Abstract
While overuse of the supraspinatus tendon is a leading factor in rotator cuff injury, the underlying biochemical changes have not been fully elucidated. In this study, torn human rotator cuff (supraspinatus) tendon tissue was analyzed for the presence of active cathepsin proteases with multiplex cysteine cathepsin zymography. In addition, an overuse injury to supraspinatus tendons was induced through downhill running in an established rat model. Histological analysis demonstrated that structural damage occurred by 8 weeks of overuse compared to control rats in the region of tendon insertion into bone. In both 4- and 8-week overuse groups, via zymography, there was approximately a 180% increase in cathepsin L activity at the insertion region compared to the controls, while no difference was found in the midsubstance area. Additionally, an over 400% increase in cathepsin K activity was observed for the insertion region of the 4-week overused tendons. More cathepsin K and L immunostaining was observed at the insertion region of the overuse groups compared to controls. These results provide important information on a yet unexplored mechanism for tendon degeneration that may operate alone or in conjunction with other proteases to contribute to chronic tendinopathy.
Keywords: supraspinatus tendon, proteases, tendon tear, proteases
INTRODUCTION
Injuries to the shoulder, particularly to the supraspinatus tendon of the rotator cuff, are significant disabilities in athletic and occupational settings.40 The factors that cause the progression of rotator cuff disorders have not been completely elucidated, but it is accepted that repetitive loading or “overuse” of the supraspinatus tendon is a major contributor to degenerative tissue changes.28 Surgical intervention has been used to treat recalcitrant pain and tendon tears; however, reinjury rates have been as high as 57%8 and do not address the underlying pathophysiology that can lead to tendon ruptures.
The etiology of overuse-based tendon injuries is complex and likely depends on both intrinsic (biologic) and extrinsic (loading) factors 7,38. However, because type I collagen is the main extracellular matrix (ECM) constituent that imparts the tensile strength necessary for tendon function18, much attention has centered on this ECM component in understanding tendinopathy. Specifically, supraspinatus tendons have shown decreased collagen content or expression as a result of degeneration or failed healing.3,36 The importance of collagen catabolism as a part of pathology progression in tendinopathy has resulted in a focus on the family of matrix metalloproteinases (MMPs). Previous studies examining the healing of tendon after tendon rupture have found increases in expression and activity of MMP-116 and MMP-13,26 but a range of proteases likely take part in tendon degeneration.
The contributions of cysteine cathepsins, a family of lysosomal and secreted proteases, are increasingly recognized in tissue maintenance and a variety of degenerative tissue diseases. They have been identified as the most potent mammalian collagenases capable of cleaving types I and II collagens both intrahelically and at the telopeptide regions, whereas other collagenases only cleave at one or the other.17 Cathepsin K, associated with bone remodeling, has been implicated in collagen proteolysis in osteoarthritis,13 while cathepsin L, potent in cleaving at collagen telopeptide regions, has been found in myopathies.14 Additionally, cathepsins can play important roles in activating other proteases such as MMPs15 and the serine proteases urokinase-type plasminogen activator.19 Sustained expression of these proteases has been linked with chronic tissue degeneration and disease progression.12 Since cathepsins have a vital role in initializing and significantly contributing to proteolytic cascades, their involvement in tendon degeneration may shed light on the mechanisms of chronic tendon diseases.
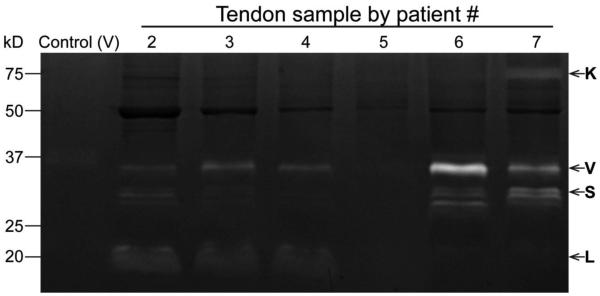
Recently, the Platt laboratory has developed a multiplex assay to determine levels of active cathepsin in tissue samples43. Multiplex cathepsin zymography is a sensitive electrophoretic technique that separates proteases and correlates the gelatin substrate digestion with proteolytic activity,43 and can be more sensitive than Western blotting.25 Using this technique, torn human supraspinatus tendon tissue was analyzed for the presence of active cathepsins in this study to represent an “end-point” of the disease state (see Fig. 1).
Figure 1.
Cathepsin activity in human chronic rotator cuff tendon tears. Zymograms depicts activity of cathepsins K, V, S, and L.
Based on these results, a more detailed study to examine the role of cathepsins in tendon overuse was performed in a rat model. In regards to animal models pertinent to the study of this pathology, the rat has been shown to have similar shoulder anatomy to humans, with a coracoacromial process that the supraspinatus tendon slides beneath, which may increase tissue damage in tendinopathy.41,39 Tendon overuse in this well-established animal model has shown altered tendon architecture and biomechanical properties42 and decreased gene expression of type I and III collagens.1 In this study, after up to 8 weeks of decline running on a treadmill, supraspinatus tendons of Dahl Salt Resistant rats were analyzed for differences between the region closest to the osseotendinous junction (the insertion region) and the region encased in muscle (the midsubstance region). Tissue damage was evaluated histologically and contralateral tendons underwent analysis via cathepsin zymography. We hypothesized that the overuse protocol would primarily cause tissue damage at the insertion region (due to possible impingement from the overlying acromion) and that there would be a concomitant increase in cathepsin activity in the overused tendon, thus implicating cathepsin upregulation and activity in degenerative tendon disorders.
METHODS
Human Tendon Tissue
Debrided fragments of tendon tissue from six patients (45-60 years of age) undergoing arthroscopic supraspinatus tendon repair for chronic tears (symptoms 3+ months) were collected by surgeons at the Emory Orthopaedics an Spine Center. Each patient provide written informed consent and only non-identifying data were collected. The study protocol, including tissue collection and storage conditions were approved by Georgia Institute of Technology and Emory University’s Institutional Review Board. Samples were assayed by cathepsin zymography as detailed below.
Rat Model
Twenty-four male Dahl Salt Resistant rats (330±20g initial weight, 12-13 weeks initial age, Harlan Labs) were used in this study, as approved by Georgia Institute of Technology’s Institutional Animal Care and Use Committee. This rat strain was selected because it is derived from the outbred Sprague-Dawley used previously,42 readily available, heavier than many other inbred strains and amenable to a running protocol.4 Twelve rats were acclimated to the running regime for 2 weeks to run at a final speed of 17m/min at a 10° decline for 1 hour/day for 5 days/week, as described previously.42 In the experimental group, rats were subjected to exercise for 4 or 8 weeks (n=6 animals/group or n=12 tendons/group). Age-matched rats that were allowed cage activity served as controls (n=6 animals/timepoint). At each endpoint, the supraspinatus muscle was detached from the scapula and the humerus was bisected by bone rongeurs. The bone-tendon-muscle units were wrapped individually in saline-soaked gauze and frozen until further processing. Samples were analyzed via, cathepsin zymography, and histology/immunohistochemistry as detailed below.
Histological Staining and Scoring
For histological evaluation, rat tendons were thawed and peritendon tissue and the majority of muscle were removed. Tendons were sharply dissected from their bony attachment. To prepare samples for cryosectioning, tendons were embedded in a 40:60 solution of 20% sucrose:optimum cutting temperature (OCT) compound (Sakura Finetek) solution, placed under vacuum for 10h to increase penetration of embedding medium, and frozen in isopentane chilled by liquid nitrogen. Tendons were sectioned with a cryostat (Thermo Scientific CryoStar NX70) into 10μm sections, longitudinal to the tendon.
Slides stained with hematoxylin (Sigma-Aldrich) and eosin (EMD Chemicals) (H&E) were imaged with a Nikon Eclipse 80i. Slides stained with picrosirius red (Sigma-Aldrich) were imaged with an Olympus BX51 polarized light microscope mounted with an Olympus Camedia C-5060 digital camera. Histological grading of H&E stained sections was carried out using a semi-quantitative scale, similar to the Bonar and Movin scales.11,27 Categories for histological scoring included regional variations in cellularity, cell shape, collagen fiber organization, and vascularity (Table 1). A 4-point scoring system was used, where 0 indicated normal appearance and 3 a markedly abnormal appearance. Four graders (JL, MG, TER, TM), blinded to the sample types, scored 4 images from each tendon (n=3 tendons/group/timepoint). Within each timepoint, the categorical scores of the control and experimental groups were compared.
Table 1.
Histological scoring matrix for H&E-stained images.
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
|
Regional
variations in cellularity |
Density of cells are uniform throughout field of view |
<25% of nuclei exhibit some disorganization in spatial arrangement |
25-50% of nuclei exhibit disorganization in spatial arrangement and <25% field of view exhibits hypercellularity |
>50% of nuclei show a disorganized arrangement and >25% field of view exhibits hypercellularity |
|
|
||||
| Cell shape | Elongated spindle shaped nuclei with no obvious cytoplasm with light microscopy |
<25% of nuclei are more ovoid to round in shape without conspicuous cytoplasm |
25-50% of nuclei are round, slightly enlarged and a small amount of cytoplasm is visible |
>50% of nuclei are round, large with abundant cytoplasm and lacuna formation |
|
|
||||
|
Collagen fiber
organization |
Collagen arranged in tight well-demarcated and large bundles, possibly with crimping |
Separation of individual fibers but demarcated bundles still evident |
Separation of fibers with loss of demarcation of bundles giving rise to expansion of the tissue |
Marked separation of fibers with complete loss of architecture |
|
|
||||
| Vascularity | Inconspicuous blood vessels between bundles |
Occasional evidence of capillaries (1 vessel per field of view) |
Evidence of capillaries in the form of a cluster of blood vessels (1 cluster per field of view) |
>2 clusters of capillaries per field of view |
Multiplex Cathepsin Zymography
For cathepsin zymography, rat tendons were isolated from muscle and bone (n=4 tendons/treatment/timepoint). The tendons were systematically divided into the insertion and midsubstance regions by transecting the tendon at 17% of the total length from the insertion end. This proportion consistently excluded the region encased in muscle from the insertion region and provided enough protein for subsequent assays. Due to overall small size of human samples, they were not divided prior to analysis.
All samples were diced and homogenized in PlusOne grinding kits (GE Healthcare) in lysis buffer (20 nM Tris–HCl at pH 7.5, 5 mM ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid (Sigma-Aldrich), 150 mM NaCl (BDH), 20 mM β-glycerolphosphate (Alfa Aesar), 10 mM NaF (Sigma-Aldrich), 1 mM sodium orthovanadate (Sigma-Aldrich), 1% Triton X-100 (EMD Chemicals), 0.1% Tween-20 (Fisher Scientific)) with freshly added 0.1 mM leupeptin (Affymetrix). Supernatants were cleared by centrifugation and collected. Protein quantification of supernatants was performed with a micro BCA kit (Pierce). Cathepsin zymography was performed on tissue lysates as previously described25,43. Briefly, non-reducing loading buffer (5X – 0.05% bromophenol blue (Fisher Scientific), 10% sodium dodecyl sulfate (SDS, Amresco), 1.5M Tris (Fisher Scientific), 50% glycerol (EMD Chemicals)) was added to 12μg of rat protein or 11μg of human protein prior to loading samples into gel. For rat samples, control and experimental samples from the same timepoint were run on the same gel (n=4/group). Samples were loaded into 12.5% SDS-polyacrylamide (Protogel) gels containing 0.2% gelatin (Sigma-Aldrich) and resolved at 110V at 4°C. Enzymes within gels were renatured in 65mM Tris buffer pH 7.4 with 20% glycerol for 3 washes, 10min each. Gels were then incubated in pH 4 activity buffer (acetate buffer, 1mM ethylenediamine tetraacetic acid (Fisher Scientific), freshly added 2 mM dithiothreitol (Sigma-Aldrich)) for 17h at 37°C. Thereafter, gels were rinsed and stained with Coomassie Blue (Sigma-Aldrich) and imaged with an ImageQuant LAS 4000 (GE Healthcare). 8.3ng cathepsin V (Enzo Chemicals) served as a positive control for all gels. For rat tendons, densitometry analysis was performed on images using ImageJ (NIH) to quantify the cleared bands that represent proteolytic activity and values were normalized to the cathepsin V band within the same gel. Values are reported as mean ± standard deviation. Immunofluorescence Staining: For immunofluorescence, slides were fixed in acetone (BDH), blocked with 2% normal goat serum (Vector Labs) for 1h and incubated with 1:100 dilution rabbit anti-rat cathepsin K (Santa Cruz Biotechnology) or L (Abcam) primary antibody overnight at 4°C in a humidified chamber. Goat anti-rabbit secondary antibody (Invitrogen) at 1:200 dilution was applied and sections were counterstained with 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI, Anaspec) and imaged with a Nikon Eclipse TE2000-U. Additional sections of two of the tendons/treatment/timepoint used for histological scoring were stained in this portion of the study.
Statistical Analysis
Categorical scores from histological evaluation were compared between the control and running groups by the non-parametric Mann-Whitney test. Data from cathepsin zymography were Box-Cox transformed and analyzed by t-tests on a per zymogram basis. All statistical analyses were evaluated at a statistical significance level of p<0.05. Statistical analysis was performed with Minitab software.
RESULTS
Human Multiplex Cathepsin Zymography
Zymography of human tendon tissues from chronic injuries demonstrated the obvious presence of active cathepsins K (75kDa), V (37kDa), S (28kDa), and L (20kDa) (Fig. 1) in 5 of 6 patient samples. Varying levels of cathepsin activity was detected in each human sample. Cathepsin L appeared more active in patients 2, 3, and 4, and patients 6 and 7 displayed high K, V, and S activity, while patient 5 displayed very little activity.
Histological Imaging and Scoring
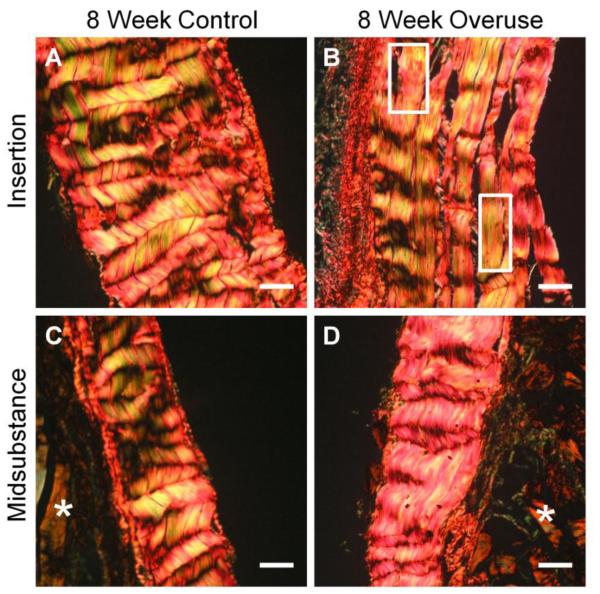
H&E stained histological sections revealed that tissue-level changes were more prominent in the insertion regions of the tendons compared to the midsubstance regions. The 4-week control tendon (Fig. 2A) showed tightly packed fibers and aligned cells at the insertion region. The 4-week overuse insertion region (Fig. 2B) showed minor collagen disorganization, as evidenced by the appearance of demarcated fibers with less dense staining between them, but collagen fiber bundles remained largely intact. The 8-week control insertion region displayed mostly intact collagen bundles with sparse and aligned tenocytes (Fig. 2C). By 8 weeks of overuse, collagen fiber thinning and separation from larger bundles was more obvious in the tendon insertion region (Fig. 2D) compared to the age-matched control (Fig. 2C). In contrast to the changes observed at the insertion regions, the midsubstance regions, where tendon is encased in muscle, exhibited similar morphology across time and condition: tightly packed collagen fibers with elongated tenocytes oriented along the axis of the tendon (Fig. 2E-H).
Figure 2.
Changes to supraspinatus tendon structure obvious by 8 weeks of overuse. The insertion region of the 4-week control (A) and 8-week control (C) tendons are contrasted with insertion region of the 4-week overuse (B) and 8-week overuse (D) tendons. The tissue structures of the midsubstance regions (E-H) were comparable across all groups. n=2, scale bar=100μm.
H&E-stained control and experimental samples evaluated within each timepoint by the semi-quantitative scoring system (Table 1) showed significant differences only at the insertion region. For the 4-week group, although the medians were the same between overuse and control group, the distribution in scores for cell shape at the insertion region was significantly different, with more instances of cell roundness (as indicated by nuclei shape) noted in the overuse group (Table 2). More regional variation in cellularity was observed in the 4-week control insertion region compared to the overuse group, suggesting that a portion of cells in control tendons were not as uniformly distributed. For the 8-week group, both cell shape and fiber organization scores were significantly different (Table 2). The insertion region of the 8-week overuse group showed more cells with increased roundness and size (Fig. 2) than the control group. Additionally, in regards to collagen fiber organization, the insertion region of the 8-week overuse group showed more instances of fiber separation than the control group. Vascularity, denoted by clusters of capillaries, was rarely noted by the observers in the tendon sections and assigned scores did not show any significant difference between overuse and control groups.
Table 2.
Summary of categorical histology scores of control and overused tendon at the insertion region.
| 4 Week Insertion | 8 Week Insertion | |||
|---|---|---|---|---|
| Control | Overuse | Control | Overuse | |
|
Regional
variations in cellularity |
2 (0, 3) |
1 (0, 3) * |
1 (0, 3) |
1 (0, 3) |
| Cell shape | 1 (0, 3) |
1 (0, 3) * |
1 (0, 3) |
2 (0, 3) * |
|
Fiber
organization |
2 (0, 3) |
2 (0, 3) |
0 (0, 2) |
1 (0, 3) * |
| Vascularity | 0 (0, 1) |
0 (0, 1) |
0 (0, 1) |
0 (0, 1) |
Data presented as median (range)
significantly different than control within the same timepoint; p<0.05
Picrosirius red stained sections imaged under circular polarized light exhibit yellow to red staining characteristic of large and organized collagen fibers (Fig. 3). While the crimping pattern of the control insertion region was uniform and uninterrupted across the section (Fig. 3A), the overuse insertion region showed less collagen packing and disturbed crimping (Fig. 3B, boxes).
Figure 3.
Picrosirius red-stained sections of tendons observed under circular polarized light microscopy. 8-week control (A) group contrasts with the overuse group (B; boxes indicate disturbed crimp pattern). The midsubstance regions of the control (C) and overuse (D) groups were comparable. * indicates muscle, n=2, scale bar=50μm.
Rat Multiplex Cathepsin Zymography
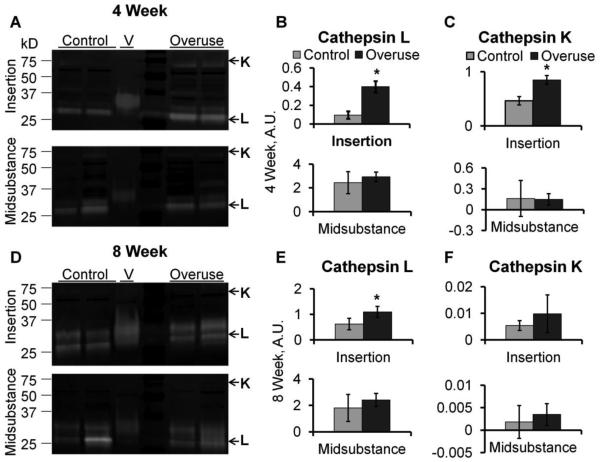
Incubation of the zymography gels in pH 4 activity buffer during the overnight assay period selects for murine cathepsin L over the other cathepsins, producing an active band between 25 – 35 kDa,43 and this activity was detected in all of the tendon samples as evidenced by the cleared white bands in the zymograms (Fig. 4A, D). Zymograms exhibited high molecular weight bands (50-75kDa) bands for some samples (Fig. 4A), which were attributed to cathepsin K bound to extracellular matrix, as was seen in osteoclasts in previous studies.34 Densitometric quantification of band intensities determined that by 4 weeks, the activity of cathepsins L and K were higher in the insertion region of the overuse group compared to the control group by 1.8-fold and 4.2-fold, respectively (Fig. 4B,C, n=3-4, p< 0.05). Additionally, by 8 weeks, cathepsin L activity was 1.8-fold higher in the insertion region of the overuse group compared to age-matched controls (Fig. 4E), although there was no longer a statistically significant increase for cathepsin K (Fig 4F). No significant differences were observed for cathepsin activity in the midsubstance regions between the overuse and control groups for either timepoint or cathepsins (Fig. 4B, E).
Figure 4.
Cathepsin activity in supraspinatus tendon. Representative zymograms (A, D) depict location of cathepsin K (higher molecular weight) and L (lower molecular weight). Cathepsin L activity in 4-week overuse (B) and 8-week overuse (E) tendons compared to age-matched controls. Cathepsin K activity in 4-week overuse (C) and 8-week overuse (F) tendons compared to age-matched controls. n=3-4, * indicates significantly greater activity over control at the same time point (p<0.05).
Immunofluorescence Staining
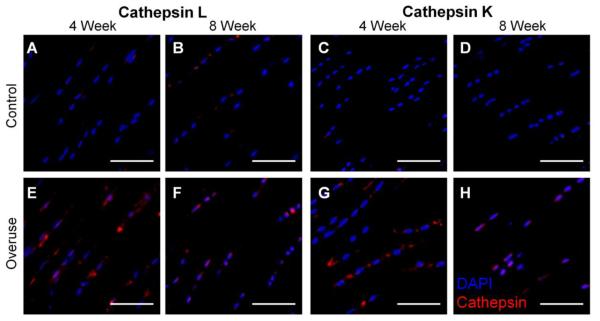
Immunofluorescence staining was employed in order to confirm the presence of cathepsin L and K. Positive controls for immunostaining were confirmed with same antibody on sections of rat spleen, and negative staining was conducted without the primary antibody (data not shown). Cathepsin staining was localized within or in close proximity to a cell. The tendon midsubstance regions showed similar positive levels of staining for overused and control tendons (data not shown), while there were more marked differences in staining at the insertion regions. For cathepsin L staining at the insertion regions, the 4- and 8- week controls showed similarly low levels of cathepsin staining (Fig. 5A, B). The 4-week and 8- week overuse group (Fig. 5E, F) showed more intense and uniformly distributed staining at the insertion region compared to their age-matched controls (Fig. 5A, B). Cathepsin K staining was slightly more variable in the 4-week overuse group (Fig. 5G), but overall showed more positive staining than seen in the controls (Fig. 5C). The 8-week overuse group (Fig. 5H) also exhibited more positive cathepsin K staining than in the controls (Fig. 5D).
Figure 5.
Immunofluorescence staining of cathepsin localization in supraspinatus tendons. Cathepsin L staining (red) in the insertion regions of the 4-week (B) and 8-week (F) overused tendons compared to controls (A, E). Cathepsin K staining (red) in the insertion regions of the 4- week (D) and 8-week (H) overused tendons compared to their controls (C, G). Cell nuclei are shown in blue. n=2, scale bar=50μm.
DISCUSSION
This study examined the presence of active cysteine cathepsins in injured supraspinatus tendon. To demonstrate clinical significance, human supraspinatus tendon tissue was collected during arthroscopic reconstructive surgery and analyzed by cathepsin zymography. The specificity and sensitivity of cathepsin zymography allows detection of femtomole quantities of cathepsin K25 and detects only the active form of enzymes, distinguishing it from antibody-based techniques and making it ideal for use in tissue samples with low protein amounts. While Cathepsin K has been examined at the gene expression level in tendon previously6,33, the presence of proteolytically active cathepsins has not been demonstrated previously within tendon tissue. In addition to tendon and muscle injuries, cathepsins have been implicated in other collagenolytic degenerative disorders6,13,14,21,33 and thus may be an important player in promoting tissue damage in tendinopathy prior to full tears. The activity of tissue-specific, highly collagenolytic cathepsins K and L, as well as V and S (Fig. 1), which have relatively weak collagenolytic ability10,23, highlights the sensitivity of zymography as well as demonstrates the complex and relatively unexplored factors causing tendon tears and the changes in proteolytic profiles from initial overuse injury to “end state” damage (full or partial tear).
Given these results, our remaining studies focused on determining the role of these cathepsins within tendon and how they may participate in the timeline of tendon injury. Due to limitations of human testing with this type of injury, an animal model able to produce tendinopathic characteristics over a period of time was required to better understand early ECM changes that might lead to end-stage disease. Thus, a well-established rat overuse protocol4,30,42 that features acromial impingement on the supraspinatus tendon was adapted for use with an inbred strain of rat (to allow for the possibility to explore autologous cell therapy for overuse injuries in future studies). The inbred Dahl Salt Resistant rat strain was chosen for this study for their greater weight compared to other inbred strains.
From histological analysis, we observed evident damage to the supraspinatus tendon after overuse. While significant damage occurred by 4 weeks of overuse in outbred strains42, only minor histological damage was observed by 4 weeks in this study, possibly due to the lower weight of inbred versus outbred strain of rats. However, by 8 weeks of overuse, the insertion region of the supraspinatus tendon displayed obvious changes to the fiber organization and cell shape (Fig. 2). Fiber separation from bundles and increased cell rounding were more evident by 8 weeks of overuse compared to the 4-week overuse group, indicating that increased time of overuse can cause accumulation of damage. Comparisons between the insertion regions of the 8- week group showed differences in crimping pattern at the insertion (Fig. 3). While the insertion region of the control tendon demonstrated tightly packed fibers with periodic and cohesive crimping, the insertion of the overuse tendon exhibited regions with flattened crimps and a loss of the periodicity of crimping. Reduction in tendon crimping can result from exercise-induced microtrauma,35 and may be a macroscopic representation of collagen disruption that can ultimately alter biomechanical properties.42 By confirming the tendon tissue damage by H&E staining and polarized light microscopy, we determined that this rat model was appropriate to study the molecular level changes in tendon overuse.
The insertion region of the tendon is clinically relevant, as most damage and tears occur in this region, reflecting the complex mechanical forces imparted to this region.5 Histological scoring displayed the differences between the overuse and control groups at the insertion region. Primary differences in scoring indicated observable changes to cell shape, which was detected by the 4-week timepoint, and fiber organization, which was observed by 8 weeks. Cell shape, reflected by the shape of nuclei, appeared to be rounder near the insertion of the overuse group, as noted previously.37 The scoring results also further confirmed the disruption in collagen organization compared to controls at the insertion region by 8 weeks of overuse, potentially contributing to the decreased alignment of cells observed (Fig. 2). The extent of tissue damage by 8 weeks indicates demonstrable overuse pathology. There was no observable neovascularization or inflammatory infiltrate in the tissue sections, which indicates that tendinopathy can occur in this model without obvious inflammation or changes to vascularity as suggested previously.1,2,11 While neovascularization has been observed in other studies, it may be a feature of a later stage of healing in response to tendon damage by other means such as tendon detachment.18
Our novel multiplex cathepsin zymography method has resulted in the first quantitative report that protease activity is upregulated specifically at the insertion region of overused tendon. The increased expression of molecules such as nitric oxide synthase4 or heat shock protein30 early in tendon overuse can help discern molecular events in disease progression. In the same vein, detection of increased cathepsin activity can also point to progressive degeneration. Previously, cathepsin D was detected only by immunohistochemistry within the granulation tissue of torn rotator cuff20 and was hypothesized to activate other chemical mediators. However, in this study, in the absence of granulation tissue, detection of increased cathepsin K and L activity over controls by 4 weeks (Fig. 4A, B, C) via zymography occurred before there was any observable tissue damage.
Other animal models have exhibited early cathepsin gene expression changes prior to the onset of orthopaedic diseases. In a transgenic Del1 osteoarthritis mouse model, higher cathepsin K mRNA levels were observed 3 months before disease onset.32 In UTU17 transgenic mice, which constitutively overexpresses the cathepsin K gene, early cathepsin K production was found in the synovial lining ahead of severe cartilage degeneration 5 months later.31 These studies suggest that cathepsin K expression precedes many observable tissue changes that describe disease onset. It has been suggested that cathepsin K plays a major role in initiating fibrillar collagen degradation by exposing a greater number of sites for other collagenases to work and sustain the degradation.17 In our study, cathepsin K exhibited a temporal response with overuse, with high activity at 4 weeks at the tendon insertion and minor activity by 8 weeks compared to age-matched controls (Fig. 4F). While cathepsin K staining was positive by immunofluorescence for both timepoints, antibodies generally do not distinguish between the inactive proform of the enzyme and the proteolytically active mature form of the enzyme.
In contrast to early cathepsin expression that may start the cascade of tissue degradation, sustained cathepsin overexpression can cause accumulation of damage over time, which has been shown in an osteoarthritis mouse model with cathepsin K overexpression.24 In the study presented here, at 8 weeks of overuse, cathepsin L activity remained higher in the overuse group than control tendons at the insertion, indicating that protein digestion remained elevated over time (Fig. 4E). These results are in general agreement with the upregulation of cathepsin L activity observed in rat supraspinatus muscles 2 weeks after supraspinatus tendon transection or suprascapular nerve injury21. This sustained higher cathepsin L activity may suggest its importance in contributing to increased extracellular matrix degradation.
Cathepsin K and L perform optimally at slightly acidic pH, which reinforces their roles in collagen degradation via local secretion or within the lysosomal compartments of tendon fibroblasts (Fig. 5). These cathepsins can have interconnected functions with each other and with other proteases such as MMPs and serine proteases.15,19 In addition, lysosomal-based enzymes such as cathepsins can play a role in cell death during tissue damage21. Thus, cathepsins may be a potential molecular target for inhibition in tendon overuse injuries, as has already been explored in other orthopaedic diseases.9 In osteoporosis, administration of cathepsin inhibitors in osteoporosis studies have shown decreased collagen resorption markers and increased bone strength,29 suggesting that similar therapeutic strategies may be efficacious in degenerative tendon disorders as well.
The results shown here represent a fundamental study on the role of a new class of enzymes within overused tendon, however, additional studies will need to be conducted to establish the specific roles and time course of cathepsins in the progression of clinical tendinopathy and correlate cathepsin activity to functional (mechanical) changes in tendon to further validate the biological and clinical relevance of these findings. In the same vein, in order to evaluate the potency of cathepsins in tendon degeneration, it will be important to study local and selective cathepsin inhibition within overused tendon. While tissue adaptation is always a possibility in these types of animal exercise models, the correlation of results in rodents with end-stage human samples, as well as the ability to detect enzymatic changes within a specific region of tendon suggest that such an animal model is useful to provide insight into mechanisms of matrix degradation with tendon overuse.
Through these studies, we have demonstrated that cathepsin activity is a novel potential mechanism for tendon matrix turnover that has not been described previously in tendon overuse injuries. In human samples representing the final stage of disease (chronic rotator cuff tears), a range of cathepsins were shown to be active via zymography. In a corresponding rodent animal model, differences in tissue structure and quantitative cathepsin activity were greatest at the insertion region, where most tendon ruptures occur.5 While it is recognized that tendinopathy represents a range of pathologies,22 in this study, cathepsin upregulation relatively early and in specific areas of the tendon suggest that these enzymes may play an important role in the cascades of events that occur prior to tendon failure. Thus, understanding the role of cathepsins in tendon overuse may help develop treatment plans to prevent tendon rupture by locally altering the activity of these potent proteases.
ACKNOWLEDGEMENTS
The authors thank Jennifer Lei for assistance in animal studies, Meredith Fay and Ang (Kevin) Li for help in tissue processing, and Bernard Kippelen’s laboratory at Georgia Tech for use of the circular polarized microscope. This study was supported by a National Football League Charities Medical Grant, a Regenerative Engineering and Medicine Seed Grant (REM) from Georgia Tech and Emory University through the Atlanta Clinical & Translational Science Institute (Advancing Translational Sciences of the National Institutes of Health, UL1TR000454) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR063692. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- MMP
Matrix Metalloproteinase
- OCT
Optimum Cutting Temperature
- H&E
Hematoxylin and Eosin
- SDS
Sodium Dodecyl Sulfate
REFERENCES
- 1.Archambault JM, Jelinsky SA, Lake SP, et al. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25(5):617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 2.Attia M, Huet E, Gossard C, et al. Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. The American journal of sports medicine. 2013;41(4):908–917. doi: 10.1177/0363546512473817. [DOI] [PubMed] [Google Scholar]
- 3.Attia M, Scott A, Duchesnay A, et al. Alterations of overused supraspinatus tendon: A possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res. 2011;30(1):61–71. doi: 10.1002/jor.21479. [DOI] [PubMed] [Google Scholar]
- 4.Barbato JC, Koch LG, Darvish A, et al. Spectrum of aerobic endurance running performance in eleven inbred strains of rats. Journal of applied physiology. 1998;85(2):530–536. doi: 10.1152/jappl.1998.85.2.530. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites ('entheses') in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund ME, Hart DA, Reno C, Wiig M. Growth factor and protease expression during different phases of healing after rabbit deep flexor tendon repair. J Orthop Res. 2011;29(6):886–892. doi: 10.1002/jor.21330. [DOI] [PubMed] [Google Scholar]
- 7.Blevins FT. Rotator cuff pathology in athletes. Sports Med. 1997;24(3):205–220. doi: 10.2165/00007256-199724030-00009. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 9.Bromme D, Lecaille F. Cathepsin K inhibitors for osteoporosis and potential off-target effects. Expert Opin Investig Drugs. 2009;18(5):585–600. doi: 10.1517/13543780902832661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromme D, Li Z, Barnes M, Mehler E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry. 1999;38(8):2377–2385. doi: 10.1021/bi982175f. [DOI] [PubMed] [Google Scholar]
- 11.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes' patellar tendons. J Orthop Res. 2004;22(2):334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Cunnane G, FitzGerald O, Hummel KM, et al. Collagenase, cathepsin B and cathepsin L gene expression in the synovial membrane of patients with early inflammatory arthritis. Rheumatology. 1999;38(1):34–42. doi: 10.1093/rheumatology/38.1.34. [DOI] [PubMed] [Google Scholar]
- 13.Dejica VM, Mort JS, Laverty S, et al. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol. 2008;173(1):161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deval C, Mordier S, Obled C, et al. Identification of cathepsin L as a differentially expressed message associated with skeletal muscle wasting. Biochem J. 360143:2001. doi: 10.1042/0264-6021:3600143. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eeckhout Y, Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu SC, Chan BP, Wang W, et al. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73(6):658–662. doi: 10.1080/000164702321039624. [DOI] [PubMed] [Google Scholar]
- 17.Garnero P, Borel O, Byrjalsen I, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273(48):32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 18.Gimbel JA, Van Kleunen JP, Mehta S, et al. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37(5):739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Goretzki L, Schmitt M, Mann K, et al. Effective activation of the proenzyme form of the urokinase-type plasminogen activator (pro-uPA) by the cysteine protease cathepsin L. FEBS Lett. 1992;297(1-2):112–118. doi: 10.1016/0014-5793(92)80339-i. [DOI] [PubMed] [Google Scholar]
- 20.Gotoh M, Hamada K, Yamakawa H, et al. Significance of granulation tissue in torn supraspinatus insertions: An immunohistochemical study with antibodies against interleukin-1b, cathepsin D, and matrix metalloprotease-1. J Orthop Res. 1997;15(1):33–39. doi: 10.1002/jor.1100150106. [DOI] [PubMed] [Google Scholar]
- 21.Joshi SK, Kim HT, Feeley BT, Liu X. Differential ubiquitin-proteasome and autophagy signaling following rotator cuff tears and suprascapular nerve injury. J Orthop Res. 2014;32(1):138–144. doi: 10.1002/jor.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 23.Kirschke H, Wiederanders B, Bromme D, Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989;264(2):467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozawa E, Nishida Y, Cheng XW, et al. Osteoarthritic change is delayed in a Ctsk-knockout mouse model of osteoarthritis. Arthritis Rheum. 2012;64(2):454–464. doi: 10.1002/art.33398. [DOI] [PubMed] [Google Scholar]
- 25.Li WA, Barry ZT, Cohen JD, et al. Detection of femtomole quantities of mature cathepsin K with zymography. Anal Biochem. 2010;401(1):91–98. doi: 10.1016/j.ab.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Lo IKY, Marchuk LL, Hollinshead R, et al. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sport Med. 2004;32(5):1223–1229. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- 27.Maffulli N, Longo UG, Franceschi F, et al. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466(7):1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med. 2004;34(14):1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- 29.Masarachia PJ, Pennypacker BL, Pickarski M, et al. Odanacatib reduces bone turnover and increases bone mass in the lumbar spine of skeletally mature ovariectomized rhesus monkeys. J Bone Miner Res. 2012;27(3):509–523. doi: 10.1002/jbmr.1475. [DOI] [PubMed] [Google Scholar]
- 30.Millar NL, Wei AQ, Molloy TJ, et al. Heat shock protein and apoptosis in supraspinatus tendinopathy. Clinical Orthop Relat Res. 2008;466(7):1569–1576. doi: 10.1007/s11999-008-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morko J, Kiviranta R, Joronen K, et al. Spontaneous development of synovitis and cartilage degeneration in transgenic mice overexpressing cathepsin K. Arthritis Rheum. 2005;52(12):3713–3717. doi: 10.1002/art.21423. [DOI] [PubMed] [Google Scholar]
- 32.Morko JP, Soderstrom M, Saamanen AM, et al. Up regulation of cathepsin K expression in articular chondrocytes in a transgenic mouse model for osteoarthritis. Ann Rheum Dis. 2004;63(6):649–655. doi: 10.1136/ard.2002.004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliva F, Barisani D, Grasso A, Maffulli N. Gene expression analysis in calcific tendinopathy of the rotator cuff. European cells & materials. 2011;(21):548–557. doi: 10.22203/ecm.v021a41. [DOI] [PubMed] [Google Scholar]
- 34.Park KY, Li WA, Platt MO. Patient specific proteolytic activity of monocyte-derived macrophages and osteoclasts predicted with temporal kinase activation states during differentiation. Integr Biol (Camb) 2012;4(12):1459–1469. doi: 10.1039/c2ib20197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson-Kane JC, Wilson AM, Firth EC, et al. Exercise-related alterations in crimp morphology in the central regions of superficial digital flexor tendons from young thoroughbreds: a controlled study. Equine Vet J. 1998;30(1):61–64. doi: 10.1111/j.2042-3306.1998.tb04089.x. [DOI] [PubMed] [Google Scholar]
- 36.Riley GP, Harrall RL, Constant CR, et al. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53(6):359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott A, Cook JL, Hart DA, et al. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56(3):871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 39.Sher JS. Anatomy, biomechanics, and pathophysiology of rotator cuff disease. In: Iannotti JP, Williams GR, editors. Disorders of the Shoulder: Diagnosis and Management. Lippincott, Williams, and Wilkins; Philadelphia: 1999. pp. 3–29. [Google Scholar]
- 40.Silverstein B, Welp E, Nelson N, Kalat J. Claims incidence of work-related disorders of the upper extremities: Washington state, 1987 through 1995. Am J Public Health. 1998;88(12):1827–1833. doi: 10.2105/ajph.88.12.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5(5):383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 42.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer award 1999 Overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9(2):79–84. [PubMed] [Google Scholar]
- 43.Wilder CL, Park KY, Keegan PM, Platt MO. Manipulating substrate and pH in zymography protocols selectively distinguishes cathepsins K, L, S, and V activity in cells and tissues. Archives of biochemistry and biophysics. 2011;516(1):52–57. doi: 10.1016/j.abb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]