Abstract
OBJECTIVE
To differentiate MRI characteristics of optic neuritis associated with neuromyelitis optica (NMO) and relapsing remitting multiple sclerosis (RRMS).
BACKGROUND
Optic neuritis is a common presenting feature of both neuromyelitis optica and multiple sclerosis. Distinguishing between NMO and RRMS is important in guiding treatment, but biomarkers of NMO and MS can be absent early in the disease process. We looked for differences in MRI characteristics of optic neuritis associated with NMO and MS that provide an early clue in the diagnostic workup.
DESIGN/METHODS
We conducted a retrospective analysis of 26 NMO and 26 RRMS patients presenting to the Johns Hopkins Hospital with MRI-confirmed acute optic neuritis. MRIs were assessed to identify the location and longitudinal extent of each contrast enhancing lesion. For the purposes of this study, the optic nerve was divided into intraorbital, canalicular, pre-chiasmal, chiasmal, and optic tract.
RESULTS
There are distinct differences in MRI characteristics between NMO- and RRMS-associated optic neuritis. The majority of NMO lesions were longitudinally extensive measuring at least 17.6 mm in length and involving at least three optic nerve segments. At a cutoff of 17.6 mm lesion length, the specificity for NMO is 76.9% with a sensitivity of 80.8% and positive likelihood ratio of 3.50. Conversely, MS lesions were more commonly focal in one optic nerve segment localized anteriorly.
CONCLUSIONS
Optic neuritis in NMO has a distinct pattern on MRI as compared with RRMS and can help differentiate these two neuroinflammatory diseases at presentation.
Keywords: neuromyelitis optica, aquaporin-4, longitudinally extensive optic neuritis, MRI, bilateral optic neuritis
Introduction
Neuromyelitis optica (NMO) and relapsing remitting multiple sclerosis (RRMS) are both recurrent immune-mediated diseases of the central nervous system (CNS) that may present with optic neuritis (ON) early in the disease course. Differentiating between the two diseases can be difficult when other clues are not available such as positive serological testing for anti-AQP4 antibodies, which are present in only 12-20% of patients with recurrent optic neuritis.1,2 Treatments for MS have been shown to worsen the disease course of NMO,3-5 and since ON is the presenting event in NMO 47% of the time,6 new biomarkers are needed to differentiate outcomes between MS and NMO after clinically isolated ON.
Attacks on the optic nerve in NMO tend to be more severe with a greater reduction in visual acuity7,8 and thinning of the retinal nerve fiber layer.9-15 NMO patients are also more likely to develop microcystic macular edema by ocular coherence tomography (OCT) in affected eyes.16, 17 In acute optic neuritis, brain MRI can be helpful in distinguishing patients with relapsing diseases from those with monophasic optic neuritis18-20. Classic MRI findings in NMO include diencephalic and brainstem lesions3,21,22 but recent work suggests NMO patients also have periependymal and subcortical white matter lesions similar to MRIs in MS.23 MRI studies of the optic nerves in the acute phase of inflammation have been small series. Khanna et al. observed that NMO lesions tended to be more posterior and more often involved the chiasm compared to MS.24 Storoni et al. showed that not only were lesions more posterior but also longer in NMO.25 The work of Pula et al. supported evidence of longitudinally extensive optic neuritis in NMO as compared to MS in a small cohort, also demonstrating a trend for bilateral and chiasmal involvement.26 However, this was not found to be the case in a pediatric cohort, where the presence of longitudinally extensive optic neuritis lesions on MRI did not differentiate MS from non-MS diseases in children, including NMO.27
In this study, we compared the length of MRI lesions of NMO and RRMS optic neuritis cases and found that NMO lesions were predominantly longitudinally extensive stretching at least 17.6 millimeters across the optic pathways as compared to MS lesions, which were < 17.6 mm and largely spared the intracranial portions of the optic nerves. Longitudinally extensive optic neuritis (LEON) is a helpful MRI finding in distinguishing NMO from MS.
Methods
We conducted a retrospective analysis of 52 patients with RRMS and NMO treated at the Johns Hopkins Hospital with MRI-confirmed acute ON. NMO diagnosis was based on the 2006 diagnostic criteria of myelitis and ON, as well as two of three supporting criteria: LETM, NMO-IgG seropositivity, and/or a brain non-diagnostic for MS. NMOSD diagnosis was based on the presence of NMO-IgG seropositivity with the presence of ON. NMO-IgG test was performed at Quest Labs and the Mayo Clinic Lab. MS diagnosis was based on the 2010 McDonald criteria. Patients were included only if the MRI imaging confirmed a gadolinium enhancing lesion within the optic nerve within 30 days of symptom onset. Length of lesions were measured based on the T1 post-gadolinium sequences to ensure that burden of disease from previous lesions was not accounted for in this cohort. MRIs were assessed to identify the location and longitudinal extent of each lesion. MRI exams were performed at either 1.5 or 3 Tesla on a Philips (Best, Netherlands), GE Healthcare (Milwaukee, WI) or Siemens (Erlangen, Germany) scanner. Fifty four percent of the NMO patient events and 41% of the MS patient events were captured using dedicated orbital imaging. For the purposes of this study, the optic nerve was divided into intraorbital (anterior), canalicular, pre-chiasmal, chiasmal, and optic tract (Figure 1). Each segment was counted separately along the two sides of the nerve: 2 intraorbital optic nerves, 2 canalicular, 2 pre-chiasmal, 1 chiasm and 2 optic tracts. An unblinded neuroradiologist measured the length of each acute ON lesion on the T1 post contrast-enhanced sequence. The total length of enhancement within the optic pathways was measured. When both the left and right optic nerves enhanced, the lengths of the lesions were added together to determine the total length of optic nerve affected. When non-contiguous enhancing lesions were noted within the optic nerve(s), the lengths of these lesions were similarly added together. Longitudinally extensive lesions are defined as those extending at least 17.6 millimeters.
Figure 1.
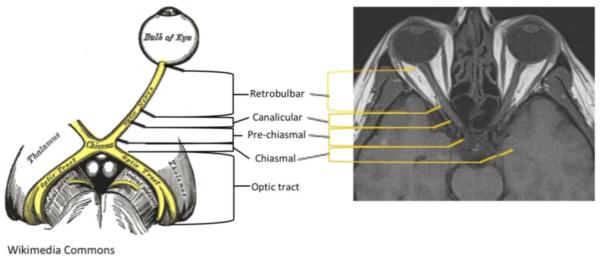
For this study, the optic nerve was divided into 5 segments, as depicted: intraorbital [extending from the optic bulb to the start of the optic canal], canalicular [the portion of the nerve that lies within the optic canal], pre-chiasmal [begins at the exit of the optic canal intra-cranially extending to the chiasm], chiasmal [across the chiasm], and optic tracts [posterior to the chiasm] (2); there were 9 segments in total. (Sketch from Wikimedia Commons.)
Statistics
Medians and Mann-Whitney U-test was used to compare lesion lengths as the distribution was not normal. To calculate the threshold for optimal distinction between NMO and MS, we used an R package "pROC" for Receiver-operator characteristic (ROC) curves analysis, an open-source package for R and S+ to analyze and compare ROC curves. The threshold for optimal sensitivity and specificity was determined as the point on the ROC curve that is closest to the perfect diagnosis, (coordinate of (1,1)). Fisher’s test was used to determine if the ratio of anterior to posterior optic nerve involvement was different between NMO and MS.
This study was approved by the Johns Hopkins Institutional Review Board.
Results
We conducted a retrospective analysis of 26 patients with RRMS and 26 patients with NMO treated at the Johns Hopkins Hospital between 1993 and 2013 with MRI-confirmed acute ON. Of the 26 NMO patients, 22 were female (85%), and 16 were African American (62%), 7 were Caucasian American (27%), and 3 (12%) were Latin American. The mean age was 35.4 years (median 36.1; range 4.0-67.4) (Table 1). Nineteen ultimately met diagnostic criteria for NMO, 12 of whom were NMO IgG seropositive, and 7 remained NMOSD with positive NMO-IgG but no spinal cord involvement. There were a total of 28 events of ON among the 26 NMO patients: 21 were the first event in the affected eye, 9 of which were the first lifetime event, 5 were the second event in the affected eye, and 2 patients had at least 2 prior events in that eye. Of the 26 MS patients, 24 were female (92%), and 9 were African American (35%), 16 were Caucasian American (62%), and 1 was Latin American (4%). The mean age was 33.6 years (median 32.0; range 15.2-54.4) (Table 1). There were a total of 27 events of ON among the 26 MS patients: 25 were the first event in the affected eye, 16 of which were the first lifetime event, 1 was a second event in the affected eye, and 1 patient had at least 2 prior events in the affected eye. Of the 28 patient events in NMO, 7 were on immunosuppressive treatment at the time of the event (2 mycophenolate mofetil, 2 azathioprine, 1 oral prednisone, 1 rituximab, 1 IVIG). Of the 27 patient events in MS, 7 were on immunomodulation/immunosuppression at the time of their event (3 glatiramer acetate, 2 interferon β-1a, 1 rituximab, 1 dimethyl fumarate).
Table 1.
Patient demographics
| NMO | MS | ||
|---|---|---|---|
| Subjects n | 26 | 26 | |
| # of Events | 28 | 27 | |
| Diagnosis | NMO Seropositive NMO Seronegative NMO |
19 (73%) 12 7 |
N/A |
| NMOSD | 7 (27%) | ||
| Female sex | 22 (85%) | 24 (92%) | |
|
Age at event
(years) |
Mean |
35.4 range 4.0-67.4 |
33.6 range 15.2-54.4 |
| Median |
36.1 years |
32.0 years |
|
| Race |
African American |
16 (61%) |
9 (35%) |
| Caucasian American | 7 (27%) | 16 (61%) | |
| Latin American | 3 (12%) | 1 (4%) | |
| ON as 1st event (%) | 35 | 61 | |
| ON as 1st event in the affected eye (%) | 75 | 93 | |
|
# untreated for disease suppression at
time of event |
23 (82%) | 22 (81%) | |
|
# MRI obtained prior to acute medication
administration |
21 (75%) | 20 (74%) | |
|
Time from first
symptom of event to MRI (days) |
Mean |
10 range 0-30 |
13 range 2-30 |
| Median |
5 |
12 |
|
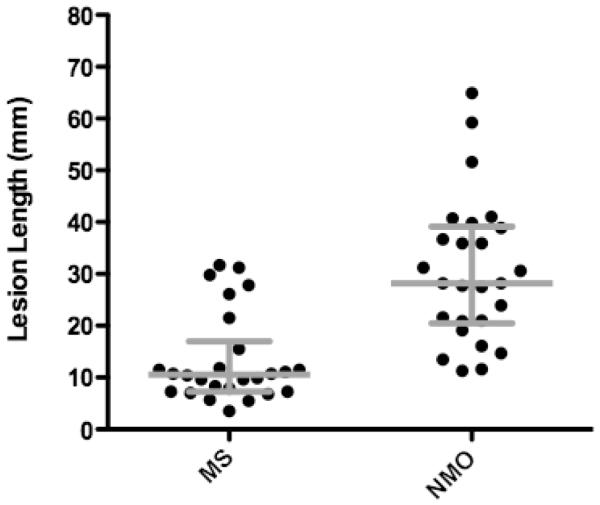
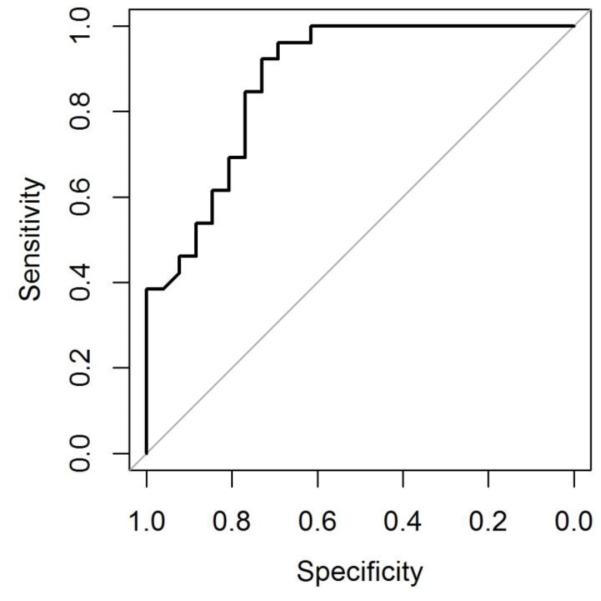
Twenty-six MRIs in NMO and 26 MRIs in MS were evaluated for measurement of the enhancing lesion. The median length of ON lesions in NMO was 28.2 mm (IQR, 18.8; range, 11.6-64.9) compared to 10.5 mm in MS (IQR, 9.7; range, 3.5-31.7) (Figure 2). Table 2 shows the sensitivities and specificities by lesion length for NMO compared to MS. The area under the ROC curve is 0.876 (95% CI: 0.782 - 0.970) (Figure 3) and the optimal threshold is 17.6 mm. At this cutoff, the specificity for NMO compared to MS is 76.9% with a sensitivity of 80.8% and positive likelihood ratio of 3.50. Longer lesions confer higher specificity for NMO. There was no difference in the length of the acute ON lesion in NMO patients who presented with their first event or a relapse (Table 3). There was also no difference in lesion length among NMO-IgG seropositive and seronegative NMO patients (Table 3).
Figure 2.
The median length of enhancing optic nerve lesions in neuromyelitis optica is 28.2 mm with IQR 18.8 compared to 10.6 mm with IQR 9.7 for multiple sclerosis lesions (Mann-Whitney U-test, p<0.0001).
Table 2.
NMO sensitivities and specificities by lesion length
| Lesion Length (mm) |
Sensitivity (%) | Specificity (%) | Likelihood ratio |
|---|---|---|---|
| ≥5 | 100 | 4 | 1.06 |
| ≥10 | 100 | 46 | 1.81 |
| ≥15 | 85 | 73 | 3.16 |
| ≥17.6 | 80.8 | 76.9 | 3.50 |
| ≥20 | 77 | 77 | 3.33 |
| ≥25 | 62 | 81 | 3.20 |
| ≥30 | 46 | 92 | 5.83 |
| ≥35 | 35 | 100 | |
| ≥40 | 19 | 100 | |
| ≥45 | 12 | 100 | |
| ≥50 | 10 | 100 |
Figure 3.
Receiver-operator characteristic (ROC) curve visualizing the tradeoff between sensitivity and specificity of optic nerve lesion length demonstrated good accuracy defined by the area under the curve. The point on the curve closest to perfect (coordinate 1,1) is 17.6 mm that correlates with a sensitivity of 80.8%, a specificity of 76.9% and a likelihood ratio of 3.50.
Table 3.
Clinical characteristics are not predictive of optic nerve lesion length.
| Number of cases |
Lesion length Mean/median (mm) |
p-value | ||
|---|---|---|---|---|
|
NMO
events |
1st ever event of NMO Recurrent NMO event |
9 (32%) 19 (68%) |
35.9/36.3 28.0/27.6 |
0.22 |
| 1st event in affected eye Recurrent event in affected eye |
21 (75%) 7 (25%) |
31.8/29.7 26.4/27.5 |
0.45 | |
|
MS
events |
1st ever event of MS Recurrent MS event |
16 (62%) 10 (38%) |
12.1/9.6 15.3/10.7 |
0.24 |
| 1st event in affected eye Recurrent event in affected eye |
24 (92%) 2 (8%) |
13.9/10.6 8.2/8.2 |
0.38 | |
|
NMO
serostatus |
NMO-IgG + NMO-IgG − |
19 (73%) 7 (27%) |
29.8/28.0 32.5/29.4 |
0.93 |
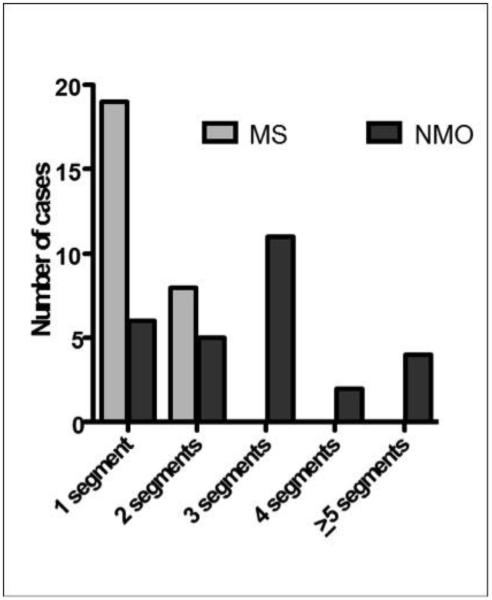
Similar to lesion length, acute ON in NMO involving at least two optic nerve segments was 79% compared to 30% in MS, and 61% of NMO cases of ON had lesions involving ≥ 3 segments versus 0% in MS (Figure 4). Conversely, MS lesions were more commonly focal in one optic nerve segment localized anteriorly, such that the entire lesion is more often contained in the intraorbital and/or canalicular regions of the optic nerve (74%, versus 33% in NMO). NMO lesions involved the posterior optic pathways in 67% of optic neuritis cases, most of which were longitudinally extensive and included all 3 regions of the optic nerve, whereas MS optic nerve lesions were more commonly anterior with only a 26% involvement of the posterior segments of the optic pathways (Table 4). NMO lesions were also more likely to be bilateral (25%, versus 0% in MS) and involve the optic chiasm (25%, versus 4% in MS) and tracts (18%, versus 0% in MS) (Table 4). There was no correlation between lesion length and age, race or symptom duration within either population.
Figure 4.
Acute optic neuritis in neuromyelitis optica involving at least two optic nerve segments was 79% compared to 30% in multiple sclerosis (Fisher’s test, p=0.0004). No lesions in multiple sclerosis extended beyond 2 segments.
Table 4.
Lesion location: Anterior versus posterior optic nerve
| NMO | MS | |
|---|---|---|
| Anterior only | 5 (18%) | 9 (33%) |
| Anterior + canalicular | 3 (11%) | 5 (19%) |
| Canalicular only | 1 (4%) | 6 (22%) |
| Canalicular + Posterior | 2 (7%) | 2 (7%) |
| Posterior only | 4 (14%) | 5 (19%) |
|
All 3 regions
(anterior+canalicular+posterior) |
13 (46%) | 0% |
| Bilateral lesions | 7 (25%) | 0 |
| Chiasmal lesions | 7 (25%) | 1 (4%) |
| Optic tract lesions | 5 (18%) | 0 |
Discussion
Longitudinally extensive optic neuritis (LEON) defined as an acute gadolinium enhancing MRI lesion extending for at least 17.6 mm may be a useful biomarker of NMO and may help distinguish NMO from RRMS early in the disease course. In this retrospective study, 81% of acute ON cases in NMO were longitudinally extensive compared with 23% for acute ON cases in MS. NMO lesions are likely to stretch from the intraorbital space through the canalicular portion and into the intracranial visual pathways including the optic chiasm and optic tracts. MS lesions tend to be focal lesions sparing the intracranial visual pathways with no MS lesions extending across all three regions of the optic nerve. These MRI features of acute ON in NMO and MS are present at the onset of disease suggesting that LEON may be prognostic for development of NMO among patients with recurrent demyelinating disease and provides a useful clue to guide preventive treatment.
LEON is not unique to NMO. In this study, six cases of ON among MS patients presented with LEON of at least 17.6 mm in length, and no commonality was found among these 6 in terms of race, sex or age. LEON may also be a feature of optic neuritis among patients with other inflammatory or infectious diseases including chronic relapsing and monophasic inflammatory optic neuropathy, as well as vascular disease of the optic nerve.28,29 The scope of this study was limited to patients with diagnoses of MS and NMO; further studies are needed to evaluate the specificity of NMO to other potential causes of LEON. This study is also limited by the modest sample size and the over-representation of African Americans in this cohort in both the NMO and MS groups, reflecting the patient population seen at the Johns Hopkins Hospital. Similarly, women were over-represented in both patient groups and may have skewed the populations towards a more favorable outcome as men have been recently shown to have generally worse outcomes after acute optic neuritis.30 Another potential limitation is the heterogeneity of the samples of both NMO and RRMS patients, which includes both first-ever and repeat optic neuritis events. Previous studies have reported no correlation between the length of an acute optic neuritis lesion by MRI and outcomes of visual acuity and function.30 Further study accounting for acute treatment protocols are needed to confirm these findings, and should include investigations of the relationship of lesion length and retinal injury as measured by optical coherence tomography.
Highlights.
There are differences in MRI features between NMO and MS-associated optic neuritis.
The majority of NMO enhancing lesions were longitudinally extensive > 17.6 mm long
NMO lesions were likely to involve the posterior optic pathways and chiasm.
MS lesions were more commonly restricted to one optic nerve segment anteriorly.
Acknowledgements
Changwan Lu, PhD, at EZBiostat.com provided assistance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Ms. Mealy received honoraria from the International Organization of Multiple Sclerosis Nurses and EMD Serono.
Ms. Whetstone has no disclosures. Dr. Orman has no disclosures.
Dr. Izbudek has received research support from Bayer.
Dr. Calabresi has received personal compensation for consulting and serving on scientific advisory boards from; Vertex, Vaccinex, Medimmune, Prothena, and Abbott; and has received research funding from companies; Biogen-IDEC and Novartis.
Dr. Levy receives research support from NIH, Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem and Genentech, and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline and Medimmune.
References
- 1.Matiello M, Lennon VA, Jacob A, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008 Jun 3;70(23):2197–200. doi: 10.1212/01.wnl.0000303817.82134.da. [DOI] [PubMed] [Google Scholar]
- 2.McKeon A, Fryer JP, Apiwattanakul M, et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol. 2009 Sep;66(9):1134–8. doi: 10.1001/archneurol.2009.178. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Kim W, Li XF, et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler. 2012;18:1480. doi: 10.1177/1352458512439439. [DOI] [PubMed] [Google Scholar]
- 4.Kleiter I, Hellwig K, Berthele A, et al. Failure of Natalizumab to Prevent Relapses in Neuromyelitis Optica. Arch. Neurol. 2012;69:239–245. doi: 10.1001/archneurol.2011.216. [DOI] [PubMed] [Google Scholar]
- 5.Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler. 201218(1):113–5. doi: 10.1177/1352458511431973. [DOI] [PubMed] [Google Scholar]
- 6.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012 Sep;69(9):1176–80. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 8.Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology. 2007;114:810–815. doi: 10.1016/j.ophtha.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 9.de Seze J, Blanc F, Jeanjean L, et al. Optical coherence tomography in neuromyelitis optica. Arch Neurol. 2008;65:920–923. doi: 10.1001/archneur.65.7.920. [DOI] [PubMed] [Google Scholar]
- 10.Merle H, Olindo S, Donnio A, et al. Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci. 2008;49:4412–4417. doi: 10.1167/iovs.08-1815. [DOI] [PubMed] [Google Scholar]
- 11.Naismith RT, Tutlam NT, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72:1077–1082. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302–308. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green AJ, Cree BA. Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. J Neurol Neurosurg Psychiatry. 2009;80:1002–1005. doi: 10.1136/jnnp.2008.166207. [DOI] [PubMed] [Google Scholar]
- 14.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteiro ML, Fernandes DB, Apostolos-Pereira SL, Callegaro D. Quantification of retinal neural loss in patients with neuromyelitis optica and multiple sclerosis with or without optic neuritis using optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:3959–3966. doi: 10.1167/iovs.11-9324. [DOI] [PubMed] [Google Scholar]
- 16.Sotirchos ES, Saidha S, Byraiah G, et al. In vivo identification of morphologic retinal abnormalities in neuromyelitis optica. Neurology. 2013 Apr 9;80(15):1406–14. doi: 10.1212/WNL.0b013e31828c2f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012 Jun;135:1786–93. doi: 10.1093/brain/aws098. Pt 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008 Mar;131:808–17. doi: 10.1093/brain/awm329. Pt 3. [DOI] [PubMed] [Google Scholar]
- 19.Tintoré M, Rovira A, Río J, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology. 2006 Sep 26;67(6):968–72. doi: 10.1212/01.wnl.0000237354.10144.ec. [DOI] [PubMed] [Google Scholar]
- 20.The Optic Neuritis Study Group Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008 Jun;65(6):727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittock SJ, Lennon VA, Krecke K, et al. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006 Mar;63(3):390–6. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 22.Kremer L, Mealy M, Jacob A, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. 2013 Oct 7; doi: 10.1177/1352458513507822. [DOI] [PubMed] [Google Scholar]
- 23.Huh SY, Min JH, Kim W, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Scler. 2014 May;20(6):695–704. doi: 10.1177/1352458513506953. [DOI] [PubMed] [Google Scholar]
- 24.Khanna S, Sharma A, Huecker J, et al. Khanna S, Sharma A, Huecker J, et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol. 2012 Sep;32(3):216–20. doi: 10.1097/WNO.0b013e318254c62d. [DOI] [PubMed] [Google Scholar]
- 25.Storoni M, Davagnanam I, Radon M, et al. Distinguishing optic neuritis in neuromyelitis optica spectrum disease from multiple sclerosis: a novel magnetic resonance imaging scoring system. J Neuroophthalmol. 2013;33:123–127. doi: 10.1097/WNO.0b013e318283c3ed. [DOI] [PubMed] [Google Scholar]
- 26.Pula JH, Kattah JC, Keung B, et al. Longitudinally extensive optic neuritis in neuromyelitis optica spectrum disorder. J Neurol Sci. 2014 Oct 15;345(1-2):209. doi: 10.1016/j.jns.2014.07.049. 12.27. [DOI] [PubMed] [Google Scholar]
- 27.Graves J, Kraus V, Soares BP, et al. Longitudinally Extensive Optic Neuritis in Pediatric Patients. J Child Neurol. 2014 Feb 20; doi: 10.1177/0883073813520500. [DOI] [PubMed] [Google Scholar]
- 28.chmalfuss IM, Dean CW, Sistrom C, Bhatti MT. Optic neuropathy secondary to cat scratch disease: distinguishing MR imaging features from other types of optic neuropathies. AJNR Am J Neuroradiol. 2005 Jun-Jul;26(6):1310–6. [PMC free article] [PubMed] [Google Scholar]
- 29.klar EM, Schatz NJ, Glaser JS, et al. MR of vasculitis-induced optic neuropathy. AJNR Am J Neuroradiol. 1996 Jan;17(1):121–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Malik MT, Healy BC, Benson LA, et al. Factors associated with recovery from acute optic neuritis in patients with multiple sclerosis. Neurology. 2014 Jun 17;82(24):2173–9. doi: 10.1212/WNL.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupersmith MJ, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain. 2002 Apr;125:812–22. doi: 10.1093/brain/awf087. Pt 4. [DOI] [PubMed] [Google Scholar]