Abstract
Context
Cancer patients experience a broad range of physical and psychological symptoms as a result of their disease and its treatment. On average, these patients report ten unrelieved and co-occurring symptoms.
Objectives
To determine if subgroups of oncology outpatients receiving active treatment (n=582) could be identified based on their distinct experience with thirteen commonly occurring symptoms; to determine whether these subgroups differed on select demographic, and clinical characteristics; and to determine if these subgroups differed on quality of life (QOL) outcomes.
Methods
Demographic, clinical, and symptom data from one Australian and two U.S. studies were combined. Latent class analysis (LCA) was used to identify patient subgroups with distinct symptom experiences based on self-report data on symptom occurrence using the Memorial Symptom Assessment Scale (MSAS).
Results
Four distinct latent classes were identified (i.e., All Low (28.0%), Moderate Physical and Lower Psych (26.3%), Moderate Physical and Higher Psych (25.4%), All High (20.3%)). Age, gender, education, cancer diagnosis, and presence of metastatic disease differentiated among the latent classes. Patients in the All High class had the worst QOL scores.
Conclusion
Findings from this study confirm the large amount of interindividual variability in the symptom experience of oncology patients. The identification of demographic and clinical characteristics that place patients are risk for a higher symptom burden can be used to guide more aggressive and individualized symptom management interventions.
Keywords: symptom clusters, latent class analysis, gender differences, age differences, symptom profiles
Introduction
Cancer patients experience a broad range of physical and psychological symptoms as a result of their disease and its treatment. On average, patients report ten unrelieved and co-occurring symptoms.1 However, clinical experience and emerging evidence2–7 suggest that a large amount of inter-individual variability exists in patients’ symptom experiences.
To develop a better understanding of this inter-individual variability, we conducted a number of studies using cluster analysis2,6 or latent class analysis (LCA)4,5 to identify subgroups of oncology patients based on their severity ratings for four common symptoms (fatigue, pain, sleep disturbance, depression). In the first two studies done in the U.S.6 and Israel,2 four distinct subgroups of oncology patients were identified using hierarchical cluster analysis. Of note, approximately 15% of these patients reported high levels (i.e., All High subgroup) and 35% reported low levels (i.e., All Low subgroup) of all four symptoms. In both of these studies, compared to the All Low subgroup, patients in the All High subgroup were significantly younger and less likely to be married or partnered. In addition, the All High subgroup reported poorer functional status and lower quality of life (QOL) scores.
In two of our recent studies, LCA was used to identify subgroups of oncology patients and their family caregivers5 or subgroups of patients with breast cancer4 based on their severity ratings for the same four symptoms. In these two studies, three distinct subgroups were identified, with between 7%4 and 12%5 of the participants being classified in the All High subgroup. Consistent with our previous reports,2,6 compared to the All Low subgroup, participants in the All High subgroup were significantly younger and had a lower functional status.
In another group of studies that used symptom occurrence ratings from the Memorial Symptom Assessment Scale (MSAS)8 or symptom severity ratings from the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30)9 to identify patients with a higher symptom burden, two10–12 or three7,13 subgroups were identified. In all five studies,7,10–13 All Low and All High symptom subgroups were identified. Although the demographic and clinical characteristics that were associated with a higher symptom burden were not consistent across these five studies, patients in the All High subgroup reported statistically significant and clinically meaningful decrements in functional status and QOL. The reasons for these inconsistent findings on number of subgroups identified, as well as the predictors of symptom subgroup membership,10–13 may relate to differences in: sample sizes; the demographic and clinical characteristics of the participants; the number of symptoms evaluated; the dimension of the symptom experience used to create the subgroups; and the statistical procedures employed.
Given the high prevalence of co-occurring symptoms and the large amount of inter-individual variability in oncology patients’ symptom experiences, findings from the studies cited above suggest that the identification of subgroups of patients with a higher symptom burden may assist clinicians to provide more aggressive and individualized symptom management. Given this promising, albeit limited amount of research, the purposes of this study were to determine if subgroups of oncology outpatients receiving active treatment (n=582) could be identified based on their distinct experience with thirteen common symptoms; to determine whether these subgroups differed on select demographic and clinical characteristics; and to determine if these subgroups differed on QOL outcomes.
Methods
Study Samples
Demographic, clinical, and symptom data from one Australian study (i.e., Symptom Clusters Study) and two U.S. studies (i.e., Fatigue, Pain, and Sleep Study (FPS Study), Symptom Prevalence Study) were combined to conduct this analysis. All three studies enrolled patients who were receiving active treatment for their cancer. Detailed information on recruitment procedures, study methods, and sample characteristics for these studies is published elsewhere.14,15 A brief summary of each of the studies is presented below. All three studies were approved by Human Subjects Committees. All of the patients signed written informed consent prior to enrollment.
Symptom Clusters Study
This study was designed to identify symptom clusters and their effects on the physical and psychological functioning of patients with metastatic disease. Patients were recruited consecutively from two major tertiary referral hospitals in Australia. Eligible patients were adults (>18 years of age) who could read, write, and understand English; had no cognitive limitations; had a primary cancer of breast, lung, colon/rectum, prostate, upper gastrointestinal (GI) tract, or ovaries; were diagnosed with metastatic disease in the past month or had clinical evidence of progressive metastatic disease; and had a prognosis between four months and two years as determined by their clinician. Questionnaires were completed during a 20 minute face-to-face interview conducted by trained interviewers. Demographic and clinical data were obtained from medical record reviews.
FPS Study
This study evaluated multiple symptoms in patients who underwent primary or adjuvant radiation therapy (RT). Patients were recruited from two RT departments and were eligible to participate if they: were ≥18 years of age; were scheduled to receive primary or adjuvant RT for breast, prostate, lung, or brain cancer; were able to read, write, and understand English; and had a Karnofsky Performance Status (KPS) score of ≥60. Patients were excluded if they had: metastatic disease, more than one cancer diagnosis, or a diagnosed sleep disorder. Patients completed the study questionnaires at the time of their simulation visit. Medical records were reviewed for disease and treatment information.
Symptom Prevalence Study
This study used self-report questionnaires to obtain information from a convenience sample of oncology outpatients. Patients were recruited from four outpatient settings and were eligible to participate if they were >18 years of age; were able to read, write, and understand English; had KPS scores of ≥50; and were receiving active cancer treatment. Patients completed the study questionnaires in their home and returned them to the research office using a postage paid envelope.
Instruments
Demographic and Clinical Characteristics
Demographic information on age, gender, marital status, and living arrangements were obtained at enrollment. Because of differences in the educational systems in Australia and the U.S., data on education were recoded into a dichotomous variable (i.e., no post high school versus post high school education). In addition, patients’ medical records were reviewed for cancer diagnosis, presence of metastatic disease, and current treatment regimens (i.e., none, chemotherapy (CTX), RT, or both CTX and RT).
In the Australian study, patient’s functional status was rated by their clinician using the Eastern Cooperative Oncology Group (ECOG) Performance Status score that ranged from 0 (fully active) to 4 (disabled).16 In the U.S. studies, patients rated their functional status using the KPS scale.17 Based on the recommendations of Verger and colleagues,18 the KPS scores were converted to ECOG scores for use in subsequent analyses.
MSAS
All three studies used the MSAS to evaluate the occurrence, severity, frequency, and distress of 32 symptoms commonly associated with cancer and its treatment.8 The MSAS is a self-report questionnaire designed to measure the multidimensional experience of symptoms. Using the MSAS, patients were asked to indicate whether or not they had experienced each symptom in the past week (i.e., symptom occurrence). If they had experienced the symptom, they were asked to rate its frequency of occurrence, severity, and distress.
From the 32 items on the MSAS, the total number of symptoms reported by each patient was calculated. In addition, three subscale scores (i.e., Global Distress Index, physical (MSAS PHYS), psychological (MSAS PSYCH)) and a total MSAS score were calculated.8 The reliability and validity of the MSAS is well established. Cronbach’s alphas for the physical subscale, psychological subscale, Global Distress Index, and total MSAS score were 0.82, 0.77, 0.83, and 0.87, respectively.
Multidimensional Quality of Life Scale- Cancer (MQOLS-CA)
In the two U.S. studies, the MQOLS-CA was used to evaluate QOL. The MQOLS-CA comprises 33 items that measure four dimensions of QOL (i.e., physical well-being, psychological well-being, social well-being, spiritual well-being) in cancer patients. Each item is rated on a 0 to 10 scale.19 A total QOL score, as well as subscale scores, were calculated, with higher scores indicating a better QOL. MQOLS-CA data from the two U.S. studies were combined and the resultant Cronbach’s alpha for the MQOLS-CA total score was 0.94.
Statistical Analysis
The three data sets were combined and data were analyzed using SPSS version 20. Descriptive statistics, means, and standard deviations for quantitative variables and frequencies and percentages for categorical variables were generated to describe various patient characteristics.
LCA was used to identify subgroups of patients (i.e., latent classes) with similar symptom experiences.20,21 Whereas the MSAS evaluates the occurrence, frequency, severity, and distress associated with 32 symptoms, for this analysis and consistent with previous studies,7,11 the LCA was performed based on patients’ ratings of symptom occurrence.
LCA identifies latent classes based on an observed response pattern.22,23 In order to have a sufficient number of patients with each symptom to perform the LCA, the symptoms that occurred in ≥40% of the patients were used to identify the distinct latent classes. A total of 13 of 32 symptoms from the MSAS occurred in ≥40% of the patients.
The final number of latent classes was identified by evaluating the Bayesian Information Criterion (BIC) and entropy. The model that fits the data best has the lowest BIC.24 In addition, well-fitting models produce entropy values of ≥0.80.25 Finally, well-fitting models “make sense” conceptually and the estimated classes differ as might be expected on variables not used in the generation of the model.24
The LCA was performed using Mplus™ Version 7.26,27 Estimation was carried out with robust Maximum-Likelihood (MLR) and the Expectation-Maximization (EM) algorithm.20 The LCA was done in two stages. First, the number of latent classes that fit the data best, without covariates (i.e., the unconditional model) was identified. Then six covariates that were associated with symptom occurrence were evaluated in the LCA (i.e., age; gender; ECOG Performance Status in two groups [i.e., high performance (ECOG 0 and 1) versus low performance (ECOG 2, 3, 4)]; diagnosis in two groups [other versus lung cancer]; radiation treatment [yes/no], CTX treatment [yes/no]). Each covariate was evaluated separately, outside the model, for its potential usefulness in improving model fit using the R3STEP procedure.26 Then the covariates that were significant predictors of latent class membership were examined within the model. That is, these covariates provided information about differences among the latent classes as part of model estimation. Initially, as part of this analysis, the complete set of covariates was examined jointly. Covariates were removed from the model if they were not significant within the model. The Wald Chi-squared statistic was used to evaluate significance at a P-value of <0.05.
After identifying the latent class solution that best fit the data, differences among the latent classes, in demographic and clinical characteristics, MSAS total and subscale scores, and QOL outcomes were evaluated using analyses of variance and Chi-square analyses. A P-value of <0.05 was considered statistically significant. Post hoc contrasts were done using a Bonferroni corrected P-value of 0.008 (0.05/6 pairwise comparisons).
Results
Latent Class Analysis
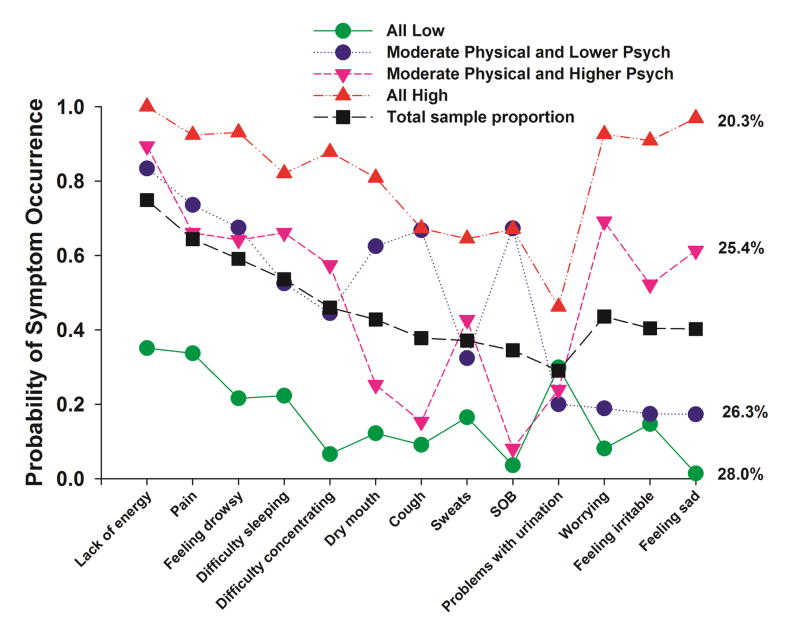
A total of 13 symptoms from the MSAS occurred in ≥40% of the patients (Fig. 1). Using LCA, four distinct subgroups of patients were identified based on their ratings of the occurrence of these symptoms. Fit indices for the candidate models are shown in Table 1. The four-class solution was selected because its BIC was lower than the BIC for both the three- and five-class solutions. The four covariates that were included in the final model were: age, ECOG Performance Status in two groups (high versus low), diagnosis in two groups (lung cancer versus other), and radiation treatment (yes versus no).
Fig. 1.
Probability of symptom occurrence for the total sample (i.e., total sample proportion) and each of the latent classes for the 13 symptoms on the Memorial Symptom Assessment Scale that occurred in ≥40% of the total sample (n=582).
Table 1.
Latent Class Fit Indices for Two- Through Five-Class Solutions
| Model | Unconditional Model | Model with Covariates | ||||
|---|---|---|---|---|---|---|
| LL | BIC | Entropy | LL | BIC | Entropy | |
| 2 Class | −4590.08 | 9352.65 | .80 | −4454.23 | 9105.81 | .80 |
| 3 Class | −4469.58 | 9201.09 | .78 | −4325.71 | 8963.38 | .79 |
| 4 Classa | −4400.73 | 9152.83 | .77 | −4230.63 | 8887.82 | .78 |
| 5 Class | −4361.80 | 9164.40 | .80 | −4176.03 | 8893.21 | .80 |
The four-class solution was selected because the BIC was lower than in both the 3- and 5-class solutions. This difference was consistent when including four covariates in the model.
Abbreviations: BIC= Bayesian Information Criterion; LL = log-likelihood
As summarized in Table 2 and illustrated in Fig. 1, the largest percentage of patients (28.0%, n=163) was classified in the “All Low” class. Probability of occurrence for the MSAS symptoms for this class ranged from 0.01 to 0.35. The second largest class (26.3%, n=153) was classified as the “Moderate Physical and Lower Psych” class. Probability of occurrence for the MSAS symptoms for this class ranged from 0.17 to 0.83. Although these patients reported moderate occurrence rates for the majority of the physical symptoms (i.e., lack of energy, pain, feeling drowsy, difficulty sleeping, difficulty concentrating, dry mouth, cough, sweats, shortness of breath, difficulty with urination), they reported relatively low occurrence rates for the psychological symptoms (i.e., worrying (0.19), feeling irritable (0.17), feeling sad (0.17)). The third class (25.4%, n=148) was classified as the “Moderate Physical and Higher Psych” class. Probability of occurrence for the MSAS symptoms for this class ranged from 0.08 to 0.89. However, the probability of occurrence of the psychological symptoms was relatively high (i.e., worrying (0.69), feeling irritable (0.52), feeling sad (0.61)). The final class consisted of 20.3% (n=118) and was called the “All High” class. Probability of occurrence for the MSAS symptoms ranged from 0.46 to 1.0.
Differences in Patient Characteristics Among the Latent Classes
Table 2 summarizes the differences in demographic and clinical characteristics among the four latent classes. Compared to the All Low and the Moderate Physical and Lower Psych classes, patients in the Moderate Physical and Higher Psych and the All High classes were significantly younger. Compared to the All Low class, a higher percentage of patients in the other three latent classes were female. A smaller percentage of patients in the Moderate Physical and Lower Psych class had completed post high school education compared to the other three latent classes. Compared to the All Low and the Moderate Physical and Higher Psych classes, a higher percentage of patients in the Moderate Physical and Lower Psych and the All High classes had a diagnosis of lung cancer, had metastatic disease, and reported a lower performance status.
Table 2.
Differences in demographic and clinical characteristics among the four latent classes
| Characteristic | All Low (1) n = 163 (28.0%) |
Moderate Physical and Lower Psych (2) n = 153 (26.3%) |
Moderate Physical and Higher Psych (3) n = 148 (25.4%) |
All High (4) n = 118 (20.3%) |
Statistics |
|---|---|---|---|---|---|
|
| |||||
| Age in years (mean ± SD) | 66.0 (10.3) | 64.3 (10.5) | 56.3 (11.9) | 56.7 (12.7) | F(3,578)=29.11 p<.0001 1 and 2 > 3 and 4 |
|
| |||||
| Age range (years) | 29 to 86 | 35 to 86 | 24 to 83 | 20 to 84 | |
|
| |||||
| % | % | % | % | ||
|
| |||||
| Female* | 37.4 | 58.2 | 60.1 | 68.6 | χ2 = 31.42 p<.0001 1 < 2, 3, and 4 |
|
| |||||
| Lives alone | 22.5 | 28.5 | 24.3 | 29.7 | NS |
|
| |||||
| Married/partnered | 69.8 | 55.9 | 61.0 | 55.6 | χ2 = 8.32 p=.040 No significant contrasts |
|
| |||||
| Education – Post high school* | 65.8 | 45.0 | 69.2 | 65.8 | χ2 = 22.93 p<.0001 2 < 1, 3, and 4 |
|
| |||||
| Diagnosis | χ2 = 120.92 p<.0001 3>1 1 > 2, 3, and 4 3 > 2 and 4 2 and 4 > 1 and 3 NS |
||||
| Breast | 23.9 | 32.0 | 45.3 | 35.6 | |
| Prostate | 47.9 | 12.4 | 25.7 | 12.7 | |
| Lung | 5.5 | 30.1 | 1.4 | 16.9 | |
| Other | 22.7 | 25.5 | 27.7 | 34.7 | |
|
| |||||
| Lung versus other | χ2 = 65.83 p<.0001 1 and 3 < 2 and 4 |
||||
| Lung cancer* | 5.5 | 30.1 | 1.4 | 16.9 | |
| Other cancer | 94.5 | 69.9 | 98.6 | 83.1 | |
|
| |||||
| Metastasis* | 22.8 | 46.4 | 25.7 | 47.5 | χ2 = 33.07 p<.0001 1 and 3 < 2 and 4 |
|
| |||||
| Treatment | χ2 = 57.56 p<.0001 2 > 1 and 3 1 > 2, 3, and 4 4 > 1 3 and 4 > 2 |
||||
| None | 7.4 | 28.1 | 12.8 | 15.3 | |
| Only radiation | 61.3 | 35.9 | 42.6 | 30.5 | |
| Only chemotherapy | 20.2 | 28.8 | 26.4 | 35.6 | |
| Both | 11.0 | 7.2 | 18.2 | 18.6 | |
|
| |||||
| ECOG Performance Status | χ2 = 87.96 p<.0001 1 and 3 > 2 and 4 |
||||
| Higher performance (ECOG 0 and 1)* | 87.1 | 52.9 | 86.5 | 49.2 | |
| Lower performance (ECOG 2, 3, 4) | 12.9 | 47.1 | 13.5 | 50.8 | |
Reference group
Abbreviations: ECOG = Eastern Coopertive Oncology Group; SD = standard deviation
Differences in MSAS Summary Scores Among the Latent Classes
Table 3 summarizes differences among the latent classes in total number of symptoms reported (out of 32), as well as differences in MSAS summary scores. In terms of total number of symptoms, compared to patients in the other three classes, patients in the All Low class reported the lowest number of symptoms. In addition, compared to the Moderate Physical and Lower Psych and the Moderate Physical and Higher Psych classes, patients in the All High class reported a significantly higher number of symptoms. For the PSYCH subscale score and the Global Distress Index, the differences among the four latent classes had the same pattern (i.e., All Low < Moderate Physical and Lower Psych < Moderate Physical and Higher Psych < All High). For the PHYS subscale score, the differences among the four classes had the following pattern: All Low < Moderate Physical and Higher Psych < Moderate Physical and Lower Psych < All High. For the MSAS total score, the post hoc contrasts demonstrated the following differences among the latent classes: 1) All Low class < Moderate Physical and Lower Psych, Moderate Physical and Higher Psych, and All High classes and 2) Moderate Physical and Lower Psych and Moderate Physical and Higher Psych classes < All High class.
Table 3.
Differences in Memorial Symptom Assessment Scale (MSAS) summary scores among the four latent classes
| MSAS scores | All Low (1) n = 163 (28.0%) |
Moderate Physical and Lower Psych (2) n = 153 (26.3%) |
Moderate Physical and Higher Psych (3) n = 148 (25.4%) |
All High (4) n = 118 (20.3%) |
Statistics |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Total number of symptoms | 3.88 (2.41) | 10.72 (3.87) | 10.74 (3.56) | 20.26 (5.24) | F(3,578)=427.54 p<.0001 1 < 2, 3, and 4 2 and 3 < 4 |
| MSAS PSYCH Subscale score | 0.17 (0.23) | 0.58 (0.46) | 1.17 (0.61) | 1.89 (0.59) | F(3,578)=327.211 p<.0001 1 < 2 < 3 <4 |
| MSAS PHYSICAL Subscale score | 0.25 (0.27) | 0.90 (0.53) | 0.75 (0.45) | 1.48 (0.51) | F(3,578)=176.08 p<.0001 1 <3 <2 <4 |
| MSAS Global Distress Index | 0.25 (0.26) | 0.85 (0.54) | 1.11 (0.51) | 1.91 (0.50) | F(3,578)=302.14 p<.0001 1 <2 <3 <4 |
| MSAS Total score | 0.22 (0.15) | 0.71 (0.34) | 0.70 (0.28) | 1.35 (0.39) | F(3,578)=328.85 p<.0001 1 < 2, 3, and 4 2 and 3 < 4 |
Abbreviation: SD = standard deviation
Differences in Quality of Life Scores Among the Latent Classes
For the two studies conducted in the U.S., differences among the latent classes in MQOLS-CA subscale and total scores are summarized in Table 4. Except for the spiritual well-being scores, the post hoc contrasts demonstrated the following differences among the latent classes for the remaining MQOLS-CA subscale scores as well as the total QOL score: 1) All Low class > Moderate Physical and Lower Psych, Moderate Physical and Higher Psych, and All High classes and 2) Moderate Physical and Lower Psych and Moderate Physical and Higher Psych classes > All High class.
Table 4.
Differences in Multidimensional Quality of Life Scale-Cancer (MQOLS-CA) subscale and total scores among the four latent classes*
| MQOLS scores | All Low (1) n = 119 (33.8%) |
Moderate Physical and Lower Psych (2) n = 56 (15.9%) |
Moderate Physical and Higher Psych (3) n = 99 (28.1%) |
All High (4) n = 78 (22.2%) |
Statistics |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Physical well-being score | 8.35 (1.35) | 6.59 (1.74) | 7.09 (1.48) | 5.58 (1.58) | F(3,348)=56.02 p<.0001 1 > 2, 3, and 4 2 and 3 > 4 |
| Psychological well-being score | 7.65 (1.49) | 6.08 (1.79) | 5.66 (1.71) | 4.43 (1.65) | F(3,346)=64.17 p<.0001 1 > 2, 3, and 4 2 and 3 > 4 |
| Social well-being score | 7.76 (1.67) | 6.25 (2.13) | 5.79 (2.07) | 4.69 (1.90) | F(3,347)=43.10 p<.0001 1 > 2, 3, and 4 2 and 3 > 4 |
| Spiritual well-being score | 5.31 (1.84) | 5.87 (2.17) | 5.26 (2.19) | 5.06 (2.21) | F(3,348)=1.76 p=.155 |
| Total quality of life score | 7.41 (1.19) | 6.16 (1.37) | 5.90 (1.34) | 4.79 (1.21) | F(3,342)=67.96 p<.0001 1 > 2, 3, and 4 2 and 3 > 4 |
Data from the two Unites States’ studies
Abbreviation: SD = standard deviation
Discussion
Rank Order of Symptom Occurrence Among the Latent Classes
This study is the first to use LCA and to incorporate clinically meaningful covariates into the LCA to identify distinct subgroups of oncology patients based on their reports of the occurrence of 13 common symptoms. In this relatively large and heterogeneous sample in terms of cancer diagnoses, the percentages of patients were distributed relatively evenly across the four latent classes. However, consistent with previous studies,7,12,13 an “All Low” and an “All High” symptom subgroup were identified. Of note, patients in the All High class reported an average of 20 of 32 MSAS symptoms, which is higher than the mean of 10 symptoms reported in a systematic review.1 These consistent findings suggest that future studies of symptom burden in oncology patients need to employ more sophisticated statistical approaches to identify higher risk patients. The reliance on mean number of symptoms will over- and underestimate symptom burden and not allow for the identification of patients who warrant more intensive symptom management.
This study is the first to identify two subgroups of oncology patients who reported moderate levels of physical symptoms but differentiated on the occurrence of three psychological symptoms (i.e., worrying, feeling irritable, feeling sad). As shown in Table 5, for patients in the Moderate Physical and Higher Psych class, worrying (0.69), feeling sad (0.61), and feeling irritable (0.52) were among the top eight symptoms. In contrast, in the Moderate Physical and Lower Psych class, worrying (0.19), feeling sad (0.17), and feeling irritable (0.17) were the three symptoms with the lowest probability of occurrence. Compared to patients in the Moderate Physical and Higher Psych class, patients in the Moderate Physical and Lower Psych class were older; less likely to have a post high school education; more likely to have a diagnosis of lung cancer; more likely to have metastatic disease, and more likely to have a lower performance status. The higher number of lung cancer patients in the Moderate Physical and Lower Psych group most likely accounts for the relatively high occurrence rates reported for cough (0.67), dry mouth (0.63), and shortness of breath (0.67). No studies were found that identified distinct subgroups of oncology patients based on the occurrence of symptoms associated with psychological distress. However, in population-based studies of depression28–30 and anxiety,28–30 older patients tend to report lower occurrence rates for both symptoms.
Table 5.
Rank order of the probability (P) of occurrence of the symptoms for the total sample and the four latent classes
| Rank | Total Sample | P | All Low | P | Moderate Physical/Lower Psych | P | Moderate Physical/Higher Psych | P | All High | P |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lack of energy | .749 | Lack of energy | .351 | Lack of energy | .834 | Lack of energy | .894 | Lack of energy | 1.00 |
| 2 | Pain | .644 | Pain | .337 | Pain | .736 | Worrying | .692 | Feeling sad | .969 |
| 3 | Feeling drowsy | .591 | Problems with urination | .299 | Feeling drowsy | .675 | Pain | .661 | Feeling drowsy | .931 |
| 4 | Difficulty sleeping | .536 | Difficulty sleeping | .223 | SOB | .673 | Difficulty sleeping | .661 | Worrying | .926 |
| 5 | Difficulty concentrating | .460 | Feeling drowsy | .216 | Cough | .668 | Feeling drowsy | .642 | Pain | .924 |
| 6 | Worrying | .436 | Sweats | .165 | Dry mouth | .625 | Feeling sad | .613 | Feeling irritable | .909 |
| 7 | Dry mouth | .428 | Feeling irritable | .147 | Difficulty sleeping | .525 | Difficulty concentrating | .574 | Difficulty concentrating | .878 |
| 8 | Feeling irritable | .404 | Dry mouth | .122 | Difficulty concentrating | .445 | Feeling irritable | .522 | Difficulty sleeping | .821 |
| 9 | Feeling sad | .402 | Cough | .091 | Sweats | .324 | Sweats | .427 | Dry mouth | .809 |
| 10 | Cough | .378 | Worrying | .081 | Problems with urination | .200 | Dry mouth | .252 | Cough | .673 |
| 11 | Sweats | .371 | Difficulty concentrating | .066 | Worrying | .189 | Problems with urination | .239 | SOB | .671 |
| 12 | SOB | .345 | SOB | .036 | Feeling irritable | .174 | Cough | .153 | Sweats | .645 |
| 13 | Problems with urination | .290 | Feeling sad | .014 | Feeling sad | .173 | SOB | .081 | Problems with urination | .462 |
Abbreviation: SOB = shortness of breath
As illustrated in Fig. 1 and Table 5, across all four classes, lack of energy was the most common symptom. While the probability of its occurrence for the total sample was 0.75, values ranged from 0.35 to 1.00. In addition, pain and feeling drowsy occurred in the top five symptoms across all four latent classes. Given their relatively high occurrence rates, clinicians need to assess and treat patients for these three symptoms on a routine basis.
When the rank order of symptom occurrence is compared between the All High and the Moderate Physical and Higher Psych classes, feeling sad (0.96), worrying (0.93), and feeling irritable (0.91) were among the top six symptoms in both classes. Additional demographic, clinical, and treatment characteristics that contribute to the high occurrence rates for psychological distress in both of these classes compared to the other two latent classes warrant investigation in future studies.
Characteristics Associated with a Higher Symptom Burden
An important component of this type of research is the identification of demographic, clinical, and treatment characteristics that are associated with a higher symptom burden. In this study, four salient characteristics were included in the LCA and provided information about differences among the latent classes as part of the model estimation. For example, compared to the All Low class, patients in the All High class were almost a decade younger. The association between younger age and higher symptom burden is consistent with previous reports.5,7,14,15 These age-related differences may be related to younger patients receiving more aggressive treatments;31,32 age-related changes in the hypothalamic-adrenal-pituitary axis that may mediate the occurrence of symptoms;33 or a “response shift” in older patients’ perceptions of symptoms.34,35
Consistent with previous LCA studies,3,5,7 compared to the All Low class, a higher percentage of women were in the other three latent classes. Additional research is warranted on gender differences in the occurrence and severity of symptoms in oncology patients because findings from primarily cross-sectional studies on gender differences are inconsistent.36–40
While a poorer performance status was associated with membership in the All High subgroup in previous studies,3,7,10,11 in the current study it was associated with membership in both the All High and the Moderate Physical and Lower Psych classes. Almost half of the patients in these two subgroups had ECOG scores of between 2 and 4. Although this repeated association between higher symptom burden and poorer performance status does not demonstrate causality, it suggests that clinicians can use KPS or ECOG scores to identify patients who may require more aggressive symptom management.
However, the reason why a higher percentage of patients in the Moderate Physical and Higher Psych class had better ECOG performance scores than patients in the Moderate Physical and Lower Psych class warrants additional investigation. Based on previously published associations between poorer performance and higher symptom burden,3,7,10,11 this finding seems somewhat contradictory. One potential explanation may be that younger patients with a better performance status find their physical symptoms more distressing, which is associated with worrying, as well as feelings of irritability and sadness. An alternative explanation is that a higher percentage of patients in the Moderate Physical and Lower Psych class had a diagnosis of lung cancer, which is a cancer diagnosis that is often associated with a poorer performance status. Because of the limited number of demographic, clinical, and treatment characteristics that were available across the three datasets, additional explanations for these subgroup differences are not readily apparent. Additional research is warranted to confirm these two subgroups and identify additional characteristics that distinguish between the subgroups.
Conflicting information exists on whether or not the presence of metastatic disease is associated with membership in the All High class, with some studies showing no2,6,7 and others showing a positive10,12 association. Given the differences among the latent classes in the current study in both the presence of metastasis and performance status scores, additional research is warranted to determine how one or both of these characteristics influence symptom burden in oncology patients.
Differences in MSAS Subscale and Total Scores
While only 13 of the 32 symptoms on the MSAS were used in the LCA, statistically significant, as well as clinically meaningful differences (i.e., range of d = 0.52 to 2.25, where d equals the difference between the two groups in standard deviation units) were found among the latent classes in the MSAS subscale and total scores (Table 3). Of note, the MSAS PSYCH, MSAS PHYS, and MSAS Global Distress Index scores differentiated among the four latent classes. However, no differences in total number of MSAS symptoms (out of 32) and MSAS Total score were found between the Moderate Physical and Lower Psych and the Moderate Physical and Higher Psych classes. Again, this finding suggests that other demographic, clinical and treatment characteristics need to be evaluated to be able to differentiate these two latent classes.
Consistent with our previous reports,2,6,7 patients in the All High class reported worse QOL outcomes compared to the other three symptom subgroups. Compared to the All Low class, the differences in QOL subscale and total scores for the other three latent classes represent not only statistically significant but clinically meaningful decrements in QOL (i.e., range of d=1.38 to d=1.64).41,42 These findings demonstrate the differential effect of symptom burden on patients’ QOL.
Strengths and Limitations
Several study strengths and limitations need to be acknowledged. The large sample size allowed for the inclusion of clinically relevant covariates within the LCA to identify distinct symptom subgroups. In addition, the heterogeneous cancer diagnoses, stages of disease, and current treatments make the findings generalizable to the majority of oncology practices. Because three data sets were combined, the analyses were limited to those characteristics that were collected across all three studies. The evaluation for differences in QOL scores among the symptom subgroups was limited to the two U.S. studies. Finally, future studies need to evaluate the impact of medications on symptom subgroup membership.
Despite these limitations, this study provides important information on subgroups of oncology patients with distinct symptom experiences. If the specific latent classes identified in this study are replicated in future studies, the phenotypic characteristics that differentiate among these classes may be useful in the development of symptom management interventions for higher risk patients. Future studies can evaluate for molecular characteristics that distinguish among the patient subgroups. These types of studies would provide insights into the mechanisms that underlie multiple co-occurring symptoms in oncology patients.
Acknowledgments
This collaborative project was funded by a grant from Atlantic Philanthropies and a Queensland University of Technology Institute of Health and Wellbeing Collaborative Grant Scheme 2010. The Symptom Clusters Study was funded under a Palliative Care National Health and Medical Research Council grant. The FPS study was funded by the National Institute of Nursing Research (NR04853).
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–736. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pud D, Ben Ami S, Cooper BA, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35:162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Dodd MJ, Cho MH, Cooper BA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum. 2011;38:33–42. doi: 10.1188/11.ONF.33-42. [DOI] [PubMed] [Google Scholar]
- 4.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs. 2014 Oct 10; doi: 10.1177/1099800414550394. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 7.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120:2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 9.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira KA, Kimura M, Teixeira MJ, et al. Impact of cancer-related symptom synergisms on health-related quality of life and performance status. J Pain Symptom Manage. 2008;35:604–616. doi: 10.1016/j.jpainsymman.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Gwede CK, Small BJ, Munster PN, Andrykowski MA, Jacobsen PB. Exploring the differential experience of breast cancer treatment-related symptoms: a cluster analytic approach. Support Care Cancer. 2008;16:925–933. doi: 10.1007/s00520-007-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder CF, Garrett-Mayer E, Blackford AL, et al. Concordance of cancer patients’ function, symptoms, and supportive care needs. Qual Life Res. 2009;18:991–998. doi: 10.1007/s11136-009-9519-6. [DOI] [PubMed] [Google Scholar]
- 13.Reese JB, Blackford A, Sussman J, et al. Cancer patients’ function, symptoms and supportive care needs: a latent class analysis across cultures. Qual Life Res. 2015;24:135–146. doi: 10.1007/s11136-014-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cataldo JK, Paul S, Cooper B, et al. Differences in the symptom experience of older versus younger oncology outpatients: a cross-sectional study. BMC Cancer. 2013;13:6. doi: 10.1186/1471-2407-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchie C, Dunn LB, Paul SM, et al. Differences in the symptom experience of older oncology outpatients. J Pain Symptom Manage. 2014;47:697–709. doi: 10.1016/j.jpainsymman.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zubrod CG, Schneiderman M, Frei E, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chron Dis. 1960;11:7–33. [Google Scholar]
- 17.Karnofsky D. Performance scale. In: Kennealey GT, Mitchell MS, editors. Factors that influence the therapeutic response in cancer: a comprehensive treatise. New York: Plenum Press; 1977. [Google Scholar]
- 18.Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer. 1992;28:1328–1330. doi: 10.1016/0959-8049(92)90510-9. [DOI] [PubMed] [Google Scholar]
- 19.Ferrell BR, Wisdom C, Wenzl C. Quality of life as an outcome variable in the management of cancer pain. Cancer. 1989;63:2321–1327. doi: 10.1002/1097-0142(19890601)63:11<2321::aid-cncr2820631142>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 21.Vermunt JK, Magdison J. Latent class cluster analyses. In: Hagenaars JA, McCutcheon AL, editors. Applied latent class analysis. New York: Cambridge University Press; 2002. [Google Scholar]
- 22.Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the social, behavioral, and health sciences. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- 23.Nylund K, Bellmore A, Nishina A, Graham S. Subtypes, severity, and structural stability of peer victimization: what does latent class analysis say? Child Dev. 2007;78:1706–1722. doi: 10.1111/j.1467-8624.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 24.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 25.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. J Classification. 1996;13:195–212. [Google Scholar]
- 26.Muthen LK, Muthen BO. Mplus user’s guide. 7. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- 27.Muthen LK, Muthen BO. Mplus (Version 7) Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- 28.Baxter AJ, Scott KM, Ferrari AJ, et al. Challenging the myth of an “epidemic” of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress Anxiety. 2014;31:506–516. doi: 10.1002/da.22230. [DOI] [PubMed] [Google Scholar]
- 29.Hinz A, Kittel J, Karoff M, Daig I. Anxiety and depression in cardiac patients: age differences and comparisons with the general population. Psychopathology. 2011;44:289–295. doi: 10.1159/000322796. [DOI] [PubMed] [Google Scholar]
- 30.Christensen H, Jorm AF, Mackinnon AJ, et al. Age differences in depression and anxiety symptoms: a structural equation modelling analysis of data from a general population sample. Psychol Med. 1999;29:325–339. doi: 10.1017/s0033291798008150. [DOI] [PubMed] [Google Scholar]
- 31.Townsley C, Pond GR, Peloza B, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–3810. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Soares HP, Balducci L, et al. Treatment tolerance and efficacy in geriatric oncology: a systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. J Clin Oncol. 2007;25:1272–1276. doi: 10.1200/JCO.2006.09.2759. [DOI] [PubMed] [Google Scholar]
- 33.Bower JE, Low CA, Moskowitz JT, Sepah S, Epel E. Benefit finding and physical health: positive psychological changes and enhanced allostasis. Soc Personal Psychol Compass. 2008;2:223–244. [Google Scholar]
- 34.Sprangers MA, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol. 1999;10:747–749. doi: 10.1023/a:1008305523548. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48:1531–1548. doi: 10.1016/s0277-9536(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin CM, Grant M, Wendel C, et al. Gender differences in sleep disruption and fatigue on quality of life among persons with ostomies. J Clin Sleep Med. 2009;5:335–343. [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung WY, Le LW, Gagliese L, Zimmermann C. Age and gender differences in symptom intensity and symptom clusters among patients with metastatic cancer. Support Care Cancer. 2011;19:417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 38.Giesinger J, Kemmler G, Mueller V, et al. Are gender-associated differences in quality of life in colorectal cancer patients disease-specific? Qual Life Res. 2009;18:547–555. doi: 10.1007/s11136-009-9468-0. [DOI] [PubMed] [Google Scholar]
- 39.Heinonen H, Volin L, Uutela A, et al. Gender-associated differences in the quality of life after allogeneic BMT. Bone Marrow Transplant. 2001;28:503–509. doi: 10.1038/sj.bmt.1703158. [DOI] [PubMed] [Google Scholar]
- 40.Miaskowski C. Gender differences in pain, fatigue, and depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:139–143. doi: 10.1093/jncimonographs/lgh024. [DOI] [PubMed] [Google Scholar]
- 41.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 42.Osoba D. Interpreting the meaningfulness of changes in health-related quality of life scores: lessons from studies in adults. Int J Cancer Suppl. 1999;12:132–137. doi: 10.1002/(sici)1097-0215(1999)83:12+<132::aid-ijc23>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]