Abstract
Objective
Limited data exist regarding the relationship between plasma 25-hydroxyvitamin D levels and duration of respiratory support. Our goal was to explore whether vitamin D status at the time of intensive care unit (ICU) admission is associated with duration of mechanical ventilation in critically ill surgical patients.
Materials and Methods
We analyzed data from a prospective cohort study involving 210 critically ill surgical patients. To explore the relationship between admission plasma 25-hydroxyvitamin D levels and duration of mechanical ventilation, we performed a Poisson regression while controlling for clinically relevant covariates. Only patients who required ≥48 hours of mechanical ventilation and survived ≥24 hours after discontinuation of respiratory support were included in the analytic cohort.
Results
Ninety-four patients met inclusion criteria. Mean (standard deviation) plasma 25-hydroxyvitamin D level was 16 (7) ng/mL and median (interquartile range) duration of mechanical ventilation was 4 (2–7) days. Poisson regression analysis, adjusted for age, sex, race, body mass index, primary surgical service, Acute Physiology and Chronic Health Evaluation II score, and season of ICU admission, demonstrated an inverse association of plasma 25-hydroxyvitamin D levels with duration of mechanical ventilation (incident rate ratio per 10 ng/mL, 0.66; 95% confidence interval, 0.54–0.82).
Conclusions
In our cohort of critically ill surgical patients, plasma 25-hydroxyvitamin D levels measured on ICU admission were inversely associated with the duration of respiratory support. Randomized controlled trials are needed to assess whether vitamin D supplementation can influence duration of mechanical ventilation in surgical ICU patients.
Keywords: vitamin D, 25(OH)D, mechanical ventilation, critical care
Introduction
In humans, vitamin D metabolites exert pleiotropic effects.1 Although the exact threshold for optimal vitamin D status remains a topic of considerable debate,2,3 circulating 25-hydroxyvitamin D (25(OH)D) levels <20 ng/mL are present in 70%–80% of patients in the intensive care unit (ICU).4-6 This is a potential cause for concern, since a growing body of evidence suggests that 25(OH)D levels are inversely associated with clinical outcomes such as infection rates, hospital length of stay, mortality, and healthcare costs.4,7-9
Among hospitalized patients who need respiratory support, duration of mechanical ventilation (MV) is an important modifiable risk factor for undesirable outcomes.10-13 While the association between 25(OH)D levels and various respiratory illnesses (eg, asthma, chronic obstructive pulmonary disease, bronchiolitis, pneumonia) has recently come to light,14-17 the relationship between vitamin D status and need for respiratory support in critically ill patients has not been well explored. Therefore, our goal was to investigate whether plasma 25(OH) D levels (the most widely accepted reflection of overall vitamin D status18) obtained within 24 hours of ICU admission are associated with duration of MV in critically ill surgical patients.
Methods
We performed a secondary analysis of the data from a prospective cohort study designed to assess vitamin D status in critically ill patients. Patients were recruited from two 18-bed surgical ICUs at the Massachusetts General Hospital (MGH) in Boston, Massachusetts. Both ICUs received admissions from all surgical services except for cardiac surgery. All patients were enrolled between June 1, 2012, and March 31, 2014. MGH is a 1052-bed teaching hospital and a level 1 trauma center, which serves a diverse population in and around eastern Massachusetts. The Partners Human Research Committee (institutional review board) approved the study protocol.
Inclusion and Exclusion Criteria
For the parent cohort, all adult men and women ≥18 years of age and who were expected to require at least 48 hours of critical care (as determined by the treating ICU team) were deemed eligible to participate. Informed consent was obtained either directly from patients or appropriate healthcare surrogates. Patients were included in the study only if blood samples to assess vitamin D status could be obtained within 24 hours of admission to the ICU. Exclusion criteria included a known history of anemia at the time of ICU admission (defined as hematocrit <25%), pregnancy or immediate postpartum status, and recent history of vitamin D supplementation (≥4000 IU/d). To minimize confounding from partially treated, new-onset illness, or chronic illnesses, we also excluded patients if they were transferred from another ICU or had been in an ICU within 1 year of the most current admission. For the present analysis, patients from the original cohort were considered for study inclusion if they required ≥48 hours of MV. To minimize confounding from patients who transitioned to comfort care measures, inclusion into the final analytic cohort required surviving ≥24 hours following discontinuation of respiratory support. We excluded patients who required only noninvasive MV, and we did not count the use of nocturnal continuous positive airway pressure therapy following MV toward the total duration of respiratory support. All patients receiving MV at the time of admission to the surgical ICU were assessed for readiness to discontinue respiratory support, and a daily protocol19 was applied to all patients to determine necessity of MV.
Blood Sample Processing and Biomarker Assays
Following informed consent, fresh blood was acquired from an indwelling arterial catheter and was collected directly into an EDTA-containing tube (lavender top). The sample was immediately stored on ice and then centrifuged within 30 minutes to separate out plasma. All samples were centrifuged at 2300 rpm for 15 minutes at 4°C. The separated plasma was immediately transferred to polypropylene tubes and stored at −80°C until biomarker testing was ready to be initiated. Assays were performed at the Harvard Medical School Clinical and Translational Science Award core laboratory at MGH. Plasma 25(OH)D (combined D2 and D3) levels were measured by enzyme-linked immunoabsorbent assay, using commercially available kits (Abbott Laboratories, Abbott Park, IL). Intraand interassay coefficients of variation were both <10%.
Clinical Data Collection
The MGH electronic medical records system was used to obtain baseline information related to age, sex, race, body mass index (BMI), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, type of surgical patient (eg, thoracic, vascular, urologic), and vitamin D supplementation status. Admission laboratory results also abstracted from the electronic medical records included hemoglobin (Hgb) level, white blood cell (WBC) count, and serum albumin level. Total body fluid balance (TBB) from admission to the time of study- related blood sample acquisition was calculated from the ICU bedside care flow sheet for each patient. Cumulative duration of MV over the first 30 days after ICU admission was calculated from individual electronic medical records. Mortality rate was determined by cross-referencing each record with the U.S. Social Security Death Index Master File.
Statistical Analysis
Descriptive statistics were calculated for patients with plasma 25(OH)D levels <20 ng/mL vs those with levels ≥20 ng/mL. Continuous data were reported as means with standard deviations (SD) or medians with interquartile ranges (IQR). Comparison of characteristics was performed using t test and Mann-Whitney analyses for normally distributed variables and nonparametric variables, respectively. Categorical values were expressed as proportions and compared using χ2 tests.
Since cumulative days on MV is typically a discrete variable with a skewed distribution, which assumed values ≥1, zero-truncated Poisson regression analysis was used to model the relationship between plasma 25(OH)D levels and duration of MV while controlling for biologically plausible covariates. In this approach, we controlled for (1) age, (2) sex, (3) race, (4) BMI, (5) primary surgical service, (6) APACHE II score, and 7) season of ICU admission. To test the robustness of the biologically plausible model, we then performed stepwise Poisson regression using forward and backward selection methods. Variables considered for entry or removal in the stepwise approach included (1) age, (2) sex, (3) race, (4) BMI, (5) primary surgical service, (6) APACHE II score, (7) season of ICU admission, (8) TBB, (9) Hbg, (10) WBC, (11) serum albumin, (12) parathyroid hormone (PTH), (13) calcium (corrected total serum value), (14) creatinine, and (15) high-sensitivity C-reactive protein. Results are reported as incident rate ratios (IRRs) with 95% confidence intervals (CIs).
We performed an a priori sample size calculation using the previously described biologically plausible model for the association between plasma 25(OH)D levels and duration of MV. Assuming that we would observe an effect size of 0.2 (comparing patients with plasma 25(OH)D levels <20 ng/mL with patients with levels ≥20 ng/mL) with a power of 0.8 and α set at 0.05, a minimum sample size of 83 patients would be required for the present study. All analyses were performed in STATA 12.0 (StataCorp LP, College Station, TX). A 2-tailed P < .05 or 95% CI that did not span 1 was considered statistically significant.
Results
From the 210 ICU patients enrolled in the parent study cohort, 94 patients met inclusion/exclusion criteria for the present analysis. A subset (n = 42) of these patients was previously described in a study that looked at the association of vitamin D status and 90-day mortality in ICU patients.20 Baseline characteristics of the analytic cohort for the present study are shown in Table 1. The mean (SD) age was 65 (16) years. Most patients were women (59%) and white (93%). Overall mean (SD) BMI was 30 (8) kg/m2, and mean (SD) APACHE II score was 18 (9). Mean (SD) 25(OH) D level for the study cohort was 16 (7) ng/mL. However, 20% of patients had 25(OH)D levels between 0 and 9.9 ng/mL, 42% had levels between 10 and 19.9 ng/mL, and 31% had levels between 20 and 29.9 ng/mL. The mean (SD) serum albumin, calcium, PTH, and creatinine levels in the cohort were 2.9 (0.5) g/dL, 9.1 (0.7) mg/dL, 80 (53) pg/mL, and 1.4 (1.2) mg/dL, respectively. Median (IQR) duration on MV was 4 (2–7) days.
Table 1.
Demographic Factors, Baseline Clinical Information, and Clinical Outcomes in Surgical ICU Patients, According to Vitamin D Status at Initiation of Care (n = 94).
| Characteristic | 25(OH)D <20 ng/mL (n = 57) |
25(OH) ≥20 ng/mL (n = 37) |
P Value |
|---|---|---|---|
| Age, y | 62 (17) | 64 (18) | .59 |
| Sex, % | .32 | ||
| Female | 61 | 54 | |
| Male | 39 | 46 | |
| Race, % | .27 | ||
| White | 91 | 95 | |
| Nonwhite | 9 | 5 | |
| BMI, kg/m2 | 29 (6) | 30 (8) | .52 |
| APACHE II | 21 (10) | 15 (6) | <.001 |
| Patient type, % | .29 | ||
| General | 19 | 32 | |
| Gynecological | 4 | - | |
| Neurosurgery | 8 | 9 | |
| Thoracic | 15 | 12 | |
| Transplant | 6 | 6 | |
| Trauma/emergent | 21 | 18 | |
| Urology | 2 | 3 | |
| Vascular | 25 | 21 | |
| TBB, mL | 2637 (2023) | 2321 (1819) | .44 |
| Hemoglobin, g/dL | 10 (3) | 11 (2) | .06 |
| WBC, ×109/L | 14 (4) | 13 (6) | .38 |
| Glucose, mg/dL | 158 (40) | 157 (44) | .91 |
| 25(OH)D, ng/mL | 12 (4) | 25 (5) | <.001 |
| Serum albumin, g/dL | 2.9 (0.5) | 2.8 (0.4) | .35 |
| PTH, median (IQR), pg/mL | 57 (30–119) | 40 (32–58) | .18 |
| Calcium, mg/dL | 8.9 (0.7) | 9.4 (0.5) | <.001 |
| Creatinine, mg/dL | 1.4 (1.0) | 1.5 (1.5) | .70 |
| hsCRP, mg/L | 177 (120) | 128 (102) | .04 |
| Season, % | .12 | ||
| Summer | 40 | 51 | |
| Winter | 60 | 49 | |
| PaO 2/FiO 2 | 307 (151) | 312 (118) | .86 |
| MV, median (IQR), d | 6 (4–9) | 3 (2–5) | .02 |
| Pneumonia, % | .52 | ||
| No | 86 | 89 | |
| Yes | 14 | 11 | |
| ICU LOS, median (IQR), d | 8 (4–10) | 5 (4–9) | .36 |
| Hospital LOS, median (IQR), d | 12 (8–25) | 10 (6–15) | .06 |
| 90-day mortality, % | <.001 | ||
| Alive | 67 | 92 | |
| Deceased | 33 | 8 |
Data are presented as mean (SD) unless otherwise indicated. Significant P values (<.05) are shown in bold. To convert ng/mL to nmol/L, please multiply by 2.496. APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; FiO 2 , fractional inspired concentration of oxygen; hsCRP, high-sensitivity C-reactive protein; ICU, intensive care unit; LOS, length of stay; MV, mechanical ventilation; PaO 2, partial pressure of oxygen; PTH, parathyroid hormone; TBB, total body fluid balance; 25(OH)D, 25-hydroxyvitamin D; WBC, white blood cell count.
The unadjusted relationship between plasma 25(OH)D levels and duration of MV in our analytic cohort is graphically represented in Figure 1. Zero-truncated Poisson regression analysis (Table 2), while controlling for biologically plausible covariates, demonstrated an inverse association between admission plasma 25(OH)D levels and duration of MV (IRR per 10 ng/mL, 0.66; 95% CI, 0.54–0.82). The stepwise Poisson regression approach did not materially change this result (IRR per 10 ng/mL, 0.54; 95% CI, 0.39–0.66).
Figure 1.
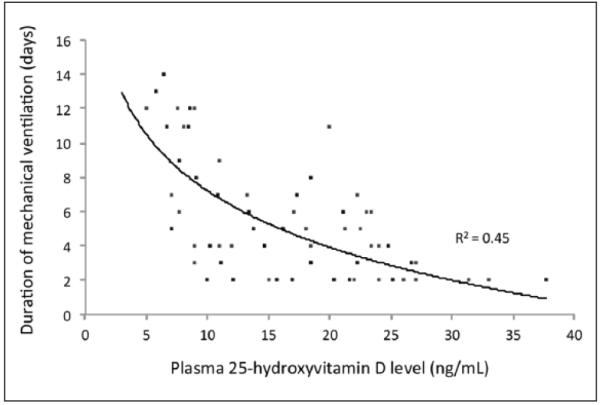
Unadjusted relationship between plasma 25-hydroxyvitamin D levels and duration of mechanical ventilation in surgical intensive care unit patients (n = 94). Logarithmic curve fitting suggests that 45% of the variation in the duration of mechanical ventilation may be explained by vitamin D status at the initiation of critical care.
Table 2.
Biologically Plausible Model to Test the Association of Admission 25-Hydroxyvitamin D Level With Duration of Mechanical Ventilation in Surgical Intensive Care Unit Patients (n = 94).
| Covariate | Incident Risk Ratio (95% Confidence Interval) |
|---|---|
| Age, y | 1.01 (1.01–1.03) |
| Sex | |
| Female | — |
| Male | 0.64 (0.46–0.87) |
| Race | |
| White | — |
| Nonwhite | 1.57 (0.96–2.56) |
| BMI, kg/m2 | 1.03 (1.01–1.05) |
| Primary surgical service | |
| General | — |
| Gynecological | 0.89 (0.55–1.48) |
| Neurosurgery | 0.21 (0.03–1.50) |
| Thoracic | 1.34 (0.77–2.34) |
| Transplant | 0.98 (0.66–1.47) |
| Trauma | 1.47 (1.13–2.27) |
| Urology | 0.55 (0.27–1.12) |
| Vascular | 1.20 (0.54–2.70) |
| APACHE II | 1.01 (0.98–1.03) |
| Season | |
| Summer | — |
| Winter | 1.02 (1.01–1.04) |
| 25(OH)D, ng/mL | 0.98 (0.98–0.99) |
Incident risk ratios are expressed per unit change in each covariate and are exponential for >1-unit change. For example, the incident risk ratio is 0.92 (0.98355) for a 5-ng/mL increase in 25(OH)D level and 0.85 (0.983510) for a 10-ng/mL increase in 25(OH)D level. Statistically significant variables are shown in bold. —, reference variable. APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; 25(OH)D, 25-hydroxyvitamin D.
Discussion
In this prospective cohort study, we investigated whether vitamin D status at initiation of care was associated with subsequent duration of MV in critically ill patients. We demonstrated that plasma 25(OH)D levels on admission to the surgical ICU were inversely associated with the need for subsequent respiratory support. Due to the observational nature of this study, a causal inference about the effect of vitamin D status on duration of MV in critically ill surgical patients is limited.
Among ICU patients, the use of MV results in cellular changes and respiratory muscle weakness,21,22 which is aggravated by malnutrition, electrolyte abnormalities, and severe infections.23 While vitamin D is widely recognized for its critical role in maintaining overall musculoskeletal health in humans,24 little is known about its effects on respiratory muscles. In animals, circulating 25(OH)D levels <20 ng/mL result in smaller diaphragm muscle fiber diameter and a reduced ability to generate inspiratory force, as compared to animals with levels ≥20 ng/mL.25 Moreover, in vitro studies suggest that vitamin D supplementation induces rapid changes in calcium metabolism of muscle cells.26 When 1,25-dihydroxyvitamin D (1,25(OH) D) (the most biologically active vitamin D metabolite) binds the vitamin D membrane receptor (VDR), it triggers second-messenger pathways in the muscle cell,27 which result in enhanced calcium uptake.28 In addition, recent studies have demonstrated that cells of the innate and adaptive immune system also express VDR.29 Macrophages activated through VDR by 1,25(OH) D upregulate expression of cathelicidin,30 which is an endogenous antimicrobial peptide with activity against a broad spectrum of infectious agents such as bacteria, viruses, fungi, and mycobacteria.31 Furthermore, low 25(OH)D levels are associated with depressed macrophage phagocytosis, attenuated chemotaxis, and proinflammatory cytokine production.32 Since optimal vitamin D status appears to be essential for musculoskeletal health and immune function, both factors that are known to influence the need for MV in ICU patients,12 our study findings raise intriguing questions that merit further investigation.
Although cohort studies provide observational evidence, they have several potential limitations, such as confounding, reverse causation, and/or the lack of a randomly distributed exposure. These issues complicate causal inferences and may decrease the generalizability of our results to all ICU patients. Despite adjustment for multiple potential covariates, there may still be residual confounding that contributed to the observed differences in outcomes. Specifically, low 25(OH)D levels may be a marker for the general condition of patients, for which we are unable to fully adjust. Moreover, our analytic cohort was selected from 2 ICUs at a single institution that is a major referral center for medically complex patients, which may further affect the generalizability of our findings. Another potential limitation is related to the fact that we assessed only plasma 25(OH)D levels within 24 hours of ICU admission. Certainly, inflammatory changes, intravenous fluid administration, and alterations in systemic protein concentrations (eg, serum albumin and vitamin D binding protein) may contribute significantly to fluctuations in circulating 25(OH)D levels during critical illness.18 As such, vitamin D status may be dramatically altered in the days following exposure to acute physiologic stress. In addition, we did not control for the use of vitamin D supplementation (since only 9 patients received a multivitamin that contained cholecalciferol), corticosteroids (since timing of initiation and rationale for use were very heterogeneous), and muscle relaxants (since only 2 patients received cisatracurium infusions). The use of these medications may indeed influence 25(OH)D levels and the duration of MV in ICU patients. While the use of sedatives (propofol and dexmedetomidine), analgesics (hydromorphone and fentanyl), and antipsychotics (haloperidol and quetiapine) was highly variable in this cohort, all patients received standardized daily sedation holidays to assess readiness for liberation from MV. And finally, patients were assessed for tracheostomy tube placement only if they had received 10 days of MV, with the actual procedure being performed between days 12 and 14 of respiratory support. Early tracheostomy tube placement is not a part of our standard protocol and was not considered in this patient cohort. These issues will need to be addressed by future studies to replicate and extend the current findings.
Conclusion
Our results suggest that vitamin D status may be a modifiable risk for prolonged duration of MV in surgical ICU patients. We hypothesize that ideal 25(OH)D levels are associated with optimal musculoskeletal heath,24 effective regulation of innate as well as adaptive immunity,29 and expression of endogenous antimicrobial peptides.30 In turn, this may attenuate the effect of respiratory muscle weakness, systemic inflammation, and infections, which have a major impact on the duration of respiratory support during critical illness.12 This is particularly important, given that suboptimal 25(OH)D levels are highly prevalent in ICU patients.4-6 Further prospective studies are needed to validate our findings, assess the potential benefit of optimizing 25(OH)D levels, and identify the mechanism by which vitamin D may reduce the duration of MV in critically ill surgical patients.
Clinical Relevancy Statement.
Suboptimal vitamin D status is highly prevalent amongst intensive care unit (ICU) patients. A growing body of evidence suggests that low 25-hydroxyvitamin D (25(OH)D) levels are associated with various undesirable outcomes in critical illness. Our results suggest that vitamin D status may be a modifiable risk factor for prolonged mechanical ventilation in surgical ICU patients.
Acknowledgments
Financial disclosure: This study was support by National Institutes of Health grants 5T32GM007592 and UL1 RR025758 (SAQ), RO1 GM81524 (JPC), and R01 AI093723 and U01 AI087881 (CAC).
References
- 1.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 4.Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15:R292. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn L, Zimmerman LH, McNorton K, et al. Effects of vitamin D deficiency in critically ill surgical patients. Am J Surg. 2012;203:379–382. doi: 10.1016/j.amjsurg.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Lucidarme O, Messai E, Mazzoni T, Arcade M, du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36:1609–1611. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 7.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204:37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair P, Lee P, Reynolds C, et al. Significant perturbation of vitamin D–parathyroid–calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39:267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 9.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12:208–211. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky MB, Gellar JE, Dinglas VD, Colantuoni E, Mendez-Tellez PA, Shanholtz C. Duration of oral endotracheal intubation is associated with dysphagia symptoms in acute lung injury patients. J Crit Care. 2014;29(4):574–579. doi: 10.1016/j.jcrc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11:367–374. doi: 10.1513/AnnalsATS.201306-210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrof BJ. Diaphragmatic dysfunction in the intensive care unit: caught in the cross-fire between sepsis and mechanical ventilation. Crit Care. 2013;17:R181. doi: 10.1186/cc12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011;2:244–253. doi: 10.3945/an.111.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quraishi SA, Bittner EA, Christopher KB, Camargo CA., Jr Vitamin D status and community-acquired pneumonia: results from the third National Health and Nutrition Examination Survey. PLoS One. 2013;8:e81120. doi: 10.1371/journal.pone.0081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15:625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care. 2011;17:43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 20.Quraishi SA, Bittner EA, Blum L, McCarthy CM, Bhan I, Camargo CA., Jr Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42:1365–1371. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 22.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17:R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel MA, Smith BK, Gabrielli A. Mechanical ventilation, diaphragm weakness and weaning: a rehabilitation perspective. Respir Physiol Neurobiol. 2013;189:377–383. doi: 10.1016/j.resp.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–615. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 25.Ray AD, Pesonius JK, Hershberger PA. Vitamin D modulation of diaphragm muscle strength in mice. FASEB J. 2013;27:1152–25. [Google Scholar]
- 26.Massheimer V, Fernandez LM, Boland R, de Boland AR. Regulation of Ca2+ uptake in skeletal muscle by 1,25-dihydroxyvitamin D3: role of phosphorylation and calmodulin. Mol Cell Endocrinol. 1992;84:15–22. doi: 10.1016/0303-7207(92)90066-f. [DOI] [PubMed] [Google Scholar]
- 27.Nemere I, Schwartz Z, Pedrozo H, Sylvia VL, Dean DD, Boyan BD. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13:1353–1359. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez G, de Boland AR, Boland R. Stimulation of Ca2+ release-activated Ca2+ channels as a potential mechanism involved in non-genomic 1,25(OH)2-vitamin D3-induced Ca2+ entry in skeletal muscle cells. Biochem Biophys Res Commun. 1997;239:562–565. doi: 10.1006/bbrc.1997.7501. [DOI] [PubMed] [Google Scholar]
- 29.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinheiro da Silva F, Machado MC. Antimicrobial peptides: clinical relevance and therapeutic implications. Peptides. 2012;36:308–314. doi: 10.1016/j.peptides.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Kankova M, Luini W, Pedrazzoni M, et al. Impairment of cytokine production in mice fed a vitamin D3–deficient diet. Immunology. 1991;73:466–471. [PMC free article] [PubMed] [Google Scholar]


