Abstract
The potential toxicity of background exposure to perfluoroalkyl substances (PFASs) is currently under active investigation. Such investigations typically rely on a single measure of PFAS concentration, yet the longer-term reliability of a single measure has not been well characterized, especially among reproductive-aged women. Our aim was to investigate the association between PFAS plasma concentrations of 100 women in two consecutive pregnancies and explore changes in plasma concentration related to reproductive factors. The women in our study were enrolled in the Norwegian Mother and Child Cohort Study (MoBa) from 2003 to 2009. About half of them breastfed exclusively for 6 months and the rest of the participants did not breastfeed between the two consecutive pregnancies (median time between pregnancies: 18 months). Maternal blood was collected at mid-pregnancy and plasma was analyzed for 10 PFASs. Statistical analyses were restricted to 6 PFASs that were quantifiable in more than 80% of the samples. We estimated the correlation between repeated PFAS measurements, the percentage change between pregnancies and the effect of several reproductive factors in multivariate linear regression models of PFAS concentration in the second pregnancy. The Pearson correlation coefficient between repeated PFAS measurements was, for perfluorooctane sulfonate (PFOS), 0.80; perfluorooctanoate (PFOA), 0.50; perfluorohexane sulfonate (PFHxS), 0.74; perfluorononanoate (PFNA), 0.39; perfluoroundecanoate (PFUnDA), 0.71; and perfluorodecanoate (PFDA), 0.60. Adjustment for maternal age, delivery year, and time and breastfeeding between pregnancies did not substantially affect the observed correlations. We found 44-47% median reductions in the concentrations of PFOS, PFOA and PFHxS between pregnancies, while the change in concentrations between pregnancies was smaller and more variable for PFNA, PFUnDA and PFDA. The variation in plasma concentrations in the second pregnancy was mainly accounted for by the concentration in the first pregnancy; for PFOS, PFOA, and PFNA, breastfeeding also accounted for a substantial proportion. In conclusion, we found the reliability of PFAS measurements in maternal plasma to be moderate to high, and in these data, several factors, especially breastfeeding, were related to plasma concentrations.
Keywords: Pregnancy, Perfluoroalkyl substances, PFOS, Correlations, MoBa
1. Introduction
Perfluoroalkyl substances (PFASs) are synthetic fluorinated organic compounds used in industrial and consumer products over the last 50 years due to their chemical and thermal stability and water and oil repellency (Buck et al., 2011). Human exposure can occur via ambient indoor air, house dust and drinking water, though the main route is through food (Fromme et al., 2009; Haug et al., 2011; Vestergren and Cousins 2009). Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) are usually the most prevalent PFASs in human blood in background-exposed populations, are highly persistent, and have half-lives (geometric means) estimated to be from 3.5 (PFOA) to 4.8 (PFOS) years ( Olsen et al., 2007). For PFOS and PFOA, after the 2000s, human exposure declined, , as a result of national and international regulations and voluntary actions to phase-out or reduce production of these compounds. Meanwhile, increasing trends have been observed for some other PFASs (Glynn et al., 2012; Haug et al., 2009b; Kato et al., 2011).
In addition to exposure, elimination from the human body is another important determinant of PFAS concentration in plasma or serum. Women have lower plasma levels of PFOS and PFOA than men and shorter elimination half-lives due to loss through menstruation (Calafat et al., 2007; Wong et al., 2014). By comparing maternal blood, cord blood, and breast milk samples studies have shown that PFASs can cross the placenta and partition into milk; hence pregnancy and breastfeeding are additional elimination pathways for women (Fromme et al., 2010; Glynn et al., 2012; Gutzkow et al., 2012). PFOS and PFOA levels are lower in pregnant women than non-pregnant women and the levels decrease across trimesters, suggesting that trans-placental transfer starts from early gestation; other physiological changes during pregnancy also contribute to this trend (Jain 2013; Javins et al., 2013; Morken et al., 2014).
In addition to the temporal trends in exposure to PFASs, parity and breastfeeding, other maternal characteristics have been found to be related to maternal PFAS levels, such as income, education, residence, ethnicity, body mass index, smoking status and diet (Brantsaeter et al., 2013; Halldorsson et al., 2008). A better understanding of how various factors, especially reproductive events, affect plasma concentration of PFAS may help improve epidemiologic study design and interpretation (Whitworth et al., 2012).
The potential for health effects from exposure to PFAS is under active investigation in various settings including occupational exposure, communities with above-average exposure, and background-exposed populations (Barry et al., 2013; Geiger et al., 2014; Raleigh et al.,2014; Steenland et al., 2013; Uhl et al., 2013; Winquist and Steenland 2014). Many of the epidemiological findings on PFAS exposure and disease are affected by the long-term reliability of a single measurement of plasma or serum PFASs concentration. The reliability of a single measure of PFASs and the factors affecting it can be important for planning studies and interpretation. The correlations between repeated measurements of PFASs at different times in pregnancy have been reported (Fei et al., 2007; Glynn et al., 2012), but the correlations between repeated measurements of PFASs over a longer period have been examined in only one study, and all the subjects were male (Nost et al., 2014).
The aim of our study was to investigate the reliability of PFAS concentrations across pregnancies and determinants of change in PFAS concentrations between pregnancies.
2. Material and Methods
2.1 Study population
Our study population included 100 women from the Norwegian Mother Child cohort study (MoBa) who were enrolled during two consecutive pregnancies. In brief, the MoBa study is a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health (Magnus et al., 2006; Ronningen et al., 2006). Pregnant women from all over Norway were recruited from 1999-2008 at 17-18 weeks of pregnancy. The cohort now includes 114,500 children, 95,200 mothers and 75,200 fathers. Data used in this study are based on version 4.301 of the quality-ensured data files, released for research in June 2010. The study was approved by The Regional Committee for Medical Research Ethics in South-Eastern Norway; 40.6% of invited women consented to participate.
Approximately 15,000 women were enrolled in MoBa for more than one pregnancy. From these, we selected women who had a mid-pregnancy plasma (weeks 17-18) specimen available for two consecutive pregnancies whose first MoBa pregnancy was in 2003 or later, when blood was drawn into EDTA tubes, yielding plasma that was preferred for the laboratory assay (heparin was used before 2003). We further restricted the eligible women to those who had not moved in the past three years before the second pregnancy, so as to focus on those for whom exposure would have been more constant over time. This left 97 women who did not breastfeed between the two pregnancies and 4770 who breastfed exclusively for at least 6 months between the two pregnancies. In order to assure that the effect of breastfeeding would be well characterized, our study population was chosen so that about 50 women were selected at random from the 97 who did not breastfed, and about 50 were selected at random from the 4770 who breastfed exclusively for at least 6 months between the two pregnancies.
2.2 PFAS measurements in maternal blood
Maternal non-fasting blood samples were collected in EDTA tubes at hospitals and maternity clinics at the time of study enrollment and shipped at ambient temperature to the MoBa biorepository in Oslo. Most samples were received and processed the day after collection (Ronningen et al., 2006). At the biorepository, plasma was separated, aliquoted, and stored at −80 degrees Celsius. Changes in PFAS concentrations in transit are believed to be negligible, as PFASs are chemically stable (Fromel and Knepper, 2010), and a recent study showed no evidence of change over time in concentrations of four PFASs in serum maintained at room temperature for 10 days (Kato et al., 2013). Additionally, the ratio of PFAS concentrations in plasma to whole blood is consistently just above 2 (Ehresman et al., 2007; Hanssen et al., 2013), indicating that PFASs do not partition into blood cells or associate with their membranes. Thus, in non-hemolyzed specimens we have no reason to be concerned about shipping effects. The differences in PFASs concentrations between non-hemolyzed and hemolyzed samples (n=22), however, were further examined. Concentrations (in ng/mL) of ten PFASs were measured in maternal plasma by high-performance liquid chromatography/tandem mass spectrometry at the Norwegian Institute of Public Health. Details of the analytic process have been published previously (Haug et al., 2009a). The measured PFASs were perfluorohexane sulfonate (PFHxS), perfluoroheptane sulfonate (PFHpS), perfluorooctane sulfonate (PFOS), , perfluoroheptanoate (PFHpA), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA) and perfluorotridecanoate (PFTrDA). For quantification of PFOS, the total area of linear and branched isomers was integrated. Statistical analyses were limited to six PFASs (PFOS, PFOA, PFHxS, PFNA, PFUnDA, and PFDA) that were quantifiable in more than 80% of the samples. The limit of quantification (LOQ) was 0.05 ng/mL for all estimated PFASs. For values below the LOQ we used the measured concentrations when a signal was observed in the instrument during the analysis (Analytical Methods 2001). Missing values were cases where no signal was observed and these values were replaced with LOQ/√2 (5 values for PFHxS, 8 for PFUnDA, and 25 for PFDA). A total of 25 quality assurance/quality control (QA/QC) plasma samples from a single pool were analyzed in batches alongside the specimens of our study participants. The laboratory technicians were blinded to their identity, and the QA/QC samples were indistinguishable from the plasma samples of the study participants. The inter-assay coefficient of variation was 11.4 for PFOS, 8.6 for PFOA, 14.6 for PFHxS, 13.3 for PFNA, 22.2 for PFUnDA, and 27.0 for PFDA (Starling et al., 2014a).
2.3 Maternal characteristics
Information on maternal and pregnancy related characteristics that have been identified as important determinants of PFAS concentrations in maternal plasma were collected (Brantsaeter et al., 2013). Thus, data on maternal age and education, pre-pregnancy body mass index (BMI), parity, and year of delivery were obtained for both pregnancies. Additionally, the time between the two consecutive pregnancies (months) was calculated. After birth, mothers were asked to report their breastfeeding practices. We retrieved information specifically for duration and exclusivity of breastfeeding between the 2 consecutive pregnancies until the age of 18 months. Information on frequency of seafood consumption was also retrieved.
Plasma creatinine was also analyzed in maternal blood in both pregnancies with an Olympus AU400e Clinical Chemistry Analyzer (Olympus America, Inc.,Irvin, TX, USA), using reagents from Beckman Coulter Inc. (Irving, TX, USA) at the National Institute of Environmental Health Sciences laboratory in Durham, NC, USA. Maternal glomerular filtration rate (GFR) was calculated for each woman in each pregnancy, based on the formula of Cockroft-Gault (GFR= (140–age) × weight(kg) × 0.85/(72 × serum creatinine (mg/dl)) (Morken et al., 2014). The average GFR of the two pregnancies was used in the models described below.
2.4 Statistical analysis
Maternal demographic, lifestyle, and pregnancy related characteristics of our study population as well as PFAS concentrations in the two pregnancies were summarized with standard descriptive statistics. Further, the percentage change of PFAS concentrations in maternal blood from the 1st to the 2nd pregnancy was calculated as: 100*[(PFAS levels in 2nd pregnancy/PFAS levels in 1st pregnancy)-1] and shown in boxplots. All PFASs failed the Shapiro-Wilk test of normality and the natural logarithm of the values was used in subsequent analyses.
As a measure of reliability, we estimated the Pearson's correlation coefficients for the ln PFAS concentrations in samples from the same women in the two pregnancies and we presented the associations between repeated PFAS measurements in scatter plots. Additionally, we performed partial correlation analysis of PFAS concentrations in two pregnancies, by adjusting for time (months) and breastfeeding for at least 6 months (yes/no) between pregnancies. Maternal age (years) and year at the 2nd pregnancy were also included to account for possible age-specific bioaccumulation effects and changes in temporal trends in PFASs exposure. The partial correlation coefficient is like a Pearson coefficient, but adjusted for the variation in the second measurement due to the included maternal and pregnancy-related factors. The contribution of each parameter to the 2nd pregnancy PFASs variability was also calculated as the squared partial correlation multiplied by 100 and is referred to as explained variation.
We also performed multiple linear regression analysis to estimate the relative change in PFAS concentrations of the 2nd pregnancy according to maternal characteristics, including GFR (in ml/min) and pre-pregnancy BMI (<25kg/m2, 25-29.9kg/m2, ≥30 kg/m2). We estimated the relative change in the PFAS concentrations of the 2nd pregnancy per 10% increase in the corresponding PFASs compound concentration in the 1st pregnancy as: (1.10(beta) −1)*100. The relative change in the PFAS plasma concentrations in the 2nd pregnancy per unit(s) increase for other parameters was calculated as: (exp(beta)−1)*100. The association between breastfeeding and PFASs concentrations in the 2nd pregnancy was examined as a categorical variable with two categories (no breastfeeding / breastfeeding for at least 6 months) and as a categorical variable with three categories (no breastfeeding / breastfeeding for at least 10 months/ breastfeeding for more than 10 months). Additionally, women were sub-categorized according to exclusivity of breastfeeding (exclusive breastfeeding at 6-8 months for duration of at least 10 months: 16 of 24 women; for duration of more than 10 months: 21 of 27 women). Finally, we tested whether fatty fish, lean fish, fish liver or shellfish consumption (in times/week) was related to PFASs concentrations in the 2nd pregnancy for 76 women who reported their fish intake.
3. Results
The women in our study were enrolled with their 1st pregnancy from 2003-2007 and their 2nd pregnancy from 2005 to 2009 (Table 1). The median time between the 1st delivery and the conception of the 2nd pregnancy was 18 months. Eighty percent did not have previous pregnancies. According to our study inclusion criteria, about half of the women had breastfed their first child exclusively for at least 6 months. Most of the 51 women reported breastfeeding for at least 6 months continued breastfeeding for over 10 months (53%). Women who breastfed were more likely to be older and of higher education compared to women who did not breastfed (data not shown).
Table 1.
Characteristics of the 100 women with two consecutive pregnancies a.
| 1st pregnancy | 2nd pregnancy | |||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Maternal age (years) | ||||
| ≤30 | 58 | (58) | 40 | (40) |
| >30 | 42 | (42) | 60 | (60) |
| Pre-pregnancy BMI (kg/m2) | ||||
| Normal (<25 ) | 67 | (70) | 61 | (65) |
| Overweight (25.0-29.9 ) | 18 | (19) | 20 | (21) |
| Obese (≥30 ) | 11 | (11) | 13 | (14) |
| Maternal education (years) | ||||
| ≤12 | 33 | (34) | 30 | (31) |
| 13-16 | 36 | (38) | 39 | (41) |
| ≥17 | 27 | (28) | 27 | (28) |
| Year of delivery | ||||
| 2002-2003 | 8 | (8) | 0 | (0) |
| 2004-2005 | 57 | (57) | 5 | (5) |
| 2006-2007 | 35 | (35) | 50 | (50) |
| 2008-2009 | 0 | (0) | 45 | (45) |
| Time between pregnancies b | ||||
| <1 year (3-11 months) | 23 | (23) | ||
| 1-2 years (12-23 months) | 55 | (55) | ||
| ≥3 years (26-53 months) | 22 | (22) | ||
| Breastfeeding between pregnancies c | ||||
| Yes | 51 | (51) | ||
| No | 49 | (49) | ||
| Number of previous children d | ||||
| 0 | 80 | (80) | ||
| 1 or more | 20 | (20) | ||
Numbers do not total to 100 for BMI and education because of missing data.
Time between pregnancies is the time between the first delivery and the conception of the second pregnancy.
Breastfeeding refers to exclusive breastfeeding for at least 6 months. No breastfeeding refers to women who did not initiate breastfeeding.
Number of children born before the 1st included pregnancy
3.1 Maternal PFAS concentrations in two consecutive pregnancies
PFOS, PFOA and PFNA were measured in all samples (Table 2). For PFHxS, PFUnDA and PFDA the percentage of samples below LOQ varied from 2 to 36, and we observed an increase in their percentage in the 2nd pregnancy compared with the 1st pregnancy. This increase was statistically significant only for PFHxS and PFUnDA (p-value for chi-square test<0.05). The maternal concentrations in the 2nd pregnancy were statistically significantly lower than the 1st pregnancy for all PFASs (p-value for Wilcoxon signed-rank test <0.05, data not shown).
Table 2.
Plasma levels of PFAS in the 100 pregnant women in two consecutive pregnancies.
| Plasma concentrations in 1st pregnancy (in ng/mL) | Plasma concentrations in 2nd pregnancy (in ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| <LOQ* | Median | IQR | P95 | <LOQ* | Median | IQR | P95 | |
| PFOS | 0 | 13.59 | 7.61 | 27.36 | 0 | 7.05 | 5.26 | 15.94 |
| PFOA | 0 | 2.57 | 1.19 | 4.15 | 0 | 1.15 | 0.91 | 2.81 |
| PFHxS | 2 | 0.58 | 0.44 | 1.64 | 11 | 0.31 | 0.30 | 1.03 |
| PFNA | 0 | 0.47 | 0.25 | 0.81 | 0 | 0.38 | 0.31 | 0.89 |
| PFUnDA | 12 | 0.18 | 0.19 | 0.50 | 24 | 0.19 | 0.19 | 0.46 |
| PFDA | 24 | 0.14 | 0.11 | 0.32 | 36 | 0.10 | 0.10 | 0.35 |
Values can be read as percent or as number of subjects.
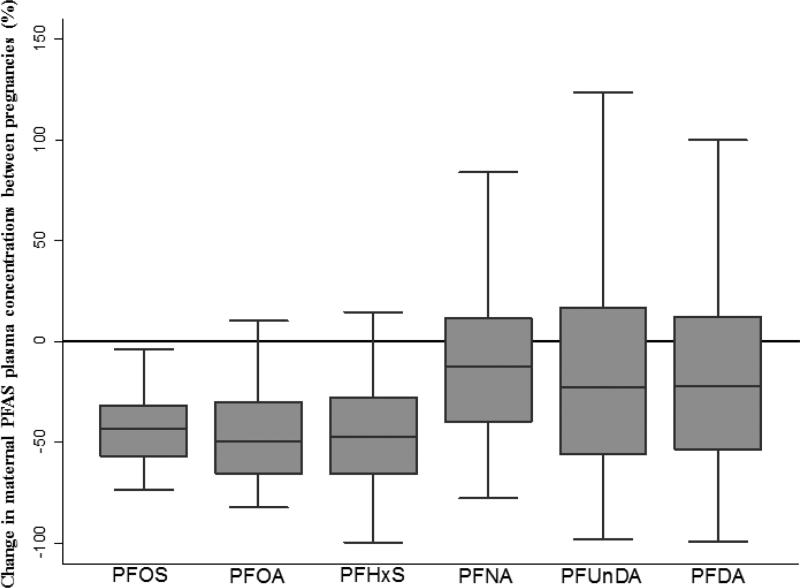
PFOS, PFOA and PFHxS plasma levels were lower in the 2nd than the 1st pregnancy for almost all women (Figure 1). However, for approximately 30 women we observed an increase of the PFNA, PFUnDA and PFDA concentrations from the 1st to the 2nd pregnancy. Notably, for six women we observed more than a 150% increase in PFNA, PFUnDA and PFDA in the 2nd pregnancy. Four of these women had a 3- to 70-fold increase for more than one compound, which suggests a common source of exposure.
Figure 1.
Percentage change in maternal PFAS plasma concentrations between consecutive pregnancies of 100 women. Values below 0 represent reduced levels in the 2nd pregnancy compared to the 1st and values above 0 represent increased levels in the 2nd pregnancy compared to the 1st. Starting from the bottom, the horizontal lines of each boxplot represent the 5th, 25th, 50th, 75th and 95th percentiles of each distribution.
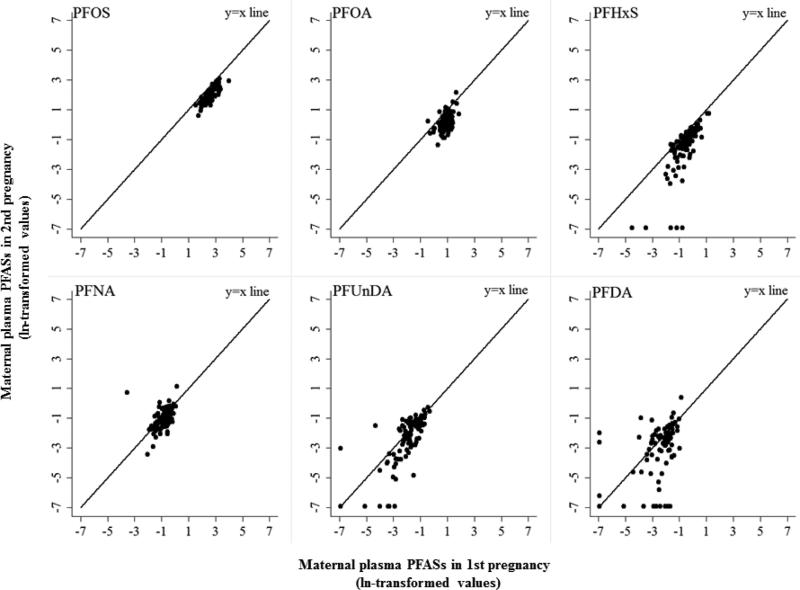
Positive and statistically significant correlations were observed between the two measurements of the corresponding compound for all PFASs, with correlations ranging from 0.39 for PFNA to 0.80 for PFOS (Table 3, and Figure 2). The partial correlation coefficients for PFOS, PFHxS, PFDA and PFUnDA were not substantially different from the crude correlations. For PFOA and PFNA the partial correlations were slightly stronger.
Table 3.
Pearson's and partial correlation coefficients between PFASs concentrations in maternal plasma of 100 women in two consecutive pregnancies.
| Plasma concentrations of PFASs in 2 consecutive pregnancies | Pearson's correlation coefficient | Partial correlation coefficient (independent of the adjusted factors) |
|---|---|---|
| PFOS | 0.80a | 0.84a |
| PFOA | 0.50a | 0.59a |
| PFHxS | 0.74 a | 0.73 a |
| PFNA | 0.39 a | 0.47 a |
| PFUnDA | 0.71 a | 0.67 a |
| PFDA | 0.60 a | 0.61 a |
The natural logarithm of PFASs concentrations is used to approximately fit a normal distribution.
Partial correlation coefficients are adjusted for maternal age at 2nd delivery, year of 2nd delivery, time between the 2 pregnancies (months), breastfeeding between pregnancies (exclusively for at least 6 months/no breastfeeding).
p-value<0.001
Figure 2.
Scatter plots of maternal PFASs in two consecutive pregnancies.
3.2 Determinants of maternal PFAS concentrations in the 2nd pregnancy
The PFAS concentrations measured in the 1st pregnancy were the main determinants for all PFASs in the 2nd pregnancy, after adjustment for other important factors (Table 4, Supplemental Table 1). Namely, a 10% increase of the respective PFAS concentration in the 1st pregnancy was significantly associated with an increase in the 2nd pregnancy of 9.0% for PFOS, 7.2% for PFOA, 14.2% for PFHxS, 6.1% for PFNA, 9.8% for PFUnDA, and 6.9% for PFDA. Regarding the year of the 2nd delivery, maternal PFOA concentrations declined by 8.7% for every year increase. On the other hand, for every year increase in maternal age at the 2nd delivery we observed a 1.5% significant increase in maternal PFOS concentrations. Breastfeeding exclusively between pregnancies was negatively associated with all PFAS concentrations in the 2nd pregnancy, while the associations were significant for PFOS, PFOA and PFNA. Notably, women who breastfed exclusively for at least 6 months between pregnancies had 40.9% lower PFOA levels compared to women who did not breastfed, independently of their PFOA levels in the 1st pregnancy. Breastfeeding between pregnancies accounted for almost as much variation in PFOA in the 2nd pregnancy (29%) as did the PFOA concentration in the 1st pregnancy (34%) (Supplemental Table 1). With categorization of breastfeeders into those breastfeeding 7-10 months or more than 10 months there was no statistically significant improvement in model fit (Supplemental Table 2). With women further categorized by both duration and exclusivity of breastfeeding, there again was no statistically significant improvement in model fit, except for PFOA. For PFOA, the breastfeeding-associated decrease was unexpectedly greater among those partially vs. exclusively breastfeeding. This finding was based on six women who breastfed > 10 months and were partial breastfeeders; due to the findings being based on a small number of subjects, the result are not shown.
Table 4.
Determinants of plasma concentrations (ng/ml) of PFASs in 2nd pregnancy of 100 women.
| Percent change in PFASs plasma concentrations in 2nd pregnancy per unit increase in determinants | ||||||
|---|---|---|---|---|---|---|
| PFOS (95%CI) | PFOA (95%CI) | PFHxS (95%CI) | PFNA (95%CI) | PFUnDA (95%CI) | PFDA (95%CI) | |
| Corresponding compound at 1st pregnancy (per 10%) | 9.0 (7.8,10.2) | 7.2 (5.1,9.3) | 14.2 (11.3,17.1) | 6.1 (3.7,8.5) | 9.8 (7.5,12.1) | 6.9 (5.0,8.9) |
| Time between pregnancies (per year) a | −5.0 (−11.6,1.9) | 12.4 (0.1,26.2) | 22.3 (−8.6,63.5) | 31.4 (10.6,56.1) | −3.7 (−30.1,32.7) | 75.4 (15.0,167.4) |
| Year of 2nd delivery (per year) | −5.0 (−9.9,0.2) | −8.7 (−16.2,−0.5) | −13.0 (−30.2,8.6) | −0.3 (−12.1,13.1) | −4.0 (−24.2,21.6) | 0.6 (−26.3,37.3) |
| Maternal age at 2nd delivery (per year) | 1.5 (0.3,2.7) | 0.1 (−1.9,2.1) | −0.8 (−5.6,4.3) | 2.3 (−0.7,5.3) | 1.1 (−4.7,7.3) | 6.1 (−1.3,14.2) |
| Breastfeeding between pregnancies b | ||||||
| No | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | −24.6 (−31.8,−16.7) | −40.9 (−50.1,−30.0) | −31.4 (−55.0,4.5) | −27.8 (−43.5,−7.6) | −16.2 (−47.7,34.3) | −30.5 (−62.2,27.7) |
Abbreviation: 95% CI: 95% Confidence Intervals.
All coefficients for a given compound are mutually adjusted.
Percent change in the PFASs plasma concentrations of the 2nd pregnancy per 10% increase in the corresponding PFASs compound concentration in the 1st pregnancy is calculated as: (1.10(beta) –1)*100.
Percent change in the PFASs plasma concentrations of the 2nd pregnancy per unit(s) increase in maternal age at 2nd pregnancy, year of 2nd delivery, time between the 2 pregnancies and breastfeeding between pregnancies is calculated as: (exp(beta)–1)*100.
Time between pregnancies is the time between the first delivery and the conception of the second pregnancy.
Breastfeeding refers to exclusive breastfeeding for at least 6 months. No breastfeeding refers to women who did not initiate breastfeeding.
Time between pregnancies was an especially important determinant for PFNA and PFDA; namely, we observed a statistically significant 31% and 75% increase for every year increase in the time between pregnancies. Time between pregnancies accounted for 10% of the variation in PFNA and 7% of the variation in PFDA.
Maternal Glomerular Filtration Rate (GFR) was a small but statistically significant predictor of PFUnDA concentration for the 86 women with available information. For every 1 ml/min increase in GFR we found a 0.40% (95% Confidence Interval (CI): −0.79, −0.01) reduction in PFUnDA levels in the 2nd pregnancy (not shown in tables). Maternal pre-pregnancy BMI of the 2nd pregnancy was also a significant predictor of PFUnDA concentrations for the 86 women (also not shown). Compared with women with normal pre-pregnancy BMI, women who were overweight before the 2nd pregnancy had 38% (95% Confidence Interval (CI): −62, 2) lower PFUnDA levels and obese women had 63% (95% CI: −80, −30) lower PFUnDA levels. When both GFR and pre-pregnancy BMI were included in the model, only pre-pregnancy BMI was a significant predictor, with obese women having a 59% reduction in PFUnDA (95% CI: −80, −17). Neither GFR nor pre-pregnancy BMI were statistically significant predictors for the other PFASs and for consistency reasons these were not included in the models presented in Table 4.
Regarding the effect of hemolysis in PFASs concentrations, in the 200 blood samples we found a statistically significant lower PFUnDA concentration in 22 hemolyzed samples (t-test p-value<0.05). Therefore, we examined the potential effect of hemolysis on PFUnDA concentrations in the 2nd pregnancy in the multivariate model and no statistically significant effect was observed. Additionally, after adjusting for hemolysis, the partial correlation coefficient was not affected (data not shown).
In these data, intake of shellfish but not other fish was an important predictor of PFUnDA – for the other PFASs no association was seen in the multivariate models. Compared with no shellfish consumption, among those consuming shellfish one or more times per week, the percent change in PFUnDA was 231 (95% CI 87, 485), and the other coefficients for predictors of PFUnDA shown in table 4 were essentially the same when shellfish was in the model (not shown).
The number of previous children and maternal education at the 2nd pregnancy were not significantly associated with any of the measured PFASs in the 2nd pregnancy and were excluded from the final models.
4. Discussion
In our study of maternal PFAS plasma concentrations in two consecutive pregnancies, the PFASs in the two pregnancies were positively and moderately to highly correlated, and adjustment for reproductive factors had little effect on the correlations. Additionally, the PFAS concentrations of the previous pregnancy were in general the main determinants of maternal PFASs in the next pregnancy, while breastfeeding, time between pregnancies, and shellfish intake were also important determinants for selected congeners.
The degree of correlation between the repeated PFAS measures (reliability coefficients, Table 3) corresponds closely with the half-lives of the compounds. Using the half-lives: PFOS, 6 years; PFOA 1.8; PFHxS, 7.1; PFNA, 1.5; PFUnDA, 4.4; PFDA, 4.2) (Zhang et al., 2013) the Spearman correlation coefficient between the reliability coefficients and half-lives is 0.94 (p value < 0.01). This high correlation is expected given that for most of the PFAS, the time between pregnancies (median: 18 months) was less than a half-life. PFOA and PFNA, compared with the other PFASs studied, have relatively short half-lives and also had the lowest crude correlation between serial measures. These two compounds also showed the greatest increase in correlation with adjustment for determinants in the second pregnancy.
Another determinant of PFASs reliability is the between-subject variability (Bland and Altman 1996), which can be assessed by the interquartile range (IQR) of the concentrations. Among PFASs that have similar half-lives, the PFAS with the higher IQR had the higher reliability. For example, for PFOS and PFHxS, PFOS had a higher IQR and a higher reliability. For PFOA and PFNA, PFOA had a higher IQR and a higher reliability.
The determinants of reliability, mentioned above, were somewhat different than the determinants of change in concentration between pregnancies. For example, though the median decrease in PFOS between pregnancies was 44%, serial measurements for a subject tended to correlate well because the variation of the decrease was relatively small (Figure 1). In contrast, the median decrease between pregnancies for PFNA was only 10%, the variation of the change was much higher, and the reliability was much lower. Decreasing exposure may have accounted for the tendency for lower levels of PFOS, PFOA, and PFHxS in the second pregnancy (Johansson et al. 2014).
4.1 Correlations and changes in maternal PFASs between pregnancies
We found moderate to high positive correlations between repeated PFAS measurements in two pregnancies. Our findings suggest that a single PFASs measurement may represent the relative level of internal exposure reasonably well over a period of several years in reproductive-aged women, especially for compounds with longer half-lives. . The correlations between repeated PFAS measurements reported by Nøst et al., in Norwegian men over a period of 7 years were generally similar to our findings in Norwegian pregnant women (Table 5). As expected, our correlations were a bit lower, probably due to the effect of the reproductive factors on PFAS levels. In line with both Glynn et al., and Nøst et al., we found the lowest correlations for PFNA (Glynn et al., 2012; Nost et al., 2014).
Table 5.
Correlations between repeated measurements of PFASs in adult men reported by Nøst et al., and in pregnant women in this study.
| Spearman's correlation coefficient between repeated PFASs measurements | ||
|---|---|---|
| N=49 Northern Norwegian men between 2001-2007 (Nøst et al.) | N=100 Norwegian pregnant women between 2003-2009 (this study) | |
| PFOS | 0.81 | 0.81 |
| PFOA | 0.75 | 0.49 |
| PFHxS | 0.81 | 0.76 |
| PFNA | 0.60 | 0.53 |
| PFUnDA | 0.79 | 0.73 |
| PFDA | 0.71 | 0.54 |
* The Spearman's correlations coefficients are based on crude data.
Maternal plasma concentrations of PFOS, PFOA and PFHxS were lower than in previous studies with women recruited in the MoBa study and lower than in the NHANES 2003-2004 survey (Starling et al., 2014a; Starling et al., 2014b; Woodruff et al., 2011). On the other hand, maternal PFASs were comparable to another Norwegian study with a similar blood collection period (Berg et al., 2014).
Having previous pregnancies is a predictor of lower PFAS concentrations in maternal blood, due to the trans-placental transfer of PFASs and elimination via breastfeeding (Beesoon et al., 2011). We estimated a crude average reduction of 44% in both PFOS and PFOA and 47% in PFHxS, while the adjusted changes, estimated with the predicted PFAS levels of the 2nd pregnancy as derived from the multivariate models, were similar (PFOS= −45%, PFOA= −48%, PFHxS= −57%). A previous study in northern Norwegian pregnant women found similar effect estimates on PFAS levels in relation to having one previous pregnancy (PFOS= −30%, PFOA= −45%, PFHxS= −37%) (Berg et al., 2014). Within the MoBa study and similar to our results, Brantsaeter et al. found 46% average reduction of PFOS concentrations for multiparous compared to nulliparous women, while for PFOA and PFHxS the estimates were very different from ours (70% and 19%, respectively) (Brantsaeter et al., 2013).
Overall, we observed a large variation in the change between pregnancies for PFNA, PFUnDA and PFDA compared to PFOS, PFOA and PFHxS. After adjusting for important determinants and using the predicted PFAS levels in the 2nd pregnancy to calculate the change between pregnancies, the variation was substantially smaller for PFUnDA, but not for PFNA and PFDA (Supplemental Figure 1). The overall decreasing trends of PFOS, PFOA and PFHxS might explain the smaller variability. For PFNA and PFDA the increasing environmental trends and the large observed variation suggest ongoing high exposure for some women and lower exposure for others. The large variation of PFNA and PFDA after adjustment for important predictors can be attributed to unknown factors or an unknown source of exposure.
In our study, obese women had 63% lower PFUnDA levels in the 2nd pregnancy compared to women of normal BMI. Interestingly, in a study by Bosma et al., BMI was positively associated with GFR (Bosma et al., 2004). We found that GFR was negatively associated only with PFUnDA levels, thus the negative association between PFUnDA and maternal BMI might be partly explained by the increased GFR in obese women. When we adjusted the PFUnDA-BMI coefficient for GFR, it decreased slightly (from 63% to 59%), which is consistent with a small portion of the association being mediated by GFR. PFASs are not lipophilic, and PFASs distribute primarily to the liver, kidneys, and blood (Fabrega et al., 2014). Since PFASs are primarily in compartments other than the adipose tissue, a positive association with BMI or change in BMI is not expected. Our finding that PFUnDA was lower in obese women compared to women with normal BMI, was similar to Berg et al., (4% reduction per unit of BMI increase) (Berg et al., 2014). A negative association between BMI and PFOS and PFOA has also been reported in women (Holzer et al., 2008), and in men (Eriksen et al., 2011). However, Brantsaeter et al., found positive relationships between BMI and PFASs in pregnant women, especially PFHxS (2% increase per unit of BMI increase) (Brantsaeter et al., 2013). In the NHANES 2003-2008 survey of 5,500 adults, BMI was positively associated with PFOA and negatively associated with PFNA (Jain 2014). Thus, overall the relationship between PFASs and BMI is not clear.
Nevertheless, it has been reported that the partition coefficient (Kow, for octanol-water) for PFUnDA is higher than that for the other PFASs we studied, thus some PFUnDA could be partitioning into the lipid compartment (Jing et al., 2009; Kim et al., 2015). However in these data, changes in BMI were unrelated to PFAS concentration in the second pregnancy and the change in BMI, when added to the model shown in table 4, did not predict PFAS concentration (data not shown).
4.2 Breastfeeding
We found that the reduction associated with exclusive breastfeeding for at least 6 months was 41% for PFOA, 25% for PFOS and 28% for PFNA, independently of the previous PFAS levels. The relatively higher transport efficiency of PFOA through lactation has been reported before by comparing the ratio of concentrations in breast milk to maternal serum (Liu et al., 2011). Additionally, Thomsen et al., reported a 46% depuration of PFOA and 18% for PFOS after 6 months of breastfeeding by analyzing repeated breast milk samples from Norwegian mothers, and these findings are comparable to our observed reductions on maternal concentrations attributed to breastfeeding (Thomsen et al., 2010). In two previous studies that used a multivariate model, the estimated reduction in maternal PFAS concentrations associated with breastfeeding was lower than in our study (Brantsaeter et al., 2013; Mondal et al., 2014). Nevertheless, our results might not be directly comparable to those in other studies due to differences in the definition of the breastfeeding variable (at least 6 months of exclusive breastfeeding vs. per month of breastfeeding). Another factor contributing to differences in results is that in typical population-based studies the multivariate models have limited potential to fully adjust for the effect of parity and breastfeeding because these determinants of PFAS concentrations are usually highly correlated. In our study, with mostly nulliparous women and an equal prevalence of breastfeeding and no breastfeeding, we were better able to disentangle the effect of breastfeeding from that of parity.
4.3 Temporal trends
As time between the two pregnancies increased (per year) we observed a 31% and 75% rise in PFNA and PFDA concentrations in the 2nd pregnancy, independently of other important predictors. A previous study also reported a positive association between maternal PFNA and time since the last pregnancy (Brantsaeter et al., 2013).
A 1-7% increase per year for PFNA, PFDA and PFUnDA in blood from 1996 to 2010 has been reported for Swedish women (Glynn et al., 2012) and Northern Norwegian men (Nost et al., 2014). The production and use of PFNA and PFDA continues, and their concentrations in the environment are increasing, and thus exposure continues and the blood levels are increasing (Wang et al., 2014). We also found a negative association between PFOS, PFOA and PFHxS with the year of delivery, while statistically significant only for PFOA. Such associations have been reported before and the decreasing temporal trends of PFOA in the environment can explain this negative association (Berg et al., 2014; Glynn et al., 2012; Haug et al., 2009b). On the other hand, we observed a small positive association between maternal age and PFOS in the 2nd pregnancy, independently of breastfeeding and previous PFOS levels. This finding might be due to higher early-life exposures for older mothers and the relatively long half-life of PFOS. Age has been positively associated with PFOS independently of other important factors in the general American population (Kato et al., 2011).
PFAS will continue to be found in the general population for a long time, and vigilance is required to assure that any new alternative agents that are used are recognized and studied, as needed (Lindstrom et al., 2011; Scheringer et al., 2014).
4.4 Seafood consumption
Shellfish consumption has been identified previously as an important determinant of PFUnDA concentrations in human blood both in Norway (Brantsaeter et al., 2013; Haug et al., 2010) and Korea (Ji et al., 2012). Data on PFUnDA in shellfish, however, appear to be limited at this time (Domingo et al., 2012; Haug et al., 2010).
4.5 Strengths and limitations
The main strength of our study is the repeated measurements of maternal PFASs in two consecutive pregnancies. To our knowledge there is no other study with serial measures of PFAS, taken several years apart, in reproductive-aged women. The adjusted reliability coefficients shown in Table 5 indicate that in a model with PFAS concentration as an independent variable and a continuous outcome the observed beta coefficient for PFAS will be 47 to 84 percent as large as the true coefficient, meaning substantial to moderate bias towards the null (White et al., 2008). Similarly, in a model with a categorical outcome, such as in a logistic regression, an odds ratio of two would be observed as 1.4 or 1.8. Limitations of our study were the small sample size and possibly limited generalizability of results. The characteristics of Norwegian women who do not breastfeed are slightly different than most women thus the determinants of PFAS concentrations in that group may not apply to all women. An additional limitation is the short period between the two blood sampling points. In addition, for PFDA, where 30% of observations were below the LOQ, our ability to assess reliability and identify important determinants might have been hindered by not having a well-quantitated or measured value. Lastly, although the participation rate in the MoBa study was relatively low, the attributes of the MoBa participants closely resembled those of the underlying population (Nilsen et al., 2009) and we have no reason to believe that the reliability of PFAS measurements would be affected by unmeasured correlates of participation.
5. Conclusions
The reliability of plasma PFAS concentrations among women of reproductive age, estimated as the correlation between repeated measurements between pregnancies, varied by specific PFASs and was not substantially affected by adjustment for reproductive factors. Nonetheless, the timing of previous pregnancies, breastfeeding, and temporal trends in exposure can have substantial effects on PFAS concentrations. Our findings suggest that a single PFASs measurement may represent the relative level of internal exposure reasonably well over a period of several years in women of reproductive age, especially for compounds with longer half-lives.
Supplementary Material
Highlights.
PFAS concentrations in consecutive pregnancies were moderately to highly correlated.
PFASs in the 1st pregnancy were the main determinants of PFASs in 2nd pregnancy.
Breastfeeding for 6 months between pregnancies was related to a 41% decrease in PFOA.
PFOA, PFNA, and PFDA levels increased with time between pregnancies.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no. N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01, grant no.2 UO1 NS 047537-06A1), and the Norwegian Research Council/FUGE (grant no. 151918/S10). We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval: The study was approved by The Regional Committee for Medical Research Ethics in South-Eastern Norway.
References
- Analytical Methods, C. Measurement of near zero concentration: recording and reporting results that fall close to or below the detection limit. The Analyst. 2001;126:256–259. doi: 10.1039/b009590g. [DOI] [PubMed] [Google Scholar]
- Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environmental health perspectives. 2013;121:1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S, Webster GM, Shoeib M, Harner T, Benskin JP, Martin JW. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: manufacturing sources and transplacental transfer. Environmental health perspectives. 2011;119:1659–1664. doi: 10.1289/ehp.1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevag OM, Odland JO, Sandanger TM. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environment international. 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measurement error and correlation coefficients. BMJ. 1996;313:41–42. doi: 10.1136/bmj.313.7048.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G. Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney international. 2004;65:259–265. doi: 10.1111/j.1523-1755.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Brantsaeter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbo M, Longnecker MP. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. EnvironInt. 2013;54:74–84. doi: 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integrated environmental assessment and management. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environmental health perspectives. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL, Ericson-Jogsten I, Perello G, Nadal M, Van Bavel B, Karrman A. Human exposure to perfluorinated compounds in Catalonia, Spain: contribution of drinking water and fish and shellfish. Journal of agricultural and food chemistry. 2012;60:4408–4415. doi: 10.1021/jf300355c. [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang SC, Butenhoff JL. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environmental research. 2007;103:176–184. doi: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Eriksen KT, Sorensen M, McLaughlin JK, Tjonneland A, Overvad K, Raaschou-Nielsen O. Determinants of plasma PFOA and PFOS levels among 652 Danish men. Environmental science & technology. 2011;45:8137–8143. doi: 10.1021/es100626h. [DOI] [PubMed] [Google Scholar]
- Fabrega F, Kumar V, Schuhmacher M, Domingo JL, Nadal M. PBPK modeling for PFOS and PFOA: validation with human experimental data. Toxicology letters. 2014;230:244–251. doi: 10.1016/j.toxlet.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environmental health perspectives. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromel T, Knepper TP. Biodegradation of fluorinated alkyl substances. Reviews of environmental contamination and toxicology. 2010;208:161–177. doi: 10.1007/978-1-4419-6880-7_3. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environmental science & technology. 2010;44:7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. International journal of hygiene and environmental health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Geiger SD, Xiao J, Ducatman A, Frisbee S, Innes K, Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere. 2014;98:78–83. doi: 10.1016/j.chemosphere.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996-2010. Environmental science & technology. 2012;46:9071–9079. doi: 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- Gutzkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. Placental transfer of perfluorinated compounds is selective--a Norwegian Mother and Child sub-cohort study. International journal of hygiene and environmental health. 2012;215:216–219. doi: 10.1016/j.ijheh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Hanssen L, Dudarev AA, Huber S, Odland JO, Nieboer E, Sandanger TM. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. The Science of the total environment. 2013;447:430–437. doi: 10.1016/j.scitotenv.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environmental science & technology. 2008;42:8971–8977. doi: 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C. Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. EnvironInt. 2011;37:687–693. doi: 10.1016/j.envint.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK. Diet and particularly seafood are major sources of perfluorinated compounds in humans. EnvironInt. 2010;36:772–778. doi: 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. Journal of chromatography A. 2009a;1216:385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environmental science & technology. 2009b;43:2131–2136. doi: 10.1021/es802827u. [DOI] [PubMed] [Google Scholar]
- Holzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, Kleeschulte P, Marschall N, Wilhelm M. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environmental health perspectives. 2008;116:651–657. doi: 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RB. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17-39 years: data from National Health and Nutrition Examination Survey 2003-2008. Journal of toxicology and environmental health Part A. 2013;76:409–421. doi: 10.1080/15287394.2013.771547. [DOI] [PubMed] [Google Scholar]
- Javins B, Hobbs G, Ducatman AM, Pilkerton C, Tacker D, Knox SS. Circulating maternal perfluoroalkyl substances during pregnancy in the C8 Health Study. Environmental science & technology. 2013;47:1606–1613. doi: 10.1021/es3028082. [DOI] [PubMed] [Google Scholar]
- Ji K, Kim S, Kho Y, Sakong J, Paek D, Choi K. Major perfluoroalkyl acid (PFAA) concentrations and influence of food consumption among the general population of Daegu, Korea. The Science of the total environment. 2012;438:42–48. doi: 10.1016/j.scitotenv.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Jing P, Rodgers PJ, Amemiya S. High lipophilicity of perfluoroalkyl carboxylate and sulfonate: implications for their membrane permeability. Journal of the American Chemical Society. 2009;131:2290–2296. doi: 10.1021/ja807961s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JH, Berger U, Vestergren R, Cousins IT, Bignert A, Glynn A, Darnerud PO. Temporal trends (1999-2010) of perfluoroalkyl acids in commonly consumed food items. Environmental pollution. 2014;188:102–108. doi: 10.1016/j.envpol.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environmental science & technology. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Basden BJ, Calafat AM. Effect of temperature and duration of storage on the stability of polyfluoroalkyl chemicals in human serum. Chemosphere. 2013;91:115–117. doi: 10.1016/j.chemosphere.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Li LY, Grace JR, Yue C. Selecting reliable physicochemical properties of perfluoroalkyl and polyfluoroalkyl substances (PFASs) based on molecular descriptors. Environmental pollution. 2015;196:462–472. doi: 10.1016/j.envpol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environmental science & technology. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, Wu Y. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environment international. 2011;37:1206–1212. doi: 10.1016/j.envint.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). IntJEpidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, Fletcher T. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environmental health perspectives. 2014;122:187–192. doi: 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morken NH, Travlos GS, Wilson RE, Eggesbo M, Longnecker MP. Maternal glomerular filtration rate in pregnancy and fetal size. PloS one. 2014;9:e101897. doi: 10.1371/journal.pone.0101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. PaediatrPerinatEpidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Nost TH, Vestergren R, Berg V, Nieboer E, Odland JO, Sandanger TM. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environment international. 2014;67:43–53. doi: 10.1016/j.envint.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh KK, Alexander BH, Olsen GW, Ramachandran G, Morey SZ, Church TR, Logan PW, Scott LL, Allen EM. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occupational and environmental medicine. 2014;71:500–506. doi: 10.1136/oemed-2014-102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. EurJEpidemiol. 2006;21:619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheringer M, Trier X, Cousins IT, de Voogt P, Fletcher T, Wang Z, Webster TF. Helsingor statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere. 2014;114:337–339. doi: 10.1016/j.chemosphere.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Richardson DB, Baird DD, Haug LS, Stuebe AM, Klungsoyr K, Harmon Q, Becher G, Thomsen C, Sabaredzovic A, Eggesbo M, Hoppin JA, Travlos GS, Wilson RE, Trogstad LI, Magnus P, Longnecker MP. Perfluoroalkyl Substances During Pregnancy and Validated Preeclampsia Among Nulliparous Women in the Norwegian Mother and Child Cohort Study. American journal of epidemiology. 2014a doi: 10.1093/aje/kwt432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbo M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD, Longnecker MP. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environment international. 2014b;62:104–112. doi: 10.1016/j.envint.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Zhao L, Winquist A, Parks C. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environmental health perspectives. 2013;121:900–905. doi: 10.1289/ehp.1206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C, Haug LS, Stigum H, Froshaug M, Broadwell SL, Becher G. Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environmental science & technology. 2010;44:9550–9556. doi: 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- Uhl SA, James-Todd T, Bell ML. Association of Osteoarthritis with Perfluorooctanoate and Perfluorooctane Sulfonate in NHANES 2003-2008. Environmental health perspectives. 2013;121:447–452. 452e441–443. doi: 10.1289/ehp.1205673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environmental science & technology. 2009;43:5565–5575. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Buck RC, Hungerbuhler K. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environment international. 2014;70:62–75. doi: 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- White E, Armstrong B, Saracci R. Principles of Exposure Measurement in Epidemiology Collecting, Evaluating, and Improving Measures of Disease Risk Factors. 2nd edition Oxford University Press; 2008. Exposure measurement error and its effects. pp. 76–78.pp. 103 [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Longnecker MP. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012;23:257–263. doi: 10.1097/EDE.0b013e31823b5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology. 2014;25:255–264. doi: 10.1097/EDE.0000000000000040. [DOI] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environmental science & technology. 2014;48:8807–8814. doi: 10.1021/es500796y. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. EnvironHealth Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environmental science & technology. 2013;47:10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.