Abstract
Objective
Narcissists behave aggressively when their egos are threatened by interpersonal insults. This effect has been explained in terms of narcissist’s motivation to reduce the discrepancy between their grandiose self and its threatened version, though no research has directly tested this hypothesis. If this notion is true, the link between narcissism and retaliatory aggression should be moderated by neural structures that subserve discrepancy detection, such as the dorsal anterior cingulate cortex (dACC). This study tested the hypothesis that narcissism would only predict greater retaliatory aggression in response to social rejection when the dACC was recruited by the threat.
Method
Thirty participants (15 females; MAge=18.86, SD=1.25; 77% White) completed a trait narcissism inventory, were socially accepted and then rejected while undergoing fMRI, and then could behave aggressively towards one of the rejecters by blasting them with unpleasant noise.
Results
When narcissists displayed greater dACC activation during rejection, they behaved aggressively. But there was only a weak or nonsignificant relation between narcissism and aggression among participants with a blunted dACC response.
Conclusions
Narcissism’s role in aggressive retaliation to interpersonal threats is likely determined by the extent to which the brain’s discrepancy detector registers the newly-created gap between the grandiose and threatened selves.
Keywords: narcissism, aggression, threatened egotism, social rejection, dACC
Aggression often results when people perceive threats to themselves (Anderson & Bushman, 2002). Identifying who is at risk for violent responses to threats remains a complex task for the scientific community. Part of the difficulty lies in myths of aggressive people as those who turn their inner hate for themselves into hate for others. The reality is that self-love often leads to aggression. Narcissists, who have grandiose and unstable high self-esteem, behave aggressively when their resplendent self-views are threatened (Bushman & Baumeister, 1998; Baumeister, Bushman, & Campbell, 2000; Baumeister, Smart, & Boden, 1996).
But why do threatened narcissists behave aggressively? Baumeister and colleagues (1996) argued that the “aggression emerges from a particular discrepancy between two views of the self: a favorable self-appraisal and an external appraisal that is much less favorable” (p. 8). A negative evaluation from another individual creates a gap between one’s internal and external appraisals and this disparity elicits negative affect (e.g., anger) towards the source of the self threat, which fosters aggression. This process refers to threatened egotism (Baumeister et al., 2000). Although a great deal of research has demonstrated the link between threatened egotism and aggression, no research has tested this discrepancy hypothesis directly. The current study fills this gap in the literature by using a likely neural marker of discrepancy detection to social threat to better understand the relationship between narcissism and aggression.
Narcissism and Aggression
Narcissism, the disposition towards grandiose and unstable global self-esteem, represents a key personality dimension related to threatened egotism and aggression. Research has shown that threatened narcissists behave aggressively (Bushman & Baumeister, 1998; Bushman, Baumeister, Phillips, & Gilligan, 1999; Bushman et al., 2009; Thomaes, Bushman, Stegge, & Olthoff, 2008; Wink, 1991). Theoretical models of narcissism argue that narcissists use their social relationships to regulate their grandiose self-views (Campbell, Brunell, & Finkel, 2006; Morf & Rhodewalt, 2001; Raskin, Novacek, & Hogan, 1991). Therefore, social rejection not only threatens narcissists need to belong, it also undermines their ability to maintain a consistent image of themselves as agentic, likeable, and dominant (Brown & Zeigler-Hill, 2004; Campbell, Rudich, & Sedikides, 2002; John & Robins, 1994). Consistent with this reasoning, socially rejected narcissists often behave aggressively, even against innocent third parties (Twenge & Campbell, 2003).
These findings identify narcissism as a reliable predictor of aggression in response to social threat. But they are mute regarding whether such aggression occurs through the detection of a disparity between the grandiose self-view and the newly created threatened self-view. Recent research suggests that individuals who tend to perseverate on the discrepancy between their ideal state and the threatened state caused by negative social feedback tend to be more aggressive (Chester, Merwin, & DeWall, 2014). However, it remains unknown if this association between discrepancy and aggression applies to narcissists. Such a disparity would be difficult to assess among people high in narcissism via conventional psychometrics because narcissists are particularly unlikely to report a threat to their self as the report itself may serve as a validation of the threatened self. This problem may be solved by turning to a neural disparity indicator to understand this process. Indeed, previous research suggests that social threats among narcissists can only be observed using neural, and not self-report, measures (Cascio, Konrath, & Falk, in press). This notion is further supported by research demonstrating that narcissists possess a physiological profile characterized by a heightened threat response that often is not reflected by self-reports. Specifically, narcissists appear to show greater cortisol reactivity to conditions of negative affect (Cheng, Tracy, & Miller, 2013). This threat orientation is mirrored in cardiovascular and autonomic functioning (Kelsey, Ordnuff, McCann, & Reiff, 2001). Thus, the inner world of narcissists may be characterized by a subjective state of threat that is unlikely to be measured by questionnaires.
The dACC: Discrepancy Detection
The dorsal anterior cingulate cortex (dACC) of the brain serves several functions that make it a likely candidate to detect discrepancies between a grandiose self-view and a threatened self-view. Once thought of as the cognitive division of the anterior cingulate, early research implicated two main functions for this area: conflict detection and distress to aversive stimuli (Eisenberger & Lieberman, 2004). Seminal work has shown that the dACC plays a crucial self-regulatory function by detecting errors and monitoring our performance for conflicts with our goal state (Botvinick, Cohen, & Carter, 2004). However, the dACC also serves affective functions. Social rejection increases dACC activation, which relates to greater self-reported distress (Eisenberger, Lieberman, & Williams, 2003). Lesions to the dACC cause individuals to report little distress from physical pain, though they still report knowledge of experiencing the pain (Foltz & White, 1968).
Both of these functions can be unified within the conceptualization of the dACC as the brain’s ‘alarm system,’ which detects discrepancies from one’s goal states (e.g., having a grandiose self-view) and elicits distress that correspond to the severity of the discrepancy (Eisenberger & Lieberman, 2004). A recent conceptualization of ACC, the Predicted Response Outcome (PRO) model, posits that the ACC tracks the probability of a given event and fires most extremely when the expectation is unmet, an experience akin to surprise (Brown, 2013). Whereas older cognitive approaches to the ACC emphasized its role in detecting error, the PRO model reappraises this role as detecting surprise in response to unlikely outcomes. The PRO model agrees with the alarm system model, indicating that the ACC, specifically its dorsal region, subserves the detection of and response to discrepancies between expected and actual outcomes.
The dACC’s function makes it the ideal candidate as an indicator of the degree to which narcissists will both perceive a threat as discrepant from their grandiose self-view and will experience distress due to it. Further, dACC activity during social rejection has been previously linked to aggressive behavior (Chester et al., 2014). As such, we hypothesized that narcissism would interact with dACC activation during social rejection to predict subsequent aggression towards the source of the threat, the rejecter. More specifically, we anticipated that aggression would be at its highest levels among highly narcissistic participants who showed the strongest dACC response to rejection. Conversely, we predicted that narcissism would not relate to aggression when dACC activation because these individuals would not experience threatened egotism. Yet what might determine whether an individual who is high in narcissism displays greater or lesser dACC reactivity to interpersonal threat or rejection?
Tuning the Alarm: The Calibrating Role of Anxious Attachment
Despite the profound threat that social threat and rejection entail, people differ in how threatening those experiences are perceived. One key individual difference dimension that modulates the threat of social rejection is attachment style (Belsky, 1997). Attachment styles are typically defined along two orthogonal dimensions: anxiety and avoidance (Fraley & Waller, 1998). Individuals high in anxious attachment are characterized by hyper-sensitivity to social threats, a tendency reflected in their exaggerated dACC response to social rejection (DeWall et al., 2012). According to the optimal calibration hypothesis, this up-regulation of dACC reactivity is an adaptation to an early life environment characterized by unpredictable social rejection from critical caregivers (Chester, Pond, Richman, & DeWall, 2012). Based on these findings and theorizing, we predicted that narcissists would display a greater dACC response to rejection when they are high in anxious attachment and show a blunted dACC response when they were low in anxious attachment. Taken together, this study sought to explicate how differential dACC reactivity to social threat (an indicator of discrepancy detection) among narcissists might differentially predict aggression and how anxious attachment style might calibrate that dACC response.
Method
Participants
Participants were 30 healthy, right-handed undergraduate students (15 females; MAge=18.86, SD=1.25; 77% White) who received course credit and monetary compensation1. Participants were screened for criteria relevant to safety and comfort in the MRI environment.
Procedure
All procedures were approved by the University of Kentucky’s ethics board. Participants arrived at the laboratory for an orientation session in which they completed a battery of questionnaires that included the 16-item Narcissistic Personality Inventory (NPI: Ames, Rose, & Anderson, 2006), the 12-item Experiences in Close Relationships – Revised scale (ECR: Wei, Russell, Mallinckrodt, & Vogel, 2007), and the 8-item Rejection Sensitivity Questionnaire (RSQ; Downey & Feldman, 1996).
Scanner task
Several days later, participants arrived at the MRI facility where they were informed that they would play a computerized ball-tossing game named Cyberball in an MRI scanner with two same-sex partners located in nearby scanners (as in Chester et al., 2014; Williams, Cheung, & Choi, 2000). The stated purpose of the task was to assess participants’ brains as they engaged in mental visualization of the virtual ball toss. To enhance the cover story, participants were assigned to one of three MRI scanners by a rigged drawing, when in fact there were no other scanners. Cyberball was then implemented as a block-design with three rounds, each lasting 60 seconds. Before each round, participants were presented with instructions to rest for 10 seconds. This was followed by a screen instructing them to “get ready” for the upcoming round (2 seconds). Acceptance was operationalized as occurring throughout rounds 1 and 2, as well as throughout the first 30 seconds of round 3, in which participants received the ball three times. Rejection was operationalized as occurring during the second half of round 3, after participants had received the ball three times and then witnessed three more ball-tosses without receiving a toss themselves for 30 seconds.
Distress measure
After a series of anatomical scans, participants were removed from the scanner and completed the 20-item Need Threat Scale which measured participants’ level of social distress due to Cyberball (Williams, 2009). Typically, higher scores on this scale represent lesser distress due to social rejection (i.e., social distress). To enhance understanding, total NTS scores were reverse-scored such that higher values corresponded to greater social distress.
Aggression measure
Participants were removed from the scanner and then were told they would play a competitive reaction-time task against one of their Cyberball partners in which the winner could deliver aversive noise blasts to the loser. Prior to each of the 9 trials, participants set the volume of the noise blast their partner would receive, ranging from Level 1 (60 decibels) to Level 10 (105 decibels) in 5 decibel intervals. A non-aggression option, Level 0, was also provided. Participants also set the duration of the noise blast, which could range from 0 seconds to 5 seconds in half-second intervals. After each competition, participants saw whether they ‘won’ or ‘lost’, as well as the volume and duration settings their partners had ostensibly set for them. Participants won five trials and lost four trials (determined randomly, despite being told that their performance was what determined the outcome of each trial). The reliability and validity of this task is well established (Anderson & Bushman, 1997; Giancola & Zeichner, 1995).
fMRI Data Analysis and Preprocessing
All images were collected on a 3 Tesla Siemens Magnetom Trio scanner. Functional images were acquired with a T2*-weighted gradient, 3D-shimmed echo sequence with the following parameters: 2.5s TR, 28ms TE, 64 × 64 matrix, 224 × 224mm FOV, 40 3.5mm axial slices acquired across the whole brain (3.5mm3 voxel size) in interleaved order. A high-resolution, T1-weighted volume was also acquired.
All preprocessing and statistical analyses were conducted using FSL [Oxford Center for Functional Magnetic Resonance Imaging (FMRIB); Smith et al., 2004; Woolrich et al., 2009]. Functional volumes were corrected for head movement to the median volume, corrected for slice-timing skew using temporal sinc interpolation, pre-whitened using FILM, and smoothed with a 5mm FWHM Gaussian kernel. To remove drifts within sessions, a high-pass filter with a cutoff period of 120s was applied. Non-brain structures were stripped from functional and anatomical volumes.
A fixed-effects analysis modeled event-related responses for each participant. Acceptance and rejection blocks were modeled as events using a canonical double-gamma hemodynamic response function with a temporal derivative. Pre-block instructions were modeled as a nuisance regressor while rest blocks were left un-modeled to account for baseline BOLD signal. The contrast of interest was rejection>acceptance. Functional volumes and first-level contrast images from this analysis were first registered to corresponding structural volumes (7 DOF) and then spatially normalized to MNI stereotaxic space (12 DOF). A top-level, mixed-effects analysis was conducted to create a group average map. Z (Gaussianized T/F) statistic images from this analysis were thresholded using clusters determined by Z>2.3 and a (family-wise error corrected) cluster significance threshold of p<.005 in the a priori region-of-interest (ROI; Heller, Stanley, Yekutieli, Rubin, & Benjamini, 2006; Worsley, 2001). Functional data from the activated voxels that comprised the main effect cluster were converted to units of percent signal change, averaged across each participant, and extracted (as outlined by Mumford, J. http://mumford.bol.ucla.edu/perchange_guide.pdf).
The dACC ROI was based on an activation cluster found in previous research on dACC activation to social rejection greater than acceptance using a similar Cyberball task (Eisenberger et al., 2003). Specifically, the ROI was an 8mm-radius sphere around the MNI coordinates x=−8, y=20, z=40.
Results
Scoring and Psychometrics
Binary responses on all 16 NPI items were scored such that narcissistic responses were coded as 1 and non-narcissistic responses were coded as 0. These responses were then summed to create a continuous narcissism index. Responses to all 20 NTS items were scored in a continuous manner such that higher scores represented greater distress due to Cyberball. Noise volume and duration levels from the aggression task were significantly correlated, r(28)=.84, p<.001. Thus, we standardized and summed all 25 intensity and 25 duration levels across all trials to create a continuous aggression index. See Table 1 for descriptive and reliability estimates of all measures.
Table 1.
Descriptive statistics for measures included in the study.
| M | SD | Minimum | Maximum | Range | α | |
|---|---|---|---|---|---|---|
| Aggression | −0.06 | 2.07 | −4.50 | 4.26 | n/a | .96 |
| Anxious Attachment | 18.00 | 7.16 | 7.00 | 34.00 | 6–36 | .78 |
| dACC | 0.02 | 0.04 | −0.11 | 0.10 | n/a | n /a |
| Narcissism | 4.80 | 3.06 | 0.00 | 13.00 | 0–16 | .70 |
| Rejection Sensitivity | 8.14 | 4.34 | 1.75 | 21.75 | 0–48 | .80 |
| Social Distress | 87.70 | 18.32 | 39.00 | 117.00 | 20–140 | .91 |
Narcissism, social distress, rejection sensitivity, and anxious attachment style measures were centered prior to entry into the multiple linear regression models described below. Outlier detection was performed based on two metrics: Cook’s leverage and distance (Cohen, Cohen, West, & Aiken, 2003; Cook & Weisberg, 1982). No outliers were detected using these metrics. All regression models summarized below met the assumptions of regression (i.e., appropriate collinearity of regressors, normally-distributed residuals, homoscedastic residuals; Cohen et al., 2003), with one exception noted below.
dACC Results
Social rejection, compared to social acceptance, was associated with increased activity in the dACC [Figure 1; 71 voxels, peak Z=3.98, MNI coordinates (x,y,z): −8, 26, 44; Rejection>Acceptance contrast]. Percent signal change units from the voxels that comprised the activated cluster within this region were unassociated with self-reported social distress, r(28)=−.04, p=.834, though this is likely due to the deflation of scores on this measure due to the extended delay between the Cyberball task and the administration of this measure (Zadro, Boland, & Richardson, 2006). See Table 2 for zero-order correlations between each variable-of-interest in this study.
Figure 1.
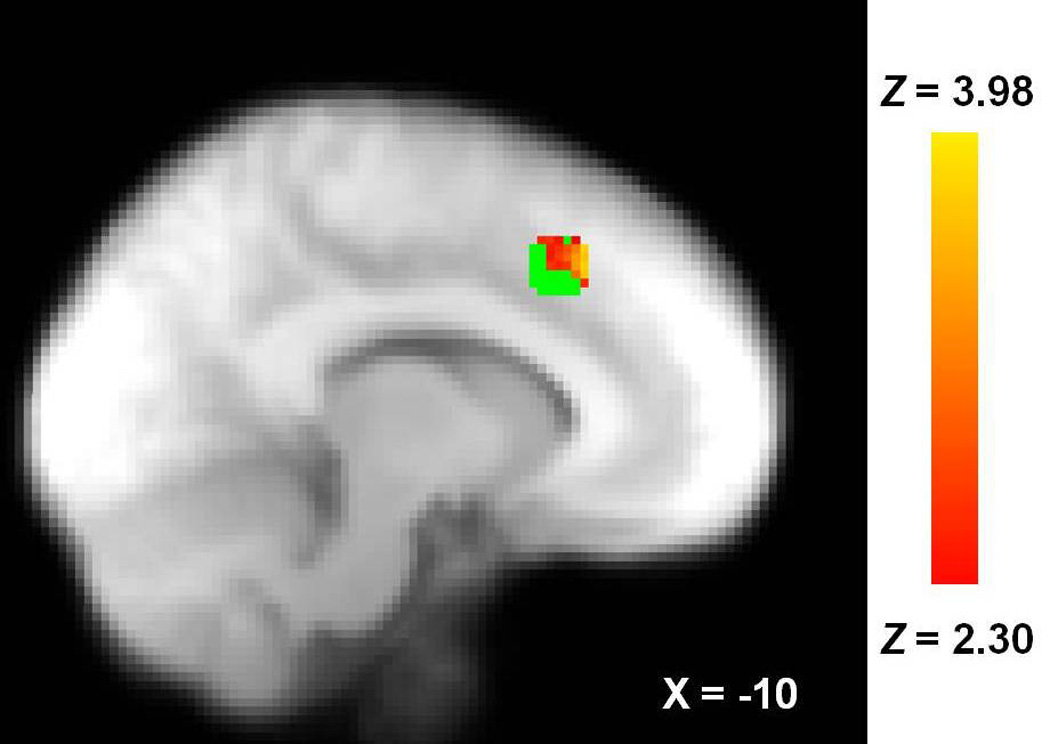
dACC activation associated with rejection>acceptance. Coordinates are in MNI space. Green voxels indicate spherical ROI extent.
Table 2.
Zero order correlations between each variable of interest.
| Aggression | Anxious Attachment |
dACC | Narcissism | |
|---|---|---|---|---|
| Anxious Attachment | .17 | |||
| dACC | −.01 | −.23 | ||
| Narcissism | .08 | −.32 | .13 | |
| Social Distress | .04 | .39* | −.04 | −.18 |
p < .05.
As predicted, narcissism interacted with dACC activation during rejection to predict aggression, β=0.70, t(26)=2.57, p=.016 (Figure 2). The main effect of trait narcissism was nonsignificant, β=−0.29, t(26)=−1.26, p=.219, whereas the main effect of rejection-specific dACC activity marginally predicted greater aggression, β=0.48, t(26)=1.82, p=.081. At low levels (−1 SD) of dACC activation, narcissism was marginally, negatively associated with aggression, β=−0.62, t(26)=−1.90, p=.067. In contrast, at high levels (+1 SD) of dACC activation, narcissism was positively associated with retaliatory aggression, β=0.56, t(26)=2.17, p=.038.
Figure 2.
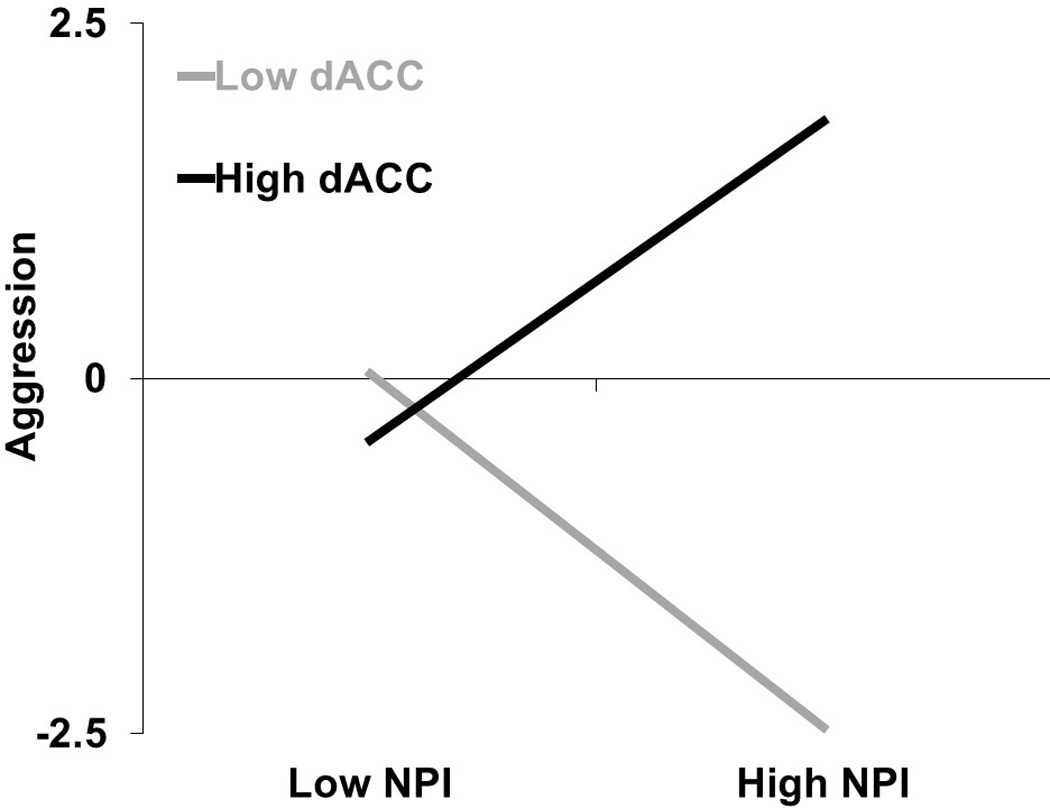
Interactive effect of narcissism scores and dACC activation associated with rejection contrasted with acceptance on standardized aggression scores.
Subsequent analyses examined the association between dACC activation on aggression at low and high levels of narcissism. At low levels (−1 SD) of narcissism, dACC activation did not correspond with aggression, β=−0.06, t(26)=−0.32, p=.752. But at high levels (+1 SD) of narcissism, dACC activation corresponded to greater aggression, β=1.08, t(26)=2.32, p=.016. The interaction term became stronger after controlling for gender, β=0.77, t(26)=2.98, p=.006, leading us to conclude that gender did not drive our pattern of results. The interaction term also remained significant after controlling for rejection sensitivity, β=0.71, t(26)=2.41, p=.023, leading us to conclude that rejection sensitivity did not drive our pattern of results.
Distress Results
Replacing dACC activity with self-reported distress, both trait narcissism, β=0.22, t(26)=1.15, p=.262, and self-reported social distress, β=−0.10, t(26)=−0.05, p=.958, were unassociated with aggression. These null main effects were qualified by an interaction between narcissism and social distress, β=0.42, t(26)=2.12, p=.044 (Figure 3). At low levels (−1 SD) of social distress, narcissism was unassociated with aggression, β=−0.23, t(26)=−0.98, p=.332. However, at high levels (+1 SD) of distress, narcissism was positively associated with retaliatory aggression, β=0.68, t(26)=2.03, p=.049. The interaction term became stronger after controlling for rejection sensitivity, β=0.46, t(26)=2.33, p=.028, leading us to conclude that rejection sensitivity did not drive our pattern of results. However, residuals from this regression were not normally distributed as indicated by a significant Kolmogorov-Smirnov test, k(30) = .17, p = .022. This interpretation of this interaction should be tempered by this assumption violation.
Figure 3.
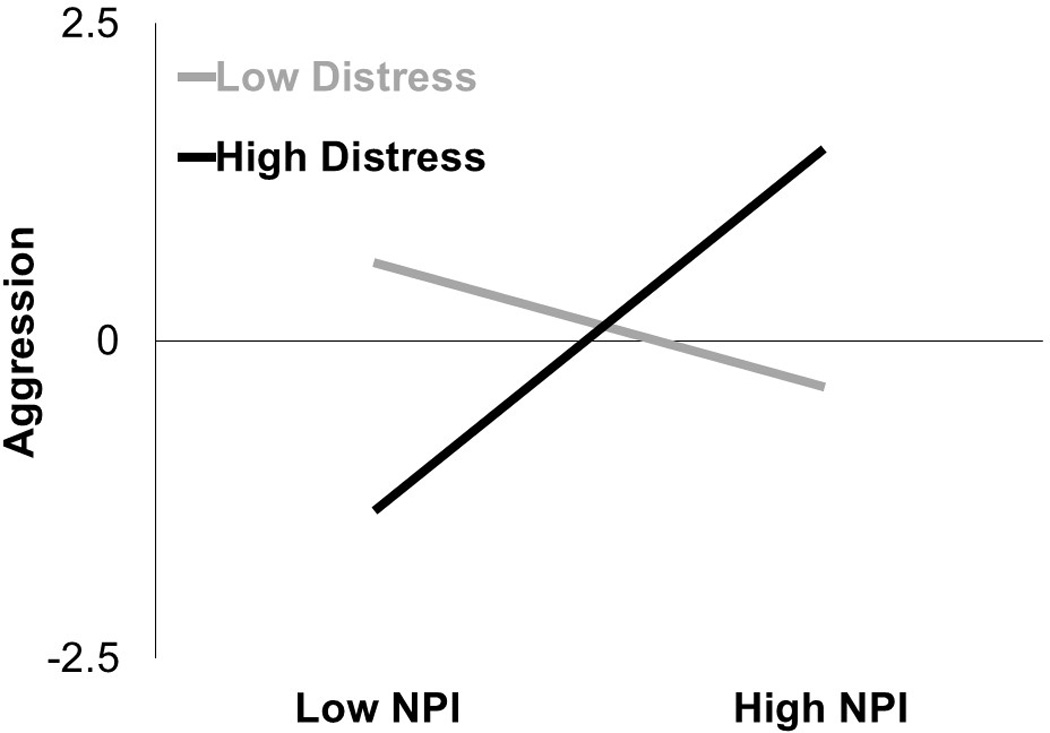
Interactive effect of narcissism scores and NTS scores (indicating distress due to rejection) on standardized aggression scores.
Attachment, Narcissism, and dACC Reactivity
The degree to which the dACC responded to rejection was not associated with either anxious attachment, β=−0.01, t(26)=−0.04, p=.970, or narcissism, β=0.24, t(26)=1.39, p=.176. However, these two personality traits did show an interaction, β=0.49, t(26)=2.74, p=.011. At low levels (−1 SD) of anxious attachment, narcissism was unassociated with dACC reactivity, β=−0.32, t(26)=−1.28, p=.212. However, at high levels (+1 SD) of anxious attachment, narcissism was positively associated with dACC reactivity, β=0.88, t(26)=2.56, p=.017. Anxious and avoidant attachment styles were uncorrelated, r(28) = .02, p = .907. Demonstrating specificity to anxious attachment style, and not avoidant attachment style, the interaction we observed between narcissism and anxious attachment increased substantially in strength after controlling for avoidant attachment, β=1.55, t(26)=3.02, p=.006. No such interaction was observed between narcissism and avoidant attachment style, β=−0.67, t(26)=−1.25, p=.224.
Discussion
Narcissists often behave aggressively, especially when their favorable self-views are threatened. The current investigation provided an initial test of a central tenet of threatened egotism theory (Baumeister et al., 1996), namely that narcissists react aggressively to interpersonal insult because of a heightened discrepancy between their grandiose self and the now threatened self. Lending direct support to this notion, narcissism related to greater aggression against a rejecter but only for those who also showed heightened activation in the dACC during social rejection. When dACC activation was low, narcissism was unrelated to aggression.
Assuming that the dACC reactivity we observed during rejection reflected, in part, a detection of a discrepancy between ideal and actual states (Brown, 2013; Eisenberger & Lieberman, 2004), the greater narcissists perceived a discrepancy between their grandiose and rejected self-views, the more aggressively they behaved. This interactive pattern of results also held for self-reported distress due to rejection, suggesting that these results are indeed due to the dACC’s alarm function and not a more ‘cold,’ cognitive process such as pure expectancy violation. Further, these results remained significant after controlling for gender and trait levels of rejection sensitivity.
Our findings add nuance to threatened egotism research by showing the importance of considering the degree to which the discrepancy between the grandiose and threatened self is realized, perceived, and elicits distress. These results relate to other narcissism research that argues that narcissists have vulnerable self-concepts that vigilantly search for threats (Miller et al., 2011), which is likely associated with heightened dACC functioning. Our results suggest that narcissism need not invariably increase the likelihood of retaliatory aggression. Future work should assess whether our effects hold among individuals with pathological narcissism, such as clients with Narcissistic Personality Disorder, and whether interventions targeted at the dACC and disparity detection are effective in reducing aggression among these populations.
When dACC activity was low, narcissism was not predictive of greater aggression. This finding is striking because most research has shown a positive association between narcissism and retaliatory aggression (e.g., Bushman & Baumeister, 1998). We argue that this typically observed main effect occludes the ability of dACC reactivity to modulate aggressive tendencies. Yet what would cause one narcissist to have a strong dACC response to rejection and a relatively weak response in another? Our finding that narcissism’s association with dACC reactivity to rejection was moderated by anxious attachment style provides some clues. Previous research has shown that anxious attachment is associated with greater dACC reactivity to social rejection (DeWall et al., 2012). This sensitization of the dACC to rejection is likely due to an early life history characterized by volatile, ‘hot-then-cold’ interactions with attachment figures (Chester et al., 2012). Based on these findings, narcissists whose ontogeny occurred largely in a largely uncertain inclusionary environment might show this exacerbated dACC response and subsequently, aggression. Narcissists who developed in a secure, inclusionary environment may then show a blunted dACC response to rejection and somewhat lower subsequent aggression. This nuanced view of developmental trajectories resulting in hostile versus less-hostile narcissists requires further research.
Personality assessment strategies may also benefit from our findings. Although our neural and self-report measures largely mirrored one another, the dACC measure yielded a larger effect size and more precise estimation of our hypotheses. Due to skew in the self-report measure, the need threat scale violated the assumption of normality of residuals. The superiority of our neural measure resonates with recent research showing that neural measures of social threat among narcissists can detect effects where self-report cannot (Cascio et al., in press). Personality researchers interested in individual differences that are characterized by under-reporting of threats to the self might benefit greatly by turning to neural indicators of threat, pain, and discrepancy instead of asking for reports.
Although the findings confirmed our hypotheses, several limitations exist. Chief amongst these is that we rely on reverse inference when interpreting the function of the dACC reactivity to rejection that we observed. Reverse inference is a problematic practice in functional neuroimaging (Poldrack, 2006). Indeed, the dACC activation we observed might represent various psychological processes (e.g., conflict, distress, interoception, pain, self-regulation, surprise; Brown, 2013; Eisenberger & Lieberman, 2004). However, each of the functions that the dACC involve discrepancy detection in some fashion. Thus, we are relatively confident that our dACC activation can be inferred as the presence of discrepancy detection, though this remains somewhat speculative.
Second, narcissism and discrepancy detection were measured and not manipulated. Hence, causal claims must be tempered by the inherent limitations of correlational findings. Experimental manipulations exist in which participants can be induced to focus and perseverate on the discrepancy between their performance and the ideal standard at which they would like to perform (e.g., Boone, Soenens, Vansteenkiste, & Braet, 2012). Crossing this manipulation with an induction of self-grandiosity is a crucial next step in determining the reliability and strength of our effect. Third, participants were given the opportunity to aggress against their rejecters and not against innocent third parties. Hence, we cannot be sure that the aggression we observed relates to retaliatory aggression or to a general aggressive disposition.
Fourth, the NPI-16 that we used to assess narcissism often loads onto the construct of grandiose narcissism, which can be juxtaposed against types of narcissism that can be characterized by more dispositional negative affect and lower extraversion (Miller et al., 2011). Future research may assess whether our interaction holds across both grandiose and vulnerable types of narcissism. Fifth, because rejection always occurred later in time than acceptance, our fMRI contrast between acceptance and rejection conditions was confounded with the inevitable changes in the MRI signal that occur over the length of a scan. To reduce the impact of this potential confound, our data were highpass filtered to remove low frequency shifts in the data over time, prewhitened to remove temporal autocorrelation, and a temporal derivative was included in the statistical model to account for time-based shifts in the hemodynamic response function (Poldrack, Mumford, & Nichols, 2011).
Our findings provide an initial empirical test that the association between threatened egotism on reactive aggression is due to a perceived disparity between the inflated self and the threatened self. We confirmed this prediction and extended it by using functional neuroimaging which had the advantage of skirting many of these inherent biases involved in self-report. By considering measures of perceived self disparity, theories of narcissism and threatened egotism may gain new insight and clinicians may explore new interventions to reduce the deleterious effects of narcissistic tendencies on aggression.
Acknowledgments
We thank David Powell for his technical help in the running of this study, as well as Richard Pond Jr. and Stephanie Richman for help with data collection.
Funding
This experiment was funded by a grant from the University of Kentucky’s Center for Drug Abuse Research Translation (Sponsor: National Institute on Drug Abuse, Grant number: DA005312) to the last author and a grant from the National Science Foundation (Grant number: BCS1104118) to the last author.
Footnotes
Portions of the current neural and aggression data have been published elsewhere from different analyses (Chester et al., 2014). No prior analyses using participant narcissism scores have been published.
References
- Ames DR, Rose P, Anderson CP. The NPI-16 as a short measure of narcissism. Journal of Research in Personality. 2006;40(4):440–450. [Google Scholar]
- Anderson CA, Bushman BJ. External validity of "trivial" experiments: The case of laboratory aggression. Review of General Psychology. 1997;1(1):19–41. [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annual Review of Psychology. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bushman BJ, Campbell WK. Self-esteem, narcissism, and aggression: Does violence result from low self-esteem or from threatened egotism? Current Directions in Psychological Science. 2000;9(1):26–29. [Google Scholar]
- Baumeister RF, Smart L, Boden JM. Relation of threatened egotism to violence and aggression: The dark side of high self-esteem. Psychological Review. 1996;103(1):5–33. doi: 10.1037/0033-295x.103.1.5. [DOI] [PubMed] [Google Scholar]
- Belsky J. Attachment, mating, and parenting. Human Nature. 1997;8(4):361–381. doi: 10.1007/BF02913039. [DOI] [PubMed] [Google Scholar]
- Boone L, Soenens B, Vansteenkiste M, Braet C. Is there a perfectionist in each of us? An experimental study on perfectionism and eating disorder symptoms. Appetite. 2012;59(2):531–540. doi: 10.1016/j.appet.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown JW. Beyond conflict monitoring: Cognitive control and the neural basis of thinking before you act. Current Directions in Psychological Science. 2013;22(3):179–185. doi: 10.1177/0963721412470685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RP, Zeigler-Hill V. Narcissism and the non-equivalence of self-esteem measures: A matter of dominance? Journal of Research in Personality. 2004;38(6):585–592. [Google Scholar]
- Bushman BJ, Baumeister RF. Threatened egotism, narcissism, self-esteem, and direct and displaced aggression: Does self-love or self-hate lead to violence? Journal of Personality and Social Psychology. 1998;75(1):219–229. doi: 10.1037//0022-3514.75.1.219. [DOI] [PubMed] [Google Scholar]
- Bushman BJ, Baumeister RF, Thomaes S, Ryu E, Begeer S, West S. Looking again, and harder, for a link between low self-esteem and aggression. Journal of Personality. 2009;77(2):427–446. doi: 10.1111/j.1467-6494.2008.00553.x. [DOI] [PubMed] [Google Scholar]
- Campbell WK, Brunell AB, Finkel EJ. Narcissism, interpersonal self regulation, and romantic relationships: An agency model approach. In: Finkel EJ, Vohs KD, editors. Self and relationships: connecting intrapersonal and interpersonal processes. New York: Guilford; 2006. [Google Scholar]
- Campbell WK, Rudich EA, Sedikides C. Narcissism, self-esteem, and the positivity of self-views: Two portraits of self-love. Personality and Social Psychology Bulletin. 2002;28(3):358–368. [Google Scholar]
- Cascio CN, Konrath SH, Falk EB. Narcissists’ social pain seen only in the brain. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsu072. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JT, Tracy JL, Miller GE. Are narcissists hardy or vulnerable? The role of narcissism in the production of stress-related biomarkers in response to emotional distress. Emotion. 2013;13(6):1004–1011. doi: 10.1037/a0034410. [DOI] [PubMed] [Google Scholar]
- Chester DS, Eisenberger NI, Pond RS, Jr, Richman SB, Dewall CN. The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Social Cognitive and Affective Neuroscience. 2014;9(5):699–704. doi: 10.1093/scan/nst038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester DS, Merwin LM, DeWall CN. The dangers of discrepancy: Maladaptive perfectionism is associated with greater aggression towards others and the self following negative feedback. Manuscript submitted for publication. 2014 [Google Scholar]
- Chester DS, Pond RS, Richman SB, DeWall CN. The optimal calibration hypothesis: How life history modulates the brain’s social pain network. Frontiers in Evolutionary Neuroscience. 2012;4 doi: 10.3389/fnevo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3 rd ed. Mahwah: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Cook RD, Weisberg S. Residuals and influence in regression. New York: Chapman and Hall; 1982. [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7(2):184–192. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70(6):1327–1343. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fraley CR, Waller NG. Adult attachment patterns: A test of the typological model. In: Simpson JA, Rholes WS, editors. Attachment theory and close relationships. New York, NY: Guilford Press; 1998. pp. 77–114. [Google Scholar]
- Giancola PR, Zeichner A. Construct validity of a competitive reaction- time aggression paradigm. Aggressive Behavior. 1995;21(3):199–204. [Google Scholar]
- Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of fMRI data. NeuroImage. 2006;33(2):599–608. doi: 10.1016/j.neuroimage.2006.04.233. [DOI] [PubMed] [Google Scholar]
- John OP, Robins RW. Accuracy and bias in self-perception: Individual differences in self-enhancement and the role of narcissism. Journal of Personality and Social Psychology. 1994;66(1):206–219. doi: 10.1037//0022-3514.66.1.206. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Ornduff SR, McCANN CM, Reiff S. Psychophysiological characteristics of narcissism during active and passive coping. Psychophysiology. 2001;38(02):292–303. [PubMed] [Google Scholar]
- Kiefer M, Ahlegian M, Spitzer M. Working memory capacity, indirect semantic priming, and stroop interference: Pattern of interindividual prefrontal performance differences in healthy volunteers. Neuropsychology. 2005;19(3):332–344. doi: 10.1037/0894-4105.19.3.332. [DOI] [PubMed] [Google Scholar]
- Miller JD, Hoffman BJ, Gaughan ET, Gentile B, Maples J, Campbell KW. Grandiose and vulnerable narcissism: A nomological network analysis. Journal of Personality. 2011;79(5):1013–1042. doi: 10.1111/j.1467-6494.2010.00711.x. [DOI] [PubMed] [Google Scholar]
- Morf CC, Rhodewalt F. Unraveling the paradoxes of narcissism: A dynamic self-regulatory processing model. Psychological Inquiry. 2001;12(4):177–196. [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE. Handbook of functional MRI data analysis. New York: Cambridge University Press; 2011. [Google Scholar]
- Raskin R, Novacek J, Hogan R. Narcissistic self-esteem management. Journal of Personality and Social Psychology. 1991;60(6):911–918. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Supplement 10):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thomaes S, Bushman BJ, Stegge H, Olthof T. Trumping shame by blasts of noise: Narcissism, self-esteem, shame, and aggression in young adolescents. Child Development. 2008;79(6):1792–1801. doi: 10.1111/j.1467-8624.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Campbell WK. “Isn’t it fun to get the respect that we’re going to deserve?” Narcissism, social rejection, and aggression. Personality and Social Psychology Bulletin. 2003;29(2):261–272. doi: 10.1177/0146167202239051. [DOI] [PubMed] [Google Scholar]
- Wei M, Russell DW, Mallinckrodt B, Vogel DL. The Experiences in Close Relationship Scale (ECR)-Short Form: Reliability, validity, and factor structure. Journal of Personality Assessment. 2007;88(2):187–204. doi: 10.1080/00223890701268041. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism: A temporal need-threat model. In: Zanna Mark P., editor. Advances in Experimental Social Psychology. Vol. 41. Philadelphia: Academic Press; 2009. pp. 275–314. [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79(5):748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wink P. Two faces of narcissism. Journal of Personality and Social Psychology. 1991;61(4):590–597. doi: 10.1037//0022-3514.61.4.590. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. NeuroImage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45(1) Supplement 10:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Functional MRI: an introduction to methods. 2001;14:251–270. [Google Scholar]
- Zadro L, Boland C, Richardson R. How long does it last? The persistence of the effects of ostracism in the socially anxious. Journal of Experimental Social Psychology. 2006;42(5):692–697. [Google Scholar]


