through ingestion of contaminated food, in general, and fish in particular. Sport caught fish from the Great Lakes have elevated levels of PCBs and other POPs and frequent consumers of these fish have higher exposures than the general population (Turyk et al. 2012). Increased risk of diabetes with elevated POP levels has been noted in Great Lakes fish consuming populations (Codru et al. 2007;Turyk et al. 2009a), but investigations of the effects of fish consumption on diabetes risk have been inconsistent (Wu et al. 2012;Zhang et al. 2013). We previously reported a relationship of DDE with incident diabetes (Turyk et al. 2009a) and of DDE and PCB 118 with prevalent diabetes (Turyk et al. 2009b) in participants in the Great Lakes Fish Consumption Study.
Hemoglobin A1c (HA1c), or glycated hemoglobin, is useful as a measure of glucose control over the lifespan of the red cell (3-4 months). It therefore represents more long-term glucose regulation than acute fluctuations and recently has been adopted as a test for diabetes (≥6.5%) and prediabetes (5.7-6.4%) (American Diabetes Association 2010). Because diabetes risk increases for persons with fasting plasma glucose values at the higher end of the normal range, it has been suggested that diabetes risk prediction may be more accurate if glycemic measures are treated as continuous rather than categorical variables (Tabak et al. 2012). Continuous glycemic measurements (i.e. fasting plasma glucose and HA1c) have been analyzed in relation to POP exposures in several investigations (Calvert et al. 1999;Grandjean et al. 2011;Henriksen et al. 1997;Jorgensen et al. 2008;Langer et al. 2014;Michalek et al. 1999;Suarez-Lopez et al. 2015), but the effects of POP exposures on continuous HA1c levels have not yet been evaluated in the Great Lakes Fish Consumption Study.
The current study measured diabetes biomarkers in participants in the Great Lakes Fish Consumption Study. Our purpose was to determine 1) if POPs or fish consumption are related to levels of adiponectin, CRP, and GGT; 2) if adiponectin, CRP, and GGT are related to incident diabetes or to continuous levels of HA1c; and 3) if the associations of POPs or fish consumption with incident diabetes and continuous HA1c are mediated or modified by adiponectin, CRP, and GGT.
2. Methods
2.1 Participants
The Great Lakes Consortium for the Health Assessment of Great Lakes Sport Fish Consumption was organized in 1992 (Anderson et al. 1996), and study design of the Great Lakes Fish Consumption Study through 2004-2005 has been previously described (Turyk et al. 2009b). Briefly, approximately 4,200 participants with frequent and infrequent Great Lakes sport fish consumption were recruited, including Great Lakes fishing charter boat captains, anglers who fished from inland Wisconsin lakes, and infrequent consumers (reporting consumption of fewer than six meals of Great Lakes sport fish in any year of the previous 20 years). Between 1994 and 2005, biological samples were collected at least once from 948 participants and tested for persistent pollutants (Anderson et al. 2008;Hanrahan et al. 1999;Persky et al. 2001). The current analysis incorporates data collected at follow up in 2004-2005 and in 2010.
2.2 Data Collection
Self-reported diagnosis of diabetes, date of diagnosis, demographics, height, weight, smoking, alcohol use, medication use, and fish consumption were assessed by survey. In 2004-2005, 515 participants were surveyed (Turyk et al. 2009b) and data on fish meals consumed in the past year was assessed, and summarized as commercial (fresh water and saltwater) fish meals and sport caught fish meals (Great Lake or other body of water). Health data assessed in 2010 was available from 598 participants, of whom 402 also participated in the 2004-2005 data collection.
In 2004-2005, non-fasting blood was collected in red-top vacutainer tubes, allowed to clot for 20 min at room temperature, centrifuged for 15 min, transferred to solvent-rinsed glass vials, and stored at -20° C until analysis. All laboratory tests were performed by technicians blinded to participant characteristics. Sera samples were analyzed for DDE, and for PCB and PBDE congeners as previously described (Anderson et al. 2008). Briefly, sera were extracted with hexanenethyl ether, with clean-up and fractionation using Florisil, silica-gel and concentrated sulfuric acid. PBDEs were analyzed by gas chromatography–mass spectrometry and PCBs and DDE by gas chromatography.
Total cholesterol and triglycerides were measured by Quest Diagnostics. Total serum lipids were calculated by the formula:
HA1c was measured in whole blood by Quest Diagnostics (Auburn Hills, MI and Wood Dale, IL) through affinity chromatography, which measured total glycosylated hemoglobin, from which HA1c is calculated.
Stored sera samples were assayed in 2010 for adiponectin, CRP, and GGT. Because sera samples had been through prior freeze-thaw cycles during testing for hormones in 2004-2005, we evaluated the stability of test results for these biomarkers after a series of freeze-thaw cycles in blood obtained from four donors. These tests did not indicate systematic decline in biomarker measurements with increasing freeze-thaw cycles.
Quality control was monitored using internal positive and negative controls. Inter-assay coefficients of variation (CVs) were calculated from repeated assays on 4-5% of the participant samples. For adiponectin and CRP, each participant sample was assayed in duplicate and CVs were calculated for each duplicate pair. The average CV was then calculated for each assay plate and the intra-assay CV was the average of all the plate averages. For GGT, a single measurement was obtained for each participant sample precluding calculation of an intra-assay CV.
Circulating serum levels of adiponectin and CRP were measured using Quantikine ELISA kits from R&D Systems (Minneapolis, MN). The human adiponectin/Acrp30 immunoassay (DRP300) recognizes recombinant and natural (low, middle, and high molecular weight) human total adiponectin, and had a sensitivity of 0.246 ng/mL. Intra and inter-assay CVs for participant samples were 4.8% and 9.9%, respectively. The human CRP immunoassay kit (DCRP00) had a sensitivity of 0.01 ng/mL. Intra- and inter-assay CVs were are 7.9% and 13.5%, respectively. GGT was measured by the University of Illinois Hospital, Pathology Laboratory Services by an enzymatic rate method using the SYNCHRON LX® System (Beckman Coulter, Inc.). The assay sensitivity was 5 U/L and the inter-assay CV for repeated measurements of participant samples was 4.6%.
2.3 Statistical Analysis
Participants were included in the analysis if data were available from 2004-2005 for POPs exposures, HA1c, serum cholesterol and triglycerides, and at least one of the diabetes biomarkers measured, as well as health data assessed in 2010. Participants were excluded because of missing data and use of hormone medications, including systemic corticosteroids (n=2), steroid hormones (n=26), steroid hormones and corticosteroids (n=1), or thyroid hormones (n=41). We excluded participants taking hormone medications (sex steroids, corticosteroid or thyroid) because of potential effects on diabetes biomarkers and HA1c (Brand et al. 2011;Fallah et al. 2012;Sargeant et al. 2000). The final sample size for analysis was 413.
HA1c% was examined as a continuous outcome in these 413 participants. Included in this sample were 349 persons without diagnosed diabetes and with HA1c<6.5%, and 64 persons with diagnosed diabetes and/or HA1c≥6.5%: 13 with HA1c≥6.5% and without diagnosis, 11 with HA1c<6.5% and with diagnosis, and 40 with HA1c≥6.5% and with diagnosis. Subgroup analyses were performed in the 349 persons without diagnosed diabetes and with HA1c<6.5%.
Incident diabetes was investigated in the subgroup of 287 participants who did not have diagnosed diabetes and did not use thyroid and steroid hormone medications at the 2004-2005 follow up and who also responded to the follow up survey in 2010. Incident diabetes (n=16) was defined as no diagnosed diabetes in 2004-2005 and reported diabetes diagnosis in 2010.
POP exposures (DDE, sum PBDEs, PBDE 47, sum PCBs and individual congeners PCB 118, 180, 132/153, 138/163), fish consumption measures, adiponectin, CRP and GGT were analyzed as continuous variables with natural log transformations (Ln). POPs and fish consumption were also modeled as quartiles or tertiles (see supplemental tables 3 and 4).
Associations of adiponectin, GGT and CRP with POPs and fish consumption were examined in linear regression models, adjusting for age, BMI, gender, and serum lipids. Education, smoking, alcohol use, and specific types of medication use were considered as covariates, but were not included in the final models because they did not confound the POP/biomarker associations. Age, BMI, and gender were evaluated for effect modification in stratified models.
Analyses of the relationships of GGT, CRP, adiponectin, POPs, and fish consumption with incident diabetes and with HA1c were conducted using logistic and ordinary least squares regression models, respectively, adjusting for gender, age, BMI, and serum lipids for POP models only. We also adjusted HA1c models for use of any diabetes medication. Education, smoking, alcohol use, serum lipids, and selected medications (beta blocker, diuretic, calcium channel blocker, antilipemic, angiotensin-converting enzyme inhibitor, any antidepressive) were included in the model if confounding was present (>10% change in effect estimate). Additional potential confounders available only for incident diabetes models were family history of diabetes and long-term prednisone use. For models with POPs as predictor variables, different types of fish meals were considered potential confounders, and the strongest confounder was selected. For models with fish meals as predictor variables, DDE and sum PCBs were considered potential confounders, and the strongest confounder was selected.
Effect modification by adiponectin, CRP, GGT and BMI was investigated using multiplicative interaction terms. For interaction terms with p-values<0.15, modification was illustrated using estimates of the exposure/outcome association at selected percentiles of the modifier. Gender and age did not modify associations of POPs and fish consumption with HA1c.
Mediation analyses were used to explore the hypothesis that these diabetes biomarkers are partial mediators in associations between POPs and diabetes. Mediation would be supported if the following factors were satisfied 1) diabetes was associated with the POP, 2) the diabetes biomarker was associated with the POP, and 3) the association of diabetes with the POP was attenuated by including the biomarker as a covariate in the regression model.
Information about fasting time prior to blood sample collection was missing for 50 persons. Adiponectin differed by fasting status prior to blood draw, with mean=10.5 mg/L for fasting ≤12 h and mean=9.0 mg/L for fasting >12 h (p=0.03). However, adjusting final adiponectin models for fasting time did not change results substantially (not shown). Fasting was not associated with the GGT, CRP, POPs and fish consumption variables, nor did adjustment for fasting substantially affect results of cross sectional or prospective models (not shown). All statistical analyses were performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC).
3. Results
3.1 Associations among Diabetes Biomarkers, Exposures and Demographics
Associations of BMI, age, gender, education, smoking, alcohol use, and serum lipids with prevalent and incident diabetes are shown in Supplemental Table 1 and associations of these characteristics with adiponectin, GGT, CRP, HA1c, POPs and fish consumption in Tables 1 and 2. Correlations among diabetes biomarkers, POPs and fish consumption variables are found in Supplemental Table 2.
Table 1.
Associations of diabetes biomarkers with participant characteristics, n=413
| Characteristic | LnAdiponectin, mg/L | LnCRP, mg/L | LnGGT, U/L | HA1c, % | |
|---|---|---|---|---|---|
| All Participants | Meana | 10.0 | 2.2 | 21.9 | 5.77 |
| 95% CI | 9.4,10.6 | 2.0, 2.5 | 20.7, 23.2 | 5.70,5.84 | |
| Men, n=313 | Meana | 8.9 | 2.4 | 24.0 | 5.8 |
| Women, n=100 | Meana | 14.0 | 1.8 | 16.7 | 5.6 |
| p-valuec | <0.0001 | 0.07 | <0.0001 | 0.008 | |
| Age <50 years, n=93 | Meana | 8.9 | 2.0 | 21.7 | 5.5 |
| Age 50-64.9 years, n=219 | Meana | 10.2 | 2.2 | 22.4 | 5.8 |
| Age ≥65 years, n=101 | Meana | 10.5 | 2.6 | 21.1 | 6.0 |
| p-valuec | 0.15 | 0.19 | 0.69 | <0.0001 | |
| BMI <25 kg/m2, n=74 | Meana | 14.2 | 1.1 | 17.3 | 5.5 |
| BMI 25-29.9 kg/m2, n=174 | Meana | 9.8 | 2.2 | 22.2 | 5.7 |
| BMI >30 kg/m2, n=167 | Meana | 8.7 | 3.0 | 24.0 | 5.9 |
| p-valuec | <0.0001 | <0.0001 | 0.0004 | <0.0001 | |
| ≤<High School, n=142 | Meana | 9.5 | 2.7 | 23.7 | 5.9 |
| Some College, n=132 | Meana | 10.9 | 2.1 | 20.5 | 5.7 |
| ≥4-year College, n=139 | Meana | 9.6 | 1.9 | 21.6 | 5.7 |
| p-valuec | 0.15 | 0.01 | 0.11 | 0.08 | |
| Smoker, n=43 | Meana | 9.5 | 2.7 | 22.2 | 5.8 |
| Non-smoker, n=370 | Meana | 10.0 | 2.2 | 21.9 | 5.8 |
| p-valuec | 0.58 | 0.22 | 0.89 | 0.93 | |
| Alcohol Use, n=301 | Meana | 10.0 | 2.2 | 23.0 | 5.7 |
| No Alcohol Use, n=107 | Meana | 9.9 | 2.1 | 19.2 | 6.0 |
| p-valuec | 0.83 | 0.77 | 0.002 | 0.003 | |
| Prevalent Diabetesd, n=64 | Meana | 8.7 | 2.2 | 23.9 | 6.9 |
| No Prevalent Diabetesd, n=349 | Meana | 10.2 | 2.4 | 21.6 | 5.6 |
| p-valuec | 0.13 | 0.44 | 0.14 | <0.0001 | |
| Incident Diabetese, n=16 | Meana | 6.8 | 4.9 | 26.4 | 6.3 |
| No Incident Diabetese, n=271 | Meana | 10.5 | 2.1 | 21.2 | 5.5 |
| p-valuec | 0.003 | <0.0001 | 0.18 | 0.003 | |
| Hemoglobin A1c, % | r | −0.15 | 0.10 | 0.10 | - |
| p-valueb | 0.003 | 0.04 | 0.05 | ||
| Cholesterol, mg/dL | r | 0.10 | 0.04 | 0.11 | −0.13 |
| p-valueb | 0.04 | 0.40 | 0.03 | 0.009 | |
| Triglycerides, mg/dL | r | −0.36 | 0.18 | 0.32 | 0.17 |
| p-valueb | <0.0001 | 0.0002 | <0.0001 | 0.0005 |
Geometric mean for adiponectin, CRP, GGT, arithmetic mean for HA1c.
P-values are for Pearson's correlation coefficients.
P-values are from Student's t-test or ANOVA.
Diagnosed diabetes reported in 2004-2005 and/or HA1c≥6.5%.
No diagnosed diabetes reported in 2004-2005 and reported diabetes diagnosis in 2010
Table 2.
Associations of POPs and fish consumption with participant characteristics, n=413
| Characteristic | LnDDE ng/g |
LnPCB 118 ng/g |
LnΣPCB ng/g |
LnΣPBDE ng/g |
LnGLSCF meals/yr |
LnSaltwater fish meals/yr |
LnTotal fish meals/yr |
|
|---|---|---|---|---|---|---|---|---|
| All Participants | Meana | 2.0 | 0.12 | 2.4 | 0.30 | 6.6 | 11.4 | 40.2 |
| 95% CI | 1.8, 2.1 | 0.11,0.13 | 2.2, 2.5 | 0.28,0.32 | 5.5, 7.9 | 9.6,13.5 | 35.7,45.3 | |
| Men, n=313 | Meana | 2.19 | 0.13 | 2.71 | 0.30 | 8.1 | 10.8 | 41.2 |
| Women, n=100 | Meana | 1.39 | 0.10 | 1.54 | 0.30 | 3.4 | 13.3 | 37.1 |
| p-valuec | <0.0001 | 0.004 | <0.0001 | 0.92 | <0.0001 | 0.31 | 0.46 | |
| Age <50 years, n=93 | Meana | 1.19 | 0.09 | 1.46 | 0.25 | 5.2 | 11.0 | 36.5 |
| Age 50-64.9 years, n=219 | Meana | 2.05 | 0.12 | 2.50 | 0.32 | 6.3 | 12.9 | 41.1 |
| Age ≥65 years, n=101 | Meana | 2.86 | 0.15 | 3.28 | 0.31 | 9.0 | 8.9 | 41.8 |
| p-valuec | <0.0001 | <0.0001 | <0.0001 | 0.02 | 0.10 | 0.23 | 0.69 | |
| BMI <25 kg/m2, n=74 | Meana | 1.27 | 0.10 | 1.95 | 0.33 | 4.1 | 9.9 | 35.9 |
| BMI 25-29.9 kg/m2, n=174 | Meana | 1.99 | 0.11 | 2.51 | 0.27 | 6.8 | 10.4 | 37.8 |
| BMI ≥30 kg/m2, n=167 | Meana | 2.34 | 0.13 | 2.42 | 0.32 | 7.9 | 13.2 | 45.0 |
| p-valuec | <0.0001 | 0.005 | 0.04 | 0.08 | 0.04 | 0.37 | 0.30 | |
| ≤High School, n=142 | Meana | 2.16 | 0.13 | 2.61 | 0.31 | 8.5 | 9.0 | 44.7 |
| Some College, n=132 | Meana | 1.97 | 0.11 | 2.22 | 0.31 | 7.1 | 13.2 | 42.5 |
| ≥4-year College, n=139 | Meana | 1.79 | 0.11 | 2.28 | 0.29 | 4.8 | 12.6 | 34.2 |
| p-valuec | 0.22 | 0.17 | 0.13 | 0.76 | 0.04 | 0.23 | 0.57 | |
| Smoker, n=43 | Meana | 1.66 | 0.10 | 2.57 | 0.34 | 15.4 | 16.0 | 57.4 |
| Non-smoker, n=370 | Meana | 2.01 | 0.12 | 2.35 | 0.30 | 6.0 | 10.9 | 38.6 |
| p-valuec | 0.20 | 0.14 | 0.44 | 0.22 | 0.001 | 0.19 | 0.02 | |
| Alcohol Use, n=301 | Meana | 2.09 | 0.12 | 2.46 | 0.30 | 7.6 | 12.2 | 44.2 |
| No Alcohol Use, n=107 | Meana | 1.76 | 0.11 | 2.15 | 0.29 | 4.3 | 9.6 | 30.9 |
| p-valuec | 0.16 | 0.10 | 0.07 | 0.79 | 0.005 | 0.25 | 0.02 | |
| Prevalent Diabetesd, n=64 | Meana | 3.28 | 0.16 | 3.03 | 0.31 | 6.6 | 11.1 | 40.4 |
| No Prevalent Diabetesd, n=349 | Meana | 1.79 | 0.11 | 2.27 | 0.30 | 6.6 | 11.4 | 40.2 |
| p-valuec | <0.0001 | 0.0009 | 0.003 | 0.78 | 0.99 | 0.91 | 0.98 | |
| Incident Diabetese, n=16 | Meana | 3.8 | 0.22 | 3.4 | 0.26 | 6.7 | 6.8 | 32.5 |
| No Incident Diabetese, n=271 | Meana | 1.8 | 0.11 | 2.3 | 0.30 | 6.3 | 12.3 | 39.7 |
| p-valuec | <0.0001 | 0.0002 | 0.03 | 0.23 | 0.89 | 0.19 | 0.53 | |
| Hemoglobin A1c, % | r | 0.30 | 0.19 | 0.17 | 0.01 | −0.04 | −0.07 | −0.08 |
| p-valueb | <0.0001 | 0.0001 | 0.0006 | 0.77 | 0.40 | 0.18 | 0.10 | |
| Cholesterol, mg/dL | r | 0.06 | 0.11 | 0.12 | 0.09 | −0.02 | 0.01 | 0.002 |
| p-valueb | 0.25 | 0.03 | 0.01 | 0.07 | 0.73 | 0.78 | 0.97 | |
| Triglycerides, mg/dL | r | 0.21 | 0.20 | 0.21 | 0.08 | 0.02 | −0.02 | −0.03 |
| p-valueb | <0.0001 | <0.0001 | <0.0001 | 0.09 | 0.76 | 0.67 | 0.54 |
Geometric mean.
P-values are for Pearson's correlation coefficients.
P-values are from Student's t-test or ANOVA.
Diagnosed diabetes reported in 2004-2005 and/or HA1c≥6.5%.
No diagnosed diabetes reported in 2004-2005 and reported diabetes diagnosis in 2010
3.2 Associations of Adiponectin, CRP, and GGT with POPs and Fish Consumption
Associations of adiponectin, CRP, and GGT with POPs and fish consumption, after adjustment for age, gender and BMI, were examined in the full cohort and in models stratified by gender, median age and median BMI (Table 3 and Supplemental Table 3). LnDDE, Lnsum PCBs and individual LnPCB congeners were not significantly associated with Lnadiponectin, LnCRP, or LnGGT (Table 3) with the exception of LnGGT, which was positively associated with tertiles of PCB 118 (Supplemental Table 3).
Table 3.
Association of POPs and Fish Consumption with Adiponectin, CRP and GGT, n=413
| Biomarker | Exposure | Beta-coefficient, p-valuea |
|---|---|---|
| LnAdiponectin | LnDDE | 0.02, 0.68 |
| LnPCB 118 | −0.01, 0.89 | |
| LnΣPCBs | 0.02, 0.68 | |
| LnPBDE 47 | 0.04, 0.26 | |
| LnΣPBDEs | 0.08, 0.07b | |
| LnGLSCF Meals | −0.01. 0.38 | |
| LnSaltwater Fish Meals | −0.01, 0.71 | |
| LnTotal Fish Meals | 0.03, 0.24 | |
| LnCRP | LnDDE | −0.01, 0.90 |
| LnPCB 118 | 0.02, 0.82 | |
| LnΣPCBs | −0.04, 0.64 | |
| LnPBDE 47 | 0.02, 0.74 | |
| LnΣPBDEs | 0.04, 0.56 | |
| LnGLSCF Meals | −0.01, 0.79 | |
| LnSaltwater Fish Meals | −0.06, 0.045 | |
| LnTotal Fish Meals | −0.03, 0.54 | |
| LnGGT | LnDDE | 0.05, 0.12 |
| LnPCB 118 | 0.07, 0.10 | |
| LnΣPCBs | 0.04, 0.33 | |
| LnPBDE 47 | 0.04, 0.13 | |
| Ln ΣPBDEs | 0.06, 0.13 | |
| LnGLSCF Meals | 0.01, 0.68 | |
| LnSaltwater Fish Meals | 0.002, 0.92 | |
| LnTotal Fish Meals | −0.02, 0.95 |
Linear regression mode s adjusted for age, gender, and BMI. Models with POPs also adjusted for serum lipids.
significant with control for fasting.
PCB132153, PCB138163, PCB180 were not associated with biomarkers and are not shown in table.
In stratified models (not shown), LnSum PBDEs and LnPBDE 47 were associated with LnGGT in persons of above median age (β=0.13, p=0.02 and β=0.11, p=0.008, respectively); Lnsum PBDEs was associated with Lnadiponectin in persons with above median BMI (β=0.11, p=0.05) and above median age (β=0.18, p=0.003); and LnPBDE 47 was associated with Lnadiponectin in persons with above median age (β=0.13, p=0.009).
Lnsaltwater fish meals were inversely associated with LnCRP in the full cohort (Table 3), and in males and persons with above median BMI (β=-0.08, p=0.005 and β=-0.08, p=0.02, respectively, not shown). Quartiles of total fish meals were inversely associated with LnCRP (Supplemental Table 3). Lntotal fish meals and LnGLSCF meals were inversely associated with LnCRP only in persons with above median BMI (β=-0.08, p=0.03, and β=-0.13, p=0.02, respectively, not shown).
3.3 Associations of Incident Diabetes and Hemoglobin A1c with Diabetes Biomarkers
After adjusting for confounding, Lnadiponectin was inversely associated with incident diabetes and HA1c; LnCRP was positively associated with incident diabetes, but not with HA1c; and GGT quartiles were positively but not significantly associated with incident diabetes, while LnGGT was positively but not significantly associated with HA1c (Tables 4 and 5).
Table 4.
Associations of Incident Diabetes with POPs, Fish Consumption, Adiponectin, GGT and CRP.
| Exposure or Biomarker | Odds of Incident Diabetes: Model 1a | Odds of Incident Diabetes: Model 2b | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | Additional confounders | |
| LnAdiponectin | 0.20 | 0.07, 0.56 | 0.002 | 0.20 | 0.07, 0.56 | 0.002 | none |
| LnCRP | 2.87 | 1.19, 6.89 | 0.02 | 3.22 | 1.25, 8.29 | 0.02 | Lnsaltwater fish meals |
| LnGGT | 1.75 | 0.77, 3.96 | 0.18 | 1.50 | 0.57, 3.90 | 0.41 | Family history diabetes |
| Quartile GGT | 1.86 | 1.04, 3.30 | 0.04 | 1.70 | 0.93, 3.11 | 0.08 | Family history diabetes |
| LnDDE | 2.08 | 1.01, 4.29 | 0.05 | 2.63 | 1.17, 5.89 | 0.02 | Calcium channel blocker use |
| LnPCB118 | 2.83 | 1.35, 5.90 | 0.006 | 3.28 | 1.50, 7.18 | 0.003 | Lntotal fish meals |
| LnPCB132153 | 2.59 | 1.05, 6.40 | 0.04 | 3.24 | 1.17, 9.03 | 0.02 | Calcium channel blocker use, Lntotal fish meals, education |
| LnPCB138163 | 2.64 | 1.12, 6.24 | 0.03 | 3.62 | 1.38, 9.52 | 0.009 | Calcium channel blocker use, Lntotal fish meals |
| LnPCB180 | 1.89 | 0.74, 4.87 | 0.19 | 2.33 | 0.86, 6.35 | 0.10 | Lntotal fish meals |
| LnΣPCB | 2.43 | 0.96, 6.12 | 0.06 | 3.38 | 1.19, 9.59 | 0.02 | Calcium channel blocker use, Lntotal fish meals |
| LnPBDE47 | 0.71 | 0.38, 1.34 | 0.29 | 0.71 | 0.38, 1.34 | 0.29 | none |
| LnΣPBDE | 0.44 | 0.16, 1.21 | 0.11 | 0.44 | 0.16, 1.21 | 0.11 | none |
| LnTotal Fish Meals | 0.79 | 0.51, 1,23 | 0.30 | 0.66 | 0.40, 1,09 | 0.10 | LnΣ PCBs |
| LnSaltwater Fish Meals | 0.80 | 0.59, 1.08 | 0.14 | 0.80 | 0.59, 1.11 | 0.18 | none |
| LnGLSCF Meals | 1.02 | 0.75 1.39 | 0.89 | 0.89 | 0.63, 1.26 | 0.50 | LnΣ PCBs |
N=287, including 16 incident diabetes cases
Model 1 adjusted for age, BMI, gender and serum lipids for POP models
Model 2 adjusted for all covariates in Model 1 and for additional covariates determined to be confounders.
Table 5.
Cross sectional Associations of Hemoglobin A1C with POPs, Fish Consumption, Adiponectin, GGT, and CRP in 2004-2005
| Group | Exposure or biomarker | Association of Exposure with Hemog obin A1c | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | |||||||
| Beta | 95% CI | P-value | Beta | 95% CI | P-value | Additional Confounders | ||
| With and without diabetes N=413 | LnAdiponectin | −0.159 | −0.246,−0.072 | 0.0004 | −0.159 | −0.246,−0.072 | 0.0004 | none |
| LnCRP | 0.027 | −0.026, 0.081 | 0.31 | 0.011 | −0.044, 0.065 | 0.70 | education, serum lipids, ace inhibitor use | |
| LnGGT | 0.100 | 0.005, 0.195 | 0.04 | 0.076 | −0.026, 0.178 | 0.15 | Alcohol use, serum lipids, antilipid use, LnDDE | |
| LnDDE | 0.099 | 0.034, 0.164 | 0.003 | 0.118 | 0.051, 0.186 | 0.0006 | LnGLSCF meals | |
| LnPCB118 | 0.055 | −0.024, 0.133 | 0.17 | 0.091 | 0.007, 0.175 | 0.03 | Alcohol use, LnGLSCF meals | |
| LnTotal Fish Meals | −0.059 | −0.102, −0.016 | 0.007 | −0.072 | −0.115, −0.029 | 0.001 | LnDDE | |
| LnSaltwater Fish Meals | −0.030 | −0.060, −0.001 | 0.045 | −0.030 | −0.060, −0.001 | 0.045 | None | |
| LnGLSCF Meals | −0.019 | −0.049, 0.010 | 0.20 | −0.039 | −0.069, −0.008 | 0.01 | smoking, LnDDE | |
| No diabetes and HA1c<6.5% N=349 | LnAdiponectin | −0.084 | −0.148, −0.019 | 0.01 | −0.074 | −0.138, −0.009 | 0.03 | Alcohol use |
| LnCRP | 0.007 | −0.030, 0.044 | 0.70 | −0.011 | −0.048, 0.026 | 0.55 | Alcohol use, smoking, lnsaltwater fish, serum lipids, beta-blocker use, ace inhibitor use, diuretic use | |
| LnGGT | 0.037 | −0.026, 0.100 | 0.25 | 0.035 | −0.034, 0.103 | 0.32 | Alcohol use, antilipid use, serum lipids | |
| LnDDE | 0.011 | −0.035, 0.057 | 0.64 | 0.025 | −0.022, 0.071 | 0.30 | smoking, Lntotal fish meals | |
| LnPCB118 | 0.010 | −0.047, 0.067 | 0.73 | 0.033 | −0.027, 0.093 | 0.27 | Alcohol use, LnGLSCF meals | |
| LnTotal Fish Meals | −0.040 | −0.070, −0.011 | 0.008 | −0.040 | −0.070, −0.011 | 0.008 | none | |
| LnSaltwater Fish Meals | −0.029 | −0.050, −0.009 | 0.005 | −0.029 | −0.050, −0.009 | 0.005 | none | |
| LnGLSCF Meals | −0.008 | −0.029, 0.012 | 0.41 | −0.014 | −0.036, 0.007 | 0.18 | Smoking, LnDDE | |
Model 1 adjusted for age, BMI, and gender, for all models; serum lipids for POP models; and diabetes medication use for models for participant group: “with and without diabetes”.
Model 2 adjusted for all covariates in model 1 and for additional covariates determined to be confounders.
Sum PCB, PCB132153, PCB138163, PCB180, Sum PBDE, PBDE47 were not associated with HA1c and are not shown in table.
3.4 Associations of Incident Diabetes with POPs and Fish Consumption: Evaluation of Mediation and Moderation by Diabetes Biomarkers
Odds of incident diabetes were significantly elevated by LnDDE, LnPCB 118, LnPCB 132153, and LnPCB 138163 exposure, controlling for age, gender, BMI, and serum lipids (Table 4). LnsumPBDEs, LnPBDE 47, Lntotal fish meals and Lnsaltwater fish meals decreased odds of incident diabetes, but these associations did not reach significance (Table 4).
We found no evidence for mediation, as no substantial changes in associations of diabetes with DDE and PCBs were seen with adjustment for CRP or GGT, and associations of diabetes with DDE, sum PCB and PCB congeners were strengthened with further adjustment for adiponectin (not shown).
Because of the small number of diabetes cases, we did not explore modification of associations of incident diabetes with POPs and fish consumption by BMI, adiponectin, CRP and GGT.
3.5 Associations of HA1c with POPs and Fish Consumption: Evaluation of Mediation and Moderation by Diabetes Biomarkers
HA1c was positively associated with LnDDE and LnPCB118, and inversely associated with Lnsaltwater fish meals, Lntotal fish meals, and LnGLSCF meals after adjustment for confounding factors (Table 5). In models that included the subgroup of participants without diabetes and with HA1c<6.5%, Lntotal fish meals and Lnsaltwater fish meals, but not LnDDE, LnPCB 118 and LnGLSCF meals, remained significantly and inversely associated with HA1c (Table 5).
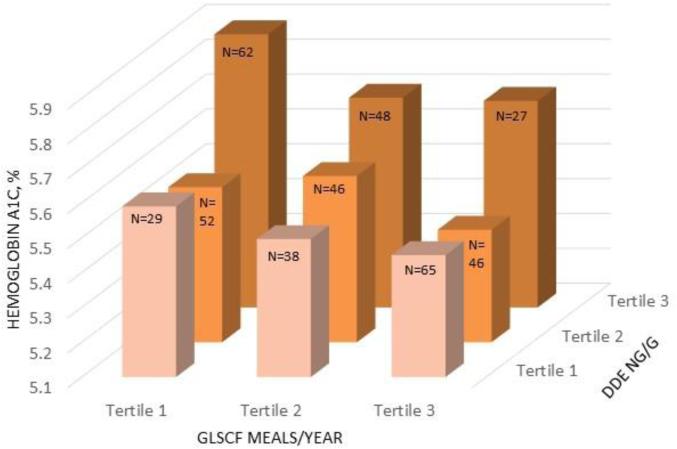
We found evidence for negative confounding, or strengthening of effect, by GLSCF meals for associations of HA1c with DDE and PCB 118. Furthermore, associations of HA1c with GLSCF and total fish meals were negatively confounded by DDE and PCBs, but not PBDEs. However, associations of saltwater fish meals with HA1c were not confounded by POPs. The joint effect of DDE and GLSCF meals on HA1c is illustrated in Figure 1. Mean percent HA1c was 0.43 higher in persons with the highest tertile of DDE and the lowest tertile of GLSCF meals compared to those with the lowest tertile of DDE and the highest tertile of GLSCF meals.
Figure 1.
Joint effect of DDE exposure and GLSCF meals in past year on HA1c levels, adjusted for age centered, gender, BMI centered, diabetes medication use, and serum lipids centered. Total N=413, number of participants in each group is shown in on bar.
Mediation was assessed by further adjusting multivariable models for Lnadiponectin, LnCRP, or LnGGT, but little change was observed in the magnitude of associations of HA1c with LnDDE, LnPCB118, and Lntotal fish, Lnsaltwater fish, and LnGLSCF meals (not shown).
GGT, CRP, adiponectin and BMI modified the positive association of LnDDE with HA1c, with stronger effects in persons with higher GGT, CRP, and BMI and with lower adiponectin (Table 6). Similarly, the effect of LnPCB118 on HA1c was stronger in persons with higher GGT. Inverse associations of HA1c with Lntotal fish meals and LnGLSCF meals were stronger in persons with higher CRP and GGT, respectively. The decline in HA1c with saltwater fish ingestion was larger in those with lower BMI and adiponectin.
Table 6.
Modification of associations of Hemoglobin A1c with POPs and fish consumption by BMI, adiponectin, CRP and GGT
| Participant Group | Exposure | Modifier | Interaction p-value | Conditional associations of Hemoglobin A1c with POPs and fish consumption at selected percentiles of modifying variables.a | ||
|---|---|---|---|---|---|---|
| 25th Percentile Betab, p-value | 50th Percentile Betab, p-value | 75th Percentile Betab, p-value | ||||
| With and Without Diabetes, n=413 | LnDDE | BMI | 0.03 | 0.08, 0.04 | 0.11, 0.002 | 0.15, <0.0001 |
| Adiponectin | 0.04 | 0.16, 0.0002 | 0.13, 0.0002 | 0.10, 0.01 | ||
| CRP | 0.05 | 0.07, 0.07 | 0.12, 0.0005 | 0.15, 0.0001 | ||
| GGT | 0.09 | 0.08, 0.05 | 0.11, 0.002 | 0.14, 0.0002 | ||
| LnPCB118 | GGT | 0.05 | 0.03, 0.56 | 0.07, 0.09 | 0.12, 0.01 | |
| LnTotal Fish Meals | CRP | 0.13 | −0.06, 0.01 | −0.08, 0.0004 | −0.10, 0.0007 | |
| LnSaltwater Fish Meals | BMI | 0.11 | −0.05, 0.01 | −0.03, 0.03 | −0.02, 0.38 | |
| Adiponectin | 0.02 | −0.06. 0.003 | −0.03, 0.03 | −0.01, 0.54 | ||
| LnGLSCF Meals | GGT | 0.14 | −0.03, 0.12 | −0.04, 0.01 | −0.05, 0.004 | |
| No Diabetes and HA1c<6.5%, n=349 | LnDDE | CRP | 0.09 | −0.001, 0.98 | 0.32, 0.22 | 0.05, 0.08 |
| LnTotal Fish Meals | GGT | 0.12 | −0.03, 0.13 | −0.04, 0.01 | −0.05, 0.001 | |
| LnSaltwater Fish Meals | BMI | 0.03 | −0.04, 0.0004 | −0.03, 0.002 | −0.02, 0.13 | |
A total of 40 mode s were examined but results shown in table only if p-value for interaction of modifier with exposure <0.15.
Adjusted for age, BMI, and gender, for all models; serum lipids for POP models; and diabetes medication use for models for participant group: “with and without diabetes”. Additional covariates were included if determined to be confounders (See Table 5).
4. Discussion
We previously reported positive associations of DDE and PCB118, but not PBDEs, with prevalent diabetes in the 2004-2005 cross-sectional data form this cohort (Turyk et al. 2009a;Turyk et al. 2009b). The current study extends our investigations of the same dataset, finding positive associations of DDE and PCB118 with a continuous measure of glycemic control, HA1c. However, associations of DDE and PCB 118 with HA1c did not reach significance in the subgroup analysis that focused on participants without diagnosed diabetes and with HA1c levels below 6.5% [the threshold used to identify potential diabetes for population screening (American Diabetes Association 2010)], suggesting that the effect of POPs on glucose dysregulation may be more important for development of diabetes than prediabetes. Analyses of cross sectional National Health and Nutrition Examination Survey (NHANES) data that defined prediabetes by HA1c% found that PCB 118 was associated with HA1c% in the higher range (5.9%-6.4%), but not in the lower range of 5.7%-5.8% (Everett and Thompson 2012), and that DDE was associated with diabetes but not prediabetes (Everett and Matheson 2010). However, a 23-year longitudinal investigation found that PCB exposure was associated with increased HA1c% during follow up in persons who became diabetic by year 20 as well as in non-diabetic controls (Suarez-Lopez et al. 2015).
Our findings on elevated incident diabetes risk with DDE exposure are consistent with published results in this cohort based on follow up from 1993 to 2005 (Turyk et al. 2009a). While we did not previously detect an association of PCBs with incident diabetes, the current analysis revealed increased diabetes risk with PCB exposure. The association of PCBs with incident diabetes is consistent with some (Lee et al. 2010;Lee et al. 2011;Vasiliu et al. 2006;Wu et al. 2013), but not all prospective investigations on this topic (Rignell-Hydbom et al. 2009;Zani et al. 2013).
Our past work did not identify any associations of years consuming sport caught fish with incident and prevalent diabetes (Turyk et al. 2009a;Turyk et al. 2009b). The current analysis focused on different fish consumption metrics, namely meals of fish consumed in the past year, including total fish meals, saltwater fish meals and GLSCF meals. Odds of incident diabetes were reduced, but not significantly, with increasing total fish meals and saltwater fish meals. HA1c was inversely and significantly associated with total fish meals and saltwater fish meals in the full cohort and remained significantly and inversely associated in the subgroup analysis that included only participants without diagnosed diabetes and with HA1c levels below 6.5%, suggesting that fish consumption may affect blood glucose levels in the normal and prediabetic range as well as the diabetic range. In persons without diabetes, HA1c was inversely associated with eating oily fish in women but not men, but the association was attenuated with adjustment for family history of diabetes, smoking, physical activity, alcohol, total energy and fruit and vegetable intake (Harding et al. 2004). Among diabetics, intake of fish has been associated with decreased HA1c (Lee et al. 2012). In the current study we were not able to determine which component of fish could account for its effect on blood glucose. Protective effects of fish consumption on diabetes have been attributed to omega-3 fatty acids, mediated in part through reduction of triglycerides and inflammation (Carpentier et al. 2006), but interventions (Akinkuolie et al. 2011;Fedor and Kelley 2009) and prospective observational studies (Wu et al. 2012;Zhang et al. 2013) have not consistently reported a beneficial effect of marine omega-3 fatty acids on insulin resistance and diabetes.
GLSCF meals were inversely associated with HA1c only after adjustment for DDE exposure, while positive associations of DDE and PCB 118 with HA1c were strengthened after control for GLSCF meals. These findings emphasize the importance of adjusting for both POP exposures and fish intake in investigations of populations ingesting contaminated fish, and are consistent with a study of sport fish consumers that noted elevated odds of diabetes with DDE and PCB exposure, but decreased odds of diabetes with increasing total fish and trout meals, with adjustment for PCBs (Philibert et al. 2009). On the other hand, a cross sectional investigation of Finnish fishermen did not find that co-adjustment for environmental contaminants, including POPs, and omega-3 fatty acids affected associations of either factor modeled separately with glucose, insulin resistance, or inflammatory markers (Turunen et al. 2013). Inconsistent effects of fish and marine omega-3 fatty acid intake on diabetes risk in prospective observational studies could potentially be confounded by unmeasured contaminant exposures (Wu et al. 2012;Zhang et al. 2013).
Our findings of elevated odds of incident diabetes with elevated CRP and GGT and decreased odds with elevated adiponectin levels were consistent with the literature (Dehghan et al. 2007;Duncan and Schmidt 2006;Nakanishi et al. 2004). However, we detected few associations of these diabetes biomarkers with POPs and fish ingestion, with the exception of positive associations of GGT with PCB 118 and with PBDEs in older persons, a positive association of adiponectin with PBDEs, and inverse associations of CRP with fish ingestion, particularly in persons with higher adiposity. Furthermore, adiponectin, CRP and GGT did not attenuate associations of POPs with either HA1c or incident diabetes, and this study does not lend support for any of these biomarkers as mediators of associations of POPs and diabetes.
We detected a positive association of adiponectin with sum PBDEs in the entire cohort, and in persons with higher body mass or older age, suggesting improved insulin sensitivity with increased PBDEs. Supporting this observation is our finding of a non-significant trend towards reduction of incident diabetes risk with PBDE exposure. To our knowledge, no other epidemiologic studies have investigated effects of PBDEs on adiponectin; however, decreased insulin-stimulated glucose oxidation was observed in adipocytes isolated from penta-BDE treated rats (Hoppe and Carey 2007). We did not observe associations of adiponectin with DDE or PCBs, overall or by age, gender, or BMI strata. In contrast, three other epidemiological investigations found inverse associations (Lim and Jee 2014;Mullerova et al. 2008) or trends (Kern et al. 2004) of adiponectin with PCBs or related chemicals, and stronger effects in subgroups with elevated BMI (Lim and Jee 2014;Mullerova et al. 2008). Experimental evidence that PCB 153 (Taxvig et al. 2012) and DDE (Howell, III and Mangum 2011) stimulate release of adiponectin from mature adipocytes supports these results. Reasons for difference in the effect of PCBs on adiponectin in these investigations and in the current study are not clear, but could be related to variability in population characteristics and exposures. Finally, fish ingestion was not related to adiponectin levels, although increased adiponectin has been detected in fish intake intervention studies (Kondo et al. 2010;Lara et al. 2007;Neale et al. 2013;Zhang et al. 2012).
POPs have been associated with increased inflammation, assessed by a variety of biomarkers (Fujiyoshi et al. 2006;Imbeault et al. 2012;Kumar et al. 2014b;Kuwatsuka et al. 2014). In the current study, we did not find any associations of CRP with POPs, and other investigators have inconsistently detected effects of POPs on CRP. Elevated CRP was associated with occupational PCB exposure in women (Persky et al. 2011), but not men (Persky et al. 2012); CRP was positively associated with organochlorine pesticides and inversely associated with non-dioxin like PCBs in NHANES participants (Ha et al. 2007;Kim et al. 2012); and dioxin-like chemicals, PCBs, and organochlorine pesticides did not affect CRP in two other studies (Kumar et al. 2014b;Turunen et al. 2013). Our findings of inverse associations of saltwater fish meals and total fish meals with CRP are consistent with observational and intervention studies (He et al. 2009;Ouellet et al. 2008;Ramel et al. 2010;Smith et al. 2009).
GGT, an independent risk factor for the development of cardiovascular disease and diabetes, is induced by oxidative stress and catalyzes the first step in the degradation of glutathione, which functions as a conjugating ligand for phase II metabolism of xenobiotics, such as POPs (Lee et al. 2008). Exposures to POPs have been associated with increased GGT, even within the normal reference range in some (Chen et al. 2006;Lee and Jacobs, Jr. 2006;Sweeney and Mocarelli 2000), but not all reports (Kumar et al. 2014a;Persky et al. 2011;Persky et al. 2012). In the current study, PCB 118 was positively associated with GGT, as were sumPBDEs and PBDE 47 in persons of above median age.
BMI, adiponectin, GGT and CRP modified the association of DDE with HA1c, with stronger effects of DDE on HA1c in persons with higher levels of risk for diabetes (higher BMI, GGT and CRP and lower adiponectin). Similarly, GGT modified the association of PCB118 with HA1c. A cross sectional analysis of NHANES data examined modification of the effect of POPs on insulin resistance by CRP, with results similar to ours, namely stronger effects of POPs on insulin resistance in persons with higher concentrations of CRP (Kim et al. 2012). There is also evidence of effect modification of associations of diabetes and POPs by body weight, with stronger effects in persons with higher adiposity (Lee et al. 2014). With respect to fish intake, our findings suggest that the protective effect of fish consumption on HA1c is stronger in persons with higher levels of the diabetes biomarkers. However, this was not the case for adiposity, as the protective effect of saltwater fish meals on HA1c was stronger in those with lower body mass, which is consistent with a meta-analysis concluding that that fish consumption was related to lower risk of diabetes in populations with lower BMI (Wu et al. 2012).
There are several limitations to this study. First, with respect to measurement of biomarkers, there may have been some loss of activity due to long term storage and prior freeze/thaw cycles. However, all of the serum samples were handled similarly, so we do not expect differential bias in statistical estimates. Second, diabetes diagnosis and fish consumption were collected by self report and may be affected by measurement error, although misclassification is unlikely to be differential. Third, there may be residual confounding for variables with measurement errors or for unmeasured variables, such as other dietary factors (Boeing et al. 2000;Harding et al. 2004;Prynne et al. 2009), physical activity, and other contaminants. Fourth, adjustment of POPs models for serum lipids is appropriate for non-fasting samples, but could potentially result in attenuated effect estimates if serum lipids are intermediates in the causal pathway between POPs and diabetes (Taylor et al. 2013). Nevertheless, this study takes advantage of an established, well characterized cohort and is one of the first studies to explore the role of diabetes biomarkers in the associations of POPs.
5. Conclusions
This investigation found that odds of incident diabetes were elevated with exposure to DDE and PCBs and that DDE and PCB 118 were positively associated with HA1c. Fish intake was inversely associated with HA1c, suggesting the presence of nutrients in fish that modify glucose levels. Co-adjustment for POPs and fish consumption strengthened associations of either factor modeled separately, highlighting the importance of assessing both POP exposures and fish intake in populations ingesting contaminated fish.
Adiponectin, CRP, and GGT were not associated with DDE and PCB congeners, with the exception of a positive association of GGT with PCB 118. CRP was inversely related to fish consumption. However, diabetes biomarkers did not mediate associations of POPs or fish consumption with HA1c or with incident diabetes. Our findings suggest that while adiponectin, CRP and GGT do not mediate the effect of POPs on HA1c, positive associations of POPs with HA1c are stronger in persons with higher levels of these diabetes risk factors.
Supplementary Material
Highlights.
Diabetes biomarkers, adiponectin, γ-glutamyl transferase and CRP, were measured
POPs were associated with incident diabetes and higher Hemoglobin A1c levels
Associations of diabetes with POPs were not mediated by diabetes biomarkers
POPs had stronger effects on blood glucose in persons with higher diabetes risk
Acknowledgments
Funding sources: This research was supported by the National Institute of Environmental Health Sciences, Grant Number R21ES017121, the U.S. Environmental Protection Agency, Grant Number RD- 83025401-1, and the Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, Grant Number H75/ ATH598322. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. Although the research described in this article has been funded in part by the U.S. Environmental Protection Agency, it has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
Human subjects research review: This study was conducted in accordance with national and institutional guidelines for the protection of human subjects. Prior to initiation of this study protocol, it was reviewed and approved by both the University of Illinois-Chicago Internal Review Board and University of Wisconsin-Madison Medical School Human Subjects Committee. Informed consent was obtained from each subject prior to participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akinkuolie AO, Ngwa JS, Meigs JB, Djousse L. Omega-3 polyunsaturated fatty acid and insulin sensitivity: a meta-analysis of randomized controlled trials. Clin Nutr. 2011;30:702–707. doi: 10.1016/j.clnu.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. doi: 10.2337/dc10-S011.: S11-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H, Falk C, Fiore B, Hanrahan L, Humphrey HE, Kanarek M, et al. Consortium for the Health Assessment of Great Lakes Sport Fish Consumption. Toxicol Ind Health. 1996;12:369–373. doi: 10.1177/074823379601200309. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Imm P, Knobeloch L, Turyk M, Mathew J, Buelow C, et al. Polybrominated diphenyl ethers (PBDE) in serum: findings from a US cohort of consumers of sport-caught fish. Chemosphere. 2008;73:187–194. doi: 10.1016/j.chemosphere.2008.05.052. [DOI] [PubMed] [Google Scholar]
- Boeing H, Weisgerber UM, Jeckel A, Rose HJ, Kroke A. Association between glycated hemoglobin and diet and other lifestyle factors in a nondiabetic population: cross-sectional evaluation of data from the Potsdam cohort of the European Prospective Investigation into Cancer and Nutrition Study. Am J Clin Nutr. 2000;71:1115–1122. doi: 10.1093/ajcn/71.5.1115. [DOI] [PubMed] [Google Scholar]
- Brand JS, Wareham NJ, Dowsett M, Folkerd E, van der Schouw YT, Luben RN, et al. Associations of endogenous testosterone and SHBG with glycated haemoglobin in middle-aged and older men. Clin Endocrinol (Oxf) 2011;74:572–578. doi: 10.1111/j.1365-2265.2010.03951.x. [DOI] [PubMed] [Google Scholar]
- Calvert GM, Sweeney MH, Deddens J, Wall DK. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med. 1999;56:270–276. doi: 10.1136/oem.56.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr. 2006;83:1499S–1504S. doi: 10.1093/ajcn/83.6.1499S. [DOI] [PubMed] [Google Scholar]
- Chen HL, Su HJ, Guo YL, Liao PC, Hung CF, Lee CC. Biochemistry examinations and health disorder evaluation of Taiwanese living near incinerators and with low serum PCDD/Fs levels. Sci Total Environ. 2006;366:538–548. doi: 10.1016/j.scitotenv.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect. 2007;115:1442–1447. doi: 10.1289/ehp.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tata V. Association of dioxin and other persistent organic pollutants (POPs) with diabetes: epidemiological evidence and new mechanisms of beta cell dysfunction. Int J Mol Sci. 2014;15:7787–7811. doi: 10.3390/ijms15057787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56:872–878. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI. The epidemiology of low-grade chronic systemic inflammation and type 2 diabetes. Diabetes Technol Ther. 2006;8:7–17. doi: 10.1089/dia.2006.8.7. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Matheson EM. Biomarkers of pesticide exposure and diabetes in the 1999-2004 national health and nutrition examination survey. Environ Int. 2010;36:398–401. doi: 10.1016/j.envint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Thompson OM. Associations of dioxins, furans and dioxin-like PCBs with diabetes and pre-diabetes: is the toxic equivalency approach useful? Environ Res. 2012;118:107–111. doi: 10.1016/j.envres.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Fallah S, Sanjary PM, Rabbani CA, Korani M. Adiponectin, leptin and lipid profiles evaluation in oral contraceptive pill consumers. Arch Gynecol Obstet. 2012;285:1747–1752. doi: 10.1007/s00404-011-2192-3. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Fedor D, Kelley DS. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care. 2009;12:138–146. doi: 10.1097/MCO.0b013e3283218299. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi PT, Michalek JE, Matsumura F. Molecular epidemiologic evidence for diabetogenic effects of dioxin exposure in U.S. Air force veterans of the Vietnam war. Environ Health Perspect. 2006;114:1677–1683. doi: 10.1289/ehp.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Henriksen JE, Choi AL, Petersen MS, Dalgard C, Nielsen F, et al. Marine food pollutants as a risk factor for hypoinsulinemia and type 2 diabetes. Epidemiology. 2011;22:410–417. doi: 10.1097/EDE.0b013e318212fab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999-2002. Environ Health Perspect. 2007;115:1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan LP, Falk C, Anderson HA, Draheim L, Kanarek MS, Olson J. Serum PCB and DDE levels of frequent Great Lakes sport fish consumers-a first look. The Great Lakes Consortium. Environ Res. 1999;80:S26–S37. doi: 10.1006/enrs.1998.3914. [DOI] [PubMed] [Google Scholar]
- Harding AH, Day NE, Khaw KT, Bingham SA, Luben RN, Welsh A, et al. Habitual fish consumption and glycated haemoglobin: the EPIC-Norfolk study. Eur J Clin Nutr. 2004;58:277–284. doi: 10.1038/sj.ejcn.1601779. [DOI] [PubMed] [Google Scholar]
- He K, Liu K, Daviglus ML, Jenny NS, Mayer-Davis E, Jiang R, et al. Associations of dietary long- chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2009;103:1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8:252–258. doi: 10.1097/00001648-199705000-00005. [DOI] [PubMed] [Google Scholar]
- Hoppe AA, Carey GB. Obesity. Vol. 15. Silver Spring; 2007. Polybrominated diphenyl ethers as endocrine disruptors of adipocyte metabolism. pp. 2942–2950. [DOI] [PubMed] [Google Scholar]
- Howell G, III, Mangum L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol In Vitro. 2011;25:394–402. doi: 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbeault P, Findlay CS, Robidoux MA, Haman F, Blais JM, Tremblay A, et al. Dysregulation of cytokine response in Canadian First Nations communities: is there an association with persistent organic pollutant levels? PLoS One. 2012;7:e39931. doi: 10.1371/journal.pone.0039931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen ME, Borch-Johnsen K, Bjerregaard P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia. 2008;51:1416–1422. doi: 10.1007/s00125-008-1066-0. [DOI] [PubMed] [Google Scholar]
- Kern PA, Said S, Jackson WG, Jr., Michalek JE. Insulin sensitivity following agent orange exposure in Vietnam veterans with high blood levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Clin Endocrinol Metab. 2004;89:4665–4672. doi: 10.1210/jc.2004-0250. [DOI] [PubMed] [Google Scholar]
- Kim KS, Hong NS, Jacobs DR, Jr., Lee DH. Interaction Between Persistent Organic Pollutants and C-reactive Protein in Estimating Insulin Resistance Among Non-diabetic Adults. J Prev Med Public Health. 2012;45:62–69. doi: 10.3961/jpmph.2012.45.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Morino K, Nishio Y, Kondo M, Fuke T, Ugi S, et al. Effects of a fish-based diet on the serum adiponectin concentration in young, non-obese, healthy Japanese subjects. J Atheroscler Thromb. 2010;17:628–637. doi: 10.5551/jat.3657. [DOI] [PubMed] [Google Scholar]
- Kumar J, Lind L, Salihovic S, van BB, Ingelsson E, Lind PM. Persistent organic pollutants and liver dysfunction biomarkers in a population-based human sample of men and women. Environ Res. 2014a;134:251–256. doi: 10.1016/j.envres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Kumar J, Lind PM, Salihovic S, van BB, Ingelsson E, Lind L. Persistent organic pollutants and inflammatory markers in a cross-sectional study of elderly swedish people: the PIVUS cohort. Environ Health Perspect. 2014b;122:977–983. doi: 10.1289/ehp.1307613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13:831–849. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwatsuka Y, Shimizu K, Akiyama Y, Koike Y, Ogawa F, Furue M, et al. Yusho patients show increased serum IL-17, IL-23, IL-1beta, and TNFalpha levels more than 40 years after accidental polychlorinated biphenyl poisoning. J Immunotoxicol. 2014;11:246–249. doi: 10.3109/1547691X.2013.835890. [DOI] [PubMed] [Google Scholar]
- Langer P, Tajtakova M, Guretzki HJ, Kocan A, Petrik J, Chovancova J, et al. High prevalence of anti-glutamic acid decarboxylase (anti-GAD) antibodies in employees at a polychlorinated biphenyl production factory. Arch Environ Health. 2002;57:412–415. doi: 10.1080/00039890209601429. [DOI] [PubMed] [Google Scholar]
- Langer P, Ukropec J, Kocan A, Drobna B, Radikova Z, Huckova M, et al. Obesogenic and diabetogenic impact of high organochlorine levels (HCB, p,p′-DDE, PCBs) on inhabitants in the highly polluted Eastern Slovakia. Endocr Regul. 2014;48:17–24. doi: 10.4149/endo_2014_01_17. [DOI] [PubMed] [Google Scholar]
- Lara JJ, Economou M, Wallace AM, Rumley A, Lowe G, Slater C, et al. Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis. 2007;193:213–221. doi: 10.1016/j.atherosclerosis.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR., Jr. Association between serum concentrations of persistent organic pollutants and gamma glutamyltransferase: results from the National Health and Examination Survey 1999-2002. Clin Chem. 2006;52:1825–1827. doi: 10.1373/clinchem.2006.071563. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DR, Jr., Salihovic S, van BB, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–1784. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR, Jr., Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35:557–601. doi: 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Jacobs DR., Jr. Can persistent organic pollutants explain the association between serum gamma-glutamyltransferase and type 2 diabetes? Diabetologia. 2008;51:402–407. doi: 10.1007/s00125-007-0896-5. [DOI] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr. Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect. 2010;118:1235–1242. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Mitochondrial dysfunction and insulin resistance: the contribution of dioxin-like substances. Diabetes Metab J. 2011;35:207–215. doi: 10.4093/dmj.2011.35.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Yoon EH, Lee HM, Hwang HS, Park HK. Relationship between Food-frequency and Glycated Hemoglobin in Korean Diabetics: Using Data from the 4th Korea National Health and Nutrition Examination Survey. Korean J Fam Med. 2012;33:280–286. doi: 10.4082/kjfm.2012.33.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- Lim JE, Jee SH. Association between serum levels of adiponectin and polychlorinated biphenyls in Korean men and women. Endocrine. 2014 doi: 10.1007/s12020-014-0231-0. [DOI] [PubMed] [Google Scholar]
- Michalek JE, Akhtar FZ, Kiel JL. Serum dioxin, insulin, fasting glucose, and sex hormone-binding globulin in veterans of Operation Ranch Hand. J Clin Endocrinol Metab. 1999;84:1540–1543. doi: 10.1210/jcem.84.5.5663. [DOI] [PubMed] [Google Scholar]
- Mullerova D, Kopecky J, Matejkova D, Muller L, Rosmus J, Racek J, et al. Negative association between plasma levels of adiponectin and polychlorinated biphenyl 153 in obese women under non- energy-restrictive regime. Int J Obes (Lond) 2008;32:1875–1878. doi: 10.1038/ijo.2008.169. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Nishina K, Li W, Ssato M, Suzuki M, Tatara K. Serum ?-glutamyltransferase and development of impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. J Intern Med. 2003;254:287–295. doi: 10.1046/j.1365-2796.2003.01198.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27:1427–1432. doi: 10.2337/diacare.27.6.1427. [DOI] [PubMed] [Google Scholar]
- Neale EP, Muhlhausler B, Probst YC, Batterham MJ, Fernandez F, Tapsell LC. Short-term effects of fish and fish oil consumption on total and high molecular weight adiponectin levels in overweight and obese adults. Metabolism. 2013;62:651–660. doi: 10.1016/j.metabol.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Weisnagel SJ, Marois J, Bergeron J, Julien P, Gougeon R, et al. Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J Nutr. 2008;138:2386–2391. doi: 10.3945/jn.108.092346. [DOI] [PubMed] [Google Scholar]
- Persky V, Piorkowski J, Turyk M, Freels S, Chatterton R, Jr., Dimos J, et al. Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Environ Res. 2011;111:817–824. doi: 10.1016/j.envres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Persky V, Piorkowski J, Turyk M, Freels S, Chatterton R, Jr., Dimos J, et al. Polychlorinated biphenyl exposure, diabetes and endogenous hormones: a cross-sectional study in men previously employed at a capacitor manufacturing plant. Environ Health. 2012;11:57. doi: 10.1186/1476-069X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky V, Turyk M, Anderson HA, Hanrahan LP, Falk C, Steenport DN, et al. The effects of PCB exposure and fish consumption on endogenous hormones. Environ Health Perspect. 2001;109:1275–1283. doi: 10.1289/ehp.011091275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert A, Schwartz H, Mergler D. An exploratory study of diabetes in a First Nation community with respect to serum concentrations of p,p′-DDE and PCBs and fish consumption. Int J Environ Res Public Health. 2009;6:3179–3189. doi: 10.3390/ijerph6123179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prynne CJ, Mander A, Wadsworth ME, Stephen AM. Diet and glycosylated haemoglobin in the 1946 British birth cohort. Eur J Clin Nutr. 2009;63:1084–1090. doi: 10.1038/ejcn.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. Effects of weight loss and seafood consumption on inflammation parameters in young, overweight and obese European men and women during 8 weeks of energy restriction. Eur J Clin Nutr. 2010;64:987–993. doi: 10.1038/ejcn.2010.99. [DOI] [PubMed] [Google Scholar]
- Remillard RB, Bunce NJ. Linking dioxins to diabetes: epidemiology and biologic plausibility. Environ Health Perspect. 2002;110:853–858. doi: 10.1289/ehp.02110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Lidfeldt J, Kiviranta H, Rantakokko P, Samsioe G, Agardh CD, et al. Exposure to p,p′-DDE: a risk factor for type 2 diabetes. PLoS One. 2009;4:e7503. doi: 10.1371/journal.pone.0007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant LA, Wareham NJ, Khaw KT. Hormone replacement therapy and glucose tolerance in EPIC-Norfolk: a population-based study. Diabetes Metab Res Rev. 2000;16:20–25. doi: 10.1002/(sici)1520-7560(200001/02)16:1<20::aid-dmrr76>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Smith KM, Barraj LM, Kantor M, Sahyoun NR. Relationship between fish intake, n-3 fatty acids, mercury and risk markers of CHD (National Health and Nutrition Examination Survey 1999-2002). Public Health Nutr. 2009;12:1261–1269. doi: 10.1017/S1368980008003844. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Lee DH, Porta M, Steffes MW, Jacobs DR., Jr. Persistent organic pollutants in young adults and changes in glucose related metabolism over a 23-year follow-up. Environ Res. 2015;137:485–494. doi: 10.1016/j.envres.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MH, Mocarelli P. Human health effects after exposure to 2,3,7,8-TCDD. Food Addit Contam. 2000;17:303–316. doi: 10.1080/026520300283379. [DOI] [PubMed] [Google Scholar]
- Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen AW, Jula A, Suominen AL, Mannisto S, Marniemi J, Kiviranta H, et al. Fish consumption, omega-3 fatty acids, and environmental contaminants in relation to low-grade inflammation and early atherosclerosis. Environ Res. 2013;120:43–54. doi: 10.1016/j.envres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009a;117:1076–1082. doi: 10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p′-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009b;75:674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Bhavsar SP, Bowerman W, Boysen E, Clark M, Diamond M, et al. Risks and benefits of consumption of Great Lakes fish. Environ Health Perspect. 2012;120:11–18. doi: 10.1289/ehp.1003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology. 2006;17:352–359. doi: 10.1097/01.ede.0000220553.84350.c5. [DOI] [PubMed] [Google Scholar]
- Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses' health study and meta-analysis. Environ Health Perspect. 2013;121:153–161. doi: 10.1289/ehp.1205248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 107 Suppl. 2012;2:S214–S227. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani C, Donato F, Magoni M, Feretti D, Covolo L, Vassallo F, et al. Polychlorinated Biphenyls, Glycaemia and Diabetes in a Population Living in a Highly Polychlorinated Biphenyls-Polluted Area in Northern Italy: a Cross-sectional and Cohort Study. J Public Health Res. 2013;2:2–8. doi: 10.4081/jphr.2013.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang C, Li L, Man Q, Meng L, Song P, et al. Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. Br J Nutr. 2012;108:1455–1465. doi: 10.1017/S0007114511006866. [DOI] [PubMed] [Google Scholar]
- Zhang M, Picard-Deland E, Marette A. Fish and Marine Omega-3 Polyunsatured Fatty Acid Consumption and Incidence of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int J Endocrinol. 2013;2013:501015. doi: 10.1155/2013/501015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.