Abstract
Navigation and the underlying brain signals are influenced by various allothetic and idiothetic cues, depending on environmental conditions and task demands. Visual landmarks typically control navigation in familiar environments but, in the absence of landmarks, self-movement cues are able to guide navigation relatively accurately. These self-movement cues include signals from the vestibular system, and may originate in the semicircular canals or otolith organs. Here, we tested the otolithic contribution to navigation on a food-hoarding task in darkness and in light. The dark test prevented the use of visual cues and thus favored the use of self-movement information, whereas the light test allowed the use of both visual and non-visual cues. In darkness, tilted mice made shorter-duration stops during the outward journey, and made more circuitous homeward journeys than control mice; heading error, trip duration, and peak error were greater for tilted mice than for controls. In light, tilted mice also showed more circuitous homeward trips, but appeared to correct for errors during the journey; heading error, trip duration, and peak error were similar between groups. These results suggest that signals from the otolith organs are necessary for accurate homing performance in mice, with the greatest contribution in non-visual environments.
Keywords: Vestibular, Path Integration, Head Direction, Navigation, Self-Movement
Introduction
Most mammals are able to navigate accurately by relying on various types of environmental cues. In familiar visual environments, allothetic cues, or landmarks, predominantly guide navigation and the underlying neural signals (Chen et al., 1994; Gallistel, 1990; Goodridge and Taube, 1995; Muller and Kubie, 1987; O'Keefe and Conway, 1978; Taube et al., 1990a; Taube et al., 1990b; Yoder et al., 2011b). In the absence of familiar visual cues or in darkness, however, navigation can be guided by an alternative strategy such as path integration which involves the use of self-movement cues to enable estimation of one’s current position (Benhamou et al., 1990; Etienne et al., 1988; Gallistel, 1990; Muller and Kubie, 1987; Stackman et al., 2003; Taube and Burton, 1995; Taube et al., 1990b; Yoder et al., 2011a). Damage to the hippocampus and associated brain regions impairs performance on both visual and non-visual navigation tasks, suggesting the hippocampus contributes to both types of navigation performance (Koppen et al., 2013; Martin and Wallace, 2007; Morris et al., 1982; Olton and Papas, 1979; Wallace and Whishaw, 2003; Whishaw and Gorny, 1999; Whishaw and Tomie, 1997). This hippocampal contribution may involve multiple sensory signals, but the importance of each sensory system in various navigation tasks is not fully known.
The known self-movement cues that contribute to path integration include optic flow, proprioception, and vestibular cues. In humans, proprioception and/or vestibular cues appear to dominantly influence path integration when these cues are available, but optic flow is capable of supporting path integration in virtual environments (Kearns et al., 2002). In rodents, bilateral vestibular lesions impair the use of self-movement cues on a food-hoarding task in darkness and on a relatively simple navigation task when no visual cues are available (Stackman and Herbert, 2002; Wallace et al., 2002; for review, see Yoder and Taube, 2014). At least a portion of this vestibular contribution to path integration appears to originate in the semicircular canals. Slow, passive rotation [below the threshold of canal activation] at the end of an outbound journey produced error during the return trip, but this error disappeared with above-threshold rotations (Mittelstaedt and Mittelstaedt, 1980). The canals do not provide the only vestibular input to path integration, however, as animals that performed a homing task on a rotating table made trajectories that were intermediate to those expected from inertial or rotatory influences alone (Mittelstaedt and Glasauer, 1991). The canal contribution may therefore be complemented by signals from the otolith organs. Further, mice with dysfunctional utricular hair cells showed much shorter trajectories than control mice in darkness, possibly the result of impaired path integration (Avni et al., 2009). The vestibular influence on path integration thus appears to include inputs from both the canals and otolith organs.
No previous studies have clearly demonstrated whether accurate path integration can occur in the absence of either the canal- or otolith-mediated signal. We therefore tested the homing ability of otoconia-deficient tilted mice on a food-hoarding task in darkness and in light. This task is known to require a functional vestibular system (Wallace et al., 2002), and the present results provide important insight into the otolithic contribution to path integration.
Materials and Methods
Subjects
All procedures involving live animals were approved by the Purdue Animal Care & Use Committee. Male tilted mice and their heterozygous littermates were used, with different mice performing in light and dark environments. C57BL/6J mice were bred with tilted mice (B6.Cg-Otop1tlt/J; Jackson Laboratories, Bar Harbor, ME) to produce heterozygous (+ / tlt) offspring. Pairs of tilted mice were bred to produce homozygous (tlt / tlt) offspring. These heterozygous and homozygous offspring were then bred to produce hetero- and homozygous offspring at a predicted 50% frequency. Offspring were screened for the tilted phenotype with a swim test, which is known to reliably detect otolith dysfunction (Ornitz et al., 1998). Briefly, mice were dropped from a height of 20 cm into a pool of water. Mice with functional otolith organs immediately resurfaced and swam with their heads above water, whereas tilted mice either failed to resurface or otherwise became submerged. Mice that were not able to swim were immediately rescued with a small net to prevent drowning. The actual frequency of tilted offspring was slightly less than 50%, as reported previously (Ornitz et al., 1998; Yoder and Kirby, 2014). Mice categorized as + / - and - / - were then selected from the entire population. All mice were 3–8 months of age at the beginning of testing.
Apparatus
The food-hoarding task was a modified version of a task designed for rats (Wallace et al., 2002; Whishaw and Tomie, 1997). A circular table (122 cm diam) with a refuge affixed to the edge was used (Fig. 1). For training trials, the table was located in the center of the room for pretraining in dim light, and the refuge was visible from all points on the table. Test trials occurred in a corner of the room, and performance was digitally recorded by an overhead color/infrared camera. Various objects around the room (a large wooden cabinet, wooden room divider, small wall cabinet) and the room geometry were visible from the table, and could serve as potential landmarks. For testing in light, the refuge (13 × 9 × 6 cm) was lowered below the edge of the table such that it was not visible from the table surface except at locations very near the box; visual detection of the box therefore would not be able to guide navigation until the animal had approached the box position. A set of stairs allowed mice to easily climb to and from the table surface. A single lamp placed behind a screen provided indirect illumination of the table and surrounding items. For testing in darkness, the refuge (13 × 11 × 9 cm, with one end open) was clamped to the edge of the table, such that the animal could easily enter the box. The table was surrounded by an opaque curtain, and all room lights were extinguished. The experimenter wore infrared goggles to allow visualization during dark testing procedures.
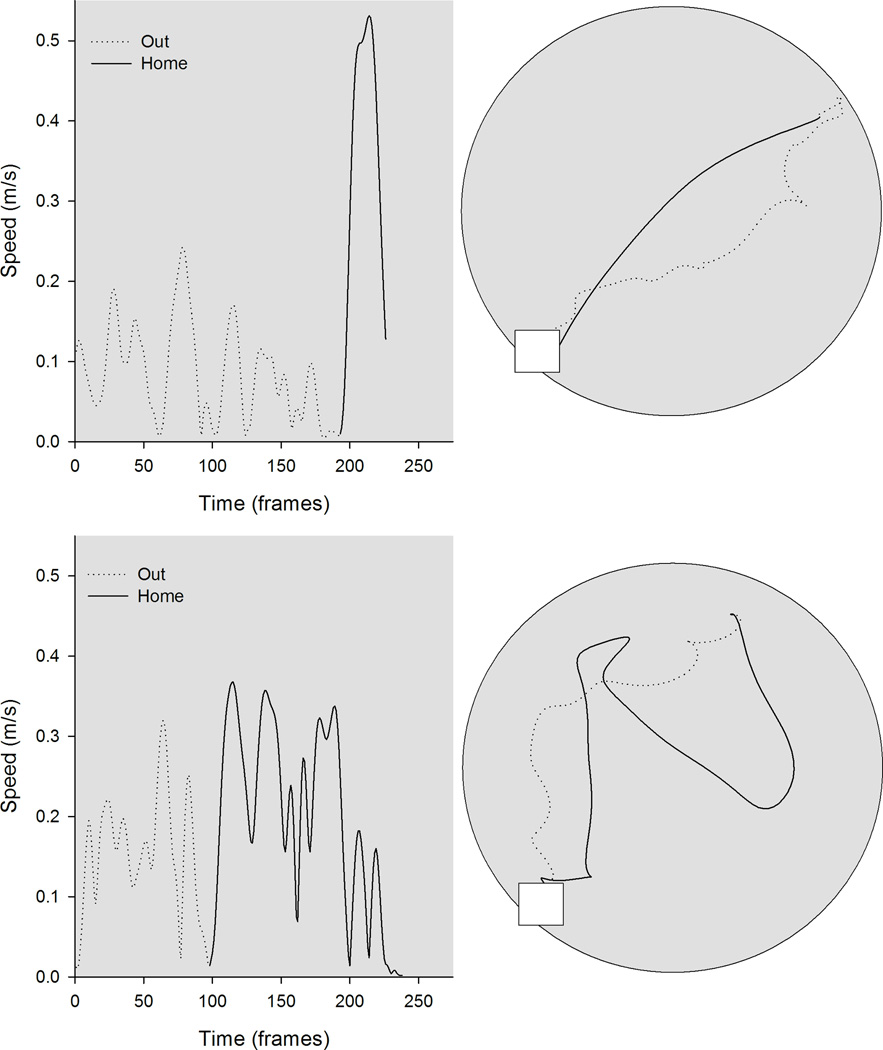
Figure 1.
Kinematic (left) and topographic (top) characteristics are plotted for a representative control (top) and tilted mouse (bottom) on the homing task in darkness. The outward path (gray line) is circuitous for both mice; the homeward segment (black line) is relatively direct for the control mouse, but is more circuitous for the tilted mouse.
Procedure
Training
Mice were food-restricted to maintain 85% of their ad lib body weight. Three daily training trials occurred across 10 days, in a lighted room. These trials involved placing a small food pellet (broken piece of rodent chow) several cm from the start box containing a small amount of bedding from the animal’s home cage. The mouse was then released to forage for food. When a mouse picked up the food, it typically carried it to the visible start box for consumption. If the mouse began to eat at the location they found the food, the experimenter activated a manual clicker, snapped their fingers, or rattled keys, etc. to encourage the mouse to return to the start box. When the mouse successfully carried the food to the start box, the distance was increased several cm on the next trial, until each mouse successfully carried the food from the opposite side of the table back to the start box. The apparatus was wiped with alcohol between each trial to remove potential odor cues.
Testing
Testing began after training was complete, and included three trials per day, across five days. The table was divided into five zones at increasing distances from the refuge (Fig. 1). A food pellet was placed within one of the three distal zones, and the mouse was released from the start box to search for food. Most mice then immediately carried the food back to the start box for consumption. In the event they did not carry the food back to the refuge, an auditory stimulus was activated to discourage consumption on the table. As with training trials, the apparatus was wiped with alcohol between each trial to remove potential odor cues.
Data analysis
The Peak Performance (Vicon, Denver, CO USA) motion capture system was used to quantify movement characteristics of food hoarding under dark and light conditions. Mouse movement was tracked by selecting one pixel that corresponded to the tip of the nose, in every fifth video frame. The resulting x- and y-coordinates were scaled to real world units and used to calculate performance measures. Each food-hoarding trip was divided into outward (searching) and homeward segments. The entire outward segment began when the mouse left the refuge and included all movements until the food pellet was located. However, most mice revisited the refuge if a search was unsuccessful, and we therefore analyzed only the movements that occurred after the last visit to the refuge. The outward segment ended when the animal picked up the food pellet. The homeward segment began at the end of the outward segment and included all movement until the mouse returned to the refuge.
Previous studies with rats revealed that the outward segment of the food-hoarding trip is typically circuitous, whereas the homeward journey is relatively direct (Koppen et al., 2013; Martin and Wallace, 2007; Wallace et al., 2010; Whishaw et al., 1995). Additionally, movement characteristics of each journey segment provide important insight into the use of self-movement cues for navigation (Martin and Wallace, 2007). These topographic and kinematic properties include: Duration was defined as the time required to find the food item (outward segment) or carry the food item to the refuge (homeward segment). Stops per trip were calculated for both outward and homeward segments by counting the number of occasions that moment-to-moment speeds dropped below 0.1 m/s on a segment and dividing by the total number of trips made across testing days. Stop duration was defined as the mean time spent below 0.1 m/s on both segments. Peak speed was defined as the maximum speed observed on a segment. Path circuity was calculated as the ratio between the distance from the start point to the end point of a segment and the actual distance traveled on the segment. A path circuity value close to 1.0 reflects a more direct path, whereas a value near 0.0 reflects a more complex path. Heading error was calculated as the angle subtended by: 1) first point of the outward segment, 2) last point of the outward segment, 3) point associated with the peak speed on the homeward segment. Peak error was calculated as the absolute difference between the mid-point of the trip (0.5) and the location of the peak speed; this value is expressed as the proportion of the trip. Each measure was averaged across test trials for each mouse; scores were then averaged for comparison between groups.
Statistical analyses were performed on a personal computer running SPSS (IBM, Armonk, NY) and Statview (SAS, Cary, NC). For each measure, a Kolmogorov-Smirnov test with Lilliefors correction was used to compare the distribution of scores to a hypothetical normal distribution, and Levene’s test was used to test for equal variances. Group performance was then compared with mixed design ANOVAs or t-tests, as appropriate. Fisher’s LSD (LSD) post hoc test was used to explore significant results of the mixed design ANOVA.
Results
Dark conditions
Control (n=10) and tilted (n=11) mice were trained to leave the refuge, search for food pellet placed at varying directions and distances from the refuge, and carry the food pellet to the refuge under dark conditions. Kinematics and topographic profiles are plotted for representative control and tilted mice (see Figure 1). Significant group differences in movement characteristics were observed on outward and homeward segments.
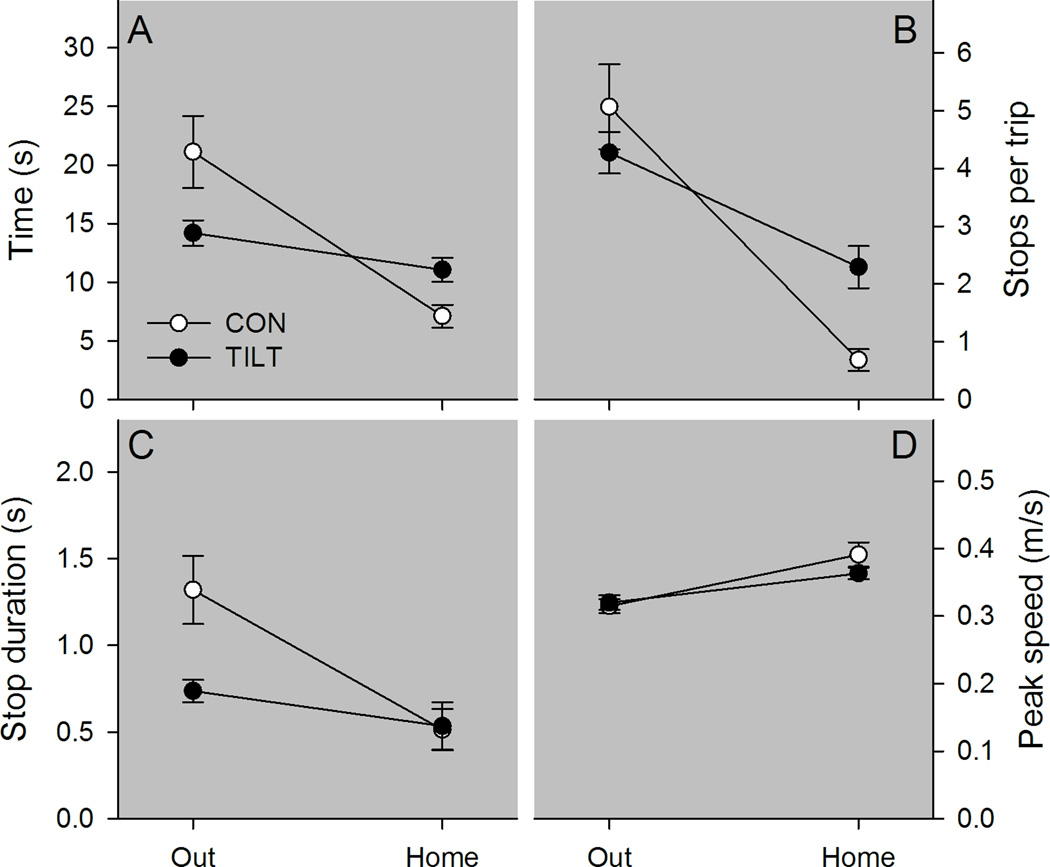
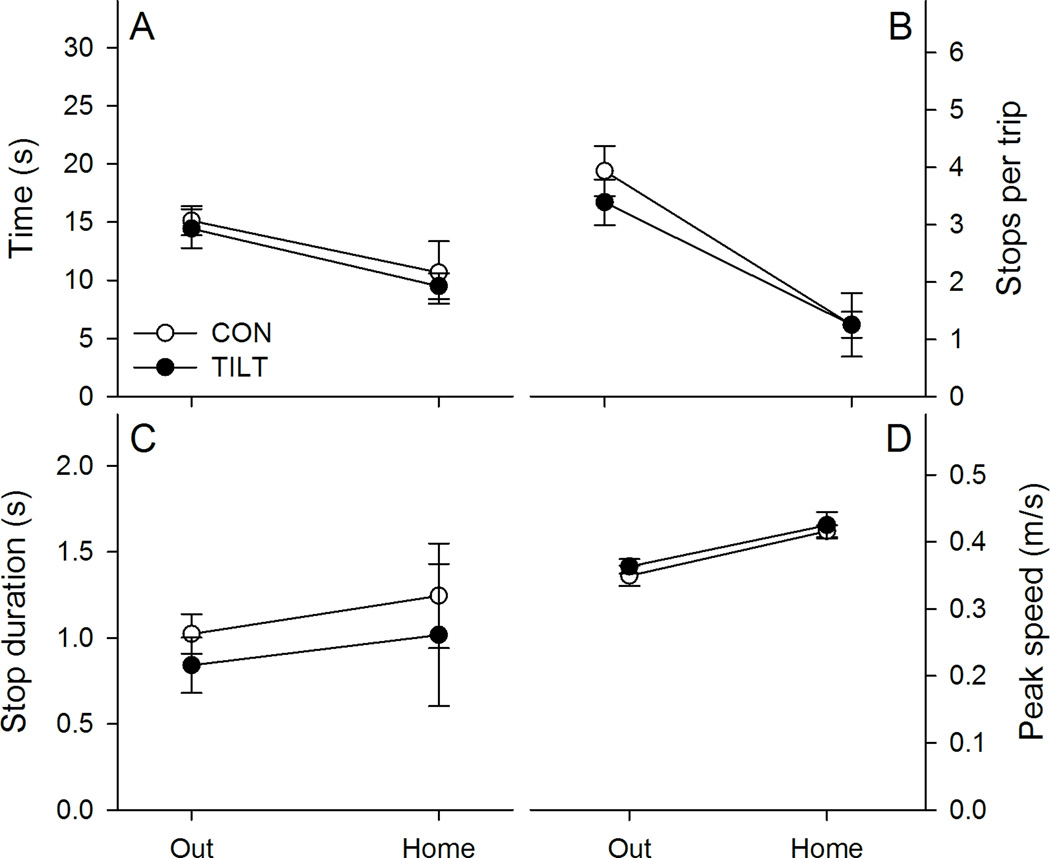
Both groups of mice took longer to find the food pellet than it took to carry the food pellet to the refuge, but group membership influenced this effect (see Figure 2 panel A). Across both trip segments, control and tilted mice showed similar trip durations, Group F(1,19)=.734, p=.402, ηp2=.037, and trip duration was different between the outward and homeward segments, Segment F(1,19)=25.955, p<.001, ηp2=.568. However, the duration of homeward and outward trip segments differed between groups, Group X Segment F(1,19)=10.035, p=.005, ηp2=.346; control mice took longer to find the food pellet than tilted mice, LSD p = .04, but the control mice took less time to return to the refuge than tilted mice, LSD p = .012. To further examine the differences in the time to locate the food pellet, the average number of stops per trip and stop duration were evaluated for outward and homeward segments (See Figure 2 panels B & C). The ANOVA conducted on average number of stops per trip revealed no difference between groups, F(1,19)=.781, p=.388, ηp2=.039; however, more stops occurred on the outward segment than on the homeward segment, Segment F(1,19)=52.839, p<.001, ηp2=.736. In addition, tilted mice exhibited more stops than control mice on the homeward segment, Group X Segment F(1,19)=7.510, p=.013, ηp2=.283, LSD p=.001. The ANOVA conducted on average stop duration revealed that control mice made longer stops relative to tilted mice, Group F(1,19)=5.056, p=.037, ηp2=.210. Pooled stop durations were longer on the outward segment relative to the homeward segment, Segment F(1,19)=12.158, p=.002, ηp2=.390. However, the group differences were marginally dependent on the trip segment, Group X Segment F(1,19)=4.351, p=.051, ηp2=.186. Control mice had significantly longer stop durations on the outward segment relative to tilted mice, LSD p=.008, but groups had similar stop durations on the homeward segment, LSD p=.91. These results may reflect impaired self-movement cue processing during outward segment stops in the tilted mice under dark conditions.
Figure 2.
Kinematic characteristics are plotted for control and tilted mice in darkness. A, Tilted mice (TILT) completed the outward segment faster than control mice (CON). However, tilted mice took longer to complete the homeward segment than control mice. B, Tilted mice’s faster performance of the outward segment did not result from fewer stops per trip. C, However, the duration of stops was shorter for tilted mice than for control mice. D, Peak speed did not differ between groups for either trip segment. *p < .05 Mean ± SEM.
Groups did not differ in peak speed, but significantly different peak speeds were observed between trip segments (see Figure 2 panel D). Peak speed was greater during the homeward segment than the outward segment, Segment F(1,19)=49.293, p<.001, ηp2=.722. However, groups showed similar peak speeds overall, Group F(1,19)=0.562, p=.463, ηp2=.029, and showed similar changes in peak speed across segments, Group X Segment F(1,19)=3.728, p=.069, ηp2=.164. These results are consistent with both groups exhibiting similar motivation to search and carry the food item to the refuge under dark conditions.
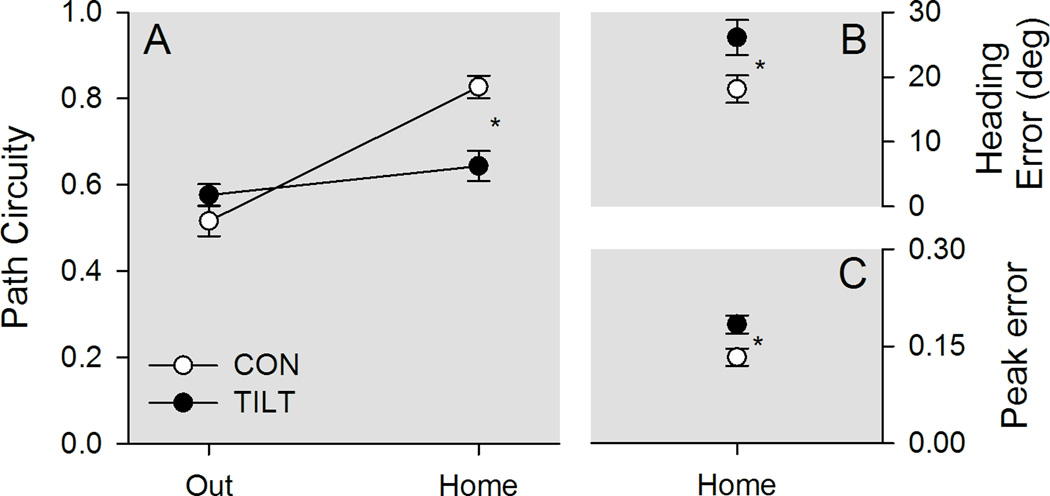
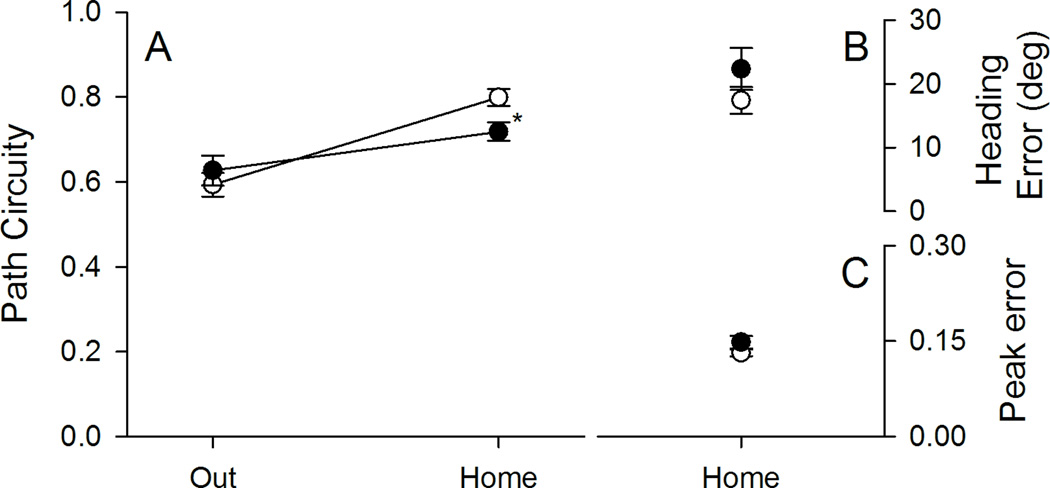
Group differences in path circuity depended on the trip segment (see Figure 3 panel A). Control and tilted mice showed similar path circuity scores across both trip segments, Group F(1,19)=3.462, p=.078, ηp2=.154, and scores differed between trip segments, Segment F(1,19)=44.816, p<.001, ηp2=.702. Importantly, groups differed in the amount of path circuity within each trip segment, Group X Segment F(1,19)=18.637, p<.001, ηp2=.495; control and tilted mice followed equivalent paths to find the food pellet, LSD, p = .17, but tilted mice followed more circuitous paths back to the refuge than control mice, LSD, p = .005. To further examine homeward segment performance, both heading and peak errors were calculated for the homeward segment (see Figure 3 Panels B & C). Tilted mice had significantly greater heading error, t(19)=2.290, p<.05, and peak error, t(19)=2.616, <.05, than control mice. These results are consistent with tilted mice exhibiting an impaired ability to estimate the direction and distance to the refuge, after finding the food pellet under dark conditions.
Figure 3.
Topographic characteristics are plotted for control and tilted mice in darkness. A, The outward segment was circuitous for both groups of mice. Upon finding the food, control (CON) mice returned relatively directly to the refuge, whereas tilted (TILT) mice took a more circuitous route. B, Tilted mice showed greater heading error than control mice. C, Peak speed occurred at a greater distance from the mid-point for tilted mice than for control mice. *p < .05 Mean ± SEM.
Light conditions
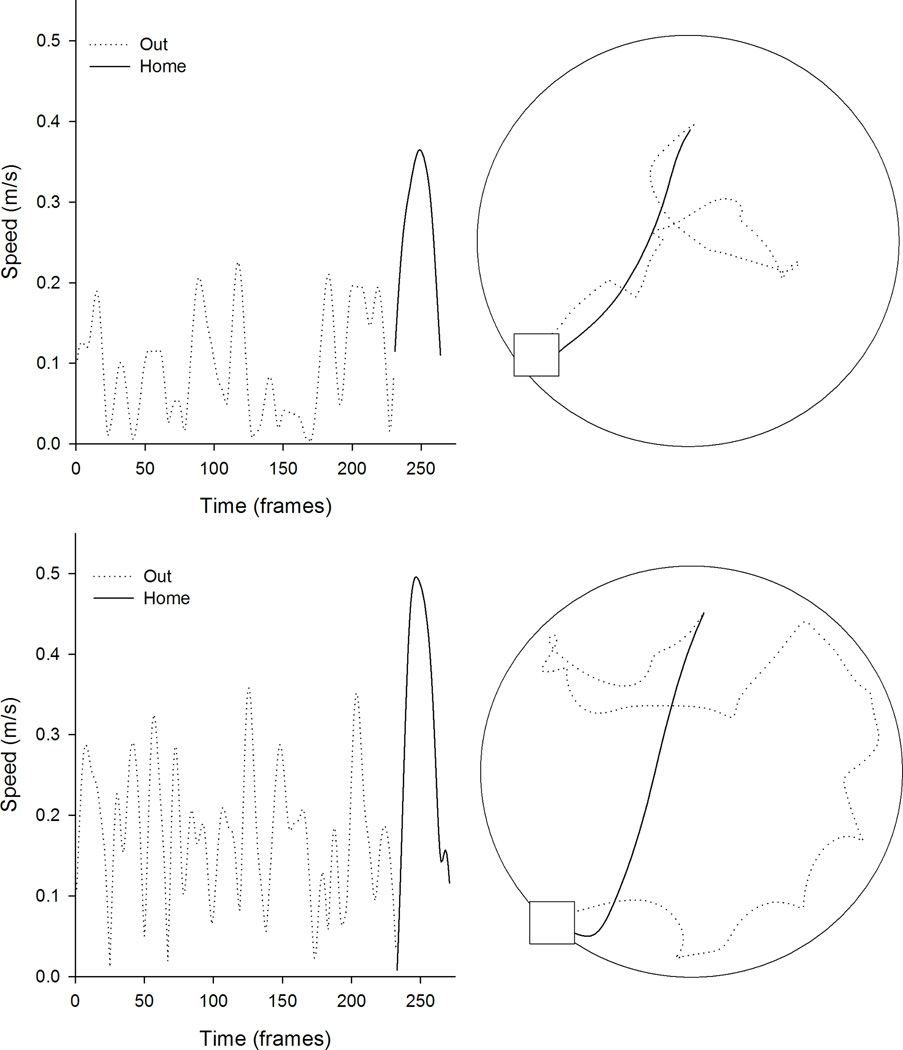
Control (n=9) and titled (n=8) mice were trained to leave the refuge, search for a randomly placed food pellet, and carry the food pellet to the refuge under light conditions. Representative kinematics and topographic profiles are plotted for representative control and tilted mice (see Figure 4). In general, food hoarding behavior was similar between groups on outward and homeward segments under light conditions.
Figure 4.
Kinematic (left) and topographic (top) characteristics are plotted for a representative control (top) and tilted mouse (bottom) on the homing task in light. The outward path (gray line) is circuitous for both mice, whereas the homeward segment (black line) is relatively direct for both mice.
Both groups exhibited a similar trend of taking longer to find the food pellet than carrying the food pellet home (see Figure 5 panel A). The homeward segment was significantly shorter than the outward segment, Segment F(1,15)=6.859, p<.05, ηp2=.314. However, groups did not differ overall, Group F(1,15)=.247, p<.626, ηp2=.016, or within each trip segment, Group × Segment F(1,15)=.019, p=.892, ηp2=.001. Recall that group differences were observed in outward segment organization under dark conditions, therefore a similar stop analysis was applied to outward and homeward segment behavior under light conditions (see Figure 5 panels B & C). The ANOVA conducted on average number of stops per trip revealed that more stops occurred on the outward segment than on the homeward segment, Segment F(1,15)=26.075, p<.001, ηp2=.635. However, control and tilted mice made similar numbers of stops overall, F(1,15)=.486, p=496, ηp2=.031, and groups did not differ across segments F(1,15)=.340, p=.568, ηp2=.022. The ANOVA conducted on average stop duration revealed that control mice made stops of similar duration, Group F(1,15)=.460, p=.508, ηp2=.030. Stop durations did not vary across segments, Segment F(1,15)=.711, p=.412, ηp2=.045, and groups did not differ within segments, Group X Segment F(1,15)=.009, p=.925, ηp2=.001. These results demonstrate that access to visual information was sufficient to organize behavior during both the outward and homeward segments.
Figure 5.
Kinematic characteristics of control and tilted mice in light. A, Both control (CON) and tilted mice (TILT) completed the homeward segment faster than the outward segment. Groups did not differ on segment duration. B, Control and tilted mice showed similar numbers of stops per trip, and C, the duration of stops was similar between groups. D, Peak speed did not differ between groups for either trip segment. Mean ± SEM.
Peak speed did not differ between groups, but significantly faster peak speeds were observed on the homeward segment relative to the searching segment (see Figure 5 panel D). Overall, peak speed was greater for the homeward segment than for the outward segment, Segment F(1,15)=50.478, p<.001, ηp2=.771. However, control and tilted mice showed similar peak speeds, Group F(1,15)=0.410, p=.532, ηp2=.027, and groups did not show different peak speeds within trip segments, Group × Segment F(1,15)=.086, p=.773, ηp2=.006. These results are consistent with both groups exhibiting similar motivation to search and carry the food item to the refuge under light conditions.
Group differences in path circuity depended on the trip segment (see Figure 6 panel A). Overall, control and tilted mice showed similar path circuity scores, Group F(1,15)=.752, p=.399, ηp2=.048. However, the homeward segment was significantly more direct than the outward segment, Segment F(1,15)=32.378, p<.001, ηp2=.683, and groups showed different path circuity scores within segments, Group × Segment F(1,15)=4.778, p<.05, ηp2=.242. Control and tilted mice followed equivalent paths to find the food pellet, LSD, p = .47, but control mice followed more direct paths to than tilted mice, LSD, p = .015. To further examine homeward segment performance, both heading and peak errors were calculated for the homeward segment (see Figure 6 Panels B & C). Groups showed similar measures of heading error, t(15)=1.291, p=.216, and peak error, t(10.960)=1.532, p=.154 (adjusted for variance heterogeneity). These results are consistent with tilted mice initially exhibiting impaired direction estimation to the refuge, but then correcting for this error as the homeward segment progressed. The use of environmental cues during the homeward journey may therefore partially compensate for tilted mice's impairments in directional heading.
Figure 6.
Topographic characteristics are plotted for control and tilted mice under light conditions. A, The outward segment was relatively circuitous for both groups of mice. Upon finding the food, control (CON) mice returned relatively directly to the refuge, whereas tilted (TILT) mice took a slightly more circuitous route. B, Both groups showed similar heading error during the homeward segment. C, Peak speed occurred relatively close to the mid-point of the homeward segment for both groups. *p < .05 Mean ± SEM.
Discussion
A food-hoarding task was used to evaluate the role of the otolith organs in navigation guided by self-movement cues alone, or by a combination of self-movement and visual cues. Otoconia-deficient mice were markedly impaired at performing the food-hoarding task in darkness, where path integration during the outward journey is necessary for accurate homing. In addition, kinematic analysis of outward segment movement in darkness revealed that otoconia-deficient mice were not impaired at movement in general, but made shorter-duration stops during the outward segment and more stops on the homeward segment. In contrast, otoconia-deficient mice showed milder impairments on the food-hoarding task in light, where navigation can be guided by allothetic or idiothetic cues, or a combination thereof.
Navigation in Darkness
The vestibular contribution to path integration includes signals from the otolith organs. In two previous studies using a food-hoarding task, control rats were able to accurately return to the refuge in darkness, whereas vestibular rats took more circuitous paths back to the refuge (Wallace et al., 2002; Zheng et al., 2009). This task requires the use of self-movement cues experienced during the outward segment, to estimate the direction to the refuge. Like vestibular rats, otoconia-deficient mice were unable to accurately return to the refuge in darkness. The tilted also made more stops than the control mice on the homeward segment. This is further evidence that tilted mice were unable to estimate their position relative to the refuge. These disruptions in performance suggest the otolith organs provide some of the self-movement cues that are essential for estimating the direction to the refuge. However, otolith dysfunction is unlikely to explain all of the path integration deficits associated with vestibular lesions, given the complementary roles of the semicircular canals and otolith organs for homing in darkness (Mittelstaedt and Glasauer, 1991).
In addition to direction estimation, the otolith organs also appear to contribute to distance estimation on the food-hoarding task . Previous studies showed that rats use distance estimates to organize the temporal pacing of moment-to-moment speeds on the homeward segment (Wallace et al., 2006; Wallace et al., 2010). Specifically, peak speed consistently occurred at the midpoint of the homeward segment. Here, we provide evidence that temporal pacing is also a characteristic of navigation in mice, as control mice exhibited a similar temporal pacing of moment-to-moment speeds on the homeward segment. The otolithic detection of linear acceleration is an ideal source of information to support online updating of distance relative to the refuge during the homeward segment. Therefore, observing the tilted mice’s greater peak error in the homeward segment, relative to control mice, is consistent with this role for the otolith organs in distance estimation. A caveat to this interpretation is that impaired distance estimation may be confounded with direction estimation on the food-hoarding task, given that an incorrect direction would place the point of peak speed at a location other than the mid-point of the journey. Regardless, the available evidence suggests an important role for otolith organs in direction estimation, and possibly distance estimation, under dark conditions.
Navigation is thought to depend on multiple neural signals that can be maintained by path integration and these signals provide insight to performance deficits in tilted mice. First, the animal's perceived directional heading is reflected by the head direction signal (for review, see Taube, 2007). A relation between the head direction signal and path integration is indicated by head direction signal maintenance by self-movement cues (Stackman et al., 2003; Taube and Burton, 1995; Taube et al., 1990b; Yoder et al., 2011a). Additionally, the head direction signal appears to update according to errors experienced during a previous foraging trip (Valerio and Taube, 2012). Further, path integration is impaired following damage to nuclei containing head direction cells (Frohardt et al., 2006; Winter et al., 2011). Therefore it is possible that the tilted mice's deficits is related to their degraded head direction cell activity (Yoder and Taube, 2009). A second brain signal that may contribute to path integration is provided by place cells, which represent the animal’s perceived location within an environment (O'Keefe and Dostrovsky, 1971), remain relatively stable even in the absence of visual landmarks (Markus et al., 1994; Muller and Kubie, 1987; O'Keefe and Speakman, 1987). Vestibular inactivation disrupted place cell activity (Russell et al., 2003b; Stackman et al., 2002), suggesting the navigation deficits associated with vestibular dysfunction may result from disrupted place cell activity. However, our preliminary results suggest that tilted mice have place cells that remain stable across trials, including in darkness (Yoder et al., 2014). The activity of grid cells represent a third possible signal to support path integration. Grid cells fire in a tessellating grid pattern even in the absence of visual information (Fyhn et al., 2004; Hafting et al., 2005; Moser and Moser, 2008; Sargolini et al., 2006). However, a vestibular contribution to grid cell activity has not been demonstrated. Thus, the performance deficits associated with otolith dysfunction may involve head direction signal degradation; however, further work is needed to characterize the role of place and grid cell signals in path integration.
Another brain signal that may process otolithic information is hippocampal theta rhythm, a robust field potential oscillation associated with movement (Vanderwolf, 1969). Vestibular involvement in theta rhythm is indicated by continuous theta during passive rotation (Gavrilov et al., 1995; Shin, 2010), and attenuated theta rhythm following vestibular lesions (Russell et al., 2006). At least some of this vestibular contribution appears to involve excitatory input from medial septal cholinergic neurons, as selective cholinergic lesions eliminated rotation-induced theta (Tai et al., 2012). Path integration deficits were observed following non-selective damage to the medial septum (Martin et al., 2007) or damage limited to medial septal cholinergic neurons (Martin and Wallace, 2007). However, theta rhythm is also influenced by medial septal GABAergic neurons (Yoder and Pang, 2005), but their role in transmitting vestibular information remains unknown. Nevertheless, path integration deficits were observed following selective GABAergic lesions (Koppen et al., 2013), suggesting they may also contribute self-movement related input to hippocampus. If the otolithic contribution to path integration involves theta rhythm, then we would expect tilted mice to exhibit deficits similar to those associated with cholinergic or GABAergic lesions. Indeed, the homing deficit of tilted mice was similar to that of rats with selective cholinergic or GABAergic lesions of the medial septum. Further, tilted mice’s kinematic characteristics during the outward journey were similar to those of rats with lesions of the fimbria/fornix or medial septum, which make shorter-duration stops during exploration than control rats (Martin et al., 2007; Whishaw et al., 1994). Thus, a portion of the otolithic information may reach hippocampus via the medial septum, and the movement deficits associated with otolith dysfunction may be related to changes in hippocampal theta rhythm.
Navigation in Light
Navigation in visual environments is typically influenced by both visual and non-visual cues. Previous studies have shown that mice predominantly rely on distal visual landmarks to guide long-distance (50 cm) journeys in a visual homing task, but preferentially use a path integration or beacon strategy for short-distance (20 cm) journeys (Alyan and Jander, 1997). In the present homing task, all homing excursions were >20 cm, and the refuge was not visible from the surface of the table. This configuration therefore favored navigation guided by distal visual cues instead of by a beacon. Despite the predominant use of distal visual cues to guide navigation on spatial tasks, signals from the vestibular system are often necessary for accurate performance. For example, bilateral vestibular dysfunction disrupted performance on working and reference memory tasks on a radial arm maze (Besnard et al., 2012; Ossenkopp and Hargreaves, 1993; Russell et al., 2003a). In contrast, vestibular dysfunction failed to disrupt rats’ homing performance on a food-hoarding task in light (Wallace et al., 2002; Zheng et al., 2009), and failed to disrupt performance on a relatively simple navigation task in light (Stackman and Herbert, 2002). Further, otoconia-deficient mice were impaired on a radial arm maze discrimination task, but were not impaired on a Barnes maze task (Yoder and Kirby, 2014). One difference between the tasks that were sensitive to vestibular dysfunction and those that were not is the number of goals; tasks that were sensitive to vestibular dysfunction included multiple goal locations, whereas those that were not sensitive to vestibular dysfunction included single goal locations. Multiple goals would have required the animal to reorient at one goal in order to navigate to the next goal, whereas a single goal location would not have required this reorientation after the goal was approached. It is therefore somewhat surprising that tilted mice showed more circuitous homeward journeys than control mice on the present homing task in light. However, tilted mice’s heading error was not different from controls, suggesting they were able to use visual cues to correct for error at some point along the homeward journey.
Several recent findings provide insight into tilted mice’s subtle visual navigation deficits on the food-hoarding task in light. The present task included a home location that remained constant across trials, relative to the room, allowing mice to efficiently return to this location by using either a relative or absolute search strategy. A relative search strategy involves moving in the direction of a learned goal relative to the apparatus, instead of to the absolute location of the goal relative to the room. Both mice and rats preferentially use relative or directional search strategies on water maze tasks (Cahill et al., 2014; Hamilton et al., 2008; Hamilton et al., 2007; Skinner et al., 2010; Stackman et al., 2012), and may therefore favor this strategy on other tasks involving open-field navigation. The relative search strategy appears to depend on the head direction signal, as the use of a relative search strategy was disrupted following anterior thalamus lesions (Stackman et al., 2012) or following damage to brain regions involved in providing visual landmark information to the head direction signal (for review, see Yoder et al., 2011b). Therefore, the degraded head direction cell activity of tilted mice (Yoder and Taube, 2009) may be related to an impaired ability to use a relative search strategy to move in the direction of the refuge. A caveat to this interpretation is that no studies have tested the search strategy used by mice on a visual homing task. Additional experimentation is therefore necessary to determine which search strategy is used to solve the homing task.
Summary and Conclusion
Mice with dysfunctional otolith organs made more circuitous homeward journeys and showed altered movement characteristics on a homing task in darkness, relative to littermate controls. In light, otolith dysfunction also resulted in more circuitous homeward journeys, but movement characteristics were similar between groups. Self-movement cues from the otolith organs therefore appear to be necessary for accurate non-visual navigation, but have a lesser role in visual navigation.
Acknowledgements
The authors thank Sarah Brockman, Ryan Harvey, and Stephanie Rutan for assistance with data collection.
Funding:
Grant Sponsor: National Institute on Deafness & Other Communication Disorders
Grant number: DC012630
References
- Alyan SH, Jander R. Interplay of directional navigation mechanisms as a function of near-goal distance: experiments with the house mouse. Behav Processes. 1997;41(3):245–255. doi: 10.1016/s0376-6357(97)00051-x. [DOI] [PubMed] [Google Scholar]
- Avni R, Elkan T, Dror AA, Shefer S, Eilam D, Avraham KB, Mintz M. Mice with vestibular deficiency display hyperactivity, disorientation, and signs of anxiety. Behav Brain Res. 2009;202(2):210–217. doi: 10.1016/j.bbr.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Benhamou S, Sauve J-P, Bovet P. Spatial memory in large scale movements: Efficiency and limitation of the egocentric coding process. J Theor Biol. 1990;145:1–12. [Google Scholar]
- Besnard S, Machado ML, Vignaux G, Boulouard M, Coquerel A, Bouet V, Freret T, Denise P, Lelong-Boulouard V. Influence of vestibular input on spatial and nonspatial memory and on hippocampal NMDA receptors. Hippocampus. 2012;22(4):814–826. doi: 10.1002/hipo.20942. [DOI] [PubMed] [Google Scholar]
- Cahill SP, Fifield KE, Thorpe CM, Martin GM, Skinner DM. Mice use start point orientation to solve spatial problems in a water T-maze. Anim Cogn. 2014 doi: 10.1007/s10071-014-0789-1. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. II. Contributions of visual and ideothetic information to the directional firing. Exp Brain Res. 1994;101(1):24–34. doi: 10.1007/BF00243213. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Maurer R, Saucy F. Limitations in the assessment of path dependent information. Behaviour. 1988;106:81–111. [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305(5688):1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Frohardt RJ, Bassett JP, Taube JS. Path integration and lesions within the head direction cell circuit: comparison between the roles of the anterodorsal thalamus and dorsal tegmental nucleus. Behav Neurosci. 2006;120:135–149. doi: 10.1037/0735-7044.120.1.135. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1990. p. 648. [Google Scholar]
- Gavrilov VV, Wiener SI, Berthoz A. Enhanced hippocampal theta EEG during whole body rotations in awake restrained rats. Neurosci Lett. 1995;197(3):239–241. doi: 10.1016/0304-3940(95)11918-m. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Preferential use of the landmark navigational system by head direction cells in rats. Behav Neurosci. 1995;109(1):49–61. doi: 10.1037//0735-7044.109.1.49. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436(7052):801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Johnson TE, Rice JP, Candelaria FT, Sutherland RJ, Weisend MP, Redhead ES. The relative influence of place and direction in the Morris water task. J Exp Psychol Anim Behav Process. 2008;34(1):31–53. doi: 10.1037/0097-7403.34.1.31. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Weisend MP, Sutherland RJ. How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J Exp Psychol Anim Behav Process. 2007;33(2):100–114. doi: 10.1037/0097-7403.33.2.100. [DOI] [PubMed] [Google Scholar]
- Harrod CG, Baker JF. The vestibulo ocular reflex (VOR) in otoconia deficient head tilt (het) mutant mice versus wild type C57BL/6 mice. Brain Res. 2003;972(1–2):75–83. doi: 10.1016/s0006-8993(03)02505-8. [DOI] [PubMed] [Google Scholar]
- Kearns MJ, Warren WH, Duchon AP, Tarr MJ. Path integration from optic flow and body senses in a homing task. Perception. 2002;31(3):349–374. doi: 10.1068/p3311. [DOI] [PubMed] [Google Scholar]
- Koppen JR, Winter SS, Stuebing SL, Cheatwood JL, Wallace DG. Infusion of GAT1-saporin into the medial septum/vertical limb of the diagonal band disrupts self-movement cue processing and spares mnemonic function. Brain Struct Funct. 2013;218(5):1099–1114. doi: 10.1007/s00429-012-0449-7. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus. 1994;4(4):410–421. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
- Martin MM, Horn KL, Kusman KJ, Wallace DG. Medial septum lesions disrupt exploratory trip organization: evidence for septohippocampal involvement in dead reckoning. Physiol Behav. 2007;90(2–3):412–424. doi: 10.1016/j.physbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Martin MM, Wallace DG. Selective hippocampal cholinergic deafferentation impairs self-movement cue use during a food hoarding task. Behav Brain Res. 2007;183(1):78–86. doi: 10.1016/j.bbr.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstaedt ML, Glasauer S. Idiothetic navigation in gerbils and humans. Zool Jb Physiol. 1991;95:427–435. [Google Scholar]
- Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften. 1980;67(11):566–567. [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. A metric for space. Hippocampus. 2008;18(12):1142–1156. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978;31(4):573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68(1):1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17(6):669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Bohne BA, Thalmann I, Harding GW, Thalmann R. Otoconial agenesis in tilted mutant mice. Hear Res. 1998;122(1–2):60–70. doi: 10.1016/s0378-5955(98)00080-x. [DOI] [PubMed] [Google Scholar]
- Ossenkopp KP, Hargreaves EL. Spatial learning in an enclosed eight-arm radial maze in rats with sodium arsanilate-induced labyrinthectomies. Behav Neural Biol. 1993;59(3):253–257. doi: 10.1016/0163-1047(93)91034-k. [DOI] [PubMed] [Google Scholar]
- Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Bilateral peripheral vestibular lesions produce long-term changes in spatial learning in the rat. J Vestib Res. 2003a;13(1):9–16. [PubMed] [Google Scholar]
- Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J Neurosci. 2003b;23(16):6490–6498. doi: 10.1523/JNEUROSCI.23-16-06490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Lesions of the vestibular system disrupt hippocampal theta rhythm in the rat. J Neurophysiol. 2006;96(1):4–14. doi: 10.1152/jn.00953.2005. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312(5774):758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Shin J. Passive rotation-induced theta rhythm and orientation homeostasis response. Synapse. 2010;64(5):409–415. doi: 10.1002/syn.20742. [DOI] [PubMed] [Google Scholar]
- Skinner DM, Horne MR, Murphy KE, Martin GM. Rats' orientation is more important than start point location for successful place learning. J Exp Psychol Anim Behav Process. 2010;36(1):110–116. doi: 10.1037/a0015773. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12(3):291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Golob EJ, Bassett JP, Taube JS. Passive transport disrupts directional path integration by rat head direction cells. J Neurophysiol. 2003;90(5):2862–2874. doi: 10.1152/jn.00346.2003. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Herbert AM. Rats with lesions of the vestibular system require a visual landmark for spatial navigation. Behav Brain Res. 2002;128(1):27–40. doi: 10.1016/s0166-4328(01)00270-4. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Jr, Lora JC, Williams SB. Directional responding of C57BL/6J mice in the Morris water maze is influenced by visual and vestibular cues and is dependent on the anterior thalamic nuclei. J Neurosci. 2012;32(30):10211–10225. doi: 10.1523/JNEUROSCI.4868-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai SK, Ma J, Ossenkopp KP, Leung LS. Activation of immobility-related hippocampal theta by cholinergic septohippocampal neurons during vestibular stimulation. Hippocampus. 2012;22(4):914–925. doi: 10.1002/hipo.20955. [DOI] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annual Review of Neuroscience. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Burton HL. Head direction cell activity monitored in a novel environment and during a cue conflict situation. J Neurophysiol. 1995;74(5):1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving ratsIDescription and quantitative analysis. J Neurosci. 1990a;10(2):420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990b;10(2):436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio S, Taube JS. Path integration: how the head direction signal maintains and corrects spatial orientation. Nat Neurosci. 2012;15:1445–1453. doi: 10.1038/nn.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Choudhry S, Martin MM. Comparative analysis of movement characteristics during dead-reckoning-based navigation in humans and rats. J Comp Psychol. 2006;120(4):331–344. doi: 10.1037/0735-7036.120.4.331. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Pellis SM, Whishaw IQ. Vestibular information is required for dead reckoning in the rat. J Neurosci. 2002;22(22):10009–10017. doi: 10.1523/JNEUROSCI.22-22-10009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DG, Koppen JR, Jones JL, Winter SS, Wagner SJ. Navigating with fingers and feet: analysis of human (Homo sapiens) and rat (Rattus norvegicus) movement organization during nonvisual spatial tasks. J Comp Psychol. 2010;124(4):381–394. doi: 10.1037/a0020546. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Whishaw IQ. NMDA lesions of Ammon's horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. Eur J Neurosci. 2003;18(3):513–523. doi: 10.1046/j.1460-9568.2003.02772.x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Cassel JC, Majchrzak M, Cassel S, Will B. "Short-stops" in rats with fimbria-fornix lesions: evidence for change in the mobility gradient. Hippocampus. 1994;4(5):577–582. doi: 10.1002/hipo.450040507. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Coles BL, Bellerive CH. Food carrying: a new method for naturalistic studies of spontaneous and forced alternation. J Neurosci Methods. 1995;61(1–2):139–143. doi: 10.1016/0165-0270(95)00035-s. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B. Path integration absent in scent-tracking fimbria-fornix rats: evidence for hippocampal involvement in "sense of direction" and "sense of distance" using self-movement cues. J Neurosci. 1999;19(11):4662–4673. doi: 10.1523/JNEUROSCI.19-11-04662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat food carrying task. Behav Brain Res. 1997;89(1–2):87–97. doi: 10.1016/s0166-4328(97)00068-5. [DOI] [PubMed] [Google Scholar]
- Winter SS, Wagner SJ, McMillin JL, Wallace DG. Mammillothalamic tract lesions disrupt dead reckoning in the rat. Eur J Neurosci. 2011;33(2):371–381. doi: 10.1111/j.1460-9568.2010.07504.x. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Brown JE, Lamia MV, Valerio S, Shinder ME, Taube JS. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. J Neurophysiol. 2011a;105(6):2989–3001. doi: 10.1152/jn.01041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Taube JS. Origins of landmark encoding in the brain. Trends Neurosci. 2011b;34(11):561–571. doi: 10.1016/j.tins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Kirby SL. Otoconia-deficient mice show selective spatial deficits. Hippocampus. 2014;24(10):1169–1177. doi: 10.1002/hipo.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Pang KCH. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15(3):381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Rutan SA, Siegel A. Neuroscience. Washington, DC: Society for Neuroscience; 2014. The place-specific activity of hippocampal place cells does not require signals from the otolith organs. 2014. [Google Scholar]
- Yoder RM, Taube JS. Head direction cell activity in mice: robust directional signal depends on intact otolith organs. Journal of Neuroscience. 2009;29(4):1061–1076. doi: 10.1523/JNEUROSCI.1679-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. The vestibular contribution to the head direction signal and navigation. Front Integr Neurosci. 2014;8:32. doi: 10.3389/fnint.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Goddard M, Darlington CL, Smith PF. Long-term deficits on a foraging task after bilateral vestibular deafferentation in rats. Hippocampus. 2009;19(5):480–486. doi: 10.1002/hipo.20533. [DOI] [PubMed] [Google Scholar]