Abstract
Background
Cortical GABA regulates a number of cognitive functions including attention and working memory and is dysregulated in a number of psychiatric conditions. In schizophrenia for example, changes in GABA neurons [reduced expression of glutamic acid decarboxylase (GAD), parvalbumin (PV) and the GABA reuptake transporter (GAT1)] suggest reduced cortical GABA synthesis and release; these changes are hypothesized to cause the cognitive deficits observed in this disorder. The goals of this experiment were to determine whether chronically reducing GAD function within the rat PFC causes attention deficits and alterations in PV and GAT1 expression.
Methods
Male Sprague Dawley rats were trained on the 5-choice serial reaction time task (5CSRTT, a task of attention) until they reached criterion performance and then were implanted with a bilateral cannula aimed at the medial PFC. Cannulae were connected to osmotic minipumps that infused the GAD inhibitor L-allylglycine (LAG, 3.2 μg/0.5 μl/hr) for 13 days. Following a 5-day recovery from surgery rats were tested on the standard 5CSRTT for 5 consecutive days and then tested on two modifications of the 5CSRTT. Finally, locomotor activity was assessed and the rats sacrificed. Brains were rapidly extracted and flash frozen and analyzed for the expression of GAD67, PV, GAT1 and the obligatory NMDA receptor subunit NR1.
Results
Chronic LAG infusions transiently impaired attention, persistently impaired impulse control and increased locomotor activity. Behavioral changes were associated with an upregulation of GAD67, but no change in PV, GAT1 or NR1 expression.
Summary
Chronic inhibition of GABA synthesis within the medial PFC, increased impulsive behavior and locomotion, but did not impair attention; results consistent with previous research following acute inhibition of GABA synthesis. Moreover, our data do not support the hypothesis that decreasing GABA synthesis and release is sufficient to cause changes in other GABA-related proteins.
Keywords: attention, schizophrenia, GABA, impulsive, GAD67, L-allylglycine
1.0 Introduction
Although GABA neurons only represent approximately 20% of cortical neurons, these neurons are involved in regulating the precise firing of excitatory pyramidal neurons, the main cortical output neuron (Markram et al., 2004). Indeed, disturbances in cortical GABA function have been found to regulate a number of neural processes including cognitive functions such as attention (e.g., Paine et al., 2011) and working memory (e.g., Auger and Floresco, 2014). Notably, alterations in cortical GABA function are observed in a number of psychiatric disorders including, but not limited to, schizophrenia (e.g., Lewis, 2014), autism spectrum disorders (e.g., Cellot and Cherubini, 2014) and depression (e.g., Sanacora and Saricicek, 2007). Furthermore, each of these cognitive disorders is associated with cognitive dysfunctions including attention deficits (Millan et al., 2014) and it has been speculated that changes in GABA function underlie the cognitive deficits observed in these disorders.
In schizophrenia, for example, alterations in fast-spiking parvalbumin (PV)-containing GABA neurons are speculated to contribute to the observed cognitive deficits (Gonzalez-Burgos and Lewis, 2008; Lewis, 2014). Fast-spiking PV-containing GABA neurons are hypothesized to underlie the generation of gamma oscillations (Gonzalez-Burgos and Lewis, 2008), a form of neural synchrony evoked during performance on cognitive tasks (Gruber et al., 1999; Steinmetz et al., 2000; Senkowski and Gallinat, 2015). People with schizophrenia, for example, exhibit reduced induced gamma oscillations during cognitive tasks and perform poorly on such tasks (Cho et al., 2006; reviewed in Senkowski and Gallinat, 2015). Furthermore, the peak gamma frequency correlates with both GABA level and working memory performance in schizophrenia (Chen et al., 2014). Combined these data have led to the GABA hypothesis of schizophrenia, namely that cortical GABA neuron dysfunction contributes to the cognitive deficits observed in schizophrenia (reviewed in Lewis, 2014).
Post-mortem analyses of schizophrenia brains support the GABA dysfunction hypothesis, finding changes that suggest a reduction in cortical GABA synthesis and release. For example, both mRNA and protein expression of the 67-kilodalton isoform of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD67) are reduced in the dorsolateral prefrontal cortex (PFC) of individuals with schizophrenia (Curley et al., 2011; Hashimoto et al., 2003; Volk et al., 2000). Notably changes in GAD67 expression are most frequently observed in fast-spiking PV-containing GABA neurons (Hashimoto et al., 2003; Curley et al., 2011), which also exhibit reduced expression of both PV (Hashimoto et al., 2003; Volk et al., 2001; Glausier et al., 2014) and the GABA reuptake transporter GAT1 (Bitanihirwe and Woo, 2014). Since both GAT1 and PV reduce synaptic GABA levels, it has been speculated that the decreased expression of GAT1 and PV are compensatory responses aimed at ameliorating the effect of reduced GABA synthesis and release caused by reduced GAD67 expression (see Curley et al., 2013; Rotaru et al., 2011). Furthermore, post-synaptic GABAA receptor α2-subunit expression is increased in the dorsolateral PFC; this may also be a compensatory response to decreased GABA levels (Volk et al., 2002; Beneyto et al., 2011). In sum, alterations in cortical GABA-related proteins in the cortex suggest a reduction in GABA synthesis and release; this may contribute to the observed cognitive deficits in schizophrenia (Lewis 2014; Tse et al., 2015).
Contrary to the GABA hypothesis of schizophrenia however, acutely decreasing cortical GABA synthesis does not appear to impair attention (Asinof and Paine, 2013; Pehrson et al., 2013). The utility of these studies in evaluating the GABA hypothesis is limited because GAD67 function is chronically decreased in psychiatric conditions including schizophrenia and, as discussed above, there are several adaptions to the reduction in GAD67 that may also contribute to the observed cognitive deficits. To date there has been little research aimed at determining whether chronically decreasing GABA synthesis is sufficient to cause cognitive deficits. Recently, Reichel et al. (2015) observed that long-term depletion of cortical GABA caused by GABA neuron ablations resulted in deficits in both sensorimotor-gating and reversal learning. These data suggest that chronic, rather than acute, decreases in GABA synthesis and release may be necessary to cause cognitive deficits.
The goals of the current experiment were to test whether chronically reducing GABA synthesis within the PFC is sufficient to cause an attention deficit and to determine if reducing GABA synthesis would cause changes in other GABA-related proteins that are consistent with those observed in schizophrenia. L-allylglycine (LAG) was used to inhibit GABA synthesis; it was delivered chronically to the medial PFC via bilateral cannulae connected to osmotic minipumps. LAG has previously been observed to reduce GABA synthesis when administered systemically (Horton et al., 1978) and intra-cranially (Cunha et al., 2010). Consistent with this, we have observed that intra-PFC infusions of LAG cause increased expression of c-fos, a marker of neuronal activation (Asinof and Paine, 2013). Visuospatial attention was measured using three different versions of the 5-choice serial reaction time task (5CSRTT), a rodent task analogous to the continuous performance task used to study attention in humans (Robbins, 2002). Because performance on the 5CSRTT can be affected by changes in locomotor activity, this was also assessed. Finally, because there has been some speculation that decreases in GABA synthesis are the proximal cause of other physiological abnormalities in the GABA system in schizophrenia (Curley et al., 2013), we also determined how chronic inhibition of GABA synthesis affects the expression of GAD67, parvalbumin (PV), the GABA reuptake transporter (GAT1) and the N-methyl-D-aspartate (NMDA) receptor NR1 subunit.
2.0 Materials and Methods
2.1 Rats
Twenty-four male Sprague-Dawley rats born at Oberlin College were used. Rats were maintained on a 14-h/10-h light-dark cycle (lights on at 0700h) and were group housed until post-natal day (PND) 55; during this time they had unlimited access to food (Purina Rat Chow) and water. On PND 55 rats were housed in pairs (until the time of surgery at which point they were housed singly) and then food restricted to ~85% of their free feeding weight. Rats were fed after daily training sessions. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996) and Oberlin College policies.
2.2 Drugs
L-Allylglycine (LAG) was purchased from Sigma-Aldrich (St. Louis MO) and dissolved in artificial cerebral spinal fluid (Na+150 mM; K+ 3.0 mM; Ca2+ 1.4 mM, Mg2+ 0.8 mM, P 1.0 mM; Cl− 155 mM) to a concentration of 6.4 μg/μl.
2.3 Surgery
Rats were anesthetized with sodium pentobarbital (65 mg/kg, IP) and implanted with a bilateral guide cannula (26-gauge; Plastics One, Roanoke, VA) aimed at the border of infralimbic (IL) and prelimbic cortex (PrL) cortices (relative to bregma: AP = + 2.8, ML = ± 0.75, DV = −3.0 mm from dura [Paxinos and Watson, 2009]). Each arm of the cannula was connected to an osmotic minipump (Alzet Model 2002); the minipumps were implanted subcutaneously at the nape of the neck. Skull screws and dental acrylic secured the guide cannulae in place. The pumps were filled with either aCSF or LAG (6.4 μg/μL) 24 hr prior to surgery; aCSF or LAG was delivered to the PFC at a rate of 0.5 μL/hr for 14 days.
2.4.1 The Standard 5-Choice Serial Reaction Time Task (5CSRTT)
Rats were trained on the 5CSRTT as described previously (Asinof and Paine, 2014; Paine et al., 2011). Sessions started with the delivery of 1 food pellet (45-mg, Bio-Serv, Frenchtown NJ); the first trial commenced upon pellet retrieval. A nose poke into the magazine initiated the first 5-sec inter-trial interval (ITI) and illumination of the house light. At the end of the ITI, a 1-sec light stimulus was presented at the rear of one of the five stimulus locations (apertures). Rats had up to 5 sec (limited hold) to make a response. A response in the illuminated aperture (correct response) triggered delivery of 1 food pellet and illumination of the magazine light, which remained illuminated for 5 sec following pellet delivery. Nose pokes in the remaining apertures were considered incorrect responses and triggered a 5-sec time-out (TO) during which the house light was extinguished. Similarly, failure to respond during the limited hold (i.e., an omission) triggered a 5-sec TO. The subsequent trial was automatically initiated at the end of the TO period or the limited hold (correct responses). Responses occurring during the ITI were considered premature responses and also triggered a 5-sec TO; the same trial was automatically re-started at the end of the TO period. Responses occurring during the TO period had no programmed consequences. Sessions ended after 90 trials or 30 min. Performance measures of interest were: % accuracy ((correct responses/ [correct + incorrect responses])*100), % omissions ([omissions/ trials completed]*100), premature responses, magazine entries, correct response latency (the time from the stimulus onset to a correct response) and reward latency (the time from a correct response to the collection of the food). Subjects were considered to have acquired the task when their accuracy was greater than 60% (chance performance in this test is 20%) and omissions were fewer than 20% for 5 consecutive days. Once all of the rats reached criterion performance, they underwent surgery to implant guide cannulae and osmotic minipumps. After a 5-day recovery period rats were tested. Approximately half of the rats (n=12) were tested on the standard 5CSRTT task once before they were sacrificed on Test Day 6, the other half of the rats (n=11) were tested for 5 consecutive days (Test Days 6-10) on the standard version of the 5CSRTT and then were tested on two modified versions of the task (see Figure 1).
Figure 1.
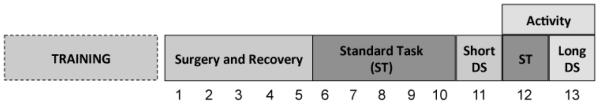
Experimental Design. Rats were trained on the standard 5CSRTT (ST) until they reached criterion performance and then were implanted with guide bilateral cannula aimed at the medial prefrontal cortex (PFC). Each cannula was connected to two osmotic minipumps that delivered either the GABA synthesis inhibitor L-allylglycine (LAG; 6.4 μg/μl) or vehicle (artificial cerebral spinal fluid) into the PFC at a rate of 0.5 μl/hr for either 6 or 13 days. After surgery rats recovered for 5 days (days 1-5) and then were tested on the ST. A subset of rats was sacrificed after a single test session, while the remainder of the rats were tested for 5 consecutive days. Next, these rats were tested on the short discriminative stimulus task (Short DS), a task in which the stimulus duration is decreased thereby increasing the attentional demands of the task. Finally, rats were tested on the long discriminative stimulus (Long DS) task, a task in which the stimulus duration is increased thereby decreasing the attentional demands of the task. Locomotor activity was measured in an open field after the last 2 days of 5CSRTT testing. Rats were sacrificed after locomotor testing on day 13.
On test day 11 rats were tested on a version of the 5CSRTT in which the stimulus duration was shortened to 0.5-sec (Short DS task); all of the other aspects of the task were unchanged from the standard task. The Short DS task has increased attentional demands relative to the standard 5CSRTT.
On test day 13, rats were tested on a version of the task in which the stimulus duration was lengthened to 5-sec (Long DS task), all other aspects of the task were unchanged. The Long DS task has decreased attentional demands relative to the standard 5CSRTT.
2.5 Locomotor Activity in an Open Field
In order to determine if chronic LAG infusions affected locomotor activity, activity was measured during two 60-min sessions on test days 12 and 13 (see Figure 1). Activity sessions took place after 5CSRTT sessions. Activity was recorded in automated (43.2 × 43.2 cm) activity chambers (MED Associates, St. Albans, VT).
2.6 Western Blots
On either test day 6 or 13 (after behavioral tests had been completed), rats were deeply anesthetized with sodium pentobarbital and then sacrificed by decapitation. Brains were rapidly extracted and frozen in isopentane kept on dry ice. Next, brains were sectioned on a microtome (Leica SM2010R, Buffalo Grove, IL) and bilateral tissue punches taken from the prelimbic cortex, infralimbic cortex (~ +3.7 relative to bregma) and the nucleus accumbens (~+2.2 relative to bregma). Each tissue punch was ~1-mm in length; midline IL and PrL punches were 2-mm in diameter (illustrated in Figure 5) and NAc tissue punches were 2 mm in diameter. Tissue punches were placed in microcentrifuge tubes and kept on dry ice. Tissue was sonicated in 100 μl of 1% sodium dodecyl sulfate (SDS) and then protein content was measured using the Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA). Sample dilutions were then adjusted to 2.0 mg/ml protein.
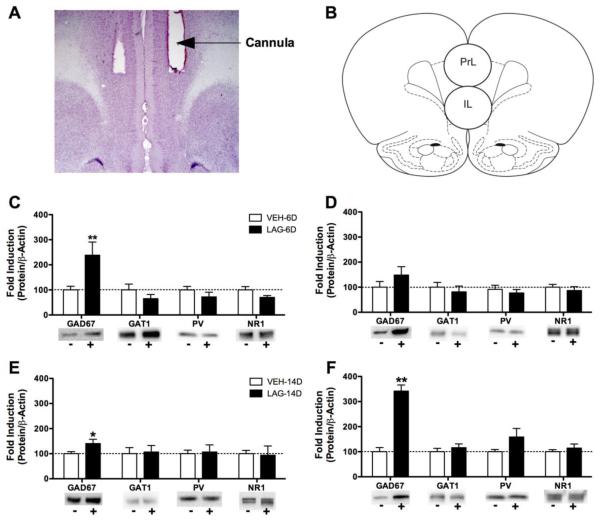
Figure 5.
Effects of chronically infusing the GABA synthesis inhibitor L-allylglycine (LAG) into the medial prefrontal cortex on expression of GAD67, GAT1, parvalbumin (PV) and the NMDA NR1 subunit. A) Representative cannula placement. B) Schematic showing approximate locations of the tissue punches for the prelimbic (PrL) and infralimbic (IL) cortices (adapted from Paxinos and Watson, 2009). C-D) 6-Day exposure to LAG increased GAD67 expression in the prelimbic (PrL, C), but not infralimbic (IL, D) cortex. E-F) 13-Day exposure to LAG increased GAD67 expression in both the PrL (E) and IL (F). GAT1, PV and NR1 expression were not affected by chronic infusions of LAG. *P < 0.05, **P < 0.001 significantly different from VEH for that protein.
Laemmli buffer (Bio-Rad) and dithiothreitol (Bio-Rad) were added to each sample prior to heating at 74°C for 10 min. Each sample (20 μg protein) was then loaded onto 4-15% Tris HCl polyacrylamide gels (Bio-Rad) for separation by gel electrophoresis. Proteins were then transferred to nitrocellulose membranes (Bio-Rad). Nonspecific binding sites on the membranes were blocked for 3 hr at room tissue in blocking buffer (5% non-fat dry milk in PBS and 0.1% Tween (PBS-T)). Blots were then incubated in primary antibody diluted in blocking buffer overnight at 4°C. Then blots were washed 3 × 10 min in PBS-T and then incubated for 3 hr in secondary antibody (1:5000 goat anti-rabbit [or anti-mouse] horseradish peroxidase-linked IgG [Vector Laboratories, Burlingame, CA]). Finally, blots were washed 3 × 10 min in PBS-T before immunological detection using SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Fisher Scientific, Rockford IL). Antibodies were stripped from the blots by incubation with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific, Rockford IL) for 15 min and then the blots were re-blocked and probed with anti-actin (1:5000 ;Sigma, St. Louis, MO). Precision Plus Protein Kaleidescope pre-stained standard (Bio-Rad) was run for molecular weight estimation. Primary antibodies used were: 1:2000 monoclonal anti-GAD67 (Sigma), 1:2000 polyclonal anti-GAT1 (Abcam, Cambridge, MA), 1:2000 polyclonal anti-parvalbumin (PV; Swant) and 1:1000 polyclonal anti-NMDA NR1 (Cell Signaling Technology, Danvers, MA). The GAD67 antibody detects a band at 67 kDa, the GAT1 antibody detects a band at ~72 kDa, the parvalbumin antibody detects at band at ~12 kDa, NMDA NR1 antibody detects a band at 120 kDa and the actin antibody detects a band at 42 kDa.
Protein immunoblots were visualized using a chemiluminescent reaction by a Kodak Gel Logic 1500 Imaging System (Kodak, Rochester, NY). Relative optical densities were determined for each band of interest (GAD67, GAT1, PV, NR1, β-actin) using ImageJ software. The optical density of each band was normalized to the corresponding optical density of β-actin in order to control for differences in protein loading. Finally, to allow for comparison amongst brain areas, data were normalized to vehicle-treated controls. Data are expressed as the mean fold induction compared to vehicle control.
2.7 Histology
Two rats were implanted with guide cannula (as above) and allowed 14 days to recover before being anesthetized with sodium pentobarbital (100 mg/kg, IP) and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde. Following perfusions, brains were removed, post-fixed for 24 h and then cryoprotected in 30% sucrose prior to slicing on a microtome. Sections (40 μm) were mounted on slides, stained with cresyl violet and used to assess cannulae placements.
2.8 Statistical Analyses
Baseline performance on the 5CSRTT was analyzed with a two-way between subjects ANOVA with Condition (VEH, LAG) and Group (6-day vs. 13-day) as between subjects factors. Test data from the 5CSRTT were analyzed using separate two-way repeated measures ANOVAs with Condition (VEH, LAG) as the between-subjects factor and Day (either baseline vs test day 6 or test days 6-10) as the within-subjects factor or with paired samples t-tests. Total locomotor activity was analyzed using a two-way repeated measures ANOVA with Condition (VEH, LAG) and Day as within-subjects factors. Significant effects were further analyzed using an estimated marginal means procedure with a least significant differences correction. The normalized optical densities of each protein were analyzed using independent samples t-tests.
3.0 Results
3.1 5-Choice Serial Reaction Time
3.1.1 Baseline Performance
Baseline performance was defined as the average performance of the three training sessions immediately preceding surgery. Prior to surgery groups did not differ on any measure of interest (all F(1,18) < 2.53, P > 0.10; see Figure 2, Table 1).
Figure 2.
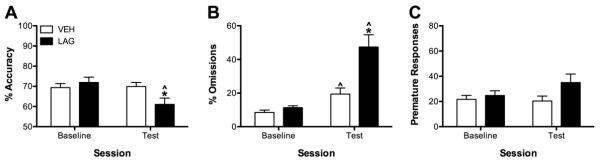
Effects of 6-day exposure to LAG on attention. Following 6-day exposure to LAG accuracy was reduced (A), omissions were increased (B), and premature responses were unchanged (C) relative to baseline and to VEH infused rats. LAG infusions did not affect premature response (C). *P < 0.05, LAG vs VEH; ^P < 0.05 test vs baseline for that condition.
Table 1.
Baseline performance on the 5-choice serial reaction time task (5CSRTT)
| % Accuracy |
% Omissions |
Premature Responses |
Magazine Entries |
Correct Latency (s) |
Reward Latency (s) |
||
|---|---|---|---|---|---|---|---|
|
6-
Day |
VEH | 68.91 ± 2.56 |
10.00 ± 1.66 |
25.50 ± 4.01 |
174.3 ± 19.9 |
0.77 ± 0.03 |
1.80 ± 0.13 |
|
| |||||||
| LAG | 68.21 ± 3.01 |
10.44 ± 1.96 |
24.73 ± 4.82 |
177.7 ± 24.0 |
0.77 ± 0.04 |
1.83 ± 0.15 |
|
|
| |||||||
|
14-
Day |
VEH | 70.82 ± 3.01 |
8.54 ± 2.78 | 19.61 ± 3.71 |
219.9 ± 36.1 |
0.80 ± 0.05 |
1.62 ± 0.07 |
|
| |||||||
| LAG | 75.69 ± 3.76 |
12.25 ± 0.92 |
24.87 ± 6.27 |
169.2 ± 35.3 |
0.75 ± 0.05 |
1.43 ± 0.13 |
|
Note: Baseline performance was the average performance of rats in the 3 training sessions prior to surgery. Rats were assigned to either the vehicle (VEH) or L-allylglycine (LAG) group based upon their performance during the baseline sessions. There were no significant differences between groups (all P > 0.05).
3.1.2 Standard Task: Test Day 6 vs. Baseline
The effects of chronic LAG administration of performance in the 5CSRTT on test day 6 are depicted in Figure 2 and Table 2. Accuracy of responding was dependent upon session and drug condition (drug × test interaction, F(1, 20) = 13.75, P < 0.01). Post-hoc analysis revealed that LAG-infused rats had significantly worse accuracy on Day 6 compared to baseline and performed worse than VEH-infused rats on Day 6 (both P < 0.01). Similarly, following cannulae implantations omissions were differentially affected by LAG and VEH infusions (drug × test interaction, F(1, 20) = 10.24, P < 0.01). Both LAG and VEH-infused rats made more omissions on Day 6 than during baseline however, the increase in omissions was significantly greater in LAG- compared to VEH-infused rats (P < 0.01). Lastly, reward retrieval latency was differentially affected by chronic LAG and VEH infusions (drug × test interaction, F(1, 20) = 4.48, P < 0.05). Comparing their performance on Day 6, LAG-infused rats took longer to retrieve rewards than they did during baseline (P < 0.01) and than VEH-infused rats (P < 0.05). Following surgery all rats took longer to respond correctly than they did prior to surgery (F(1, 20) = 10.09, P < 0.01), but this was not affected by condition (F(1, 20) = 3.04, P > 0.05). There was no effect of test day or infusion on either premature responses or magazine entries (all F < 2.52, P > 0.10).
Table 2.
Effects of short-term LAG infusions on 5CSRTT performance
| Measure | Condition | Baseline | Day 6 |
|---|---|---|---|
| Magazine Entries |
VEH | 213.96 ± 19.44 | 203.83 ± 24.07 |
|
| |||
| LAG | 173.46 ± 20.17 | 177.70 ± 28.11 | |
|
| |||
| Correct Latency |
VEH | 0.83 ± 0.03 | 0.88 ± 0.06^ |
|
| |||
| LAG | 0.76 ± 0.03 | 0.95 ± 0.07^ | |
|
| |||
| Reward Latency |
VEH | 1.83 ± 0.21 | 1.91 ± 0.11 |
|
| |||
| LAG | 1.63 ± 0.23 | 2.31 ± 0.12*^ | |
Note: Rats were implanted with chronic indwelling cannula connected to minipumps that infused either vehicle (VEH; artificial cerebral spinal fluid) or L-allylglycine (LAG; 3.2 μg/0.5 μL/hr). On day 6 following surgery, all rats (n=12 VEH, n=10 LAG) were tested on the standard 5-choice serial reaction time task (5CSRTT).
P < 0.05, significantly different from baseline for that drug condition;
P < 0.05, significantly different from VEH.
3.1.3 Standard Task: Comparison between Test Days 6-10
A subset of rats (n=6 VEH, n=5 LAG) continued to be tested on the standard 5CSRTT for additional four days (Days 7-10); data comparing baseline performance to performance on Days 6-10 are presented in Figure 3. With prolonged testing there was no effect of chronic LAG infusions on accuracy of performance (all F < 1.0, P > 0.05). In order to confirm the effect observed with short-term tests we conducted a two-way ANOVA comparing performance of VEH and LAG across baseline and Day 6. In this subset of rats, there was a trend towards a day × condition interaction (F(1,10) = 3.40, P < 0.10); that was caused by a reduction in accuracy from baseline to test for the LAG condition (P=0.07).
Figure 3.
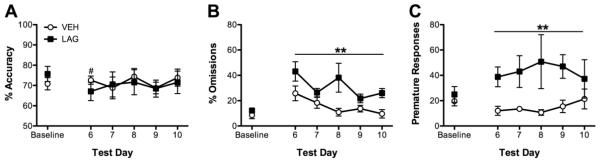
Effects of chronic LAG infusions on performance in the standard version of the 5CSRTT. Chronic infusions of LAG transiently affected accuracy (A) and increased both omissions (B) and premature responses (C). #P < 0.05 relative to baseline for LAG condition; **P < 0.01 from VEH.
Compared to VEH-infused rats, rats infused chronically with LAG omitted more trials (F(1, 9) = 13.68, P < 0.01) and the number of trials omissions differed across days of testing (F(5, 45) = 6.20, P < 0.01). Regardless of condition, rats omitted more trials on Day 6 and Day 10 than they did baseline (P<0.05). LAG-infused rats also made more premature responses than VEH-infused rats (F(1, 9) = 66.45, P < 0.01), regardless of day. Finally, correct response latency was affected by day of testing (F(1, 5) = 3.00, P < 0.05), but no day was significantly different from any other day (all P > 0.05). All other main effects and interactions were not significant (all F < 1.75, all P > 0.05, see Figure 3 and Table 3). In order to confirm the effect of LAG-exposure on reward latency observed following 6-day exposure to LAG, a two-way ANOVA comparing performance of VEH and LAG across baseline and test day 6 was conducted on data from the 13-day group. A significant day × condition interaction (F(1, 9) = 8.00, P < 0.05) was observed. Post-hoc analyses revealed that LAG animals took longer to retrieve the sugar pellets on day 6 than they did during the baseline session.
Table 3.
Effects of prolonged LAG infusion on performance in the 5CSRTT
| Measure | Condition | Baseline | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|---|
| Magazine Entries |
VEH | 219.8 ± 88.4 | 211.0 ± 36.1 | 203.5 ± 36.2 | 181.2 ± 25.5 | 222.8 ± 34.3 |
|
| ||||||
| LAG | 169.2 ± 79.0 | 224.6 ± 41.4 | 204.0 ± 23.0 | 273.2 ± 88.6 | 253.2 ± 89.3 | |
|
| ||||||
| Correct Latency |
VEH | 0.80 ± 0.12 | 0.88 ± 0.08 | 0.88 ± 0.05 | 078 ± 0.08 | 0.79 ± 0.04 |
|
| ||||||
| LAG | 0.75 ± 0.11 | 1.00 ± 0.16 | 0.90 ± 0.08 | 0.88 ± 0.05 | 0.77 ± 0.2 | |
|
| ||||||
| Reward Latency |
VEH | 1.62 ± 0.18 | 1.97 ± 0.09 | 2.17 ± 0.25 | 1.91 ± 0.09 | 1.72 ± 0.05 |
|
| ||||||
| LAG | 1.43 ± 0.30 | 2.47 ± 0.25 | 2.36 ± 0.20 | 2.54 ± 0.54 | 1.65 ± 0.08 | |
Note: Rats were implanted with chronic indwelling cannula connected to minipumps that infused either vehicle (VEH; artificial cerebral spinal fluid) or L-allylglycine (LAG; 3.2 μg/0.5 μL/hr). After a 5-day recovery, a subset of rats (n=6 VEH, n=5 LAG) were tested on the standard version of the 5-choice serial reaction time task (5CSRTT) for 5 consecutive days. Chronic infusion of LAG did not affect magazine entries, correct response latency or reward retrieval latency (all P > 0.05).
3.1.3 Short DS Task
Chronic LAG infusions increased omissions relative to VEH infusions (t(9) = 2.28, P < 0.05), but did not affect any other performance measures in the short DS task (all t(9) < 1.46, P > 0.10; see Table 4).
Table 4.
Performance on the short stimulus duration version of the 5-choice serial reaction time task
| % Accuracy |
% Omissions |
Premature Responses |
Magazine Entries |
Correct Latency (s) |
Reward Latency (s) |
|
|---|---|---|---|---|---|---|
| VEH | 58.58 ± 3.47 |
14.63 ± 6.24 |
18.83 ± 2.52 | 232.2 ± 43.0 |
0.74 ± 0.06 |
1.90 ± 0.18 |
| LAG | 66.24 ± 3.98 |
35.11 ± 6.38* |
24.80 ± 5.30 | 222.8 ± 86.2 |
0.69 ± 0.07 |
1.62 ± 0.15 |
Note: The stimulus duration in the long stimulus duration task is decreased from 0.5 sec to 0.25 sec thereby minimizing the attentional demands of the task.
P < 0.05, significantly different from VEH.
3.1.4 Long DS Task
Chronic LAG infusions of did not affect performance in the long DS task (all t(9) < 1.33, P > 0.10; see Table 5).
Table 5.
Performance on the long stimulus duration version of the 5-choice serial reaction time task
| % Accuracy |
% Omissions |
Premature Responses |
Magazine Entries |
Correct Latency (s) |
Reward Latency (s) |
|
|---|---|---|---|---|---|---|
| VEH | 84.39 ± 3.77 |
1.48 ± 0.62 | 16.50 ± 4.81 | 203.50 ±31.44 |
1.00 ±0.06 |
1.94 ± 0.12 |
|
| ||||||
| LAG | 90.40 ± 2.96 |
2.89 ± 2.10 | 15.20 ± 3.21 | 202.20 ± 47.85 |
1.12 ±0.08 |
1.74 ± 0.11 |
Note: The stimulus duration in the long stimulus duration task is increased from 1.0 sec to 5.0 sec thereby minimizing the attentional demands of the task. Chronic LAG did not affect performance on this task (all P > 0.05).
3.2 Locomotor Activity
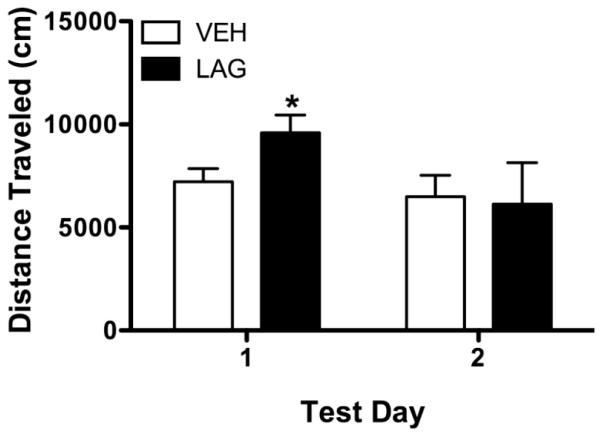
There was a trend for a day × condition interaction (F(1, 9) = 3.84, P = 0.08, see Figure 4), but no main effect of either condition (F(1,9) = 0.58, P > 0.05) or day (F(1,9) = 1.66, P > 0.05). Based on our previous research (Asinof and Paine, 2013), we hypothesized that LAG infusions would increase locomotor activity; thus we further explored the day × condition interaction. Rats with chronically infused with LAG had greater activity than rats chronically infused with VEH during the first activity (i.e., test day 12; P = 0.05) test, but not during the second activity test (i.e., test day 13; P >0.05).
Figure 4.
Effects of chronic LAG infusions on locomotor activity. Activity was assessed for 60-min after 5CSRTT testing on test days 12 and 13. During the first test rats chronically infused with LAG exhibited more activity than rats chronically infused with VEH, a similar effect was not seen during the second test. * indicates significantly different from VEH, P < 0.05.
3.3 Protein Expression
Because all data were normalized to actin expression, differences in actin expression were analyzed first. In the blots from the IL and PrL, actin expression did not differ between the VEH and LAG groups in either the 6-day or 13-day groups (all t < 1.71, all P > 0.1). In the blots from the NAc however, actin expression was significantly higher in 6-day LAG rats relative to 6-day VEH rats (t(10) = 3.19, P < 0.05, not shown).
Exposure to LAG for 6 days increased GAD67 expression in the PrL (t(9) = 3.71, P < 0.01 see Figure 5) and decreased GAD67 expression in the NAc (t(9) = −2.39, P < 0.05). Problematically, the NAc blot associated with reduced GAD67 expression was also associated with increased actin expression; the difference in actin expression may have contributed to the group difference in GAD67 expression in the LAG. All other comparisons were not significant (all t(9) < 1.65, all P > 0.05).
13-day exposure to LAG increased the GAD67 expression of in both the PrL and IL (both t(9) > 2.27, P < 0.001, see Figure 5). All other comparisons were not significant (all t(9) < 1.83, all P > 0.05).
4.0 Discussion
Chronic delivery of the GABA synthesis inhibitor LAG to the medial PFC caused a transient deficit in attention as measured by a decrease in accuracy and an increase in omissions and a more persistent change in premature responses and locomotor activity. Also, exposure to LAG lead to time-dependent increases in the expression of GAD67 in the PrL and the IL: shorter exposures caused increases in the IL while more prolonged exposure resulted in increases in both medial PFC subregions. Contrary to our hypothesis, chronic exposure to LAG did not, however, affect expression of GAT1, PV or the NDMA receptor NR1 subunit. Thus, contrary to our hypotheses decreasing GABA synthesis is not sufficient to cause changes in the expression of other GABA-related proteins nor lead to long-term changes in attention.
4.1 Effect of Chronic LAG on 5CSRTT Performance
Chronic infusions of the GABA synthesis inhibitor LAG into the medial PFC had a transient effect on attention as measured by the accuracy of responding and the omissions. Accuracy was decreased and omissions were increased on Day 6 (the first test day following surgery) but the accuracy deficit did not persist beyond the initial day of testing on the standard task. Furthermore, following 11 days of LAG exposure when rats were challenged with a more difficult version of the 5CSRTT, the short DS version of the task, LAG-treated rats did not exhibit an accuracy deficit. Combined these data suggest that there was not a persistent change in attention following prolonged exposure to LAG. In contrast, omissions were fairly consistently elevated across days of testing (with the exception that there was no difference in omissions during testing on the Long DS task). Moreover, the omissions did not increase when rats were challenged with the short DS task, suggesting that the difference in omissions is not a reflection of an attention deficit per se (see Asinof and Paine, 2014). These data are consistent with our previous work showing that acute administration of LAG increased omissions but did not affect accuracy in the standard or modified versions of the 5CSRTT (Asinof and Paine, 2013).
In addition to reflecting an attention deficit, increased omissions in the 5CSRTT can reflect changes in motivation to respond or alterations in locomotor activity (Asinof and Paine, 2013; Asinof and Paine, 2014; Robbins, 2002). It is possible that the initial increase in omissions reflects a decrease in motivation as the reward retrieval latency was also increased on Day 6 of testing. However, the reward retrieval latency in the LAG group returned to VEH levels by Day 10 of testing and the reward retrieval latency of the two groups did not differ during the Short DS test suggesting that a change in motivation cannot account for the prolonged increase in omissions. In addition, it is possible that increased locomotor activity caused the increase in omissions (see Asinof and Paine, 2014). Increased locomotor activity can interfere with a rats’ ability to engage in the task thereby increasing the number of missed trials. Although locomotor activity was not measured on Day 6 of testing, it was increased on Day 12 of testing. Furthermore, we have previously observed that acute intra-cortical LAG infusions increase locomotor activity (Asinof and Paine, 2013).
Although prolonged exposure to LAG did not affect attention as measured by a change in accuracy, it did increase impulsive responding. Following prolonged chronic LAG infusions, rats made more premature responses in the standard task than did rats in control group. This is consistent previous observations of increased premature responding following acute administration of GABA synthesis inhibitors (Asinof and Paine, 2013; Pehrson et al., 2013). Consist with these findings, Reichel et al (2015) observed an impairment in reversal learning in mice with medial PFC GABA neuron lesions. Moreover, Jupp et al. (2013) recently found that trait-like impulsivity is associated with decreases in GABAA receptor expression in regions of the medial PFC. Similarly, decreases in cortical GABA levels have been associated with increased impulsivity in humans (Boy et al., 2011; Silveri et al., 2013). Combined, these data suggest that a reduction in GABA synthesis is associated with enhanced impulsive behavior.
4.2 Effect of Chronic LAG on Protein Expression
It has been hypothesized that decreased expression of GAD67 in PV-containing interneurons in the dorsolateral PFC underlies other alterations in GABA transmission observed in the brains of people with schizophrenia (Curley et al., 2011; Hashimoto et al., 2003; Volk et al., 2000). Moreover, it has been speculated changes in GAD67 expression lead to the observed changes in GAT1 and PV expression in this disease (see Curley et al., 2013; Rotaru et al., 2011). For example, the observed reduction in PV has been speculated to be a compensatory response to decreased GAD67 function since increased levels of intracellular PV are associated with decreased GABA release (see Rotaru et al., 2011 and references there in). Here, we find that decreasing GABA synthesis with LAG is not sufficient to cause changes in the expression GAT1, PV or NR1 within the PrL or IL. Problematically, GAD67 expression was increased following both 6-day and 13-day exposure to LAG, suggesting that GABA function may have been at least partially restored at the time point that the protein analyses were conducted. Restored GABA function may have limited the impact that blocking GABA synthesis had on the expression of these other GABA-related proteins. That said, the current data are consistent with that of Curley et al (2013) who found that mice with heterozygous knockout of GAD67 or those with a partial knockout of GAD67 in the PV-containing neurons did not exhibit changes in the expression of GAT1 or PV. Combined, these two studies suggest that reduced GAD function is not sufficient to alter other proteins commonly observed to show changes in expression in schizophrenia and thus imply that some other factor(s) causes the changes in the expression of these other proteins.
As mentioned above, there were time-dependent increases in the expression of GAD67 in the IL and PrL. Conversely, there was a modest decrease in GAD67 expression in the NAc, suggesting that GAD67 was not uniformly increased by intra-PFC LAG infusions. It is unclear why the pattern of GAD67 expression in IL and PrL in the 6-day and 13-day groups is different (a greater increase in the PrL in the 6-day group and a greater increase in the IL in the 13-day group). It is however, possible that the cannulae placements in the 13-day group were deeper than those in the 6-day group; because tissue punches were collected we were unable to assess the relative depth of the cannulae. Nevertheless, it is likely that the increase in GAD67 expression reflects a compensatory upregulation of GAD67 aimed at normalizing GABA synthesis and release that is inhibited by LAG. We chose to look at protein expression at the 6-day and the 13-day time points because these time points correspond to the beginning and ending of testing. Moreover, we chose to begin behavioral testing on Day 6 in order to allow for a) recovery from surgery and b) sufficient time for the decrease in GAD function to cause changes on the expression of other proteins (e.g., PV, GAT1, NR1). That said, even following 6 days of LAG exposure there is a compensatory upregulation in GAD67 in the PrL.
Interestingly, the increase in GAD67 does not appear to be sufficient to compensate for behavioral deficits caused by chronic LAG infusions. Attention was impaired following 6-day of LAG exposure and response inhibition was impaired following more prolonged exposure to LAG. It should also be noted, the there was a greater increase in GAD67 in the PrL in the 6-day exposure group, the time point when attention was impaired. Notably, lesions and inactivation of the PrL are consistently associated with impaired attention in the 5CSRTT (Chudasama et al., 2003; Passetti et al., 2002). In contrast, GAD67 was increased in the IL in the 13-day exposure group, the time point when impulsivity was increased. Lesions or inactivation of the IL are associated with increases in premature responses in the 5CSRTT (Chudasama et al., 2003; Murphy et al., 2012). Given this association, it is possible that the different behavioral effects observed in the 6-day and 13-day exposure groups is a consequence of the location where GAD67 was maximally upregulated. It seems likely that the area of maximal GAD67 change is associated with the largest disruption in GABA function. Future research is needed to test this hypothesis.
4.3 Limitations and Conclusions
This was a proof-of-concept study aimed at determining whether chronically reducing GAD67 function was sufficient to cause changes in attention and other GABA-related proteins. The approach taken, namely using LAG to block GAD67 is not without its limitations. First, LAG inhibits the action of both the 65-kilodalton isoform of GAD (GAD65) and GAD67, while only reductions in GAD67 expression are commonly observed psychiatric illnesses such as schizophrenia (Glasier et al., 2015; Lewis, 2014). Moreover, LAG affects GAD67 and GAD65 function in all varieties of cortical GABA neurons, while PV-containing GABA neurons are most commonly implicated in schizophrenia (Hashimoto et al., 2003; Curley et al., 2011). Thus, some caution must be used in extending these findings to psychiatric conditions. Indeed, global changes in GABA concentrations, per se, have not been consistently found in psychiatric conditions such as schizophrenia (Kegeles et al., 2012) suggesting that more subtle changes in the release characteristics of GABA rather than widespread decreases in GABA synthesis may contribute to the cognitive deficits observed. Finally, because tissue punches were taken from the IL and PrL, we were unable to verify the cannulae placement and thus the exact location where GAD function was compromised; this may impact that pattern of cognitive deficits that we observed. That said, the findings from current experiment are corroborated, at least to some degree, but findings of other experiments using other techniques to decrease GABA function (Curley et al., 2013; Reichel et al., 2015). In conclusion, prolonged changes in GABA synthesis in the medial PFC do not alter the expression of PV, GAT1 or NR1 or cause attention deficits; these changes do however, cause a deficit in impulse control.
Highlights.
Chronic inhibition of GABA synthesis increased impulsivity and locomotor activity.
Chronic inhibition of GABA synthesis only transiently impaired attention.
Chronic inhibition of GABA synthesis did not affect PV, GAT1 or NR1 expression.
Acknowledgements
This work was supported by NIH grant R15MH098246 awarded to TAP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 References
- Asinof SK, Paine TA. The 5-choice serial reaction time task: a task of attention and impulse control for rodents. Journal of Visualized Experiments. 2014;90:e51574. doi: 10.3791/51574. doi: 10.3791/51574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asinof SK, Paine TA. Inhibition of GABA synthesis in the prefrontal cortex increases locomotor activity but does not affect attention in the 5-choice serial reaction time task. Neuropharmacology. 2013;65:39–47. doi: 10.1016/j.neuropharm.2012.09.009. doi: 10.1016/j.neuropharm.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger ML, Floresco SB. Prefrontal cortical GABA modulation of spatial reference and working memory. Int J Neuropsychopharmacol. 2014;18:1–11. doi: 10.1093/ijnp/pyu013. doi: 10.1093/ijnp/pyu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU. Transcriptional dysregulation of γ-aminobutyric acid transporter in parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. Psychiatry Res. 2014;220:1155–1159. doi: 10.1016/j.psychres.2014.09.016. doi: 10.1016/j.psychres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Lawrence AD, Singh KD, Husain M, Sumner P. Dorsolateral prefrontal γ-aminobutyric acid in mend predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr. 2014;2:70. doi: 10.3389/fped.2014.00070. doi: 10.3389/fped.2014.00070. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-MA, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, Schroeder CE, Kegeles LS. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. doi: 10.1016/j.nicl.2014.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Zanoveli JM, Ledvinka-Filho E, Brandão ML. L-allylglycine dissociates the neural substrates of fear in the periaqueductal gray of rats. Brain Res Bull. 2010;81:416–423. doi: 10.1016/j.brainresbull.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Eggan SM, Lazarus MS, Huang J, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: Implications for schizophrenia. Neurobiol Dis. 2013;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Kimoto S, Fish KN, Lewis DA. Lower glutamic acid decarboxylase 65-kDa isoform messenger RNA and protein levels in the prefrontal cortex in schizoaffective disorder but not schizophrenia. Biol Psychiatry. 2015;77:167–176. doi: 10.1016/j.biopsych.2014.05.010. doi: 10.1016/j.biopsych.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Fish KN, Lewis DA. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36. doi: 10.1038/mp.2013.152. doi: 10.1038/mp.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mol Psychiatry. 2014;19:140. Erratum in: [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gruber T, Müller MM, Keil A, Elbert T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol. 1999;110:2074–2085. doi: 10.1016/s1388-2457(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RW, Chapman AG, Meldrum BS. Regional changes in cerebral GABA concentration and convulsions produced by D and by L-allylglycine. J Neurochem. 1978;30:1501–1504. doi: 10.1111/j.1471-4159.1978.tb10484.x. [DOI] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Saigal N, Revert I, Shrestha S, Cumming P, Everitt BJ, Robbins TW, Dalley JW. Dopaminergic and GABA-ergic markers of impulsivity in rats: evidence for anatomical localization in ventral striatum and prefrontal cortex. Eur J Neurosci. 2013;37:1519–1528. doi: 10.1111/ejn.12146. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joëls M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando ABP, Urcelay GP, Robinson ESJ, Mar AC, Theobald DEH, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. (2012) DOI 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Slipp LE, Carlezon WA., Jr Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th Ed Academic Press; Amsterdam, Netherlands: 2009. [Google Scholar]
- Pehrson AL, Bondi CO, Totah NK, Moghaddam B. The influence of NMDA and GABA(A) receptors and glutamic acid decarboxylase (GAD) activity on attention. Psychopharmacology (Berl) 2013;225:31–39. doi: 10.1007/s00213-012-2792-z. doi: 10.1007/s00213-012-2792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel JM, Nissel S, Rogel-Salazar G, Mederer A, Käfer K, Bedenk BT, Martens H, Anders R, Grosche J, Michalski D, Härtig W, Wotjak CT. Distinct behavioral consequences of short-term and prolonged GABAergic depletion in prefrontal cortex and dorsal hippocampus. Front Behav Neurosci. 2015;8:452. doi: 10.3389/fnbeh.2014.00452. doi: 10.3389/fnbeh.2014.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Galliant J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.034. in press. doi: 10.1016/j.biopsych.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, Rosso IM, Jensen JE. Frontal lobe γ-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol Psychiatry. 2013;74:296–304. doi: 10.1016/j.biopsych.2013.01.033. doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Tse MT, Piantadosi PT, Floresco SB. Prefrontal Cortical Gamma-Aminobutyric Acid Transmission and Cognitive Function: Drawing Links to Schizophrenia from Preclinical Research. Biol Psychiatry. 2015;77:929–939. doi: 10.1016/j.biopsych.2014.09.007. doi: 10.1016/j.biopsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy J-M, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: Decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase 67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]