Abstract
The purpose of this study was to investigate the mechanical consequences of proteoglycan 4 (Prg4) deficiency on intervertebral disc mechanics using a Prg4 knockout mouse model. Prg4, also called lubricin, was first identified as the boundary lubricant in synovial fluid but has subsequently been localized within a number of musculoskeletal tissues in areas subjected to shear and tensile stresses, including the intervertebral disc. The function of lubricin in the intervertebral disc has not been determined. Lumbar level 1–2 vertebral body-disc-vertebral body motion segments were isolated from Prg4 null mice and wild type (WT) litter mate controls. Disc dimensions were measured and motion segments were tested in axial loading and torsion. Torque measurements and disc dimensions were used to calculate the torsional apparent modulus for discs from Prg4 null and WT discs. Discs from Prg4 null mice had a significantly smaller mean transverse disc area (p=0.0057), with a significantly larger proportion of this area occupied by the nucleus pulposus (p<0.0001), compared to WT specimens. Apparent torsional moduli were found to be elevated in Prg4 null lumbar discs compared to WT controls at 10–10° (p=.0048) and 10–30° (p=0.0127) rotation. This study suggests a functional role for Prg4 in the murine intervertebral disc. The absence of Prg4 was associated with an increased apparent torsional modulus and the structural consequences of Prg4 deficiency in the intervertebral disc, with expansion of the area of the nucleus pulposus relative to the transverse disc area in Prg4 null specimens.
Keywords: Disc, lubricin, apparent torsional modulus, proteoglycan 4, Prg4
1. Introduction
Synovial joints and intervertebral discs are uniquely adapted for the demands of their specific articulations (Pattappa et al., 2012). An interesting similarity between synovial joint articular cartilage and intervertebral discs is the selective localization of the mucinous glycoprotein lubricin (Shine et al., 2009; Shine and Spector, 2008). Lubricin, also called superficial zone protein (SZP) or proteoglycan 4 (Prg4), was first identified as the boundary lubricant in synovial fluid (Rhee et al., 2005). In synovial joints, lubricin is produced by superficial zone chondrocytes and synovial cells; it is present in synovial fluid and deposited as a protective layer over the articular cartilage surface and synovium (Rhee et al., 2005). Lubricin is also present within the superficial zone of articular cartilage (Ateshian and Hung, 2005). It has been proposed that lubricin facilitates microscale lubrication between collagen fibrils when the surface is subjected to compression and shear. This hypothesis is supported by the selective localization of lubricin in other areas of elevated shear and tensile stresses: the collagen bundles throughout the entire knee meniscus, the tendon fascicles in digital flexor tendons, and the rotator cuff (Funakoshi et al., 2010; Sun et al., 2006; Zhang et al., 2011).
Lubricin has also been identified in the lumbar intervertebral discs of goats (Shine and Spector, 2008) and humans (Shine et al., 2009). Notably, while lubricin immunostaining in caprine discs identified lubricin primarily in the outer annulus fibrosus (Shine and Spector, 2008), lubricin was identified in the cells, matrix, and tissue surfaces of the annulus and nucleus pulposus in human intervertebral discs (Shine et al., 2009). The consequences of lubricin deficiency for the functional integrity of the intervertebral disc, and the potential role of insufficient lubricin in degenerative disc disease, have not been investigated.
Camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome is an autosomal recessive syndrome resulting from loss of function mutations of Prg4, the gene coding for lubricin. Patients affected with CACP have normal-appearing joints at birth but develop early joint degeneration (Bahabri et al., 1998). In a longitudinal case series of patients affected by CACP, the development of scoliotic and kyphotic deformities were noted in some individuals in adolescence and adulthood (Faivre et al., 2000). Potential mechanisms by which lubricin deficiency might impact intervertebral disc mechanics include direct effects on tissue mechanical properties, accumulated damage in the setting of inadequate natural lubrication, and indirect effects on the disc from altered motion and loading in the setting of widespread, early onset degenerative spondylosis.
A Prg4 knockout mouse was developed to study the mechanisms by which lubricin deficiency results in the phenotype of CACP syndrome (Rhee et al., 2005). Prior studies using the Prg4 knockout mouse have focused on the consequences of lubricin deficiency in articular joints. The objective of this study was to investigate the mechanical consequences of lubricin deficiency on intervertebral disc mechanics using the Prg4 knockout mouse model. Our hypothesis was that the apparent torsional modulus is elevated in the intervertebral discs of young adult Prg4 null mice compared to wild-type (WT) littermates.
2. Materials and methods
Lumbar disc specimens were obtained from an active breeding colony of Prg4 −/− mice maintained on the BL6 background strain. For mechanical testing, 26 male mice, 10 weeks of age, formed the two groups, Prg4 −/− (KO) and PRG4 +/+ (WT) with n=13 specimens per group. Following harvest, each specimen was sealed in an airtight container and frozen at −20° C until the day of testing. All animals were euthanized according to an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
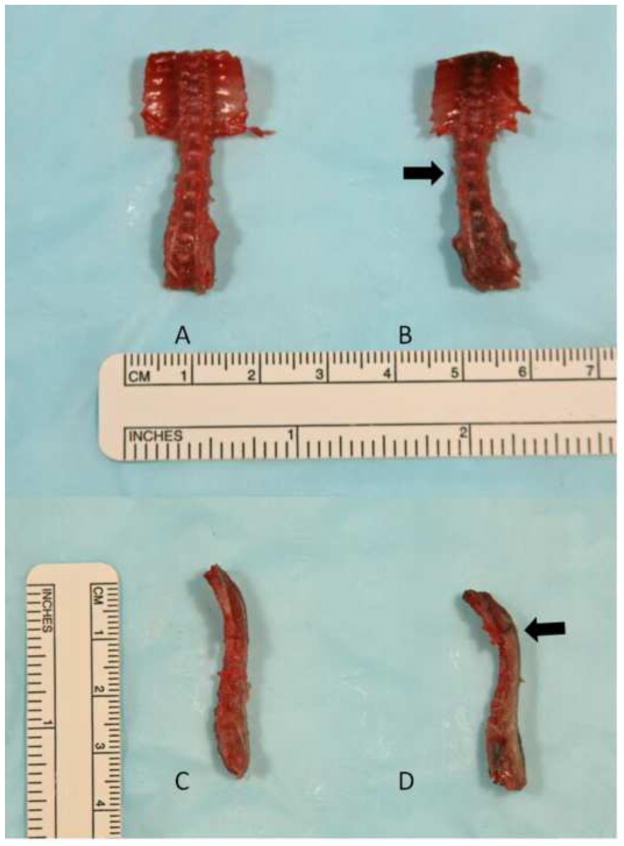
On the day of testing, each specimen was thawed for two hours at room temperature. The lumbar spine was dissected free (Figure 1), and the lumbar L1–L2 disc and its adjacent vertebral bodies were identified. The posterior elements including the facet joints and surrounding soft tissues were dissected off of the sample to isolate a vertebral body-disc-vertebral body motion segment.
Figure 1.
Gross dissection of the thoracolumbar spine for 10-week-old male mice, WT (A, C) and PRG4 −/−(B, D). Note the scoliosis (B, arrow) and kyphosis (D, arrow) of the PRG 4 −/− specimen.
Mechanical testing was a destructive process. In order to estimate the dimensions of the nucleus pulposus relative to total disc dimensions, matched L1–L2 discs were obtained from another five Prg4 null and five WT 10-week-old male specimens. Disc height, transverse disc major axis, and transverse disc minor axis were measured using a digital caliper (Mitutoyo Corporation, Kawasaki, Japan). Discs were sectioned axially at midheight so that the nucleus pulposus transverse major and minor axes could be measured. These values were averaged for each group and the averaged values were used to estimate the dimensions of the nucleus pulposus relative to the total disc area in the tested specimens.
2.1 Mechanical Testing
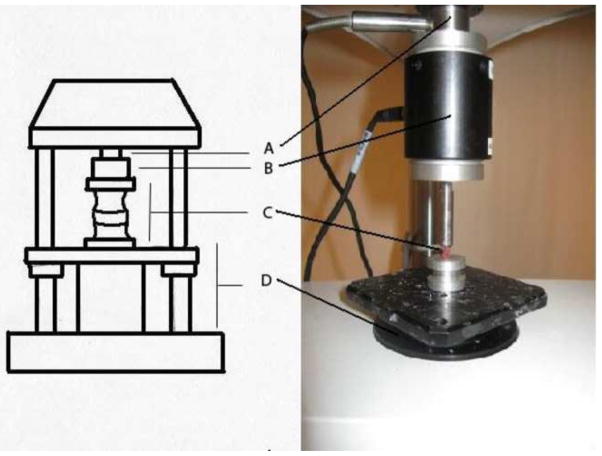
Following specimen preparation, the vertebral body-disc-vertebral body superior and inferior end plates were secured in the testing frame using cyanoacrylate glue (Super Glue, Pacer Technology, Rancho Cucamonga, CA). Specimens were kept moist by regular applications of normal saline. Mechanical testing was performed using an ELF 3200 material testing system (Bose, Framingham, MA). A 1.5 Newton-meter torsion load cell and 22 Newton axial load cell were used in series to apply a constant compressive force of 0.35 Newtons (approximately 1.5 times mouse bodyweight) across the testing segment while the torque-rotation data were recorded. The torsional actuator was located at the base of the testing frame and rotated the inferior vertebra relative to the fixed superior vertebra under rotational angle control (Figure 2). Prior to data collection, the motion segment was loaded with 0.35 Newtons of compression and rotated from 5 to −5 degrees for 34 cycles at 0.07 Hz to establish a reproducible torque-rotation response. A rotation from 10 to −10 degrees was then exerted on the disc. Following a sequence of three rotations from 10 to −10 degrees, a single rotation from 0–30 degrees was performed while torque and angular rotation were recorded. Torsional modulus calculations were performed using the torque versus angular rotation data from the first rotation and the final rotation from 0 to 30 degrees (Supplement Figure 1).
Figure 2.
Testing apparatus for torsional modulus calculations: Axial load cell (A), Torsion load cell (B), Mounted vertebral body-disc-vertebral body segment (C), and Torsional actuator (D).
Torsional apparent modulus was calculated according to the method of Elliott and Sarver (Elliott and Sarver, 2004) from moment-angular deflection (M-Θ) curves that were constructed for the first 10 to −10 degree and the 10 to 30 degree data sets. Torsional stiffness (K, N-mm/deg) was calculated by linear regression using the moment-angular deflection curves. Apparent torsional modulus (GA, MPa) was calculated from torsional stiffness (K), disc height (h), and the polar moment of inertia of the disc (J, mm4), where GA = Kh/J and J = π([WAPWL3-WAP3WL] − [NAPNL3+NAP3NL])/64. WAP and WL represent the anteroposterior and lateral disc diameters, and NAP and NL represent the diameters of the nucleus pulposus.
In the referenced method for calculating the polar moment of inertia (Elliott and Sarver, 2004), the nucleus pulposus was assumed to be 0.2 of total disc area. In this study, measurements were taken to estimate the dimensions of the nucleus pulposus relative to the area of the disc.
2.2 Statistical Analysis
Generalized linear models for Gaussian data were used to compare genotypes on all dependent variables. Classical sandwich estimation was used to adjust for any model misspecification. Alpha was set to 0.05 per dependent variable.
3. Results
For specimens sectioned to calculate the relative volume of the nucleus pulposus, significant differences were observed between the Prg4 +/+ and Prg4 −/− specimens in the dimensions of the disc, nucleus pulposus, and ratio of the area of the nucleus to the total disc area (Table 1). Significant differences were observed between the mechanically tested Prg4 +/+ and Prg4 −/− discs in transverse disc area (p=0.0057), transverse disc major axis (p=0.0045), and disc height (p=0.0002) (Table 2). There was a trend towards difference in disc minor transverse axis dimensions (p=0.0574). Polar moment of inertia for the annulus, which predicts an object’s resistance to torsional stress, was significantly different between Prg4 +/+ and Prg4 −/− subjects (p<0.0001) (Table 2). The observed range of rotation during the first −10 to 10 degree testing cycle was from −13 to 11 degrees. Rotational stiffness values for Prg4 +/+ and Prg4 −/− discs did not show significant differences for either −10–10 or 10–30 degrees of rotation. Apparent torsional modulus, which is stiffness corrected for disc dimensions, showed significant differences between Prg4 +/+ and Prg4 −/− specimens for both −10° to 10° (p=0.0048) and 10° to 30°(p=0.0127)(Figure 3).
Table 1.
Disc Dimensions Measured for the Estimation of Nucleus Pulposus Volume
| n=5 per group | Prg4 +/+ (±stdev) | Prg4 −/− (±stdev) | p-value |
|---|---|---|---|
| Nucleus Pulposus Major Transverse Axis (mm) | 1.10(±0.24) | 1.53(±0.22) | *p=0.0272 |
| Nucleus Pulposus Minor Transverse Axis (mm) | 0.57(±0.08) | 0.67(±0.04) | *p=0.0105 |
| Disc Major Transverse Axis (mm) | 2.12(±0.14) | 1.91(±0.18) | *p=0.0025 |
| Disc Minor Transverse Axis (mm) | 1.30(±0.08) | 1.10(±0.08) | *p=0.0450 |
| Ratio Nucleus to Total Transverse Disc Area | 0.23(±0.08) | 0.49(±0.02) | *p<0.0001 |
Table 2.
Disc Dimensions and Polar Moments of Inertia for Mechanically Tested Specimens
| n=13 per group | PRG4 +/+ (±stdev) | PRG4 −/− (±stdev) | p-value |
|---|---|---|---|
| Transverse Disc Area (mm2) | 1.76 (±0.39) | 1.40 (±0.20) | *p=0.0057 |
| Disc Major Transverse Axis (mm) | 2.06 (±0.15) | 1.86(±0.17) | *p=0.0045 |
| Disc Minor Transverse Axis (mm) | 1.08 (±0.18) | 0.96(±0.14) | p=0.0574 |
| Disc Height (mm) | 0.86(±0.10) | 0.68(±0.11) | *p=0.0002 |
| Polar Moment of Inertia (J, mm4) | 0.58(±0.23) | 0.28(±0.07) | *p<0.0001 |
Figure 3.
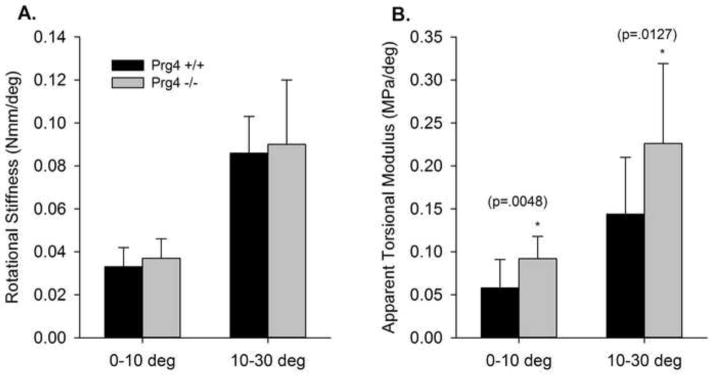
Rotational Stiffness (A) and Apparent Torsional Modulus (B).
4. Discussion
Mechanical testing of Prg4 +/+ and Prg4 −/− intervertebral discs demonstrated significantly greater apparent torsional modulus of the L1–L2 intervertebral disc in Prg4 null mice compared to WT litter mates. Lubricin null discs were significantly smaller with respect to transverse major diameter and height, compared to Prg4 +/+ specimens, and within these dimensions, the nucleus pulposus occupied a relatively greater portion in Prg4 −/− subjects compared to Prg4 +/+.
Our results supported a mechanical function for lubricin within the intervertebral disc, which is consistent with studies of articular cartilage showing increased articular surface friction and early progressive surface damage in the absence of lubricin (Elsaid et al., 2007; Rhee et al., 2005). These results are also aligned with another study showing increased gliding resistance in tendon fascicles in mouse tail tendons lacking a functional Prg4 gene (Reuvers et al., 2011). A previous study by Shine and colleagues identified lubricin in the outer layers of the annulus fibrosus in caprine intervertebral discs (Shine and Spector, 2008). In human discs, lubricin was found within the annulus fibrosis and nucleus pulposus (Shine et al., 2009). It is notable that lubricin localization in the disc varies between quadrupeds and bipedal humans, a finding consistent with the difference in lumbar spine mechanical demands. Further study is needed to determine if the distribution of lubricin in the disc changes with age, injury, or degeneration.
The functional importance of lubricin is supported by the early onset flexor tendon contractures and progressive degenerative joint disease observed in patients with CACP. In this study, the absence of lubricin in the disc was associated with the finding of a relatively thinner annulus fibrosus. A recent study by Waller et al. found increased chondrocyte apoptosis in cartilage bearings lubricated by saline (Waller et al., 2013) compared to solutions containing lubricin. It is possible that the absence of lubricin in the intervertebral discs of Prg4 null subjects results in progressive tissue damage and loss of tissue volume of the annulus fibrosus. Regarding these measurements, it is worth noting that in the Prg4 +/+ specimens, measurements to estimate the volume of the nucleus pulposus produced a mean area ratio of 0.23, which is close to the assumption for this area of 0.2 which was reported by Elliott and Sarver (Elliott and Sarver, 2004). The mean area ratio in Prg4 −/− animals was found to be 0.49 in this study. In addition, while mean body weights have not been found to be significantly different between the PRG4 null and WT controlled populations, disc height and transverse major diameter were also found to be signficantly smaller in PRG4 null subjects.
An important limitation of our study is the small size of the disc specimens, which restricts our ability to analyze the annulus fibrosus. This testing protocol also looked only at the properties of the disc in axial rotation after removal of the posterior elements, which serve as stabilizers and restraints on rotational motion, in vivo. Additional studies are needed in order to characterize the full impact of PRG4 deficiency on lumbar range of motion and flexibility. Another limitation is the lack of an antibody to localize the expression of lubricin within different regions of the mouse intervertebral disc. Future directions for this work include histologic analysis of human intervertebral disc specimens in degenerative disc disease to determine if decreased or altered expression of lubricin is a feature of lumbar disc disease.
Supplementary Material
Supplemental Figure: Online Appendix
Acknowledgments
Funding for this project was received from the National Institutes of Health P20-GM104937 (COBRE Bioengineering Core); NIAMS grants R21-AR055937, R01-AR050180; and NIH T32-AR055885-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: G.D.J. has authored patents on lubricin and PRG4. Patent numbers are USPTO#6743774, 6960562, and 7001881. Other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ateshian GA, Hung CT. Patellofemoral joint biomechanics and tissue engineering. Clinical orthopaedics and related research. 2005:81–90. doi: 10.1097/01.blo.0000171542.53342.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis and rheumatism. 1998;41:730–735. doi: 10.1002/1529-0131(199804)41:4<730::AID-ART22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29:713–722. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis and rheumatism. 2007;56:108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- Faivre L, Prieur AM, Le Merrer M, Hayem F, Penet C, Woo P, Hofer M, Dagoneau N, Sermet I, Munnich A, Cormier-Daire V. Clinical variability and genetic homogeneity of the camptodactyly-arthropathy-coxa vara-pericarditis syndrome. American journal of medical genetics. 2000;95:233–236. doi: 10.1002/1096-8628(20001127)95:3<233::aid-ajmg9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Martin SD, Schmid TM, Spector M. Distribution of lubricin in the ruptured human rotator cuff and biceps tendon: a pilot study. Clinical orthopaedics and related research. 2010;468:1588–1599. doi: 10.1007/s11999-009-1108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. Journal of anatomy. 2012;221:480–496. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuvers J, Thoreson AR, Zhao C, Zhang L, Jay GD, An KN, Warman ML, Amadio PC. The mechanical properties of tail tendon fascicles from lubricin knockout, wild type and heterozygous mice. Journal of structural biology. 2011;176:41–45. doi: 10.1016/j.jsb.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, Carpten JD. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. The Journal of clinical investigation. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine KM, Simson JA, Spector M. Lubricin distribution in the human intervertebral disc. The Journal of bone and joint surgery. 2009;91:2205–2212. doi: 10.2106/JBJS.H.01344. [DOI] [PubMed] [Google Scholar]
- Shine KM, Spector M. The presence and distribution of lubricin in the caprine intervertebral disc. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2008;26:1398–1406. doi: 10.1002/jor.20614. [DOI] [PubMed] [Google Scholar]
- Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connective tissue research. 2006;47:215–221. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5852–5857. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Cheriyan T, Martin SD, Gomoll AH, Schmid TM, Spector M. Lubricin distribution in the torn human anterior cruciate ligament and meniscus. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29:1916–1922. doi: 10.1002/jor.21473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Online Appendix