Abstract
Background
Disparate lower extremity ultrasound (LUS) screening practices among trauma institutions reflect lack of consensus regarding screening indications and whether screening improves outcomes. We hypothesized that LUS screening for DVT is not associated with reduced incidence of pulmonary embolism (PE).
Methods
The 2012 ACS National Trauma Data Bank Research Data Set was queried to identify 442,108 patients treated at institutions reporting at least one LUS and at least one DVT. Institutions performing LUS on more than 2% of admitted patients were designated high screening (HS) facilities and remaining institutions were designated low screening (LS) facilities. Patient characteristics and risk factors were used to develop a logistic regression model to assess the independent associations between LUS and DVT, and between LUS and PE.
Results
Overall, DVT and PE were reported in 0.94% and 0.37% of the study population, respectively. DVT and PE were more commonly reported in HS than LS (DVT: 1.12% vs. 0.72%, p<0.0001; PE: 0.40% vs. 0.33%, p=0.0004). Multivariable logistic regression demonstrated that LUS was independently associated with DVT (OR=1.43, CI 1.34-1.53) but not PE (OR=1.01, CI 0.92-1.12) (c-statistic 0.86 and 0.85, respectively). Sensitivity analyses performed at various rates for designating HS facilities did not alter the significance of these relationships.
Conclusions
LUS in trauma patients is not associated with a change in the incidence of pulmonary embolism. Aggressive LUS DVT screening protocols appear to detect many clinically insignificant DVTs for which subsequent therapeutic intervention may be unnecessary, and the use of these protocols should be questioned.
Background
Venous thromboembolism (VTE) commonly occurs in patients hospitalized for traumatic injuries, and pulmonary embolism (PE) has been cited as the third leading cause of death for trauma patients who survive beyond the first day.1 Accordingly, there is significant interest in measures that may prevent PE. Many centers perform routine screening of high-risk trauma patients for lower extremity deep venous thrombosis (DVT) using duplex ultrasonography (DUS) because detection of asymptomatic DVT may allow early initiation of therapy to prevent PE.2 However, contradictory evidence about the utility of routine DVT screening to decrease the incidence of PE is reflected by differences and ambiguity in practice guidelines and variability in practice patterns.1–3. Previous literature demonstrates that surveillance bias accounts for much of the variability in reported rates of DVT, however, it remains unclear whether aggressive screening practices affect the incidence of the key clinical outcome of interest – pulmonary embolism. We sought to characterize the relationship between LUS screening and PE and hypothesized that aggressive DVT screening is not associated with a reduction in the incidence of PE in trauma patients.
Methods
Data Source
The National Trauma Data Bank (NTDB) Research Data Set (RDS) for admission year 2012 was utilized for this study with approval by the American College of Surgeons. The University of Virginia Institutional Review Board exempted this study from formal review as the NTDB contains de-identified data, of which the use is not considered human subject research. The NTDB is a multi-institutional clinical outcomes database that combines trauma registry data from over 900 trauma centers in the United States. The RDS contains all records submitted to the NTDB for a particular admission year and is an appropriate data set for studying specific procedures and conditions among trauma patients. Detailed descriptions of the NTDB data collection and handling, as well as limitations of the data set, have been extensively described in the literature and in publically-available user manual distributed by the American College of Surgeons.4
Patients and Outcomes
The 2012 NTDB RDS contained records for 833,311 trauma admissions to participating institutions. Data for 442,108 patient admissions from institutions who reported performing at least one LUS and at least one episode of DVT to the NTDB were included for study. The remaining records were excluded from study because the admitting institution did not report performing at least one LUS or at least one DVT. The primary outcome of interest was the risk-adjusted association between institutional ultrasound rate and PE, while a secondary outcome was the risk-adjusted association between institutional ultrasound rate and DVT.
Patient Characteristics and Risk Factors
Independent, a priori variables previously shown to predispose trauma patients to VTE, as described in earlier literature, were included for analysis.5–7. These risk factors included age≥40, Injury Severity Score (ISS)≥9, head injury with AIS≥3, lower extremity fracture with AIS≥3, pelvic fracture, spinal cord injury with neurologic deficit, vertebral column fracture, solid organ injury, venous injury, ventilator days≥3 days, and major surgery.
Statistical Analysis
Hospital ultrasound rate was calculated by dividing patients who underwent at least one LUS at an institution by the total number of admissions5,8. Hospitals that performed LUS on at least two percent of admitted patients were designated as “high screening” facilities, replicating methodology previously described by Haut et al.5,8
Data analyses were designed to test the null hypothesis that hospital ultrasound rate is not associated with PE or DVT. Statistical significance was determined using the standard alpha value of <0.05. All data analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC).
Descriptive, univariate analysis was performed to characterize baseline injury characteristics and outcome frequencies and stratified by hospital screening designation. Bivariate analysis was utilized to describe outcome frequencies at high screening (HS) versus low screening (LS) facilities by risk factor. Categorical values are reported as a percentage of the total population of each group, and were compared using the Chi-square test. Finally, multivariate logistic regression was performed to determine the independent, risk-adjusted associations between hospital screening status, risk factors, and outcome measures. Modeled factor likelihood ratios (Wald 2 statistic) were utilized to estimate the predictive strength and relative contribution of each covariate with the odds of DVT and PE. Results are reported as adjusted odds ratios (AOR) with 95% confidence intervals (CI). Model performance was assessed using the calculated Area Under the Receiver Operating Characteristic Curve. All calculated test statistics were used to derive reported two-tailed p-values.
Sensitivity Analysis
Sensitivity analysis was performed to determine the association of primary and secondary outcomes with various LUS rate thresholds used to designate facilities as HS versus LS facilities. Multivariate logistic regressions were repeated using screening thresholds of 1%, 5%, 10%, 20%, and 30%.
Results
Patient and injury characteristics, as well as unadjusted outcomes, are listed in Table 1, and stratified by hospital screening status. More than half of study patients were admitted to HS facilities, using the 2% designation rate. Patients admitted to HS facilities more commonly had ISS>9 and other injury characteristics associated with the development of VTE.
Table 1.
Risk Factors Stratified by Admission to High Screening vs. Low Screening Facility
| Risk Factor | n | Low Screening | High Screening | P |
|---|---|---|---|---|
| Total | 442,108 | 43.9% | 56.1% | |
| Number of DVT Diagnosed | 4,171 | 0.7% | 1.1% | <0.0001 |
| Number of PE Diagnosed | 1,642 | 0.3% | 0.4% | 0.0004 |
| Age ≥ 40 | 230,440 | 48.7% | 54.8% | <0.0001 |
| Injury Severity Score >9 | 164,604 | 34.9% | 39.1% | <0.0001 |
| Head injury (AIS≥3) | 89,095 | 18.9% | 21.1% | <0.0001 |
| Lower extremity fracture | 95,691 | 21.2% | 22.0% | <0.0001 |
| Pelvic Fracture | 29,195 | 6.1% | 7.0% | <0.0001 |
| Spinal Cord Injury | 8,238 | 1.7% | 2.0% | <0.0001 |
| Vertebral Column Fracture | 65,389 | 13.5% | 15.8% | <0.0001 |
| Solid Organ Injury | 25,101 | 5.5% | 5.8% | 0.0002 |
| Major venous injury | 2,226 | 0.5% | 0.5% | 0.31 |
| Ventilator Days ≥ 3 | 26,457 | 5.7% | 6.2% | <0.0001 |
| Major surgery | 157,673 | 34.5% | 36.6% | <0.0001 |
Table 2 displays results of bivariate analysis comparing rates of DVT by risk factor at HS and LS institutions. Individual risk factors were associated with higher rates of DVT in HS facilities versus LS facilities, except for spinal cord injury. In contrast, Table 3 demonstrates that only three risk factors – solid organ injury, ventilator days≥3, and major surgery – were associated with higher rates of PE at HS institutions.
Table 2.
Unadjusted DVT Rates at High Screening vs. Low Screening Facilities by Risk Factor
| Risk Factor | DVT - LS | DVT - HS | P |
|---|---|---|---|
| Age ≥ 40 | 0.99% | 1.43% | <0.0001 |
| Injury Severity Score >9 | 1.50% | 2.27% | <0.0001 |
| Head injury (AIS≥3) | 1.44% | 2.19% | <0.0001 |
| Lower extremity fracture | 1.19% | 1.66% | <0.0001 |
| Pelvic Fracture | 1.97% | 2.99% | <0.0001 |
| Spinal Cord Injury | 3.52% | 4.38% | 0.05 |
| Vertebral Column Fracture | 1.73% | 2.44% | <0.0001 |
| Solid Organ Injury | 2.16% | 3.12% | <0.0001 |
| Major venous injury | 5.39% | 7.84% | 0.02 |
| Ventilator Days ≥ 3 | 6.13% | 9.09% | <0.0001 |
| Major surgery | 1.76% | 2.65% | <0.0001 |
Table 3.
Unadjusted PE Rates at High Screening vs. Low Screening Facilities by Risk Factor
| Risk Factor | DVT - LS | DVT - HS | P |
|---|---|---|---|
| Age ≥ 40 | 0.47% | 0.50% | 0.4443 |
| Injury Severity Score >9 | 0.67% | 0.74% | 0.0626 |
| Head injury (AIS≥3) | 0.46% | 0.51% | 0.3134 |
| Lower extremity fracture | 0.66% | 0.71% | 0.3721 |
| Pelvic Fracture | 1.05% | 1.12% | 0.5735 |
| Spinal Cord Injury | 1.19% | 1.37% | 0.50 |
| Vertebral Column Fracture | 0.81% | 0.81% | 0.9758 |
| Solid Organ Injury | 1.01% | 1.40% | 0.0063 |
| Major venous injury | 0.90% | 1.71% | 0.10 |
| Ventilator Days ≥ 3 | 2.25% | 2.69% | 0.026 |
| Major surgery | 0.78% | 0.91% | 0.0057 |
Table 4 presents results of multivariate regression examining the independent, adjusted associations between risk factors; and DVT and PE at a 2% screening designation rate. All risk factors were associated with elevated risk for DVT, with ventilator days≥3 conferring the largest increase in risk. In addition, hospitalization at a HS facility was found to confer a 44% increase in risk for the discovery of a DVT. Similarly, most risk factors were independently associated with the development of PE on multivariate regression. However, admission to a HS facility was not associated with a change in incidence of PE.
Table 4.
Results of Multivariate Regressions with Outcomes of DVT and PE
| Deep Venous Thrombosis* | Pulmonary Embolism† | |||||
|---|---|---|---|---|---|---|
| Risk Factor | Odds Radio (95% CI) | Wald χ2 | p-value | Odds Radio (95% CI) | Wald χ2 | p-value |
| Admission to High Screening Facility | 1.44 (1.34-1.53) | 114.40 | <0.0001 | 1.01 (0.92-1.12) | 0.06 | 0.81 |
| Diagnosis of DVT | -- | -- | -- | 8.27 (7.22-9.47) | 932.87 | <0.0001 |
| Age ≥ 40 | 1.80 (1.68-1.97) | 284.75 | <0.0001 | 1.65 (1.48-1.84) | 84.20 | <0.0001 |
| Injury Severity Score >9 | 1.94 (1.77-2.12) | 207.47 | <0.0001 | 1.96 (1.72-2.24) | 99.02 | <0.0001 |
| Head injury (AIS≥3) | 1.16 (1.07-1.24) | 14.27 | 0.00 | 0.65 (0.57-0.74) | 45.00 | <0.0001 |
| Lower extremity fracture | 1.61 (1.50-1.72) | 179.35 | <0.0001 | 1.86 (1.67-2.06) | 134.57 | <0.0001 |
| Pelvic Fracture | 1.36 (1.25-1.49) | 45.39 | <0.0001 | 1.36 (1.18-1.56) | 18.86 | <0.0001 |
| Spinal Cord Injury | 1.57 (1.39-1.78) | 52.20 | <0.0001 | 1.24 (1.01-1.54) | 4.12 | 0.04 |
| Vertebral Column Fracture | 1.24 (1.15-1.33) | 33.36 | <0.0001 | 1.13 (1.00-1.27) | 4.03 | 0.04 |
| Solid Organ Injury | 1.12 (1.02-1.23) | 5.79 | 0.02 | 1.42 (1.24-1.63) | 24.57 | <0.0001 |
| Major venous injury | 2.98 (2.49-3.57) | 139.07 | <0.0001 | 0.94 (0.64-1.38) | 0.09 | 0.76 |
| Ventilator Days ≥ 3 | 5.37 (4.99-5.79) | 1988.17 | <0.0001 | 3.00 (2.65-3.40) | 297.29 | <0.0001 |
| Major surgery | 4.46 (4.05-4.90) | 936.55 | <0.0001 | 3.62 (3.15-4.16) | 324.77 | <0.0001 |
c-statistic = 0.863
c-statistic = 0.847
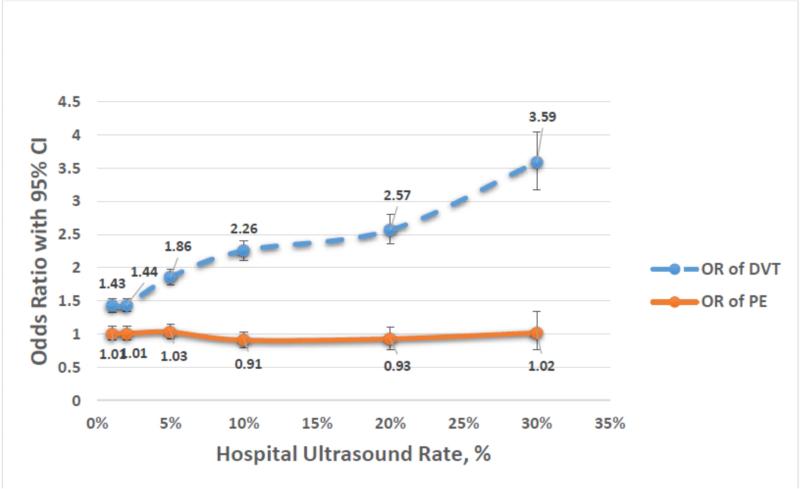
Table 5 and Figure 1 describe results of the sensitivity analysis in which the screening threshold designation rate was varied between 1% and 30%. Approximately 66% of included institutions performed LUS on 1% or more of trauma admissions, while only seven institutions (2.6%) performed LUS on 30% or more of admitted patients. The odds of diagnosis of DVT increase as the screening designation rate increases, yet the odds of PE does not change.
Table 5.
Sensitivity Analysis of Threshold Rates for Designating LUS Screening Facilities.
| Screening Rate | 1% | 2% | 5% | 10% | 20% | 30% |
| Screening institutions (n, %) | 177 (65.8%) | 146 (54.3%) | 78 (29.0%) | 44 (16.4%) | 17 (6.3%) | 7 (2.6%) |
| Patients (n, %) | 284,442 (64.3%) | 247,982 (56.1%) | 132,962 (30.1%) | 76,821 (17.4%) | 30,621 (6.9%) | 11,511 (2.6%) |
| OR of DVT (95% CI) | 1.43 (1.33 - 1.53) | 1.44 (1.34 - 1.53) | 1.86 (1.75 - 1.98) | 2.26 (2.11 - 2.41) | 2.57 (2.36 - 2.81) | 3.59 (3.18 - 4.05) |
| OR of PE (95% CI) | 1.01 (0.91 - 1.12) | 1.01 (0.92 - 1.12) | 1.03 (0.93-1.15) | 0.91 (0.80 - 1.03) | 0.93 (0.77 - 1.11) | 1.02 (0.77 - 1.34) |
Figure 1.
Odds Ratios DVT and PE by Hospital Ultrasound Rate
Discussion
In this study, we sought to characterize the relationship between routine screening for DVT in trauma patients and the incidence of PE. Our results show that the rate of ultrasound performance is associated with DVT but not a reduction in the incidence of PE, after appropriate risk-adjustment. These findings corroborate existing evidence that surveillance bias accounts for much of the variability in the incidence of DVT by institution. Furthermore, these results suggest that routine screening protocols may not affect the most important reason for screening – prevention of pulmonary embolism.
Routine DVT screening in trauma patients may be performed with the rationale that early detection of DVT will allow therapeutic intervention – including early mobilization, therapeutic anticoagulation, and placement of inferior vena cava filters (IVCF) – to reduce the likelihood of PE. Indeed, some literature has supported screening for DVT among asymptomatic, high-risk trauma patients, which is reflected in recommendations by the most recent consensus guidelines from the Eastern Association for the Surgery of Trauma (EAST) and the American College of Chest Physicians (CHEST). However, the supporting evidence for these recommendations is based on small, single-institution series or cost-effectiveness studies that rely on untested assumptions about the frequency of asymptomatic DVT leading to PE3,9–13. Moreover, other studies, albeit with similar limitations, have suggested that DVT screening is not cost-effective or is less effective in preventing PE than adherence to prophylaxis protocols14–17.
Our results corroborate previous findings by Haut et al. that a hospital's DVT rate reflects screening practice rather than quality of care. After patient-level adjustment for known risk factors for development of DVT, HS designation was associated with a 43% increased odds of DVT diagnosis. This relationship persisted in our sensitivity analysis in which LUS threshold rate was varied from 1-30%, demonstrating a positive association between screening rate and DVT diagnosis.
Our results extend the known effects of surveillance bias on DVT rates to demonstrate that aggressive screening practices do not appear to improve clinical outcomes by reducing PE. Despite increased odds of finding DVT at hospitals that perform more screening, HS status is not independently associated with a reduction in the incidence of PE.
A potential explanation for these findings may result from overzealous or inappropriate application of DVT screening practices by some trauma physicians to patients not meeting high-risk criteria, thus obscuring the benefit of DVT screening in reducing PE among true high-risk patients. Controversy within the trauma community about the role of DVT screening is likely responsible for this observation and has resulted in widely divergent practice patterns among providers. A survey of trauma providers reported that 53% of respondents endorsed DVT screening of asymptomatic trauma patients while 36% disagreed 2. The results further signaled that trauma providers disagree widely on high-risk variables for the development of VTE, the appropriate frequency for DVT screening, the utility of DVT screening in improving outcomes, and the cost-effectiveness of DVT screening.2 Indeed, our sensitivity analysis shows wide disparity in screening rates. While most patients are treated at institutions that perform LUS in less than one percent of patients, sizeable numbers of patients are admitted to facilities that perform LUS at much higher rates.
Improved adherence to prophylaxis strategies in recent years may also be responsible for preventing PE in trauma patients, obviating the need for DVT screening in patients who previously may have received suboptimal prophylaxis and would have benefitted from LUS screening. Evidence suggests that DVT screening may be unnecessary and not cost-effective in patients who receive appropriate prophylaxis15–17. Furthermore, trauma centers are increasingly providing appropriate prophylaxis to trauma patients as efforts to reduce venous thromboembolism have received greater attention18,19.
This study has several important limitations. First, our designation of screening institutions is based on the reported institutional rate of patients who undergo a LUS using arguably arbitrary thresholds. While institutions with high LUS rates presumably perform a higher percentage of screening ultrasounds versus exams performed for clinically-indicated reasons, the NTDB RDS does not report indications for the LUS. As a result, differences in patient acuity could result in institutions being improperly classified as HS or LS facilities, confounding the observed effect of LUS rate on PE. Second, the NTDB RDS reports only the performance of at least one LUS but not the frequency, timing, or total count of LUS's performed in an encounter. This lack of detail could obscure important differences in outcomes due to variability in screening protocols not captured by the NTDB. Third, NTDB RDS contains observational data which cannot be used to establish causal relationships between DVT screening and outcomes. Finally, the NTDB RDS is a large, clinical database that may contain errors or omissions that would distort or alter our findings.
Nevertheless, the present findings should prompt more critical evaluation of whether routine screening protocols are warranted in trauma patients. Common therapeutic interventions for patients with DVT, such as early mobilization and therapeutic anticoagulation, may be contraindicated in some trauma patients due to immobility or risk of hemorrhage. Another alternative – placement of prophylactic IVCF – has recently been shown to offer limited, if any, benefit in reducing PE, and this benefit may be outweighed by risks of the procedure20. Thus, if DVT screening offers any clinical benefit in reducing PE, it may only be for highly selective groups of trauma patients where intervention can be shown to reduce the incidence of PE. Indeed, with no difference in the odds of PE between institutions with LUS rates of 1% and as high as 30%, our results suggest that optimal screening rates should be quite low.
In conclusion, we have shown that surveillance bias accounts for wide disparities in the incidence of DVT among trauma institutions, yet our evidence does not support the aggressive use of LUS screening for DVT as a means to reduce PE. Furthermore, these data demonstrate that DVT rates in trauma patients reflect variable usage patterns of LUS, not necessarily differences in quality of care. While the described limitations of this study limit the ability to make causal inferences between screening and PE, we believe that trauma institutions should review their practice of DVT screening and promote adherence to best-practice recommendations for the use of LUS in appropriate high-risk patients. Quality-improvement efforts should focus on compliance with DVT prophylaxis guidelines, which may better reflect quality of care and allow for institution-level comparisons.
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health grants T32 AI078875 and T32 CA163177.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentation: Presented at the 10th Annual Academic Surgical Congress in Las Vegas, NV, February 3-5, 2015.
Disclosure: Committee on Trauma, American College of Surgeons. Chicago, IL, October 2013. The content reproduced from the NTDB remains the full and exclusive copyrighted property of the American College of Surgeons. The American College of Surgeons is not responsible for any claims arising from works based on the original data, text, tables, or figures.
References
- 1.Geerts WH, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Haut ER, et al. Duplex ultrasound screening for deep vein thrombosis in asymptomatic trauma patients: a survey of individual trauma surgeon opinions and current trauma center practices. J. Trauma. 2011;70:27–33. doi: 10.1097/TA.0b013e3182077d55. discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 3.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J. Trauma. 2002;53:142–64. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 4.National Trauma Data Bank User Manual. 2013 at < https://www.facs.org/quality programs/trauma/ntdb/datasets>.
- 5.Haut ER, et al. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors-an analysis of the National Trauma Data Bank (NTDB). J. Trauma. 2009;66:994–9. doi: 10.1097/TA.0b013e3181991adc. discussion 999–1001. [DOI] [PubMed] [Google Scholar]
- 6.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism After Trauma. Ann. Surg. 2004;240:490–498. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shackford SR, Cook A, Rogers FB, Littenberg B, Osler T. The increasing use of vena cava filters in adult trauma victims: data from the American College of Surgeons National Trauma Data Bank. J. Trauma. 2007;63:764–9. doi: 10.1097/01.ta.0000240444.14664.5f. [DOI] [PubMed] [Google Scholar]
- 8.Pierce C. a, et al. Surveillance bias and deep vein thrombosis in the national trauma data bank: the more we look, the more we find. J. Trauma. 2008;64:932–6. doi: 10.1097/TA.0b013e318166b808. discussion 936–7. [DOI] [PubMed] [Google Scholar]
- 9.Burns G, Cohn S. Prospective ultrasound evaluation of venous thrombosis in high-risk trauma patients. J. Trauma. 1993 doi: 10.1097/00005373-199309000-00012. at < http://journals.lww.com/jtrauma/Abstract/1993/09000/Prospective_Ultrasound_Evaluation_of_Venous.12.aspx>. [DOI] [PubMed]
- 10.Napolitano LM, et al. Asymptomatic deep venous thrombosis in the trauma patient: is an aggressive screening protocol justified? J. Trauma. 1995;39:651–7. doi: 10.1097/00005373-199510000-00006. discussion 657–9. [DOI] [PubMed] [Google Scholar]
- 11.Meythaler JM, DeVivo MJ, Hayne JB. Cost-effectiveness of routine screening for proximal deep venous thrombosis in acquired brain injury patients admitted to rehabilitation. Arch. Phys. Med. Rehabil. 1996;77:1–5. doi: 10.1016/s0003-9993(96)90210-5. [DOI] [PubMed] [Google Scholar]
- 12.Brasel KJ, Borgstrom DC, Weigelt JA. Cost-effective prevention of pulmonary embolus in high-risk trauma patients. J. Trauma. 1997;42:456–60. doi: 10.1097/00005373-199703000-00013. discussion 460–2. [DOI] [PubMed] [Google Scholar]
- 13.Piotrowski JJ, et al. Is deep vein thrombosis surveillance warranted in high-risk trauma patients? Am. J. Surg. 1996;172:210–3. doi: 10.1016/s0002-9610(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 14.Cipolle MD, Wojcik R, Seislove E, Wasser TE, Pasquale MD. The role of surveillance duplex scanning in preventing venous thromboembolism in trauma patients. J. Trauma. 2002;52:453–62. doi: 10.1097/00005373-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Meyer CS, Blebea J, Davis K, Fowl RJ, Kempczinski RF. Surveillance venous scans for deep venous thrombosis in multiple trauma patients. Ann. Vasc. Surg. 1995;9:109–14. doi: 10.1007/BF02015324. [DOI] [PubMed] [Google Scholar]
- 16.Spain DA, et al. Venous thromboembolism in the high-risk trauma patient: do risks justify aggressive screening and prophylaxis? J. Trauma. 1997;42:463–7. doi: 10.1097/00005373-199703000-00014. discussion 467–9. [DOI] [PubMed] [Google Scholar]
- 17.Satiani B, Falcone R, Shook L, Price J. Screening for major deep vein thrombosis in seriously injured patients: a prospective study. Ann. Vasc. Surg. 1997;11:626–9. doi: 10.1007/s100169900101. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira PGR, et al. Measurable outcomes of quality improvement using a daily quality rounds checklist: two-year prospective analysis of sustainability in a surgical intensive care unit. J. Trauma Acute Care Surg. 2013;75:717–21. doi: 10.1097/TA.0b013e31829d27b6. [DOI] [PubMed] [Google Scholar]
- 19.Haut ER, et al. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Arch. Surg. 2012;147:901–7. doi: 10.1001/archsurg.2012.2024. [DOI] [PubMed] [Google Scholar]
- 20.Haut ER, et al. The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta-analysis. JAMA Surg. 2014;149:194–202. doi: 10.1001/jamasurg.2013.3970. [DOI] [PubMed] [Google Scholar]