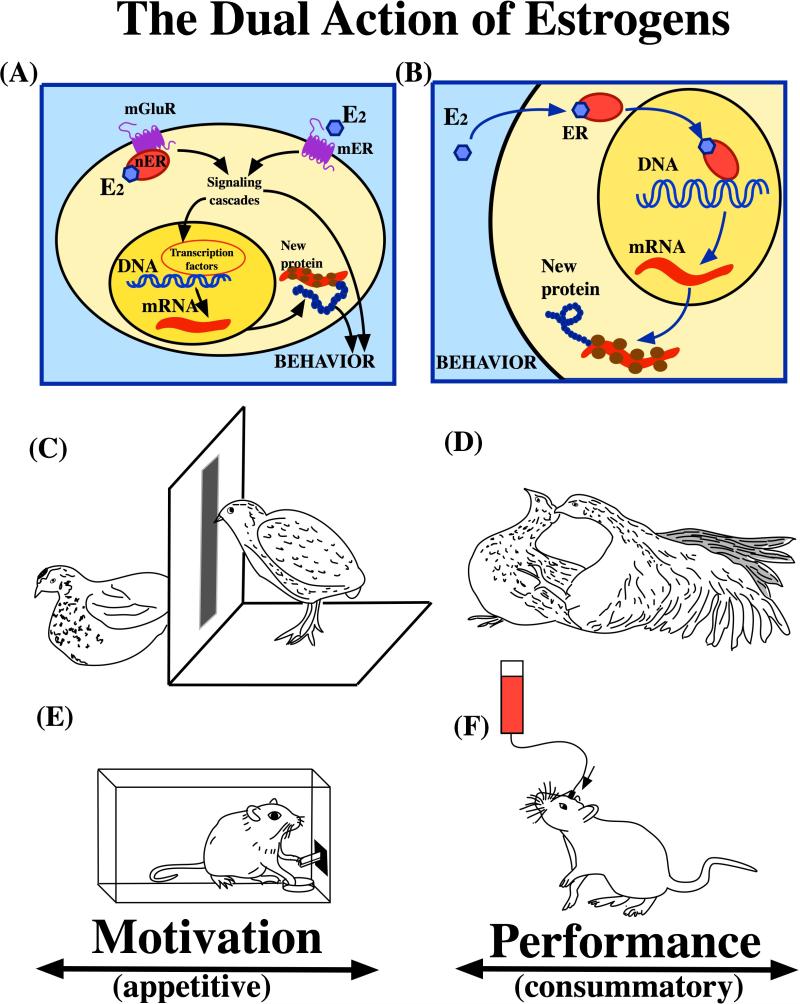
Abstract
Estradiol can act in the brain in a relatively fast manner (i.e. seconds to minutes) usually through signaling initiated at the cell membrane. Brain-derived estradiol has thus been considered as another type of neurotransmitter. Recent work found that behaviors indicative of male sexual motivation are activated by estrogenic metabolites of testosterone (T) in a fast manner while sexual performance (copulatory behavior per se) is regulated by brain estradiol in a slower manner via nuclear-initiated actions. This functional division between these two types of action appears to generalize to other behavioral systems regulated by estradiol. We propose the Dual Action Hypothesis of Estrogen Action to explain this functional distinction between these two different modes of action.
Keywords: Estrogens membrane-initiated action; estrogens nuclear action; motivation; performance; appetitive behavior, consummatory behavior
Introduction: Neuroestrogens vs. Ovarian estrogens
Estrogens are steroid hormones initially isolated and characterized in the late 1920ies by Adolf Butenandt (Nobel laureate for this discovery) and Edward A. Doisy [1]. They are present in all vertebrates and some invertebrates, and traditionally considered as the primary female sex hormone since they are produced by the ovary, released into the blood and play a key role in the control of female reproduction. Estrogens are secreted in the developing ovarian follicles by aromatization of androgenic substrates that have been produced locally. Aromatization is a complex enzymatic reaction catalyzed by aromatase (also known as estrogen synthase or EC 1.14.14.1, the product of gene CYP19), an enzyme of the cytochrome P450 family. Aromatization of androgens includes three successive hydroxylations resulting in the elimination of one methyl group and acquisition of a fully saturated (aromatic) “A” ring [2]. The main substrates of aromatization are the androgens testosterone and androstenedione that are transformed by aromatase into 17β-estradiol (E2: see Glossary) and estrone (E1) respectively.
In the late 1960ies, a marked level of aromatase activity was discovered in the brain of mammalian species including humans [3] and this finding was soon generalized to representative species in the major vertebrate classes (i.e., fish, reptiles, birds [4]). These claims were based on data from radioenzyme assays quantifying the transformation of radioactive testosterone into radioactive estradiol that were later supplemented by the identification of the presence of the relevant mRNA (by PCR or in situ hybridization) and of the enzymatic protein (by immunohistochemistry or Western blot). This finding of widespread brain aromatase opened an entirely new field of investigation that resulted over the years in the demonstration that estrogens produced in the brain either by the aromatization of testicular testosterone or via a series of enzymatic steps from the precursor of all steroids, cholesterol [5, 6] also called neuroestrogens, play a decisive role in a) the sexual differentiation of brain and behavior and b) the activation of male sexual behavior in rodents and many other vertebrate species [7]. Thus the notion that estrogens should be considered a “female” hormone is an over-simplification. Estrogens have important roles in males as well. During the last few decades, it was discovered that estrogens additionally control a host of physiological and pathological traits ranging from bone formation, lipid metabolism and brain plasticity and repair to tumor growth. They are now considered as pleiotropic signaling factors whose action extends well beyond the control of reproduction.
The dual controls of estrogen synthesis
Ovarian and brain estrogens have the exact same chemical structure and are produced by the same enzyme, aromatase. Two fundamentally different controls of brain aromatase activity have been identified that operate in distinct time domains. In the long term, steroids increase transcription of the aromatase gene resulting in an increased enzyme concentration and thus activity (genomic control). In a shorter time scale, post-translational modifications of the enzymatic protein drastically modify its activity within minutes without changing its concentration (post-translational control) (See Box 1).
Fast vs. slow actions of estrogens
Like other steroid hormones, estrogens are classically considered to exert their biological effects by binding to their cognate nuclear receptors (e.g., Estrogen receptor α, ERα; and Estrogen receptor β, ERβ), which then act as ligand-activated transcription factors to regulate transcription of specific genes associated with an estrogen-responsive element (ERE) or other types (e.g. AP1, Sp1, NF-κB; [8]) of response elements. These biological effects are thus mediated via transcription of genes, translation of corresponding mRNAs into proteins, and their integration into functional pathways; they take hours to days to develop.
As early as 1976, it was however observed that in the brain estrogens exert, in addition to these relatively slow effects, electrophysiological effects with latencies of only a few seconds [9]. This discovery was at the origin of a whole new line of research that resulted in the identification of a second mode of estrogen action usually initiated at the cell membrane and associated with shorter latencies in the second to minute range. These rapid membrane-initiated effects of E2 are mimicked by E2 associated with a larger molecule that prevents its entry into the cells (e.g., E2-BSA or E2 biotin; [10]). They are mediated by the activation of intracellular signaling cascades resulting in changes in intracellular calcium concentration or, in a slightly slower manner, in the phosphorylation of various proteins including transcription factors such as the cyclic AMP response-element-binding protein, CREB, inducing in this way the so-called indirect genomic effects that appear with longer latencies than real non-genomic effects [11].
More recently, research has also implicated at the organismal level this mode of estrogen action in the control of a variety of behaviors including male and female sexual behavior, aggression, nociception, learning and memory, auditory signal processing (see for review [12, 13]). The first of these behavioral studies in the context of sexual behavior reported that a bolus of estradiol activates within 35 min anogenital sniffing and mounting in castrated male rats, Rattus norvegicus [14]. Subsequent studies extended this notion of rapid effects of E2 on sexual behavior to Japanese quail (Coturnix japonica) and mice (Mus musculus) by showing that a single injection of the aromatase inhibitor rapidly (within 10-30 min) and transiently reduces various components of male sexual behavior [15, 16]. Additional experiments also demonstrated that in these two species, a single E2 injection increases male sexual activity within 10-15 min [16, 17].
These experiments in quail had two important features in common. First, the rapid effects of estrogens on sexual behavior were easier to demonstrate when males were in addition exposed to a weak stimulation by androgens (a low dose of testosterone; [17]). Second, there was a clear suggestion that these rapid effects concerned mostly the initial phases of the interaction with the females rather than the copulatory performance per se. In the study assessing rapid behavioral effects of aromatase inhibition, the decrease in two aspects of appetitive sexual behavior, the rhythmic contractions of the cloacal sphincter muscles (RCSM) and the learned social proximity response (LSPR) in a two compartment chamber (behaviors described in Box 2), was for example more reliably inhibited (effect size = 0.408 and 0.534 respectively) than the copulatory act per se (effect size=0.144 or 0.251 for the low and high dose of inhibitor respectively; [15]).
Distinct controls of behavioral motivation and performance
This distinction was confirmed and reinforced by more recent experiments during which brain estrogen bioavailability was pharmacologically manipulated by intracerebroventricular (ICV) injections. For these studies, castrated male quail that were chronically treated with exogenous testosterone (Subcutaneous Silastic™ implant) were implanted with an ICV cannula in the third ventricle and then used as their own control in successive behavior trials after injections of aromatase inhibitors or various forms of estrogens [18].
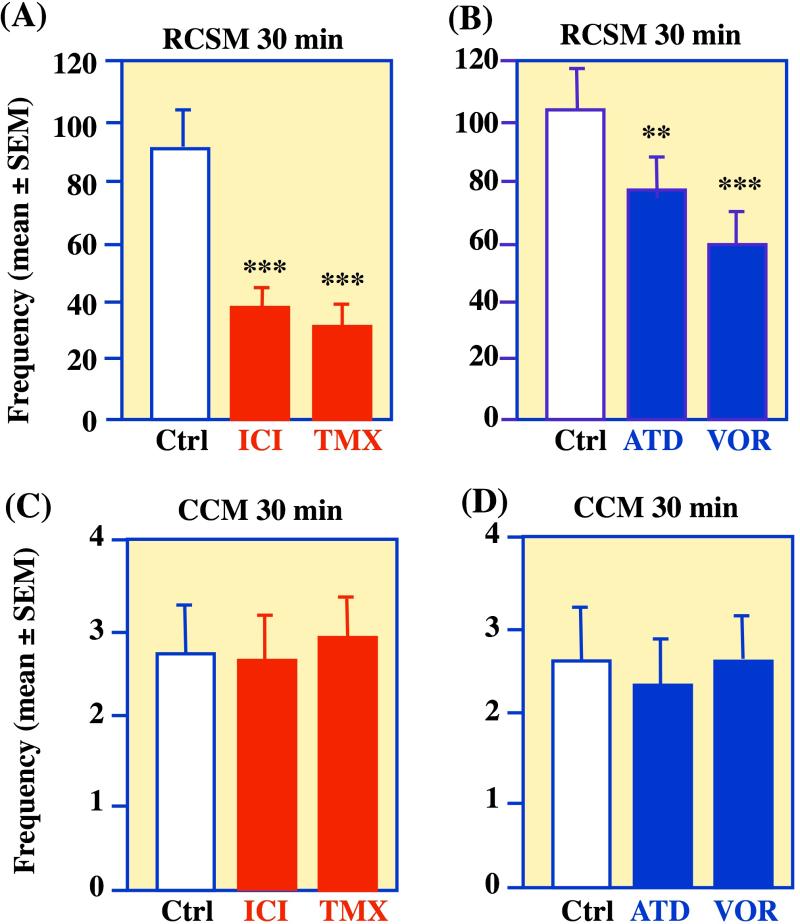
The frequency of RCSM was reduced by more than 50 percent, 30 minutes after a single ICV injections of either tamoxifen or ICI 182,780 (i.e., Fulvestrant or Faslodex™), two anti-estrogens, as well as of Vorozole™ or ATD (1,4,6-androstatriene-3,17-dione), two aromatase inhibitors (Figure 1A-B). A very significant inhibition of RCSM was already observed 15 minutes after injection of tamoxifen or ICI 182,780. In contrast, the frequency of cloacal contact movements (CCM), the actual copulatory pattern (see Box 2) was completely unaffected by all these manipulations (Figure 1C-D).
Figure 1. Rapid effects of neuroestrogens on quail sexual behavior.
Blockade of estrogen action or of estrogen production by intracerebroventricular injections of antiestrogens (panel A; ICI 182,780, ICI or tamoxifen, TMX) or of aromatase inhibitors (panel B; 1,4,6-androstatriene-3,17-dione, ATD or Vorozole™, VOR) markedly inhibits within 30 min the expression of rhythmic contractions of the cloacal sphincter muscles (RCSM), a form of appetitive sexual behavior reflecting sexual motivation, but does not affect the frequency of copulatory behavior sensu stricto, represented here by the frequency of cloacal contact movements (CCM, panels C and D). **=p<0.01, ***=p<0.001 compared to the control (Ctrl) condition (Newman-Keuls post hoc tests following a significant overall ANOVA). Redrawn from data in [18].
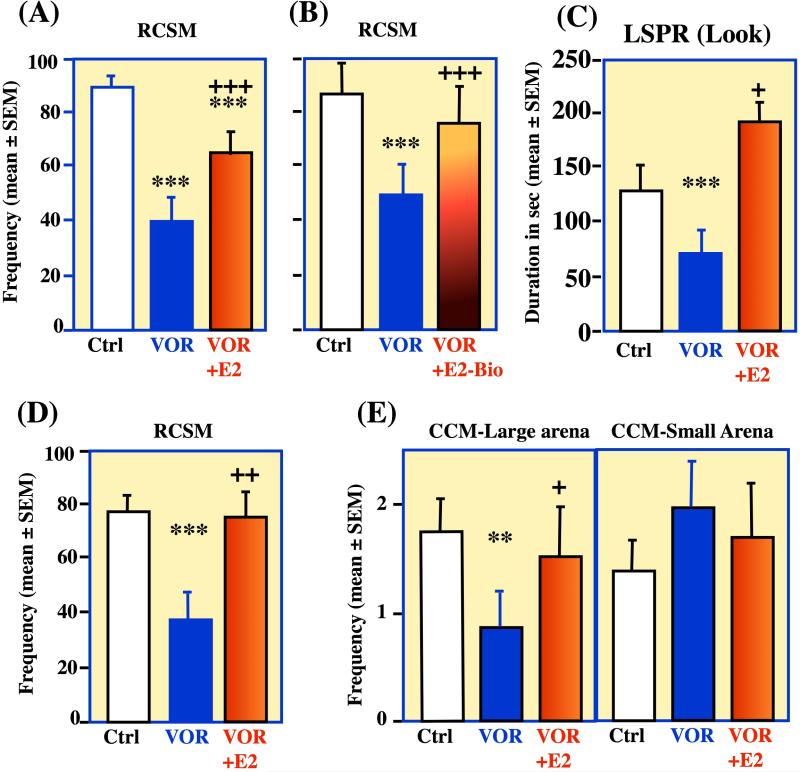
The behavioral inhibitions observed after injection of aromatase inhibitors were clearly related to the depletion of local estrogen synthesis since they could be prevented by a single injection of exogenous E2 (50 μg/bird 15 min. before testing i.e., 15 min after injection of the aromatase inhibitor; Figure 2A). The short latency of this behavioral effect suggested it did not rely on new protein synthesis and was thus mediated by non-genomic mechanisms and this was confirmed by the fact that the Vorozole-induced inhibition of RCSM could similarly be prevented by an acute injection of E2-biotin (Figure 2B).
Figure 2. Specificity of the rapid, membrane-initiated effects of neuroestrogens on the motivational and performance aspects of male sexual behavior in quail.
A-B. The aromatase inhibitor Vorozole™ (VOR) inhibits within 30 min expression of rhythmic contractions of the cloacal sphincter muscles (RCSM) and this behavior is restored by a single injection 15 min before the behavioral test of estradiol (E2; A) or estradiol coupled to biotin (E2-Bio; B). C. A similar inhibition after VOR injections and recovery after E2 injection affects the expression of another form of appetitive sexual behavior, the learned social proximity response (LSPR) illustrated here by the time the male spends looking at the female through a narrow window. D-E. In another group of birds, the acute inhibition of aromatase activity by VOR reduced RCSM frequency and recovery was observed after E2 treatment (D). No effect of CCM frequency was again observed when tests were performed in a small arena (E, right), but in a large arena where male had to pursue the female to be able to copulate, the treatments modulated CCM frequency (decrease after VOR, recovery after E2; E, left). **=p<0.01, ***=p<0.001 compared to the control (Ctrl) condition; += p<0.05, ++=p<0.01, +++=p<0.001 compared to the VOR group (Newman-Keuls post hoc tests following a significant overall ANOVA). Redrawn from data in [18].
The blockade of RCSM, but not copulation, following inhibition of neuroestrogens production or action suggested dissociation between mechanisms controlling sexual motivation and performance. To test whether this distinction could be generalized, we assessed the effects of these treatments on another measure of sexual motivation, the learned social proximity response (LSPR; see Box 2). Here again aromatase inhibition acutely (within 30 min) inhibited expression of the behavior and it was restored by a single injection of E2 given 15 min. before the behavioral test (Figure 2C).
Finally, we wondered how copulatory behavior could be left unaltered by treatments that were markedly suppressing two independent measures of sexual motivation. One explanation could reside in the fact that copulatory behavior was tested in a small arena where the copulatory sequence was triggered reflexively due to close proximity of the partners so that decreases in motivation would not matter much. To test this interpretation, rapid effects of estrogen receptor antagonists and aromatase inhibitors were tested again but this time in a larger arena where females could more easily escape so that males had to actively pursue them to copulate successfully. In these conditions the two aromatase inhibitors Vorozole™ and ATD and the antiestrogen tamoxifen significantly inhibited within 30 min the expression of CCM and the antiestrogen ICI 182,780 reduced their frequency although the decrease was not significant.
These differences in the control by neuroestrogens of sexual motivation or performance and the influence of testing conditions on the latter behavior were confirmed in another group of subjects that were sequentially tested for RCSM and for CCM in a small and then a big arena. Vorozole™ inhibited RCSM in small arena within 30 min, an effect that was prevented by E2 injected 15 min before testing. Acute aromatase inhibition had no effect on CCM frequency when tested in the small arena but inhibited this behavioral response measured in the larger arena. In this condition, E2 counteracted Vorozole's effect (Figure 2D-E).
Together, these results demonstrate that acute changes in local estrogen bioavailability do not alter the ability of male quail to produce a highly coordinated sexual motor response but modulate the expression of two behaviors that reflect the sexual motivation of the subjects. Based on these data we hypothesize that estrogens have a dual action of sexual behavior. They would act rapidly in a membrane-initiated and non-genomic manner to modulate sexual motivation but may only affect sexual performance after a longer latency, implying the existence of nuclear (genomic) mechanisms. This conclusion was further supported by an experiment performed on castrated T-treated males injected chronically with the aromatase inhibitor Vorozole™ so that both sexual motivation and performance were drastically inhibited. In these birds, a single injection of E2 or of E2-BSA largely restored within 15 min. the expression of RCSM but had no effect of the CCM frequency [18].
Towards a generalization?
We think that the Dual Action hypothesis has wide applicability. It is hard to be certain at present because investigators studying estrogen actions in other behavioral systems have not designed their studies to address questions related to the dual action view as we have done. But a consideration of some other examples of estradiol action on motivated behaviors is illustrative.
Three studies identified rapid effects of estrogens on male sexual behavior in rodents (rats: [14, 19] and mice: [16]). In all cases, the behaviors that were most markedly affected are ano-genital olfactory investigations and mounts, plus in one case intromissions [16]. For unknown reasons, one classically accepted measure of sexual motivation, the anticipatory level changes in a bi-level chamber, was however not increased after E2 injections in the study of Kaufman and collaborators. Data on ejaculations are presented in only one of these studies and this behavior was not rapidly affected following injection of E2 [19]. These data are thus broadly consistent with the distinction between effects of estrogens on motivation (i.e., ano-genital investigations) and performance if we accept that mounts (and possibly intromission) are at the interface between the appetitive and consummatory phase of male sexual behavior ([20]; See [21-23] for a detailed discussion on this topic). Note also that in quail the performance aspects of male sexual behavior are rapidly affected as an indirect consequence of the changes in motivation following changes in estrogens bioavailability if, and only if, the behavioral tests take place in a large arena where the male has to actively pursue the female to perform the copulatory sequence [18]. One might thus argue that this was the case for mounts and intromissions in the behavioral tests reported in rats and mice but answering this question would require additional experiments.
The separate effects of estrogens on motivational and performance aspects of behavior might also apply to other aspects of brain activity. For example, there are prominent female-biased sex differences in the response to and abuse of drugs such as amphetamine and cocaine. These differences are mediated by sex differences in estradiol secretion (e.g., [24, 25]) and estradiol seems to have differential effects on the motivational and consummatory aspects of drug seeking and consumption. Estradiol has rapid effects on striatal dopaminergic function that can be linked to some behaviors that exhibit female-biased sex differences such as stereotypic behavioral responses to amphetamine (e.g., [26, 27]).
In the case of cocaine, rats will lever press on a progressive ratio schedule of reinforcement, an appetitive response that will result in the consummatory event of cocaine self administration. This appetitive response is more pronounced in females than in males and involves rapid effects of estradiol on striatal dopamine release [27]. Female rats will also exhibit increases in locomotor activity in response to cocaine. This occurs 30 min. after E2 treatment and this effect is blocked by co-administering antagonists to mGluR5 receptors consistent with an estradiol effect on ERα receptors fused to the membrane [29]. Thus sex-specific effects of E2 on appetitive measures of drug seeking do seem to involve fast actions of E2 working at the membrane.
Behavior that involves self-administering cocaine on an FR1 schedule of reinforcement (i.e. every response immediately results in cocaine being administered) can be considered as the consummatory component of drug taking behavior ([30, 31]). The acquisition of self-administration of cocaine by rats is enhanced by estradiol but requires several days of estradiol administration and experience to be acquired [32, 33]. These data thus suggest that appetitive aspects for drug consumption are rapidly enhanced by actions of estrogens presumably initiated at the membrane level whereas the actual drug consumption itself would require slower estrogen actions presumably mediated by nuclear mechanisms. The applicability of the dual action hypothesis to estrogenic facilitation of appetitive and consummatory aspects of drug abuse is not definitive but the existing data are highly suggestive and warrant further investigation of the relevance of this hypothesis to these behaviors.
Selective aspects of other behavioral systems also are regulated by estradiol in an intriguing fashion implicating at the same time effects that seem to depend on acute membrane-initiated effects and longer term (presumably genomic) actions. This is the case, for example, for the activation of female sexual behavior in rats [34, 35], the control of auditory processing in songbirds [36, 37] and of learning and memory [38-41]. One might wonder to what extent this dichotomy in temporal characteristics may also reflect a dissociation between motivational and performance aspects of these behaviors.
Multiple neuroanatomical targets
The separation of estrogens’ effects on motivation and performance may possibly relate to a neuroanatomical specificity in the site of steroid action on sexual behavior in quail and other vertebrates. Several studies that we previously reviewed [42] have indeed suggested dissociations between the neuronal circuits underlying the expression of appetitive sexual behavior (ASB) reflecting motivation and consummatory sexual behavior (CSB) indicative of performance.
In quail, detailed analysis of the extent of stereotaxic lesion studies aimed at the medial preoptic area (mPOA) and of the related behavior deficits suggested that more rostral parts of the mPOA might be preferentially associated with the expression of ASB whereas more caudal parts of this region would control CSB [43]. This conclusion was independently supported by an immunohistological study analyzing c-Fos expression in castrated male quail treated with testosterone who had been allowed to freely copulate with a female (CSB) or to only see her and demonstrate signs of sexual motivation in the form of increased frequencies of RCSM. The performance of CSB was associated with an increased c-Fos expression compared to control un-manipulated birds throughout the medial preoptic nucleus while in contrast in the ASB group the increased c-Fos expression was limited to the rostral part of this nucleus [44].
Since aromatase-immunoreactive cells and both ERα– and ERβ–expressing cells are present throughout the entire rostro-caudal extent of the mPOA, even if it is with a heterogeneous density [42, 45, 46], it can be hypothesized that the anatomical specificity in the control of these two aspects of male sexual behavior correlates with a membrane-initiated action of estrogens on motivation in the rostral mPOA and a slower nuclear action on performance in its caudal part.
Such an anatomical separation between brain regions controlling motivation and performance also seems to be present in mammalian species. Lesions of the mPOA impair copulatory behavior in rats and many other mammals as well as in all species of birds, reptiles, amphibians and fishes that have been investigated (see [47] for review). Conversely, a large number of studies in a variety of species indicate that stereotaxic implants of testosterone in the mPOA activate most if not all aspects of male copulatory behavior [47]. There is however a pronounced neurochemical and functional heterogeneity within this brain region such that the whole area does not seem to be implicated in the control of copulation (see [42] for review). For example, one study showed that lesions of the caudal mPOA and anterior hypothalamus in rats block the expression of copulatory behavior but more rostral lesions in the mPOA had little or no effect [48] (see [42] for other examples). The studies of Barry Everitt and his colleagues additionally suggested a double dissociation between brain regions controlling in male rats appetitive and consummatory sexual behavior in rats. Males bearing lesions in the mPOA were no longer copulating but were still working actively in an operant conditioning procedure to get access to a female, thus indicating that they were still sexually motivated [49].
The anatomical dissociation between controls by steroids of motivational and performance aspects of behavior is also clearly illustrated in studies of the endocrine control of singing in canaries (Serinus canaria). It is well established that testosterone activates singing in canaries and other songbirds and that part at least of this effect results from the action of estrogenic metabolites of the androgen [50]. Because song control nuclei such as HVC contain both androgen and estrogen receptors it was initially assumed that steroids act at this level to activate behavior [50, 51]. However things are not so simple [52]. Blocking T action in HVC reduces song quality but does not affect song rate [53]. Conversely, implanting T in the HVC of castrated white-crowned sparrows does not activate singing [54]. Our recent study indicated that stereotaxic implantation of testosterone in the mPOA of castrated male canaries fully restores singing activity indicating that this brain region controls the singing motivation [51]. Three aspects of song quality, its energy, bandwidth and entropy, were however poorer in these birds than in subjects exposed to systemic testosterone indicating that the performance is controlled by steroids acting at another locus. This separation might again reflect the dual action of estrogenic metabolites of testosterone on the motivation to sing and song quality (performance aspect).
The different estrogen receptors
The two types of behavioral effects of estrogens could also be potentially differentiated based on the different receptors mediating the responses (see Box 3). Several studies have implicated the receptors ERα and ERβ in the control of sexual behavior by nuclear mechanisms but there is no published study on the type of estrogen receptor mediating rapid membrane-initiated effects on male sexual behavior. One study recently reported that the Gq protein-coupled membrane ER (Gq-mER) does not seem to play a role in the activation of male sexual behavior in rats [19]. More work on this topic is thus needed.
An important related question is whether these membrane effects are taking place in the same neural circuits as the nuclear ones, and at a finer level in the same cells. The neural distribution of nuclear sex steroid receptors has in the past been valuable in guiding research on the neural mechanisms of sexual and social behavior. Determining where, in the brain, estrogens act at the membrane level is however more problematic. Visualizing these types of receptors by immunohistochemistry has turned out to be technically challenging and correlating these neurochemical results to function is difficult because there are few specific blockers of these receptors and these often interfere with nuclear signaling as well. One option is of course to map the activation of ER-dependent intracellular signaling pathways initiated at the membrane level but since these pathways are often rather ubiquitous and not clearly identified for most behaviors; this approach would require acquisition of essential new knowledge. Furthermore even when critical brain areas will have been uncovered, one key remaining question will be whether membrane and nuclear initiated events take place, within these areas, in the same or in distinct cell populations. Both possibilities appear at this stage equally possible.
Conclusion: the dual action of estrogen hypothesis
The dual action theory of estrogen action provides a useful way to organize recent somewhat surprising findings about estrogens, their regulation of behavior and their mechanisms of action. We were among those who had previously proposed that brain estrogens should be considered more like a neurotransmitter than a traditional hormone [55, 56]. These observations stimulated additional research on the various modes by which estrogens can act on behavior. These data in turn stimulated the posing of the dual action hypothesis. Using functional criteria to propose such a hypothesis at first might not seem wise. However, the functional criteria for the dual action (appetitive/motivational vs. consummatory/performance) does seem to map onto a variety of physiological criteria (fast action/membrane vs. slower action/nuclear; Figure 3). The entire goal of proposing this hypothesis is to test how consistently these criteria will separate out different aspects of estrogen action. There are many systems that one could utilize to test this theory and research conducted in light of this paradigm will be helpful in furthering our understanding of the multiple ways brain estrogens can act.
Figure 3. Schematic presentation of the dual action of estrogens hypothesis.
The figure illustrates the overlapping dichotomies between the cellular mechanisms mediating estrogens action (A-B), the separate controls of sexual motivation and sexual performance (C-D) in male quail, and the appetitive and consummatory phases of drug addition in rats and mice (E-F). A. Estrogens such as estradiol-17β (E2) signal at the membrane level by binding to estrogen receptors located at this level only (GPR30, Gq-mER) or to a receptor that is found both in the nucleus and at the membrane (ERα, ERβ) but is associated at the membrane with a metabotropic glutamate receptor (mGluR). This modifies the activity of intracellular signaling cascades that affect behavior either directly or via indirect genomic effects. B. Alternatively, steroids including E2 bind to intracellular receptors that will then act as ligand-activated transcription factors to modulate production of new mRNA and new proteins that will affect behavior. C. E2 rapidly modulates appetitive sexual behavior in male quail such as the rhythmic contractions of the cloacal gland or the learned social proximity response illustrated here (male looking at a female through a narrow “window”) by acting at the neuronal membrane level. D. A more prolonged action of E2 presumably at the nuclear level is needed to activate copulatory behavior sensu stricto illustrated here by the cloacal contact of a male with a female. E. The appetitive responses displayed by rodents to have access to a drug, such as lever pressing on a progressive ratio of operant conditioning, are increased within 30 minutes by an E2 treatment acting presumably via an ER associated at the membrane level with a mGluR. F. In contrast, consummatory self-administration of drug by intracerebral injections as observed in a FR1 schedule of operant conditioning (each lever pressing results in drug delivery) seems to be affected after only several days of exposure to E2, suggesting a genomic control by the steroid. See text for additional explanations.
Our thinking was also influenced by why such a dual mode of action of estrogens might have evolved. One hallmark of steroid hormones, like the estrogens, is that they can coordinate suites of morphological, physiological and behavioral traits into an organized adaptive response. Sexual and other social behaviors have to be carefully coordinated with physiological and morphological changes for successful reproduction to occur. Behavior of course is regulated at several different time scales, e.g., ontogenetic, seasonal and seconds-minutes. It therefore makes sense that a chemical messenger that acts in the brain would be able to regulate behavioral activation both at a relatively fast as well a longer term time scale. However, the well-known sluggishness of the nuclear actions of steroids made many assume that steroids could not be involved in significant short-term behavioral regulation (as Wilson famously declared in 1975; see p6 in [57]). We now know that estrogens can act in the brain on several different time scales. Is this variance functionally organized in the manner proposed by the Dual Action Hypothesis? This is an idea worth testing.
Box 1. Two independent controls of aromatase activity.
The genomic controls of aromatase synthesis are very different from one tissue to another. The human aromatase gene is composed of 9 coding exons and multiple alternative non-coding first exons that regulate this tissue-specific expression [2, 58]. A similar situation has been identified in other species including rodents [58]. Depending on which of the alternative un-translated first exons is used for transcription, aromatase can be up-regulated by androgens (brain), cyclic AMP (ovary, testis, placenta), dexamethasone (liver, adipose and vascular tissue, skin) or retinoic acid (bone) among others [58]. Aromatase activity (AA) and estrogen production are consequently modified in parallel with changes in aromatase concentration. In the brain for example, AA increases after a few hours to a few days following exposure to testosterone [59, 60].
An alternative mechanism of aromatase activity control was identified more recently that does not involve changes in enzyme concentration and takes place more rapidly (5-15 min). We serendipitously discovered that AA in quail (Coturnix japonica) brain homogenates is rapidly (within min) inactivated by phosphorylating conditions (high but physiological concentrations of ATP, Mg2+ and Ca2+; [61, 62]), an effect that is blocked in the presence of kinase inhibitors. Subsequent investigations performed on preoptic-hypothalamic explants maintained in vitro indicated a similar rapid inactivation of the enzyme activity by phosphorylations themselves under the control of glutamatergic transmission [63]. This phosphorylation-dependent inactivation of AA was also demonstrated in various cell lines transfected with the human aromatase gene as well as in quail ovaries [64]. Work conducted in the zebra finch (Taeniopygia guttata) telencephalon indicated that this acute modulation of AA by phosphorylating conditions occurs specifically at the level of synaptic terminals but not in the perikaryon [65]. In vivo dialysis in the auditory cortex of the same species provided converging evidence that glutamate rapidly decreases local estrogen concentrations [66] and this effect results from changes occurring at the level of synapses [67]. Social interactions with a congener of the opposite sex and acute stress also modulate within minutes brain AA and/or local estrogen concentration in a sex- and anatomically-specific manner [68-71].
Box 2. Independent measures of sexual motivation and sexual performance.
Separate independent measures of sexual motivation and performance have been developed and validated for male quail. They are briefly described below (see [72] for a full description).
Rhythmic contractions of the Cloacal Sphincter muscles (RCSM)
When visually exposed to a female, male quail display RCSM that produce a meringue-like foam which is transferred to females during copulation and enhances the probability that sperm will fertilize the egg [73]. Several lines of evidence indicate that this behavioral response reflects the sexual motivation of the male: (1) it is rapidly upregulated in sexually active males visually exposed to a female but not a male [73], (2) it is displayed by naive males but can also be conditioned to an arbitrary stimulus previously associated with the presentation of a female [74], (3) it decreases after castration but is restored by treatments with exogenous testosterone [43], and (4) is inhibited by lesions of the preoptic area, a brain region known to control male sexual behavior [43].
The learned social proximity response (LSPR): Approach and Look
The LSPR is a form of associative learning in which a male learns to stand in front of a narrow window where he had previously been able to see a sexually receptive female and later allowed to copulate with her [75]. The behavior is easily quantified in a two compartment chamber where the male placed in the larger compartment is allowed to see the female placed in a smaller adjacent compartment through a narrow vertical slit (the “window”; see Figure 3C). After quantifying the time the male spends looking at the female, the separation between the two compartments is removed and the male is allowed to freely copulate with the female. The duration of looking reflects male sexual motivation since 1) it is only displayed by males exposed to testosterone [76], 2) it is inhibited by lesions of the preoptic area [43].
The copulatory sequence sensu stricto
Sexual performance is then measured by the actual copulatory sequence during which the male grabs the female's neck feathers, mounts her and then performs the cloacal contact movements (CCM) during which sperm transfer occurs.
Box 3. Multiple estrogen receptors mediating nuclear and membrane-initiated effects.
Two receptors, ERα and ERβ, that mediate nuclear actions of estrogens have been identified [77] and implicated in the control of various aspects of estrogen action. After experiencing post-translational modifications such as palmitoylation, these receptors can also be translocated to the cell membrane [78, 79] and signal via their association with a G-protein coupled seven transmembrane domains receptor (GPCR) such as the metabotropic glutamate receptors, mGluRs [80]. One additional estrogen receptor called GPER or GPR30 has also been formally identified at the membrane level [81] and two additional ones have been postulated based on pharmacological and functional studies, Gq-mER and ER-X [82, 83] but their chemical nature has not been identified.
At the organismal level, rapid actions of E2 have now been ascribed to the first 4 of these ER located at least in part at the membrane (ERα, ERβ GPR30 and Gq-mER). Most advances in this context have been made in our understanding of the estrogenic regulation of learning and memory [40, 41, 84, 85] as well as nociception [86-88]. Limited information is however available concerning the specific ER implicated in the control of reproduction and it does not directly concern male sexual behavior. For example, membrane-initiated effects of estrogens on lordosis behavior in rat occurring within 30 hours or so would depend on membrane-associated ERα transactivating an mGluR [35] and rapid changes in LH secretions would be controlled by E2 acting on GPR30 [89].
Outstanding question: Is the duality of action limited to estrogens?
Estrogens are without a doubt the steroid hormones for which the largest amount of literature has accumulated on the topic of fast action. We would like to suggest however that the dichotomy between rapid membrane-initiated and slower nucleus-initiated effect applies to several other steroids such as progesterone, corticosterone and testosterone. These steroids are indeed classically assumed to exert their effects via slow mechanisms that are mediated by nuclear receptors and presumably require regulation of the transcription of multiple genes. This was in fact formally demonstrated in a substantial number of cases [90, 91].
However in addition, progesterone enhances the lordosis behavior of female rodents via a fast action in the mesencephalic periaqueductal gray that synergizes with its nuclear action in the ventromedial hypothalamus ([92-94]). Testosterone has been shown to exert rapid behavioral effects on mounting behavior and on penile reflexes in rodents but also on anxiety, rewarded behaviors and pain sensitivity. Some of these effects might be mediated by the aromatization of testosterone into an estrogen but others are clearly androgen-dependent (see [95] for review and discussion). Effects of testosterone on appetitive and consummatory components of sexual behavior also seem to imply its action at different brain sites ([96]; See [42] for review). Finally, besides its classical nuclear actions, corticosterone also acts rapidly at the membrane level to modulate namely sexual behavior in male newts (see for review:[97]).
There is thus evidence concerning several other steroids for a separated action at the nuclear and at the membrane level, overlapping with an action in different time domains (slow [hours to days] vs. rapid [in the minutes range]) and in some cases with a separation in the neuroanatomical target sites of action. Data are however generally not available to decide whether this dichotomy also covers the notion of motivation versus performance presented here that would be necessary to support a broader application of the dual action of estrogens to other hormones but we certainly hope that the present opinion paper will promote research on this topic.
Highlights.
- Estrogens affect behaviors by slow nuclear- and faster membrane-initiated mechanisms
- Sexual motivation is rapidly controlled by estrogens acting at the cell membrane
- Sexual performance requires action of estrogens during a longer period
- This differential control extends to controls by estrogens of other behaviors
Acknowledgments
Research in our laboratories mentioned here and the preparation of this review were supported by NIH grant RO1 MH50388. CAC is F.R.S.-FNRS Research Associate. We thank Dr. Margaret M. McCarthy for useful comments on an earlier version of this article.
Glossary
- AA
aromatase activity
- ASB
appetitive sexual behavior
- ATD
1,4,6-androstatriene-3,17-dione
- BSA
bovine serum albumin
- E1
estrone
- CCM
cloacal contact movements
- CREB
cyclic AMP response-element-binding protein
- CSB
consummatory sexual behavior
- E2
estradiol-17β
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- ERE
estrogen-responsive element
- GPCR
G-protein coupled seven transmembrane domains receptor
- HVC
used as proper name for a song control nucleis in song birds ((previously High Vocal Center)
- ICV
intracerebroventricular
- LPSR
learned social proximity response
- mGluRs
metabotropic glutamate receptors
- mPOA
medial preoptic area
- RCSM
rhythmic contractions of the cloacal sphincter muscles
- T
testosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tata JR. One hundred years of hormones. EMBO reports. 2005;6:490–496. doi: 10.1038/sj.embor.7400444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson ER, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr.Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 3.Naftolin F, et al. The formation of estrogens by central neuroendocrine tissues. Rec. Prog. Horm. Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 4.Callard GV, et al. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- 5.Hojo Y, et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fester L, et al. Estrogen synthesis in the hippocampus. Cell and tissue research. 2011;345:285–294. doi: 10.1007/s00441-011-1221-7. [DOI] [PubMed] [Google Scholar]
- 7.Balthazart J, Ball GF. Brain aromatase, estrogens and behavior. Oxford University Press; 2013. [Google Scholar]
- 8.McDevitt MA, et al. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly MJ, et al. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Research. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 11.Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Frontiers in neuroendocrinology. 2014;35:530–549. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornil CA, et al. Rapid control of male typical behaviors by brain-derived estrogens. Frontiers in neuroendocrinology. 2012;33:425–446. doi: 10.1016/j.yfrne.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laredo SA, et al. Rapid effects of estrogens on behavior: environmental modulation and molecular mechanisms. Frontiers in neuroendocrinology. 2014;35:447–458. doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross E, Roselli CE. 17b-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 15.Cornil CA, et al. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taziaux M, et al. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornil CA, et al. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006;66:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Seredynski AL, et al. Neuroestrogens rapidly regulate sexual motivation but not performance. J Neurosci. 2013;33:164–174. doi: 10.1523/JNEUROSCI.2557-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman KR, et al. Rapid effects of 17beta-estradiol on male copulatory behaviors are not elicited by the novel membrane active estrogenic compound STX. Behav Neurosci. 2013;127:598–605. doi: 10.1037/a0032950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaus JG. Frank A. Beach Award - Homologies of animal and human sexual behaviors. Horm.Behav. 1996;30:187–200. doi: 10.1006/hbeh.1996.0024. [DOI] [PubMed] [Google Scholar]
- 21.Sachs BD. A contextual definition of male sexual arousal. Horm Behav. 2007;51:569–578. doi: 10.1016/j.yhbeh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Hormones and Behavior. 2008;53:307–311. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sachs BD. The appetitive-consummatory distinction: Is this 100-year-old baby worth saving? Reply to Ball and Balthazart. Horm Behav. 2008;53:315–318. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 25.Carroll ME, et al. Sex and estrogen influence drug abuse. Trends in pharmacological sciences. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neuroscience Letters. 1990;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 27.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J.Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, et al. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez LA, et al. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wise RA. Intravenous drug self-administration: a special case of positive reinforcement. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; 1987. pp. 117–141. [Google Scholar]
- 31.Roberts DC, et al. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neuroscience and biobehavioral reviews. 2013;37:2026–2036. doi: 10.1016/j.neubiorev.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch WJ, et al. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 33.Hu M, et al. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 34.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewing P, et al. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remage-Healey L, et al. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harburger LL, et al. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inagaki T, et al. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacome LF, et al. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiology of learning and memory. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phan A, et al. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 42.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Frontiers in neuroendocrinology. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balthazart J, et al. Appetitive and consummatory male sexual behavior in japanese quail are differentialy regulated by subregions of the preoptic medial nucleus. Journal of Neuroscience. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taziaux M, et al. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur J Neurosci. 2006;23:1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- 45.Balthazart J, et al. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chem Neuroanat. 1996;11:147–171. doi: 10.1016/0891-0618(96)00149-4. [DOI] [PubMed] [Google Scholar]
- 46.Voigt C, et al. Sex differences in the expression of sex steroid receptor mRNA in the quail brain. J Neuroendocrinol. 2009;21:1045–1062. doi: 10.1111/j.1365-2826.2009.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hull EM, Dominguez JM. Male sexual behavior. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's physiology of reproduction. 4th Edition Elsevier; 2015. pp. 2211–2285. [Google Scholar]
- 48.Van de Poll NE, Van Dis H. The effect of medial preoptic-anterior hypothalamic lesions on bisexual behavior of the male rat. Brain Res. Bull. 1979;4:505–511. doi: 10.1016/0361-9230(79)90035-2. [DOI] [PubMed] [Google Scholar]
- 49.Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neuroscience and biobehavioral reviews. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 50.Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, et al., editors. Hormones, Brain and Behavior. Academic Press; 2009. pp. 898–941. [Google Scholar]
- 51.Alward BA, et al. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc Natl Acad Sci U S A. 2013;110:19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnold AP. Logical levels of steroid hormone action in the control of vertebrate behavior. Amer.Zool. 1981;21:233–242. [Google Scholar]
- 53.Meitzen J, et al. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27:12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenowitz EA, Lent K. Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci U S A. 2002;99:12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Saldanha CJ, et al. Synaptocrine Signaling: Steroid Synthesis and Action at the Synapse. Endocr Rev. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson EO. Sociobiology: The new synthesis. Belknap Press of Harvard University Press; 1975. [Google Scholar]
- 58.Harada N, Honda SI. Molecular mechanisms controlling brain aromatase. In: Balthazart J, Ball GF, editors. Brain aromatase, estrogens and behavior. Oxford University Press; 2013. pp. 138–154. [Google Scholar]
- 59.Balthazart J, et al. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiology and Behavior. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Roselli CE, Resko JA. The distribution and regulation of aromatase activity in the central nervous system. Steroids. 1987;50:495–508. doi: 10.1016/0039-128x(87)90034-1. [DOI] [PubMed] [Google Scholar]
- 61.Balthazart J, et al. Rapid and reversible inhibition of brain aromatase activity. J.Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 62.Balthazart J, et al. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 63.Balthazart J, et al. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 64.Charlier TD, et al. Human and Quail Aromatase Activity Is Rapidly and Reversibly Inhibited by Phosphorylating Conditions. Endocrinology. 2011;152:4199–4210. doi: 10.1210/en.2011-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornil CA, et al. Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology. 2012;153:2562–2567. doi: 10.1210/en.2011-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Remage-Healey L, et al. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Remage-Healey L, et al. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. Journal of Neuroscience. 2011;31:10034–10038. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dickens MJ, et al. Acute Stress Differentially Affects Aromatase Activity in Specific Brain Nuclei of Adult Male and Female Quail. Endocrinology. 2011;152:4242–4251. doi: 10.1210/en.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dickens MJ, et al. Relationships between rapid changes in local aromatase activity and estradiol concentrations in male and female quail brain. Horm Behav. 2014;65:154–164. doi: 10.1016/j.yhbeh.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Bournonville C, et al. Dynamic changes in brain aromatase activity following sexual interactions in males: where, when and why? Psychoneuroendocrinology. 2013;38:789–799. doi: 10.1016/j.psyneuen.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Remage-Healey L, et al. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. Journal of neurophysiology. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2010;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seiwert CM, Adkins-Regan E. The foam production system of the male japanese quail: characterization of structure and function. Brain Behav.Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- 74.Cornil CA, Ball GF. Effects of social experience on subsequent sexual performance in naive male Japanese quail (Coturnix japonica). Horm Behav. 2010;57:515–522. doi: 10.1016/j.yhbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. Journal of Comparative Psychology. 1986;100:59–67. [PubMed] [Google Scholar]
- 76.Balthazart J, et al. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behavioral Neuroscience. 1995;109:485–501. [PubMed] [Google Scholar]
- 77.Kuiper GGJM, et al. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc.Natl.Acad.Sci.USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Acconcia F, et al. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Molecular biology of the cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pedram A, et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. The Journal of biological chemistry. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 80.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Frontiers in neuroendocrinology. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toran-Allerand CD, et al. ER-X : a novel membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. Journal of Neuroscience. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly MJ, Ronnekleiv OK. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Frontiers in neuroendocrinology. 2012;33:376–387. doi: 10.1016/j.yfrne.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu F, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 85.Boulware MI, et al. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuhn J, et al. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 87.Small KM, et al. Activation of membrane estrogen receptors attenuates opioid receptor-like1 receptor-mediated antinociception via an ERK-dependent non-genomic mechanism. Neuroscience. 2013;255:177–190. doi: 10.1016/j.neuroscience.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu Y, et al. 17beta-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERalpha and GPR30. Endocrinology. 2013;154:2421–2433. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- 89.Noel SD, et al. Involvement of G Protein-Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 92.Pleim ET, et al. Facilitation of sexual receptivity in hamsters by simultaneous progesterone implants into the VMH and ventral mesencephalon. Horm.Behav. 1990;24:139–151. doi: 10.1016/0018-506x(90)90001-e. [DOI] [PubMed] [Google Scholar]
- 93.Frye CA, et al. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- 94.DeBold JF, Frye CA. Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm.Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 95.Nyby JG. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Frontiers in neuroendocrinology. 2008;29:199–210. doi: 10.1016/j.yfrne.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matochik JA, et al. Intracranial androgenic activation of male-typical behaviors in house mice: Motivation versus performance. Behav.Brain Res. 1994;60:141–149. doi: 10.1016/0166-4328(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 97.Moore FL, Orchinik M. Membrane receptors for corticosterone: A mechanism for rapid behavioral responses in an amphibian. Horm.Behav. 1994;28:512–519. doi: 10.1006/hbeh.1994.1049. [DOI] [PubMed] [Google Scholar]