Abstract
Obesity is among the most pressing health concerns in the world since it is increasingly common even in the developing world, and is clearly associated with increased risk for chronic debilitating diseases and death. Furthermore, obesity can influence the pathogenesis of infectious diseases by affecting the balance of pathogen clearance and pathological inflammation. The mechanisms that result in enhanced inflammation in obese individuals are poorly understood. Clostridium difficile is a major cause of nosocomial infections worldwide. Recent studies have shown that obesity is associated with increased risk of C. difficile infections. In this review, we will discuss our current knowledge of the role of obesity in determining risk of C. difficile infections, and focus on the role of the adipose tissue-derived cytokine leptin in C. difficile infections.
Obesity and infections
Obesity is an emerging health problem and worldwide > 500 million people are obese (body mass index, BMI ≥ 30) and > 1.4 billion are overweight (BMI 25–30) (1). Obesity is clearly associated with a higher risk of cardiovascular diseases and metabolic syndrome, and high BMI is associated with increased all-cause mortality (2). In terms of infectious diseases, observational studies have shown that obesity is associated with an increased risk of both nosocomial and community-acquired infections: increased surgical site infections (3), cellulitis (4) and community-acquired pneumonia (5). Interestingly, while obese patients with community-acquired pneumonia have higher systemic inflammation – higher serum C-reactive protein levels (a marker of inflammation) and higher frequency of sepsis (based on systemic inflammatory response syndrome criteria) (6), the 30-day mortality is lower in obese patients (6, 7). In H1N1 influenza virus infections, both obesity (BMI ≥ 30) and morbid obesity (BMI ≥ 40) increases the risk of ICU admissions by two-fold, with morbid obesity (BMI ≥ 40) being associated with a higher risk of death (8). The impact of obesity on infectious diseases outcomes is thus likely to be dependent on the specific pathogenic insult, the associated immune response and the degree of obesity.
While the mechanisms of obesity-mediated effects during infections are poorly understood, obesity-associated immune dysregulation likely plays an important role in infectious disease pathogenesis and outcomes. For example, in genetically obese mice (leptin deficient, ob/ob mice), infection with gram negative bacteria shows impaired macrophage responses, increased pathogen burden and higher mortality (9). Mycobacterial infection of ob/ob mice is also associated with impaired immune response, as seen by decreased IFNγ production, impaired granuloma formation and higher pathogen burden (10). In diet-induced obese mice, a model system that more closely resembles human obesity, H1N1 influenza virus infection leads to higher pro-inflammatory cytokine/chemokine production, severe pulmonary inflammation, lung injury and higher mortality (11) but impaired influenza-specific memory T cell function in obese animals (12). Thus, obesity could have differential impact on the adaptive and innate immune systems.
Obesity and C. difficile infections
The epidemic of C. difficile has reached alarming proportions and is a leading cause of healthcare-associated infections in community hospitals in U.S. and Europe (13). Antibiotic exposure, proton pump inhibitors and H2 blockers, old age, underlying chronic disease, recent hospitalization, gastrointestinal surgeries and tube feeds (14, 15), have all been associated with increased risk of C. difficile infections. The role of BMI was unknown until recently when studies showed that high BMI and obesity is associated with a higher risk of acquiring C. difficile infections in hospitalized patients (16, 17). To the best of our knowledge, effects of obesity and BMI on disease severity or outcomes after C. difficile infection have not been studied.
The spectrum of C. difficile-associated disease extends from asymptomatic colonization to self-limited diarrheal illness, to severe colitis and sometimes death. This variability in disease course and outcomes suggests that host factors play an important role in disease pathogenesis. In fact, recent studies suggest that host inflammation is an important determinant of outcomes in C. difficile infections (18). Since obesity is a state of persistent, low-grade inflammation (19), obesity-associated inflammation will likely have an impact on both the risk and outcomes in cases of C. difficile infections. Since obesity affects the composition of gut microbial communities (20, 21) and disruption of gut microbiota is an essential first step in C. difficile infection, obesity-associated differences in host microbiome could be another factor affecting risk and outcomes of C. difficile infections.
Leptin and inflammation
While obesity is the result of dysregulation of multiple physiologic processes and no single molecule is causative, obesity-associated inflammation is believed to be both initiated and propagated by adipose tissue (19). Adipose tissue secretes a number of adipocytokines (e.g. leptin, adiponectin, apelin, omentin, IL-6, TNF and MCP-1) during critical illness that affect both metabolism and host inflammation (22, 23). Leptin is one such adipocytokine that is elevated during early sepsis (24). Leptin is a member of the IL-6 family and was initially discovered for its role in regulation of metabolism and satiety (25). It is primarily secreted by fat cells (minor contributions from gut and placenta tissue) and leptin receptors are expressed on many different cell types: immune cells, epithelial cells and neurons (26). Leptin has pleotropic functions and affects multiple physiological processes including angiogenesis (27), hematopoiesis (28), lipid (26), glucose (26) and bone metabolism (29), reproduction (30) and both adaptive and innate immune responses (31, 32).
In terms of inflammation and immunity, leptin has a pro-inflammatory mode of action. Leptin induces inflammatory cytokine and chemokine production, increases neutrophil chemotaxis, enhances NK cell cytotoxicity and activates dendritic cells, neutrophils and NK cells (32–36). Leptin also regulates adaptive immunity by suppressing regulatory T cells (Tregs) and increasing Th1 cellular responses (31, 32). Presumably via increasing the inflammatory response, leptin enhances the host defense during infections. Thus, humans with leptin deficiency have a higher incidence of infections (37) and mice deficient in leptin (ob/ob) and leptin receptor (db/db) are more susceptible to pneumonia (9, 10), listeriosis (38) and amebic colitis (39). Congruent with leptin’s pro-inflammatory role, mice lacking leptin (ob/ob mice) have significantly less inflammation and colonic cytokine production as compared to wildtype control mice in models of inflammatory colitis (DSS- and TNBS-induced colitis) (40). In the gastrointestinal tract, leptin also increases mucin production by activating Protein Kinase C-, phosphatidylinositol 3-kinase- and MAP kinase-dependent pathways (41, 42), enhances epithelial cell turnover (43) and maintains intestinal epithelial barrier integrity (44).
In humans, serum leptin levels are proportional to the amount of adipose tissue (45) and thus obesity is a state of hyper-leptinemia (45). Interestingly, the high serum leptin levels in obese patients fails to control their body weight, a condition termed as “leptin resistance” (46). While this phenomenon is clinically observed, due to the varied effects of leptin on multiple physiologic processes, the underlying molecular mechanisms of leptin resistance have not been defined. Specifically the affect of high leptin levels on immune cells in obese individuals and in turn response to infectious and inflammatory challenge is not known.
Leptin and infections
Alterations in leptin signaling pathway do play a role in influencing the risk and outcomes of infectious diseases. We have shown that a particular single nucleotide polymorphism (SNP rs1137101) located at amino acid position 223 in leptin receptor, encoding for arginine (223R) instead of glutamine (223Q) increases the risk of amebiasis in children in Bangladesh (OR = 3.91; p = 0.007) (47). The homozygous 223R allele is also associated with increased susceptibility to amebic liver abscess in an independent cohort of adults (47). Other studies have shown that homozygosity for 223R allele is linked to increased risk of death in cases of non-appendicular secondary peritonitis (48) and increased risk of developing chronic bronchitis (49). While the functional consequences of LEPR Q223R polymorphism have not been completely elucidated, our studies have shown that the polymorphism affects downstream STAT3 signaling with the wild type 223Q allele having a more robust STAT3 signal after leptin stimulation as compared to the mutant 223R allele (50), without any affects on the binding kinetics of leptin to it’s receptor (51). Recently, we have shown that the odds of having C. difficile infection are increased in individuals homozygous for the 223R allele as compared to heterozygous or those homozygous for the 223Q allele (OR = 3.03; p = 0.01) (52).
Previous studies have investigated the role of leptin during C. difficile disease pathogenesis and showed an increase in both serum leptin levels and gut mucosal leptin receptor mRNA expression in mice after Toxin A challenge (53). Mechanistically, both leptin- and leptin receptor-deficient (ob/ob or db/db respectively) mice were resistant to toxin A-induced ileal fluid secretion (53). The role of leptin signaling during infection (rather than intoxication) with C. difficile had not been studied until our recent work, which demonstrated that leptin signaling leads to increased inflammation and clinical signs of disease acutely after C. difficile infection (52). This heightened response to infection was associated with better control of pathogen burden during acute infection (Day 2, peak disease) as measured by levels of Toxin A/B and C. difficile-specific glutamate dehydrogenase (GDH) in the cecal contents (52). Notably, mice with uncoupled leptin receptor-STAT3 signaling had diminished colonic inflammation, decreased colonic chemokine and immune cell recruitment, and less severe clinical signs of disease (52). Thus leptin-signaling acting via STAT3 promotes an early inflammatory response after C. difficile infection that manifests as worse clinical disease, but also enhances bacterial clearance during acute infection. The role of leptin signaling later during the course of C. difficile disease has not yet been defined.
Possible mechanisms of leptin-mediated effects during C. difficile infection
The tempo, vigor and character of the inflammatory response to noxious challenge is an important determinant of immune homeostasis and disease course. Early (or delayed) inflammation or an over-exuberant inflammatory response can thus be critical in determining the balance between protection and disease. Leptin is a pleotropic cytokine with effects on different organ systems and thus there are multiple possible mechanisms of leptin-mediated actions during C. difficile infection including effects on immune cell recruitment and function (directly via leptin receptor expression on immune cells and indirectly via leptin receptor expression on non-immune cells like intestinal epithelial cells or neurons) or by effects on host microbiota composition.
Leptin is a pro-inflammatory molecule and is a potent chemoattractant for neutrophils (34, 35, 54), monocytes and macrophages (55). In mouse models of acute lung injury, neutrophil recruitment to the lungs of db/db (leptin receptor-deficient) mice is delayed (54). The effects of leptin during C. difficile colitis could thus be secondary to accelerated kinetics of inflammatory cell recruitment, which could conversely explain the diminished clinical signs and bactericidal activity we observed in the absence of leptin signaling (52). Apart from cellular recruitment, leptin functions in immunity include promoting Th1 and Th17 responses (32, 56). Leptin could thus affect the character and composition of the immune response during C. difficile colitis. C. difficile infection leads to a profound inflammatory response: upregulation of numerous pro-inflammatory cytokines and chemokines, anti-microbial peptide secretion, and polymorphonuclear cell recruitment (57–59). Of these multiple inflammatory mediators, the ones that are induced by leptin have been only partially described in our recent studies but need further definition (52). As an example, leptin enhances IL-17 induction (56), and IL-17A can support neutrophil infiltration as well as induce mucin and anti-microbial peptides (60). IL-17-mediated inflammation could thus be a common mechanism driving divergent leptin actions. It is important to note that in addition to cellular recruitment, leptin also affects inflammatory cell function. Leptin induces oxidative species and inflammatory cytokines/chemokines production, and enhances the phagocytic capacity of neutrophils, monocytes and macrophages (34, 35, 61). Thus another possible mechanism of leptin actions could be affects on the functional capacity of neutrophils (enhanced reactive oxygen species production, phagocytosis and other downstream effectors of neutrophil function including pro-inflammatory cytokines and chemokines).
Leptin also regulates the composition of gut microbiota: both leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice have significantly altered gut microbial communities as compared to wild type littermates (20, 21). Antibiotics that alter the microbiome are of course the major risk factor for C. difficile infections in humans (62). In animal models, antibiotic pre-treatment reduces the overall mass of bacteria (63, 64) and alters the composition of the gut flora: increased abundance of Proteobacteria and Enterobacteriaceae and loss of Firmicutes, Lachnospiraceae and Barnesiella (62), thus predisposing mice to C. difficile infection. Microbiota-directed changes in host immunity, particularly effects on gut mucosal defenses, have been shown previously (65). As an example, colonization of germ-free mice with segmented filamentous bacteria induces anti-microbial peptide production, pro-inflammatory cytokines and Th17 cells in the lamina propria (66, 67). Thus, differences in susceptibility to C. difficile due to leptin could well be acting at the level of the microbiome: directly via affecting C. difficile colonization resistance or indirectly via affecting the gut mucosal immune responses.
Remaining Questions
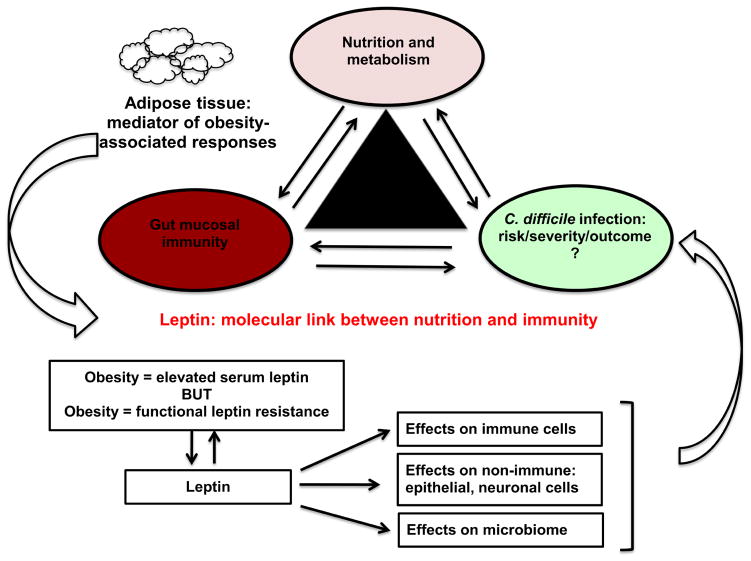
BMI and obesity clearly affect the risk as well as outcomes of infections in patients, however the underlying mechanisms are unknown. Leptin, produced by adipose tissue (45) and acting on immune cells (32), serves as a molecular link between nutrition and immunity (Figure 1). Leptin is also a key obesity-associated molecule. While many studies have shown that complete leptin deficiency and leptin receptor polymorphisms (affecting downstream signaling) modify the risk and outcome of infectious diseases (9, 37, 47, 48, 52), the mechanism(s) by which leptin modifies host inflammation during an infection in obese individuals needs to be further elucidated.
Figure 1. Model of leptin-mediated inflammatory response during C. difficile infections.
Adipose tissue acts as a sensor of host nutritional status and secretes leptin (proportional to body fat). Leptin acts as a molecular link between nutrition and immunity and affects the risk, severity and outcomes of C. difficile infections. The mechanism of leptin actions could be via immune cells, non-immune cells or changes in the host microbiome and since obesity is associated with both elevated leptin levels but also “leptin resistance”, the effects of leptin-mediated actions could lead to diverse effects on the outcomes of C. difficile infections and needs further investigation.
It is possible that the high circulating leptin levels in obese patients, acting on immune cells could lead to enhanced inflammation, however if there is a common molecular mediator of “leptin resistance” that affects both the satiety and immunologic signals, then obese patients may behave as functionally leptin deficient and thus have a diminished inflammatory response (Figure 1). Further, due to the pleotropic nature of leptin’s actions, other mechanisms like leptin effects on host microbial ecology and on non-immune cells (intestinal epithelial cells, neuronal cells etc.) could play an important role in determining C. difficile disease severity and outcomes (Figure 1). With an increase in worldwide population of obese and overweight individuals, patients with high BMI will form a large part of the hospitalized patient population in the near future. Thus understanding the role of leptin in modulating disease pathogenesis has the potential to unravel novel targets for therapeutic intervention and provide key insights in determining therapies and treatment principles for this rapidly expanding patient group.
Highlights.
Obesity is associated with an increased risk of C. difficile infection.
Role of obesity in C. difficile disease outcomes needs further investigation.
High serum leptin levels are seen in obese individuals.
Leptin enhances colonic inflammation after C. difficile infection.
Lack of leptin signaling alters indigenous host microbiota.
Acknowledgments
This work was supported by NIH RO1 AI-26649-25 to W. A. Petri Jr.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. WHO Fact Files: Ten facts on obesity. Geneva: WHO; 2013. [Google Scholar]
- 2.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hourigan JS. Impact of obesity on surgical site infection in colon and rectal surgery. Clin Colon Rectal Surg. 2011;24:283–90. doi: 10.1055/s-0031-1295691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karppelin M, Siljander T, Vuopio-Varkila J, Kere J, Huhtala H, Vuento R, Jussila T, Syrjanen J. Factors predisposing to acute and recurrent bacterial non-necrotizing cellulitis in hospitalized patients: a prospective case-control study. Clin Microbiol Infect. 2010;16:729–34. doi: 10.1111/j.1469-0691.2009.02906.x. [DOI] [PubMed] [Google Scholar]
- 5.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–8. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 6.Singanayagam A, Chalmers JD. Obesity is associated with improved survival in community-acquired pneumonia. Eur Respir J. 2013;42:180–7. doi: 10.1183/09031936.00115312. [DOI] [PubMed] [Google Scholar]
- 7.Corrales-Medina VF, Valayam J, Serpa JA, Rueda AM, Musher DM. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. 2011;15:e54–7. doi: 10.1016/j.ijid.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Fezeu L, Julia C, Henegar A, Bitu J, Hu FB, Grobbee DE, Kengne AP, Hercberg S, Czernichow S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–9. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 10.Wieland CW, Florquin S, Chan ED, Leemans JC, Weijer S, Verbon A, Fantuzzi G, van der Poll T. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int Immunol. 2005;17:1399–408. doi: 10.1093/intimm/dxh317. [DOI] [PubMed] [Google Scholar]
- 11.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, Zheng BJ, Hung IF, Lam KS, Xu A, Yuen KY. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–80. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–33. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 13.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–90. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 14.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 15.Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012–9. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bishara J, Farah R, Mograbi J, Khalaila W, Abu-Elheja O, Mahamid M, Nseir W. Obesity as a risk factor for Clostridium difficile infection. Clin Infect Dis. 2013;57:489–93. doi: 10.1093/cid/cit280. [DOI] [PubMed] [Google Scholar]
- 17.Leung J, Burke B, Ford D, Garvin G, Korn C, Sulis C, Bhadelia N. Possible association between obesity and Clostridium difficile infection. Emerg Infect Dis. 2013;19:1791–8. doi: 10.3201/eid1911.130618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Feghaly RE, Stauber JL, Deych E, Gonzalez C, Tarr PI, Haslam DB. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis. 2013;56:1713–21. doi: 10.1093/cid/cit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 20.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 21.Geurts L, Lazarevic V, Derrien M, Everard A, Van Roye M, Knauf C, Valet P, Girard M, Muccioli GG, Francois P, de Vos WM, Schrenzel J, Delzenne NM, Cani PD. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:149. doi: 10.3389/fmicb.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marques MB, Langouche L. Endocrine, metabolic, and morphologic alterations of adipose tissue during critical illness. Crit Care Med. 2013;41:317–25. doi: 10.1097/CCM.0b013e318265f21c. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235:1412–24. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 24.Yousef AA, Amr YM, Suliman GA. The diagnostic value of serum leptin monitoring and its correlation with tumor necrosis factor-alpha in critically ill patients: a prospective observational study. Crit Care. 2010;14:R33. doi: 10.1186/cc8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 26.Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56:s38–46. doi: 10.1111/j.1753-4887.1998.tb01685.x. discussion s54–75. [DOI] [PubMed] [Google Scholar]
- 27.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 28.Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–80. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 29.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 30.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–20. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 31.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 32.Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Moore SI, Huffnagle GB, Chen GH, White ES, Mancuso P. Leptin modulates neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2003;71:4182–5. doi: 10.1128/IAI.71.7.4182-4185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414–8. [PubMed] [Google Scholar]
- 35.Caldefie-Chezet F, Poulin A, Vasson MP. Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res. 2003;37:809–14. doi: 10.1080/1071576031000097526. [DOI] [PubMed] [Google Scholar]
- 36.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 37.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 38.Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005;54:182–9. doi: 10.2337/diabetes.54.1.182. [DOI] [PubMed] [Google Scholar]
- 39.Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA., Jr Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2011;4:294–303. doi: 10.1038/mi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–25. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- 41.El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, Bado A, Scoazec JY, Plaisancie P. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am J Physiol Gastrointest Liver Physiol. 2007;293:G365–73. doi: 10.1152/ajpgi.00091.2007. [DOI] [PubMed] [Google Scholar]
- 42.Plaisancie P, Ducroc R, El Homsi M, Tsocas A, Guilmeau S, Zoghbi S, Thibaudeau O, Bado A. Luminal leptin activates mucin-secreting goblet cells in the large bowel. Am J Physiol Gastrointest Liver Physiol. 2006;290:G805–12. doi: 10.1152/ajpgi.00433.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sukhotnik I, Coran AG, Mogilner JG, Shamian B, Karry R, Lieber M, Shaoul R. Leptin affects intestinal epithelial cell turnover in correlation with leptin receptor expression along the villus-crypt axis after massive small bowel resection in a rat. Pediatr Res. 2009;66:648–53. doi: 10.1203/PDR.0b013e3181be9f84. [DOI] [PubMed] [Google Scholar]
- 44.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 45.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 46.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–51. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC, Jr, Myers MG, Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA., Jr A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121:1191–8. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bracho-Riquelme RL, Loera-Castaneda V, Torres-Valenzuela A, Loera-Castaneda GA, Sanchez-Ramirez JP. Leptin and leptin receptor polymorphisms are associated with poor outcome (death) in patients with non-appendicular secondary peritonitis. Crit Care. 2011;15:R227. doi: 10.1186/cc10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B, Fu E, Cao Y, Zhong Y, Fu G, Tian X, Li S. Effect of leptin receptor mutation on the development of chronic bronchitis. Asia Pac J Public Health. 2013;25:80S–7S. doi: 10.1177/1010539513497218. [DOI] [PubMed] [Google Scholar]
- 50.Marie CS, Verkerke HP, Paul SN, Mackey AJ, Petri WA., Jr Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect Immun. 2012;80:1934–43. doi: 10.1128/IAI.06140-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verkerke H, Naylor C, Zabeau L, Tavernier J, Petri WA, Jr, Marie C. Kinetics of leptin binding to the Q223R leptin receptor. PLoS One. 2014;9:e94843. doi: 10.1371/journal.pone.0094843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madan R, Guo X, Naylor C, Buonomo EL, Mackay D, Noor Z, Concannon P, Scully KW, Pramoonjago P, Kolling GL, Warren CA, Duggal P, Petri WA., Jr Role of leptin-mediated colonic inflammation in defense against Clostridium difficile colitis. Infect Immun. 2014;82:341–9. doi: 10.1128/IAI.00972-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mykoniatis A, Anton PM, Wlk M, Wang CC, Ungsunan L, Bluher S, Venihaki M, Simeonidis S, Zacks J, Zhao D, Sougioultzis S, Karalis K, Mantzoros C, Pothoulakis C. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 2003;124:683–91. doi: 10.1053/gast.2003.50101. [DOI] [PubMed] [Google Scholar]
- 54.Kordonowy LL, Burg E, Lenox CC, Gauthier LM, Petty JM, Antkowiak M, Palvinskaya T, Ubags N, Rincon M, Dixon AE, Vernooy JH, Fessler MB, Poynter ME, Suratt BT. Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am J Respir Cell Mol Biol. 2012;47:120–7. doi: 10.1165/rcmb.2011-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293:C1481–8. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 56.Deng J, Liu Y, Yang M, Wang S, Zhang M, Wang X, Ko KH, Hua Z, Sun L, Cao X, Lu L. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 2012;64:3564–73. doi: 10.1002/art.34637. [DOI] [PubMed] [Google Scholar]
- 57.Sadighi Akha AA, Theriot CM, Erb-Downward JR, McDermott AJ, Falkowski NR, Tyra HM, Rutkowski DT, Young VB, Huffnagle GB. Acute infection of mice with Clostridium difficile leads to eIF2alpha phosphorylation and pro-survival signalling as part of the mucosal inflammatory response. Immunology. 2013;140:111–22. doi: 10.1111/imm.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect Immun. 2012;80:2989–96. doi: 10.1128/IAI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madan R, WA Immune responses to Clostridium difficile infection. Trends Mol Med. 2012;18:658–66. doi: 10.1016/j.molmed.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004;287:L497–502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 62.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145–58. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009;77:2367–75. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]