Abstract
Ticks are obligate blood-feeders and serve as vectors of human and livestock pathogens worldwide. Defining the tick microbiome and deciphering the interactions between the tick and its symbiotic bacteria in the context of tick development and pathogen transmission, will likely reveal new insights and spawn new paradigms to control tick-borne diseases. Descriptive observations on the tick microbiome that began almost a century ago serve as forerunners to the gathering momentum to define the tick microbiome in greater detail. This review will focus on the current efforts to address the microbiomes of diverse ticks, and the evolving understanding of tick microbiomes. There is hope that these efforts will bring a holistic understanding of pathogen transmission by ticks.
Keywords: tick endosymbionts, tick microbiome
Tick microbiome: old players hold new hope
The collection of commensal, symbiotic, and pathogenic microorganisms that occupy various niches of our body is called the microbiome - a term coined originally by Joshua Lederberg [1]. Our perception of the microbiome has undergone a radical change in the last decade or so, humbled by the understanding that our phenome is really shaped by our microbiome [2]. The major focus of the microbiome is currently on its Eubacterial members, but the microbiome is also composed of Archaea, virus, and eukaryotic microbes such as protozoa, nematodes and fungi and their interactions both within and across kingdoms might additionally modulate human health [3]. While the lack of standard marker genes and reference database makes it more laborious to define the identities of the non-bacterial members of the microbiome at this juncture, it is expected that rapidly evolving molecular techniques will help to realize this understanding.
All metazoans have partnered with small or large consortia of microbes to enhance health and survival on this planet. Arthropods are no exception and the literature is rich with examples of various arthropod-microbiota associations that modulate essential aspects of the arthropod life cycle including reproductive fitness, survival and vectorial competence [4-6]. Arthropods vector human, livestock and plant pathogens worldwide and pose a tremendous health and economic burden [7, 8]. It is anticipated that understanding the arthropod microbiome in the context of arthropod survival, and pathogen transmission might spur a new generation of arthropod and arthropod-borne pathogen control strategies. This review will focus on ticks that vector human and livestock pathogens worldwide [9] and summarize our current understanding of the microbiome of ticks. Throughout this review we will use the term ‘microbiome’ to indicate the bacterial members of the microbial consortia and emphasis will be on bacteria that are not established vertebrate pathogens.
Ticks: vectors of mammalian pathogens
Ticks belong to the order parasitiformes and are divided into four families, Nuttalliellidea and Laelaptidae (that each comprises of one single species), Ixodidae or hard tick (that includes about 700 species) and Argasidae or soft tick (that includes about 200 species) [10, 11]. Ticks are distributed across the world from tropics to subarctic regions, with greatest species diversity in the tropics and subtropics [11]. Several of these serve as vectors of pathogens with up to 28 species transmitting human pathogens worldwide [11]. A detailed geographic distribution of the species and their role in pathogen transmission to humans and livestock are published [9, 12] (www.ct.gov/caes). The list of pathogens transmitted by ticks to mammalian hosts is increasing [13-15] in part to climatic changes [16] and there is an urgency to develop new strategies to control ticks, prevent infection prevalence and impair tick-borne pathogen transmission. Ticks are obligate blood-feeders; they have three life stages (larvae, nymphs and adults) and while hard ticks require one blood meal at each stage for development, soft ticks require multiple blood meals at each stage [11]. The tick stages thus have ‘on-host’ and ‘off-host’ phases in their life stages and off-host phases often impose inhospitable conditions of temperature and humidity. Intertwined in this rather challenging life style is the pathogen that cycles between the tick and the vertebrate host. The pathogen enters the tick gut when the larval tick takes a blood meal on an infected vertebrate host and colonizes the tick gut as in the case of Borrelia burgdorferi, the agent of Lyme disease [17]; exits the gut and infects the tick salivary glands as in the case of Anaplasma phagocytophilum that causes anaplasmosis [18]; or Borrelia hermsii, the causative agent of tick-borne relapsing fever [19]. Once the larval tick is infected, the pathogen is maintained through subsequent developmental stages (nymph and adult) of the tick, and the tick essentially remains infected for life [20]. Unlike soft ticks that feed multiple times in a given developmental stage and thus have the potential to transmit and acquire harbored pathogens to and from multiple hosts in each developmental stage, hard ticks feed only once at each developmental stage thus have only a limited opportunity to transmit and acquire pathogens in each stage. Additionally, success of pathogen transmission and acquisition at each stage are likely influenced significantly by the health of the host. Further, the molting success of each developmental stage, and the maintenance of the pathogen during molting would determine the continuing cycle of vector-pathogen burden in the enzootic cycle. Molting is a complex process involving tissue autolysis and regeneration [20, 21]. How the pathogen is maintained in the tick through the developmental stages and how it evades tick immune responses during colonization of the gut, and migration to salivary glands remains to be fully understood. There is an increasing interest in determining whether members of the tick microbiome play a key role in these events that are central to vectorial competence.
Intracellular endosymbionts of ticks
The associations of ticks with non-pathogenic bacteria was recognized definitively by Cowdry at the beginning of the 20th century [22] who showed, using light microscopy, that a wide variety of ticks (at least 16 species of ticks, including both hard and soft ticks) harbored Gram-negative Rickettsia-like bacteria in various tissues including eggs, ovaries, malphigian tubules and intestinal epithelial cells of unfed larvae, with slight differences in the morphology of the bacteria among the tick species. Cowdry also noted that, in some instances, the morphology of the bacteria correlated with tick species, presciently suggesting the occurrence of tick-specific microbiomes. Interestingly, Cowdry also noticed different species of bacteria within the same cell that did not associate with each other, but tended to form distinct clumps [22] and their presence did not appear to have any deleterious effects on the cells. These early findings have been corroborated since, and several tick bacterial endosymbionts with commensal, mutualistic or parasitic interactions have been identified [23-26]. Noda et al. [24] exploited bacterial species-specific 16S ribosomal DNA (rDNA) primers and showed that only bacteria closely related to Rickettsia rickettsii of the spotted fever group, members of the class α-Proteobacteria, were associated with the ovaries in Ixodes scapularis. While PCR amplicons specific to Rickettsia species were detected in all I. scapularis larvae examined, only 50 % of the nymphal stage retained the rickettsial bacteria suggesting that the endosymbiont was either cleared or diminished in male nymphs during molting. The pathogenic potential of these rickettsial endosymbionts remains unknown [27]. Rickettsial endosymbionts are thought to alter tick physiology and transmission of other rickettsial pathogens as seen by the inverse relationship between the infection prevalence of R. rickettsii (pathogenic) and Rickettsia peacockii (symbiotic) in Dermacentor andersoni [28, 29]. These provocative observations allude to the possibility that the presence of specific endosymbionts might modulate the vectorial capacity of the tick. Field and lab studies focused on deciphering these correlations would doubtless help define “biomarkers” of infection prevalence and transmission in endemic areas, and will be one of the goals of this field (Box 1). Noda et al. [24] also showed using 16S rDNA amplicon sequencing that in Ornithodoros moubata, Rhipicephalus sanguineus, and Hemaphysalis longicornis the bacterial symbiont found in ovaries and malphigian tubules were closely related to Coxiella burnetii, a mammalian pathogen. In addition, O. moubata ovaries and malphigian tubules also harbored an endosymbiont closely related to Francisella tularensis, a mammalian pathogen [30] of the class γ-Proteobacteria. This was consistent with an earlier study [31] that observed two kinds of bacteria in the ovaries and malphigian tubules of O. moubata. Francisella-like endosymbionts have also been identified in several Dermacentor species [26]. While these bacteria are transovarially transmitted and potentially obligate endosymbionts, the functional consequence of these on the tick vector is not fully understood. Zhong et al. [32] showed, using antibiotics to cure the tick of endosymbionts, that Coxiella endosymbionts of Amblyomma americanum were likely critical for the survival and fitness of the tick. Further, the presence of the Coxiella endosymbionts in the salivary glands of A. americanum was suggested to impair the transmission of horizontally acquired pathogens such as Ehrlichia chaffiensis [33]. Phylogenetic analysis of the Coxiella species isolated from different ticks showed distinct phylogenetic clades of Coxiella spp., with each clade specific to the tick species regardless of the geographic location of the tick [29].
Box 1. Outstanding questions.
What is the origin of the tick microbiome?
What external factors shape the tick microbiome composition?
Can we define specific tick endosymbionts that correlate directly or inversely with infection and transmission of specific pathogens?
Answers to these nested questions are pivotal to transform our mechanistic understanding of the tick microbiome into strategies to control ticks and tick-transmitted pathogens.
In Ixodes ricinus, bacteria were observed in the mitochondria of ovaries [34]. These bacteria of the class α-Proteobacteria named Candidatus Midichloria mitochondrii [35] entered the mitochondria of cells lining the oocysts and propagated within the inner and outer membranes of the mitochondria and apparently consumed the organelle [25]. About 94 -100 % of field-collected adult female I. ricinus were infected with this bacterium [34, 36], and showed a 100 % transovarial transmission rate. Despite the apparent parasitism, the oocysts developed normally in M. mitochondrii-infected ticks. The distribution, prevalence and transovarial transmission of this endosymbiont in I. ricinus suggested that this association might be obligate, and have a potential role in the fitness of the vector. Interestingly, in lab-raised colonies of I. ricinus, the prevalence of this mitochondrial endosymbiont was considerably decreased [36] suggesting that the advantage to the tick vector might be revealed only in a field setting.
Ticks of the genera Ixodes, Rhipicephalus, Amblyomma and Dermacentor [37], have also been shown to harbor related endosymbiotic bacteria that infect the ovarian mitochondria. More importantly, the endosymbiont thought to be restricted to the ovaries was also observed in the salivary glands of some I. ricinus ticks. Moreover, humans and animals bitten by these ticks were seropositive to the endosymbiont bacterial antigens [38, 39]. Further, Budachtri et al. [40] showed that Amblyomma maculatum were predominantly infected with Francisella, Wolbachia and rickettsial species and sequences corresponding to rickettsial outer membrane protein-encoding genes (ompA and ompB) were also observed in salivary glands suggesting that these endosymbionts had the potential for transmission to the vertebrate host. While most tick-borne endosymbionts were thought to be innocuous passengers [22, 41], these observations question that presumption. Another endosymbiont frequently identified in hard ticks including I. ricinus [42], Amblyomma and Dermacentor species is Arsenophonus-like symbionts [43, 44]. Arsenophonus spp. belong to the γ-Proteobacteria class, are widely distributed in insects [45] and presumed to be involved in sex-ratio distortion [46]. Recent studies also demonstrate the presence of Wolbachia spp. in I. ricinus [47], I. scapularis [48], and A. americanum [49].
Many vertically, potentially transovarially-transmitted endosymbionts of ticks are very similar to tick-transmitted pathogens (Coxiella-like, Rickettsia-like or Francisella-like) and suggest that the ancestral origin of these endosymbionts might have been vertebrate pathogens acquired by the tick while feeding on an infected host. These pathogens are suggested to have evolved along two lines, one that adapted specifically to the tick environment and became confined to tick tissues, and one that adapted to both tick and vertebrate host and became pathogenic [24]. We must also consider the possibility that endosymbionts might have evolved to become virulent mammalian pathogens [50]. Under conditions that remain to be understood, commensal endosymbionts might emerge as vertebrate pathogens. This would suggest that these apparently benign endosymbionts must really be regarded as potential pathobionts awaiting a molecular trigger to realize their pathogenic potential. The presence of commensal endosymbionts closely related to pathogenic bacteria has also been proposed to serve as a barrier to infections by related pathogenic bacteria [29, 51, 52]. Understanding the mechanisms by which endosymbionts might offer this privilege to the tick might reveal new strategies to control tick-transmitted pathogens.
Beyond the intracellular endosymbionts
Advancements in DNA and RNA sequencing platforms and data analysis tools [53, 54] have served as key drivers in our ability to realize the full depth of the composition of the tick microbiome in greater detail. The unfolding picture of the tick microbiome suggests that it is complex and is composed of more than a handful of intracellular symbionts. Sanger sequencing of full-length 16S rDNA clones, 454-pyrosequencing, Ion torrent, or Illumina based sequencing of 16S rDNA hypervariable regions as well as whole genome shot-gun approach in conjunction with a novel data analysis approach [55] have been utilized to define the microbiomes of various tick species. Focus, understandably, has been on the hard tick since a larger number of genera in this family (Ixodidae) transmit diverse pathogens to humans and live stock [9]. Different studies have used different hypervariable regions of the 16S rDNA [56] (V1-V3, V4, V5, or V6, regions) and examined different tick stages from diverse geographic locations in the world (Table 1). Further, while most studies have utilized whole ticks, only a handful has utilized specific tissues dissected from the ticks [40, 57-59]. The limitation of using whole ticks is that it does not allow one to define the tissue-specific microbiome [60] and this understanding might be pertinent to derive functional inferences in the context of the biology of the tick and its interactions with tick-borne pathogens. Also, when whole ticks are assessed, one cannot discern exoskeleton-associated environmental contaminants from bona-fide tick tissue-associated bacteria. Exoskeleton-associated bacteria should, however, not be dismissed for these might provide additional barrier surveillance strategies as seen with the microbiome of mammalian skin [61]. While a diverse variety of bacterial genera have been identified in each of these studies, observations potentially influenced by parameters specific to each study, some unifying patterns emerge. Across all genospecies of hard ticks, bacteria of the phylum Proteobacteria are predominant followed by Actinobacteria, Firmicutes and Bacteroidetes. Bacterial members of the phylum Acidobacteria, Cyanobacteria, Fusobacteria and TM7 are also observed at lower levels in some studies [40, 58]. Both aerobic and anaerobic bacteria were observed, and Gram-negative bacteria were predominant [48]. At the genus level, some members were representative of tick species regardless of their geographic locations -including Rickettsia and Coxiella in Amblyomma americanum [44, 62, 63] and Rhipicephalus species [64]; Rickettsia in almost all Ixodes species [48, 55, 58, 59, 65-68]; and Wolbachia in Ixodes ricinus [66, 67], Amblyomma maculatum and Rhipicephalus microplus [40, 57], suggesting that these might be obligate endosymbionts of tick species. Narasimhan et al. [58] and Moreno et al. [65] have examined I. scapularis nymphs (lab colonies and wild-caught ticks from Connecticut and New York, respectively) and despite the use of very different techniques to address the microbiome (454 pyrosequencing and temporal temperature gradient gel electrophoresis respectively) identified many common genera such as Stenotrophomonas, Sphingobacterium, Pseudomonas, and Acinetobacter in nymphal and adult stages. Importantly, Narasimhan et al. [58] utilized dissected gut tissues, while Moreno et al. [65] utilized whole ticks. Therefore, the identification of several common bacterial genera in both studies suggests that these bacteria are likely bona-fide tick gut residents. Overall, in addition to obligate intracellular endosymbionts, several bacterial genera are observed more frequently across several hard tick species and include Pseudomonas, Sphingobacterium, Acinetobacter, Eneterobacteria, and Stenotrophomonas. These might represent bacterial genera with a greater propensity to colonize tick species (Figure 1).
Table 1.
A brief overview of tick microbiomes assessed in the last ten years.a
| Genus/species & Reference cited | Target gene | Approach | Geographic location of ticks | Developmental stage | Tissue assessed | Ref |
|---|---|---|---|---|---|---|
| Ixodes ricinus | 16S | Sanger sequencing of DGGE fragments | Austria | Adults | Whole ticks | [60] |
| Ixodes scapularis | 16S | Sanger sequencing of clones | USA | Nymphs | Whole ticks | [47] |
| Ixodes scapularis | 16S | Sanger sequencing, TGGE | USA | Adult and nymphs | Whole ticks | [65] |
| Ixodes ricinus | 16S | Sanger sequencing of clones | Netherlands | Nymphs | Whole ticks | [67] |
| Amblyomma americanum | 16S | Sanger sequencing of clones | USA | Adults, larvae and eggs | Whole ticks | [44] |
| Rhipiciphalus microplus | 16S | 454 pyrosequencing | USA | Adults | Whole ticks, midguts, ovaries eggs | [57] |
| Ixodes ricinus | 16S | 454 pyrosequencing | Italy | Nymphs and adults | Whole ticks | [66] |
| Amblyomma americanum | 16S | Ion torrent | USA | Nymphs and adults | Whole ticks | [70] |
| Amblyomma americanum | 16S | 454 pyrosequencing | USA | Adults and nymphs | Whole ticks | [63] |
| Amblyomma, Ixodes, Haemaphysalisb | Whole genome | 454 pyrosequencing | Japan Netherlands; | Adults and nymphs | Whole ticks | [55] |
| Ixodes ricinus | Whole transcriptome | Illumina | France | Nymphs | Whole ticks | [68] |
| Ixodes scapularis | 16S | 454 pyrosequencing | USA | Larvae, and nymphs | Whole ticks and guts | [58] |
| Ixodes Haemaphysalisb | 16S | 454 pyrosequencing | Japan | Adults | Salivary glands | [59] |
| Amblyomma maculatum | 16S | 454 pyrosequencing | USA | Adult ticks | Guts, salivary glands, and saliva | [40] |
| Ixodes persulcatus | 16S | Illumina sequencing | China | Adult ticks | Flat and fed whole ticks | [72] |
Studies focused on specific bacterial genera in ticks are not listed here.
When several tick species were assessed in a single study, only the tick genus is listed.
Figure 1. Predominant bacterial members of the tick microbiome.
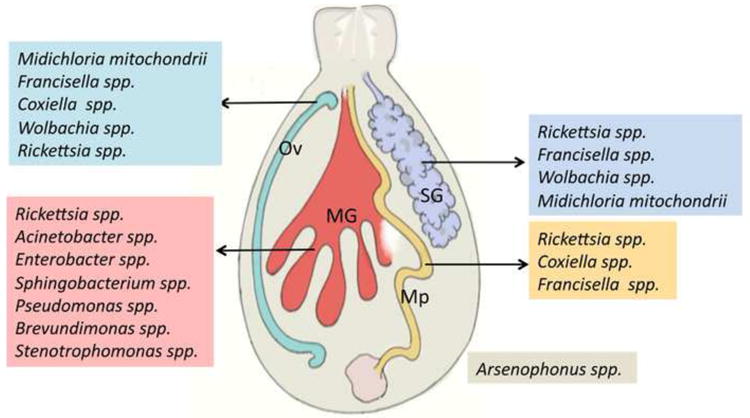
A schematic representation of bacterial genera frequently observed in the salivary glands (SG), midgut (MG), Ovary (Ov), and Malphigian tubules (Mp) of ticks. Arsenophonus species have been frequently identified in ticks, but the tissue distribution is not determined.
A non-traditional approach to the identification of tick-associated bacteria comes from a study by Villar et al [69]. Using a combination of transcriptomic analysis by paired-end RNA sequencing and proteomic analysis by reverse phase liquid chromatography coupled with tandem mass spectroscopy, this study assessed stress response genes and proteins in unfed Dermacentor reticulatus larvae. Interestingly, along with the increases in D. reticulatus stress response genes and proteins, the study also identified 16 transcripts and 14 proteins that corresponded to Rickettsia spp.[69]. Thus, in addition to genomic approaches, this approach offers an opportunity to define tick-associated pathogenic and commensal bacteria at the transcriptome and proteome level.
Laboratory and wild-caught ticks showed similarities as well as differences in microbiome composition, potentially due to environmental factors including temperature, light-dark cycles, host availability, and vegetation, as well as development stage and feeding/nutritional status of the tick [44, 65-67, 70, 71]. Several studies suggest that feeding increased the bacterial diversity of the tick microbiome [58, 65, 72]. In a study of the microbiome of Ixodes persulcatus from woodland areas of China [73], up to 200 genera in different stages of I. persulcatus were observed. When the blood of rats on which these ticks fed was assessed, several bacterial genera found in ticks were also found in rat blood, indicating that at least some of these bacterial members of the tick microbiome were also likely transmitted to the mammalian host [73]. It is likely that only some bacterial taxa are bona-fide members of the tick microbial consortium and many are environmental contaminants. We must bear in mind that increased sensitivity of the sequencing platforms comes with the pitfalls of identifying minor environmental contaminants. It will become important to adhere to protocols that would minimize errors both in DNA preparations and analysis [3, 74] and critical to outline experimental procedures that would facilitate the research community to glean and define core microbiomes representative of the tick species. Comparisons of the microbiome of field-collected ticks to that of lab-reared ticks might additionally serve to highlight core microbiota inherent in specific tick species, and changes in specific bacterial taxa and in proportions of core members between field and lab-reared ticks might provide the context to infer the functional roles of specific members. Age, tick stage, feeding status, rearing procedures of lab colonies, and geography, time of year and day of field-collected ticks, will all likely influence the microbiome profile and recording all co-variates would be more conducive to a unified understanding of the core microbiome of ticks.
The spatial organization
Several questions arise from these tick microbiome studies. Are the consortia of bacteria on the epithelial cell surface, are they intracellular, or are they in the lumen of the gut, and salivary glands? Of special interest is the gut microbiota, as the gut is the site of the first encounter between the tick and the incoming pathogen. Ticks have a unique mode of blood-meal digestion. The gut lumen is alkaline, and enzyme-mediated lysis of red blood cells and release of hemoglobin takes place in the gut lumen. Hemoglobin is taken up by receptors on digestive cells of the tick gut and broken down intracellularly by a series of hemoglobinases in the acidic environment (pH 3.5-4.5) of the digestive vesicles [75]. The lumen serves as a ‘holding place’ for the blood meal as the tick engorges and the uptake of nutrients commences during engorgement and proceeds through the molting process. Digestion products including heme are transported back into the lumen and defecated. So, if the gut lumen contains extracellular symbionts as observed in mammals [76], the resident bacteria must be capable of surviving the heme-filled lumen, the toxic reactive oxygen species (ROS) from neutrophils and macrophages and complement proteins and proteases in the bloodmeal of the host. Indeed, Budachetri et al. [40] observed that the guts of A. maculatum predominantly harbored Enterobacteria species, known to generate ROS and survive ROS-mediated killing [77]. As digestion proceeds through the molt, the lumen of the gut might become congested with digested peptides and debris and undergoes structural and functional changes [78]. The profiles of the gut bacteria, be it in the lumen or in the epithelial cells, must therefore undergo dramatic shifts in composition to take advantage of the changing milieu. Consistent with this assumption, Heise et al. [72], observed in A. americanum females that, upon feeding, proportions of Coxiella spp. decreased, Rickettsia spp. increased and genera not detectable in the unfed tick such as Pseudomonas increased. Do luminal bacteria come in contact with the epithelial cells of the gut, or are they separated by the peritrophic matrix (PM) that separates the lumen from the digestive cells of the gut [79]? A visible PM is shown to be formed around 6-12 hours after tick feeding [80], so are the luminal bacteria in brief contact with the epithelial cells during the pre-PM formation stage? If they are, then the immunome of the tick gut [81, 82] must respond to this contact and the resulting immune milieu must influence the ability of the incoming pathogens to colonize the tick. The microbiome composition might determine the potency of the immune response, potentially detrimental to incoming pathogens. Microbiome profiles might possibly serve as biomarkers of infection prevalence in ticks in endemic areas. The tick gut is also unique in that, in contrast to other structures of the tick that are formed de novo at each ontogenic stage, the gut epithelial cells do not undergo histolysis and are retained from the previous developmental stage [21]. This must have a critical impact both on the survival and stability of gut microbiota and gut colonizing pathogens such as B. burgdorferi and Babesia microti. Detailed ultrastructural examination of the tissue-specific tick microbiome at various stages of tick feeding and development will enhance our functional understanding of tick microbiome.
The functional role of tick microbiome
Understanding the functional consequence of tick–microbiome interactions is fundamental to developing new paradigms based on the microbiome to control ticks and tick-transmitted pathogens. Zhong et al. [32] showed that curing Amblyomma ticks of their endosymbiont resulted in prolonged time to oviposition, decreased hatching of the eggs, and decreased larval survival. In contrast, curing Ixodes pacificus of its rickettsial endosymbiont had no apparent impact on oviposition or tick survival [83]. In the study by Narasimhan et al. [58] the tick microbiota and predominantly the gut microbiota, was shown to play a role in maintaining the integrity of the PM. When ticks were treated with gentamicin or when ticks were raised in a sterile environment that did not allow a normal tick microbiome to develop, the integrity of the PM was compromised and B. burgdorferi colonization was impaired. Borrelia colonizes the gut by adhering tightly to the gut epithelial cells [84, 85] and is likely shielded from the deleterious elements of the gut lumen by the PM that forms at the apical side of the epithelial cell [80] during tick feeding. The PM thus served a non-canonical role by facilitating B. burgdorferi colonization of the tick gut [58], in contrast to the traditional barrier role to preclude pathogen entry suggested for the PM in Drosophila [86] and for the mucus layer in the mammalian gut [87]. Alterations in the microbiome composition might also result in differences in the levels of activation of immune response molecules such as Toll and immune deficiency (IMD) in the gut epithelium [88], and influence pathogen survival and infection (Figure 2). Effector molecules including reactive oxygen species generated upon immune activation maintain bacterial homeostasis, but also impose collateral damage on the gut epithelium [89]. At least in Drosophila, epithelial damage has been shown to signal the secretion of Unpaired3 Upd3, a cytokine-like molecule that activates the Janus kinase/Signal Transducer and Activator of Transcription JAK-STAT pathway [90]. Functional orthologs of Upd3-like molecules in the tick gut might be regulated as part of normal gut microbiota-tick interactions to ensure bacterial homeostasis and maintenance of a viable epithelial barrier offered by the PM (Figure 2). Tick-borne pathogens like B. burgdorferi might have evolved to exploit this normal friction that accompanies the tick-microbiota dialogue (Figure 2). It will be important to define the gut microbial profiles that favor or impair pathogen colonization, as shown for Plasmodium survival in the mosquito gut [77]. This understanding is paramount to fully exploit the microbiome to control ticks and tick-transmitted pathogens (Box 1) and remains one of many challenges that tick microbiome research faces (Box 2).
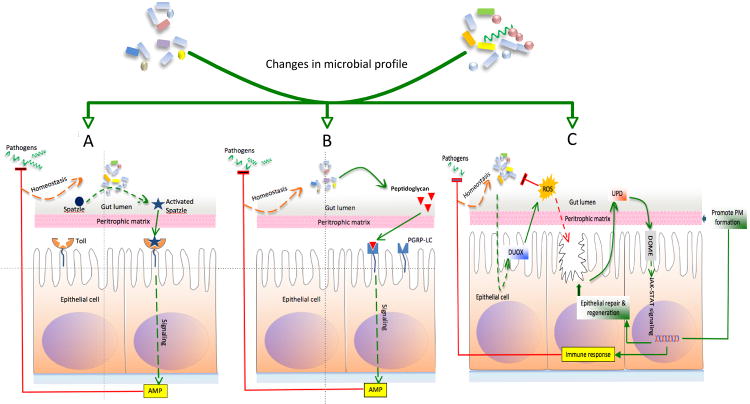
Figure 2. Changes in the microbial composition and the consequent immune responses in the tick gut.
Alterations in the gut microbiome associated with feeding, development and infection could modulate the following immune response pathways in ticks: A. Toll pathway. Microbiota-induced activation of Spatzle, enabling Spatzle-Toll interaction and initiation of the signaling cascade resulting in the production of antimicrobial peptides (AMPs). B. Immunodeficiency (IMD) pathway. Sensing of Gram-negative peptidoglycan (Peptidoglycan) by the peptidoglycan recognition protein (PGRP-LC) to activate the signaling cascade leading to AMP production. C. Janus kinase/Signal transducer and activator of transcription JAK-STAT pathway. Microbiota and pathogen-induced activation of Dual oxidase (DUOX) results in the reactive oxygen species (ROS) production to control bacteria. ROS-mediated collateral damage to the gut epithelial cells initiates the release of cytokine-like molecule Unpaired 3 Upd that engages with its receptor, DOME, a signal transducing transmembrane protein receptor, to activate the JAK-STAT signaling pathway. STAT transcriptionally regulates pathways leading to immune responses, epithelial regeneration and repair, and peritrophic membrane integrity. AMPs and immune responses generated by Toll, IMD and JAK-STAT pathways influence pathogen survival, and also facilitate bacterial homeostasis (Based on citations 58, 87, 88 and 89).
Box 2. Tick microbiome-challenges ahead.
Germ-free ticks
An understanding of the functional consequence of core microbial members of the microbial consortium would require that we generate ticks that harbor no bacteria - i.e., germ-free ticks. The ability to generate germ-free mice has contributed greatly to an overall understanding of how members of the microbial consortia might regulate host biology (1, 75, 86). Such an opportunity remains to be developed for ticks. Current strategies utilize antibiotics (32,58), but the introduction of antibiotics into ticks is tedious, transient and subject to potential side effects of the antibiotic. We would require germ-free facilities to hatch eggs, and feed the subsequent stages on germ-free mice. While the operational logistics is overwhelming, developing such a protocol would enable great strides in our understanding of tick-microbiome interactions.
Gnotobiotic ticks
Once germ-free ticks become available, the ability to generate gnotobiotic ticks, i.e., ticks that harbor one or more specific bacteria, would be the next goal. While it is possible to artificially feed ticks (references cited in 58) and potentially introduce specific bacteria into the tick, this requires further optimization to feed ticks to repletion. Essential requisites to achieve this goal are the ability to fully define tick microbiomes at the species level, and the ability to grow axenic cultures of the core bacteria. Integrated into these achievements would be the futuristic hope of paratransgenesis, i.e., the potential use of these core bacteria to deliver anti-tick or anti-pathogenic gene products.
Genetically engineered ticks
The ability to generate stable knockout and knock-in ticks is currently not available for tick research. RNA interference-mediated knockdown of gene expression (58) is handicapped by the transient nature of the silencing. As we begin to uncover molecular interactions between the tick and its microbiome, the ability to generate germ-line knockouts of specific tick genes would help better understand how tick gene products shape the tick microbiome.
Origin of the tick microbiome
In humans and mice, it has been shown that the maternal microbiota (in the birth canal) is inoculated into the offspring and lays the critical foundation for a healthy microbiome [91]. In addition to the transovarially-transmitted endosymbionts of ticks, it is conceivable that the mother's microbiota might serve as the first inoculum in eggs and the developing larvae. The observation by Narasimhan et al. [58] that larvae hatched in a sterile environment harbored a significantly different microbiome composition would suggest that I. scapularis larvae, at least partially, acquire their microbiota from the environment, including bacteria entering through openings such as the spiracles, oral or the anal pore. Copulation can also serve as an additional route to augment bacterial inoculation (paternal transmission) of the tick microbiome [92]. Ticks are obligate hematophagous arthropods and microbiota on the host skin might also contribute to the microbial diversity of the tick gut. Nevertheless, of all the diverse environmental microbiota that might gain access into the tick, only a few get to be bona-fide members of the tick. Since diet plays a central role in shaping the composition of the mammalian microbiome [93], it is likely that hematophagy might select for certain bacterial genera. Bacteria that can either supplement the deficiencies of mammalian blood, such as the tsetse endosymbiont that provides vitamin B to the tsetse fly [94] or those that encode functions to lyse red blood cells or metabolize nutrients in the blood might be selected for [95]. If diet were the only deciding factor, then we must observe a similar microbiome in all hematophagous arthropods. A dedicated meta-analysis might help address this possibility. Interestingly, the microbiome of Drosophila melanogaster, despite feeding on a variety of decaying matter with ample opportunity to harbor a complex microbiome, has a very low diversity and the core members are Acetobacter, Gluconobacter and Lactobacillus species [96]. In contrast, despite a restricted diet, the microbiome of the tick appears complex [55], suggesting that the arthropod taxonomy must additionally impose genetic constraints on who stays and who does not. Indeed, data supporting this possibility is presented by Hawlena et al. [97], who examined the microbiota of several vectors including Dermacentor variabilis and I. scapularis from about 200 rodents in southern Indiana. Meta sequence analysis revealed that Rickettsia phylotype 1 was always observed in I. scapularis, and Francisella phylotypes were seen only in D. variabilis. Understanding how the tick acquires its microbiota and how the microbiome composition is shaped by various environmental and genetic factors would be essential to begin to exploit the microbiota of the tick to control the prevalence of ticks and derail transmission of pathogens by ticks (Box 1).
Concluding remarks
Ticks have evolved over the last 5-65 million years to circumvent the many challenges they had to encounter [98]. A recent study [99] showed that I. scapularis has colluded with its microbial partners and borrowed the tae (Type VI amidase effector) genes, that encode enzymes to degrade cell walls of competing bacteria, in a trans-kingdom transfer event several million years ago and exploits it to control the growth of pathogens like B. burgdorferi. On the down side, the prevalence of antibiotic resistance genes encoded by several members of the tick microbiome [55] presents a potential opportunity for tick-borne pathogens to pilfer these genes by horizontal gene transfer [100] and result in the emergence of antibiotic-resistant tick-borne pathogens. It is therefore important to address and comprehend the ‘yin and yang’ of the tick microbiome - those that might confound pathogen transmission and those that might enhance pathogen transmission. As the tick microbiome research comes to center-stage poised with powerful molecular and technological advancements, it offers a new vantage point to understand the biology of the tick and its interactions with the microbes it harbors, and this really is the exciting promise of the tick microbiome.
Highlights.
Tick microbiome is predominantly composed of Gram-negative bacteria of the phylum Proteobacteria.
Intracellular bacterial endosymbionts modulate reproductive fitness and vectorial competence of ticks.
Bacterial endosymbionts stabilize the peritrophic matrix and maintain the epithelial barrier integrity of the gut of ticks.
Bacterial symbionts modulate the immune status of the gut and influence the vectorial competence of the tick.
Acknowledgments
We sincerely thank Dr. Madan. K. Anant for the illustrations. Research funding from NIAID/NIH and HHMI helped support some of our efforts highlighted in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 2.Kinross JM, et al. Gut microbiome-host interactions in health and disease. Genome medicine. 2011;3:14. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodrich JK, et al. Conducting a microbiome study. Cell. 2014;158:250–262. doi: 10.1016/j.cell.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends in parasitology. 2011;27:514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zug R, Hammerstein P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological reviews of the Cambridge Philosophical Society. 2015;90:89–111. doi: 10.1111/brv.12098. [DOI] [PubMed] [Google Scholar]
- 6.Dennison NJ, et al. The mosquito microbiota influences vector competence for human pathogens. Current opinion in insect science. 2014;3:6–13. doi: 10.1016/j.cois.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goddard J. Infectious Diseases and Arthropods. Humana Press; 2008. [Google Scholar]
- 8.Beugnet F, Marie JL. Emerging arthropod-borne diseases of companion animals in Europe. Veterinary parasitology. 2009;163:298–305. doi: 10.1016/j.vetpar.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Goodman JL, Dennis DT, Sonenshine DE. Tick-Borne Diseases of Humans. ASM Press; Washington, DC: 2005. [Google Scholar]
- 10.Vesco U, et al. An integrated database on ticks and tick-borne zoonoses in the tropics and subtropics with special reference to developing and emerging countries. Experimental & applied acarology. 2011;54:65–83. doi: 10.1007/s10493-010-9414-4. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JF, Magnarelli LA. Biology of ticks. Infectious disease clinics of North America. 2008;22:195–215. v. doi: 10.1016/j.idc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Sonenshine DE, Roe MR. Biology of ticks. Vol. 2. Oxford University Press; 2014. [Google Scholar]
- 13.Spolidorio MG, et al. Novel spotted fever group rickettsiosis, Brazil. Emerging infectious diseases. 2010;16:521–523. doi: 10.3201/eid1603.091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gugliotta JL, et al. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. The New England journal of medicine. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masters EJ, et al. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infectious disease clinics of North America. 2008;22:361–376. viii. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Gray JS, et al. Effects of climate change on ticks and tick-borne diseases in europe. Interdisciplinary perspectives on infectious diseases. 2009;2009:593232. doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radolf JD, et al. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kocan K, et al. Advances toward understanding the molecular biology of the Anaplasma tick interface. Frontiers in bioscience. 2008;13:7032–7045. doi: 10.2741/3208. [DOI] [PubMed] [Google Scholar]
- 19.Raffel SJ, et al. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS pathogens. 2014;10:e1004056. doi: 10.1371/journal.ppat.1004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonenshine DE. Biology of ticks. Vol. 1. Oxford University Press; 1991. [Google Scholar]
- 21.Balashov YS, Grigoryeva LA. Cytological changes in the midgut of tick females of the genus Ixodes during and after feeding. Doklady biological sciences : proceedings of the Academy of Sciences of the USSR, Biological sciences sections / translated from Russian. 2003;393:527–530. doi: 10.1023/b:dobs.0000010314.97335.2c. [DOI] [PubMed] [Google Scholar]
- 22.Cowdry EV. A Group of Microorganisms Transmitted Hereditarily in Ticks and Apparently Unassociated with Disease. The Journal of experimental medicine. 1925;41:817–830. doi: 10.1084/jem.41.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes SF, Burgdorfer W. Reactivation of Rickettsia rickettsii in Dermacentor andersoni ticks: an ultrastructural analysis. Infection and immunity. 1982;37:779–785. doi: 10.1128/iai.37.2.779-785.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noda H, et al. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Applied and environmental microbiology. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacchi L, et al. A symbiont of the tick Ixodes ricinus invades and consumes mitochondria in a mode similar to that of the parasitic bacterium Bdellovibrio bacteriovorus. Tissue & cell. 2004;36:43–53. doi: 10.1016/j.tice.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Scoles GA. Phylogenetic analysis of the Francisella-like endosymbionts of Dermacentor ticks. Journal of medical entomology. 2004;41:277–286. doi: 10.1603/0022-2585-41.3.277. [DOI] [PubMed] [Google Scholar]
- 27.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 28.Childs JE, Paddock CD. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? The American journal of tropical medicine and hygiene. 2002;66:450–457. doi: 10.4269/ajtmh.2002.66.450. [DOI] [PubMed] [Google Scholar]
- 29.Ahantarig A, et al. Hard ticks and their bacterial endosymbionts (or would be pathogens) Folia microbiologica. 2013;58:419–428. doi: 10.1007/s12223-013-0222-1. [DOI] [PubMed] [Google Scholar]
- 30.Burgdorfer W, Owen CR. Experimental studies on argasid ticks as possible vectors of tularemia. The Journal of infectious diseases. 1956;98:67–74. doi: 10.1093/infdis/98.1.67. [DOI] [PubMed] [Google Scholar]
- 31.Reinhardt C, et al. Distribution of Rickettsia-like microorganisms in various organs of an Ornithodorus moubata laboratory strain (Ixodoidea, Argasidae) as revealed by electron microscopy. Zeitschrift fur Parasitenkunde. 1972;39:201–209. doi: 10.1007/BF00329456. [DOI] [PubMed] [Google Scholar]
- 32.Zhong J, et al. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PloS one. 2007;2:e405. doi: 10.1371/journal.pone.0000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klyachko O, et al. Localization and visualization of a Coxiella-type symbiont within the lone star tick, Amblyomma americanum. Applied and environmental microbiology. 2007;73:6584–6594. doi: 10.1128/AEM.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beninati T, et al. A novel alpha-Proteobacterium resides in the mitochondria of ovarian cells of the tick Ixodes ricinus. Applied and environmental microbiology. 2004;70:2596–2602. doi: 10.1128/AEM.70.5.2596-2602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sassera D, et al. ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. International journal of systematic and evolutionary microbiology. 2006;56:2535–2540. doi: 10.1099/ijs.0.64386-0. [DOI] [PubMed] [Google Scholar]
- 36.Lo N, et al. Widespread distribution and high prevalence of an alpha-proteobacterial symbiont in the tick Ixodes ricinus. Environmental microbiology. 2006;8:1280–1287. doi: 10.1111/j.1462-2920.2006.01024.x. [DOI] [PubMed] [Google Scholar]
- 37.Epis S, et al. Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology. 2008;135:485–494. doi: 10.1017/S0031182007004052. [DOI] [PubMed] [Google Scholar]
- 38.Mariconti M, et al. Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: is Midichloria a novel pathogen, or just a marker of tick bite? Pathogens and global health. 2012;106:391–396. doi: 10.1179/2047773212Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazzocchi C, et al. Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales: Midichloriaceae) in different mammalian species. Parasites & vectors. 2013;6:350. doi: 10.1186/1756-3305-6-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budachetri K, et al. An insight into the microbiome of the Amblyomma maculatum (Acari: Ixodidae) Journal of medical entomology. 2014;51:119–129. doi: 10.1603/me12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgdorfer W, et al. Isolation and characterization of symbiotes from the Rocky Mountain wood tick, Dermacentor andersoni. Journal of invertebrate pathology. 1973;22:424–434. doi: 10.1016/0022-2011(73)90173-0. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian G, et al. Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks and tick-borne diseases. 2012;3:406–410. doi: 10.1016/j.ttbdis.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Dergousoff SJ, Chilton NB. Detection of a new Arsenophonus-type bacterium in Canadian populations of the Rocky Mountain wood tick, Dermacentor andersoni. Experimental & applied acarology. 2010;52:85–91. doi: 10.1007/s10493-010-9340-5. [DOI] [PubMed] [Google Scholar]
- 44.Clay K, et al. Microbial communities and interactions in the lone star tick, Amblyomma americanum. Molecular ecology. 2008;17:4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- 45.Novakova E, et al. Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC microbiology. 2009;9:143. doi: 10.1186/1471-2180-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor GP, et al. The host range of the male-killing symbiont Arsenophonus nasoniae in filth fly parasitioids. Journal of invertebrate pathology. 2011;106:371–379. doi: 10.1016/j.jip.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Hartelt K, et al. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia spp.), Wolbachia spp., Rickettsia spp., and Babesia spp. in Southern Germany. International journal of medical microbiology : IJMM. 2004;293(Suppl 37):86–92. doi: 10.1016/s1433-1128(04)80013-5. [DOI] [PubMed] [Google Scholar]
- 48.Benson MJ, et al. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Applied and environmental microbiology. 2004;70:616–620. doi: 10.1128/AEM.70.1.616-620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, et al. Distribution and molecular characterization of Wolbachia endosymbionts and filarial nematodes in Maryland populations of the lone star tick (Amblyomma americanum) FEMS microbiology ecology. 2011;77:50–56. doi: 10.1111/j.1574-6941.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weller SJ, et al. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. Journal of clinical microbiology. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azad AF, Beard CB. Rickettsial pathogens and their arthropod vectors. Emerging infectious diseases. 1998;4:179–186. doi: 10.3201/eid0402.980205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de la Fuente J, et al. Infection exclusion of the rickettsial pathogen Anaplasma marginale in the tick vector Dermacentor variabilis. Clinical and diagnostic laboratory immunology. 2003;10:182–184. doi: 10.1128/CDLI.10.1.182-184.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuczynski J, et al. Experimental and analytical tools for studying the human microbiome. Nature reviews Genetics. 2012;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walters WA, Knight R. Technology and techniques for microbial ecology via DNA sequencing. Annals of the American Thoracic Society. 2014;11(Suppl 1):S16–20. doi: 10.1513/AnnalsATS.201306-160MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakao R, et al. A novel approach, based on BLSOMs (Batch Learning Self-Organizing Maps), to the microbiome analysis of ticks. The ISME journal. 2013;7:1003–1015. doi: 10.1038/ismej.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrosino JF, et al. Metagenomic pyrosequencing and microbial identification. Clinical chemistry. 2009;55:856–866. doi: 10.1373/clinchem.2008.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andreotti R, et al. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC microbiology. 2011;11:6. doi: 10.1186/1471-2180-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narasimhan S, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the lyme disease spirochete. Cell host & microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu Y, et al. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PloS one. 2014;9:e103961. doi: 10.1371/journal.pone.0103961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schabereiter-Gurtner C, et al. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. Journal of microbiological methods. 2003;52:251–260. doi: 10.1016/s0167-7012(02)00186-0. [DOI] [PubMed] [Google Scholar]
- 61.Belkaid Y, Segre JA. Dialogue between skin microbiota and immunity. Science. 2014;346:954–959. doi: 10.1126/science.1260144. [DOI] [PubMed] [Google Scholar]
- 62.Jasinskas A, et al. Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum. Applied and environmental microbiology. 2007;73:334–336. doi: 10.1128/AEM.02009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponnusamy L, et al. Diversity of Rickettsiales in the microbiome of the lone star tick, Amblyomma americanum. Applied and environmental microbiology. 2014;80:354–359. doi: 10.1128/AEM.02987-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lalzar I, et al. Tissue tropism and vertical transmission of Coxiella in Rhipicephalus sanguineus and Rhipicephalus turanicus ticks. Environmental microbiology. 2014 doi: 10.1111/1462-2920.12455. [DOI] [PubMed] [Google Scholar]
- 65.Moreno CX, et al. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environmental microbiology. 2006;8:761–772. doi: 10.1111/j.1462-2920.2005.00955.x. [DOI] [PubMed] [Google Scholar]
- 66.Carpi G, et al. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PloS one. 2011;6:e25604. doi: 10.1371/journal.pone.0025604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Overbeek L, et al. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. FEMS microbiology ecology. 2008;66:72–84. doi: 10.1111/j.1574-6941.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 68.Vayssier-Taussat M, et al. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PloS one. 2013;8:e81439. doi: 10.1371/journal.pone.0081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villar M, et al. A systems biology approach to the characterization of stress response in Dermacentor reticulatus tick unfed larvae. PloS one. 2014;9:e89564. doi: 10.1371/journal.pone.0089564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lalzar I, et al. Composition and seasonal variation of Rhipicephalus turanicus and Rhipicephalus sanguineus bacterial communities. Applied and environmental microbiology. 2012;78:4110–4116. doi: 10.1128/AEM.00323-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menchaca AC, et al. Preliminary assessment of microbiome changes following blood-feeding and survivorship in the Amblyomma americanum nymph-to-adult transition using semiconductor sequencing. PloS one. 2013;8:e67129. doi: 10.1371/journal.pone.0067129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heise SR, et al. Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. Journal of medical entomology. 2010;47:258–268. doi: 10.1603/me09197. [DOI] [PubMed] [Google Scholar]
- 73.Zhang XC, et al. The composition and transmission of microbiome in hard tick, Ixodes persulcatus, during blood meal. Ticks and tick-borne diseases. 2014;5:864–870. doi: 10.1016/j.ttbdis.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Huttenhower C, et al. Advancing the microbiome research community. Cell. 2014;159:227–230. doi: 10.1016/j.cell.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sojka D, et al. New insights into the machinery of blood digestion by ticks. Trends in parasitology. 2013;29:276–285. doi: 10.1016/j.pt.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cirimotich CM, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grigor'eva LA. Morphofunctional changes in the midgut of Ixodid ticks during the life cycle. Entomological Review. 2010;90:405–409. [Google Scholar]
- 79.Sojka D, et al. IrAE: an asparaginyl endopeptidase (legumain) in the gut of the hard tick Ixodes ricinus. Int J Parasitol. 2007;37:713–724. doi: 10.1016/j.ijpara.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grigor'eva LA, Amosova LI. Peritrophic matrix in the midgut of tick females of the genus Ixodes (Acari: Ixodidae) Parazitologiia. 2004;38:3–11. [PubMed] [Google Scholar]
- 81.Kopacek P, et al. Tick innate immunity. Advances in experimental medicine and biology. 2010;708:137–162. [PubMed] [Google Scholar]
- 82.Smith AA, Pal U. Immunity-related genes in Ixodes scapularis--perspectives from genome information. Frontiers in cellular and infection microbiology. 2014;4:116. doi: 10.3389/fcimb.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurlovs AH, et al. Ixodes pacificus ticks maintain embryogenesis and egg hatching after antibiotic treatment of Rickettsia endosymbiont. PloS one. 2014;9:e104815. doi: 10.1371/journal.pone.0104815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunham-Ems SM, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. The Journal of clinical investigation. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amosova LI. An electron microscopic study of Borrelia in the body of the female ixodid tick Ixodes persulcatus. Parazitologiia. 2000;34:234–240. [PubMed] [Google Scholar]
- 86.Kuraishi T, et al. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15966–15971. doi: 10.1073/pnas.1105994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wlodarska M, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buchon N, et al. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell host & microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Lee WJ. Bacterial-modulated signaling pathways in gut homeostasis. Sci Signal. 2008;1:e24. doi: 10.1126/stke.121pe24. [DOI] [PubMed] [Google Scholar]
- 90.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 91.Friswell MK, et al. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PloS one. 2010;5:e8584. doi: 10.1371/journal.pone.0008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Afzelius B, Alberti G, Dallai R, Godula J, Witalinski W. Virus-infected and Rickettsia-infected sperm cells in arthropods. J Inv Path. 1989;53:365–377. [Google Scholar]
- 93.Carmody RN, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell host & microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, et al. Tsetse fly microbiota: form and function. Frontiers in cellular and infection microbiology. 2013;3:69. doi: 10.3389/fcimb.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annual review of entomology. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 96.Broderick NA, Lemaitre B. Gut-associated microbes of Drosophila melanogaster. Gut microbes. 2012;3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hawlena H, et al. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. The ISME journal. 2013;7:221–223. doi: 10.1038/ismej.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de la Fuente J. The fossil record and the origin of ticks (Acari: Parasitiformes: Ixodida) Experimental & applied acarology. 2003;29:331–344. doi: 10.1023/a:1025824702816. [DOI] [PubMed] [Google Scholar]
- 99.Chou S, et al. Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature. 2014 doi: 10.1038/nature13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perry JA, Wright GD. Forces shaping the antibiotic resistome. BioEssays : news and reviews in molecular, cellular and developmental biology. 2014;36:1179–1184. doi: 10.1002/bies.201400128. [DOI] [PubMed] [Google Scholar]