Abstract
Opioid effects are potentiated by cannabinoid agonists including anandamide, an endocannabinoid. Inter-individual variability in responses to opioids is a major clinical problem. Multiple deaths and anoxic brain injuries occur every year in due to opioid induced respiratory depression in surgical patients and drug abusers of opioids and cannabinoids. This study aimed to determine specific associations between genetic variants of fatty acid amide hydrolase (FAAH) and postoperative central opioid adverse effects in children undergoing tonsillectomy. This is a prospective genotype blinded observational study 259 healthy children between 6 and 15 years that received standard perioperative care with a standard anesthetic and an intraoperative dose of morphine were enrolled. Associations between frequent polymorphisms of FAAH and central postoperative opioid adverse effects including, respiratory depression (RD), postoperative nausea and vomiting (PONV) and prolonged stay in Post Anesthesia Recovery Room (PACU) due to RD and PONV were analyzed. Five specific FAAH SNPs had significant associations with more than 2 fold increased risk for refractory PONV (adjusted p<0.0018), and nominal associations (p<0.05) with RD and prolonged PACU stay in white children undergoing tonsillectomy. FAAH SNP, rs324420 is a missense mutation with altered FAAH function and it is linked with other FAAH SNPs associated with PONV and RD in our cohort; association between PONV and rs324420 was confirmed in our extended cohort with additional 66 white children. Specific FAAH polymorphisms are associated with refractory PONV, opioid-related respiratory depression, and prolonged PACU stay due to opioid adverse effects in white children undergoing tonsillectomy.
Introduction
Opioids are commonly used analgesics to manage surgical pain. However, effective and safe postoperative pain management with opioids is an unmet perioperative clinical need. This is mainly because of narrow therapeutic indices and large inter-individual variations in opioid responses. Morphine is one of the commonly used perioperative opioids. Similar to other opioids, clinical doses of morphine can cause significant respiratory depression, along with other adverse effects such as Postoperative Nausea and Vomiting (PONV). Genetic factors contribute to significant variability in opioid induced respiratory depression, nausea and analgesia in twin studies.1, 2 Important genetic risk factors for increased opioid induced postoperative respiratory depression and other adverse effects are currently not well known.
Endocannabinoids play a significant role in pain modulation and inflammation.3 Anandamide, an endogenous cannabinoid, has been demonstrated to have analgesic properties in several different models of pain mostly by activation of cannabinoid receptors, CB1 and CB2. However, the intense analgesic actions of anandamide are short lived because of its rapid catabolism by fatty acid amide hydrolase (FAAH).4–6 The current literature suggests that FAAH inhibition enhances analgesia by increasing the bioavailability of anandamide7 and this is a promising strategy to treat certain types of pain and inflammation.8–13 Considering remarkable regulation of anandamide’s duration of action and amplitude by FAAH and tight control of fast catabolism of fatty acid amides by a single enzyme, inhibitors of FAAH have been targeted as valuable pharmaceutical agents for the treatment of pain and inflammation.6, 14 In addition, evidence suggests that human FAAH genetic variants modulate pain15 but their clinical role in surgical pain management is not well studied.
Endogenous cannabinoid receptors are widely distributed throughout the CNS, including the brainstem, and modulate a variety of functions, including breathing. In addition to effects on pain sensitivity, endogenous cannabinoids have been shown to mediate the antinociceptive effects of opioids.16 It had been shown that cannabinoid receptor CB1 are involved in morphine’s central nociception and mediate the influence via μ-opioid receptor agonistic action.17 In addition, anandamide if protected from degradation by FAAH, acts via the CB1 receptor and modulate morphine’s analgesia by interactions with kappa opioid receptors (Supplemental Figure 1).18 In neonatal mice, activation of cannabinoid CB1 receptor with anandamide had been shown to depress the medullary respiratory rhythm generator, probably via the catecholaminergic system.19 This could potentially explain increased mortality20 and morbidity21, 22 in infants exposed to substance abuse including cannabinoid during the perinatal period and opioid/marijuana abusers.
Opioid and cannabinoid systems reciprocally and synergistically modulate functions at multiple levels. However, effects of genetic variants of FAAH on clinical pain management with opioids are not well studied. We hypothesized that genetic variations in FAAH significantly influence the safety and efficacy of morphine in children undergoing surgery. The purpose of this study was to investigate the associations between common genetic polymorphisms of FAAH and opioid related effects and adverse effects following tonsillectomy in a large pediatric population. Such knowledge will help advance the ultimate goal of individualizing perioperative pain management in children.
Patients and Methods
Study Design and Setting
This is a prospective, genotype blinded, clinical observational study in a large cohort of children undergoing outpatient adenotonsillectomy with standard perioperative anesthetic, surgical and nursing care. The study is part of a larger ongoing clinical study, entitled Personalizing Perioperative Morphine Analgesia in Children, which is registered with clinicaltrials.gov, NCT01140724. This large prospective clinical study with standard perioperative care (with the clinical care team blinded to patients’ genotypes) evaluates factors contributing to inter-individual variations in analgesic and adverse effect responses to perioperative opioids in children. The study was approved by the institutional review board and written informed consent was obtained from parents and assent obtained when appropriate from children > 7 years of age before enrollment.
Participants
Children 6 – 15 years undergoing elective outpatient tonsillectomy or adenotonsillectomy were recruited for the study on the day of surgery. Sample inclusion criteria were children designated to have an American Society of Anesthesiologists (ASA) physical status 1 or 2 scheduled for tonsillectomy or adenotonsillectomy because of recurrent tonsillitis, adenotonsillar hypertrophy or obstructive sleep apnea (OSA). Sleep disordered breathing with history of snoring plus respiratory pauses during sleep lasting more than 10 seconds or daytime drowsiness were considered to constitute the clinical diagnosis of OSA. Accordingly, the indication for tonsillectomy in these children was documented as OSA. In addition, the Pediatric Sleep Questionnaire (PSQ),23, 24 a validated tool was used to assess children for sleep disorders. If the parent of study child reports “yes” to 8 or more of the 22 questions in the PSQ, the child was considered to have OSA.
Children were excluded if they or their parents were non-English speaking. Children allergic to study medications or who had developmental delay, liver or renal diseases, or preoperative pain requiring analgesics (e.g. chronic tonsillitis) were excluded. Due to limited availability of research coordinators for this study, we were not able to recruit all eligible subjects which resulted in convenience sampling (Figure 1).
Figure 1. The consort diagram.

illustrates the flow of study participants through this clinical trial. Eligible participants, reasons for exclusions, enrolled and analyzed patients are reported. IRB = institutional review board.
Standard Care and Study Procedures
All participants received standard perioperative care, including standardized surgical (electrocautery based) and anesthetic techniques. Anesthesia was induced using sevoflurane followed by a propofol (2 mg/kg) bolus to facilitate endotracheal intubation. Anesthesia was maintained with sevoflurane without the use of neuromuscular blockade. Patients received morphine prior to surgical incision. Children with OSA history received 0.1 mg/kg morphine while those without OSA diagnosis received 0.2 mg/kg. If there were any signs suggestive of pain (clinically significant increase in heart rate and blood pressure) following surgical incision and cauterization, the clinical anesthesia team provided additional morphine at 0.05 mg/kg increments intraoperatively as necessary. All children receive prophylactic ondansetron (0.1 mg/kg) and dexamethasone (0.1 mg/kg) intraoperatively. Significant postoperative pain measured with facial expression; leg movement; activity; cry; and consolability (FLACC) pain score25 ≥ 4/10 was managed in the postoperative anesthesia care unit (PACU) with rescue doses of morphine (0.05mg/kg increments).
Duration of PACU stay (time to achieve PACU discharge readiness) was defined as the duration in PACU before achieving the following discharge criteria. Level of consciousness: easily arousable or awake, airway: patent with adequate air exchange, core body temperature: ≥ 36·3 degrees Celsius, acceptable pain level (pain score <4), hemodynamically stable, no significant opioid related adverse effects such as PONV and respiratory depression, and surgical site without any bleeding or complications. This is discharge readiness time is different from actual PACU discharge time as we did not want to include delays due to social (non-medical) reasons (e.g. waiting for car ride). If a patient required more than 90 minutes to meet PACU discharge criteria following tonsillectomy, it was defined as a prolonged PACU stay.
Clinical Outcome Measures
Metrics for analgesic effectiveness and opioid-related adverse effects were recorded for each participant. For this paper, we focused on two opioid-related adverse effect outcomes: clinical respiratory depression (RD) and refractory Post-Operative Nausea and Vomiting (PONV). Total morphine requirement (mg/kg of body weight) was also examined as a measure of analgesic effectiveness. In our study, we defined clinical RD as a persistent (more than a minute) oxygen desaturation <90% or respiratory rate <8 breaths per minute or oxygen desaturation <94% along with respiratory rate <10 per minute requiring supplemental oxygen to maintain SpO2 >94% in the absence of clinically obvious upper airway obstruction. We defined PONV as an actual episode of emesis and/or episode of self-reported persistent nausea needing an antiemetic intervention. Prolonged PACU stay (>90 minutes) secondary to respiratory depression and refractory PONV were assessed consistently by the research coordinator. Total morphine dose was total amount of morphine used (in mg/kg) intraoperatively and immediate postoperative period in PACU.
Genotyping
Blood was drawn for DNA in the operating room upon intravenous line placement under anesthesia for genotyping of FAAH single nucleotide polymorphisms (SNPs). DNA was isolated on the same day and, frozen at −20°C. Six previously studied common SNPs were selected to be genotyped using TaqMan allelic discrimination system assays (Life Technologies, Applied Biosystems, USA). These included rs932816, rs4141964, rs3766246, rs324420, rs324419, and rs2295632. In addition, a genome-wide genotyping was performed on the Illumina Human OMNI-5 genotyping array using the iScan System (Illumina) and Infinium2 chemistry. Genotypes were called using the Gentrain2 algorithm within Illumina Genome Studio. Samples with call rates below 95% and SNPs with call rates below 95% or a HWE p-value less than 0.001 were dropped from the study. We identified 39 SNPs in the FAAH gene location and within 5kb upstream or downstream of FAAH. Two of the SNPs, rs3766246 and rs324420 were included in TaqMan and Illumina Omni5 GWAS assays with 100% concordance in genetic result reports. In addition, we used 244 validated ancestry informative markers (AIMs) for population stratification. Since genotyping was done after clinical care and clinical data collection, perioperative care providers and researchers were blinded to genotypes when clinical care was delivered.
Statistical analysis
To assess whether self-reported white and black races match well to genetic ancestry, we used 1397 HapMap subjects as our reference populations. Out of the 244 AIMs genotyped, 218 were found in the HapMap data. Therefore, we performed principal component analysis with 218 AIMs using SVS 7.7.6 (Golden Helix, Bozeman, MT). Up to 10 PCs were also used in the assessment of the potential confounding by population stratification. The genomic inflation factor (λ) was estimated from the median χ2 statistic in PLINK.
Other statistical analyses were performed using Statistical Analysis Software (SAS), version 9.3, JMP Genomics, version 6.0 (SAS Institute Inc., Cary, NC), and R.
Prior to analyses, quality of the data was checked. Characteristics of the patients and properties of the SNPs were examined in African American and Caucasian children respectively. Hardy Weinberg equilibrium (HWE) was tested. To analyze binary outcomes RD and PONV, logistic regressions were performed. To analyze total morphine requirement, linear regression was used. Prior to evaluation of FAAH variants, the effects of covariates were tested. For total morphine dose, age, sex, BMI z scores and OSA were evaluated. For adverse effect outcomes RD and PONV, total morphine was considered as an additional covariate. To select the best fitting model, log likelihood, Akaike and Bayesian Information criterion were compared, and residuals were examined. Covariates that significantly improved model fitting (p<0.05) were retained for subsequent genetic analyses. To assess the single SNP association with the outcomes, we used additive models, in which the genotypes were recoded and tested as continuous variables. Genotypes were recoded to 0, 1 and 2 according to the number of minor alleles of the entire cohort. Statistical modeling was conducted with white and black patients separately.
In this study, we focused on the association of two adverse outcomes respiratory depression and PONV with 14 FAAH SNPs that had minor allele frequency (MAF) ≥5% in both black and white children. The SNPs were linked with mean D’ of 0.967 (whites: 0.976; blacks: 0.9) and mean R2 of 0.289. Though there was significant correlations between both opioid adverse effects (Spearman correlation), in order to not overestimate associations, we performed a conservative simple Bonferroni correction for multiple comparisons, which yielded a significance threshold of 0.0018 [p=0.05/(2 outcomes x14 SNPs)]. We also report associations reaching the threshold of 0.05 as nominally associated.
Power analysis
Prior to analysis, we estimated statistical power to detect a genetic effect using Quanto. We varied MAF from 0.2 to 0.5 to capture the expected frequency range of our variants and held α to 0.0006 to account for multiple testing. Assuming a frequency of 17% for PONV and 32% for RD in white, we were 80% powered to detect odds ratios ranging between 3.3 and 3.8 for PONV and 2.6 to 3.1 for RD using our sample of 216 white children. As we were seeking to identify clinically relevant genetic associations, these effect sizes were reasonable.
Results
Demographics
A consort diagram illustrates eligible, approached and enrolled study subjects (Figure 1). Participants were primarily white with slightly more girls. Compared to white children, black children were slightly heavier and had higher OSA frequencies (Table 1).
Table 1.
Characteristics of participants
| Whites (N=216) | Blacks (N=43) | |
|---|---|---|
| Age (year) (median (IQR)) | 8.4 (7.1–11.0) | 8.8 (7.1–11.2) |
| Weight (Kg) (median (IQR)) | 33.8 (25.8–46.3) | 34.7 (26.2–54.1) |
| BMI z scores | 0.7 (−0.2–1.6) | 1.2 (0.0–2.0) |
| Intra-operative morphine requirement (mg/kg) (median (IQR)) | 0.19 (0.17–0.21) | 0.20 (0.16–0.20) |
| Sex (N, %) | ||
| Male | 105 (49%) | 19 (44%) |
| OSA (N, %) | ||
| Yes | 93 (43%) | 29 (67%) |
| PONV (N, %) | ||
| Yes | 36 (17%) | 3 (7%) |
| Respiratory Depression (N, %) | ||
| Yes | 68 (32%) | 17 (40%) |
| Total morphine requirement (mg/kg) | 0.25 (0.08) | 0.30 (0.09) |
Age, weight, BMI z score and intra-operative morphine requirement are shown as median and inter-quartile range (IQR); total morphine requirement is shown as mean (standard deviation); sex and Obstructive Sleep Apnea (OSA) are shown as frequencies and proportions. BMI z scores were calculated using CDC growth charts.
FAAH SNPs description
A total of 39 SNPs in the FAAH gene were genotyped by TaqMan and/or Illumina Human Omni 5 array techniques. Two FAAH SNPs, rs3766246 and rs324420 were genotyped by both methods; both methods yielded identical genotypes for all subjects, suggesting high reliability of our genotype data. Among the 39 SNPs, 14 had minor allele frequency (MAF) of 5% or more in both white and black children. Tests on Hardy Weinberg equilibrium (HWE) showed that these 14 SNPs were all in HWE at alpha=0.004 level (Bonferroni correction of 14 tests). Therefore, 14 SNPs were included in genetic association analyses.
Self-reported race and genetic ancestry
We compared self-reported white and black races with genetic ancestries estimated from 218 AIMs. In 250 out of the total of 259 patients (>95%), self-reported races clustered well with European and African ancestry; the remaining 9 subjects had genetic marker admixture. Principal component (PC) 1 and 2 successfully separated white and black races (data not shown). In this study, we stratified the analyses by self-reported races, as race differences in opioid effects have been reported in children,26 and self-reported races are readily available to clinicians compared to genetic AIMs.
Genetic association with Clinical Outcomes
Black children required higher total morphine dose (p<0.05, t test) and tended to have lower incidence of PONV (p=0.159, Fisher’s exact test), but the incidences of RD were comparable between black and white children (p=0.376) (Table 1). Overall the incidence of RD was more than PONV in our study population (Table 1). Before testing the genetic effect, we evaluated the effects of co-variables on PONV or RD. For PONV, significant sex and morphine dose effects were detected; for RD, significant effects of morphine dose and BMI z score were detected. No significant OSA effect was detected for either PONV or RD. The significant co-variables were then included in the genetic models, in which single SNP association with PONV or RD was tested in whites and blacks respectively. The results were summarized in Table 2 and Figures 2A and 2B.
Table 2.
Single FAAH SNP associations with PONV and Respiratory Depression
| outcome | SNP | location | white | black | Putative function | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| minor allele (%) | p HWE | p association | OR (95% CI) Beta ± SE | minor allele (%) | p HWE | p association | OR (95% CI) Beta ± SE | ||||
| PONV | rs4141964 | 46865040 | A (0.39) | 0.127 | 0.0014 | 2.42 (1.41, 4.16) | G (0.27) | 0.472 | intron | ||
| rs3766246 | 46865671 | T (0.39) | 0.127 | 0.0014 | 2.42 (1.41, 4.16) | C (0.27) | 0.134 | intron | |||
| rs324420 | 46870761 | A (0.23) | 0.008 | 0.0003 | 2.73 (1.58, 4.73) | A (0.41) | 0.939 | missense | |||
| rs2295633 | 46874383 | A (0.38) | 0.103 | 0.0028 | 2.26 (1.32, 3.85) | G (0.43) | 0.980 | intron | |||
| rs11576941 | 46875067 | A (0.31) | 0.867 | 0.0311 | 0.49 (0.26, 0.94) | A (0.10) | 0.389 | intron | |||
| rs2295632 | 46879562 | A (0.29) | 0.029 | 0.0005 | 2.61 (1.52, 4.47) | C (0.35) | 0.606 | downstream | |||
| kgp12517369 | 46882118 | A (0.29) | 0.029 | 0.0005 | 2.61 (1.52, 4.47) | G (0.42) | 0.359 | N/A | |||
| RD | rs4141964 | 46865040 | A (0.39) | 0.127 | 0.0402 | 1.57 (1.02, 2.41) | G (0.27) | 0.472 | intron | ||
| rs3766246 | 46865671 | T (0.39) | 0.127 | 0.0402 | 1.57 (1.02, 2.41) | C (0.27) | 0.134 | intron | |||
| rs324420 | 46870761 | A (0.23) | 0.008 | 0.0473 | 1.61 (1.01, 2.59) | A (0.41) | 0.939 | missense | |||
| rs2295632 | 46879562 | A (0.29) | 0.029 | 0.0343 | 1.62 (1.04, 2.54) | C (0.35) | 0.606 | downstream | |||
| kgp12517369 | 46882118 | A (0.29) | 0.029 | 0.0343 | 1.62 (1.04, 2.54) | G (0.42) | 0.359 | N/A | |||
Note: effects were shown as odds ratio (OR) and 95% CI for Postoperative Nausea and Vomiting (PONV) and respiratory depression (RD). OR indicated the odds ratio when minor allele increased by one copy; effect for total morphine was shown as dose increase for one copy increase of the minor allele.
In white children, tests on genetic association with PONV were adjusted for sex and total morphine dose; tests on genetic association with RD were adjusted for total morphine dose and BMI z score. In black children, no statistically significant co-variables were detected; therefore no co-variables were included in the genetic association tests.
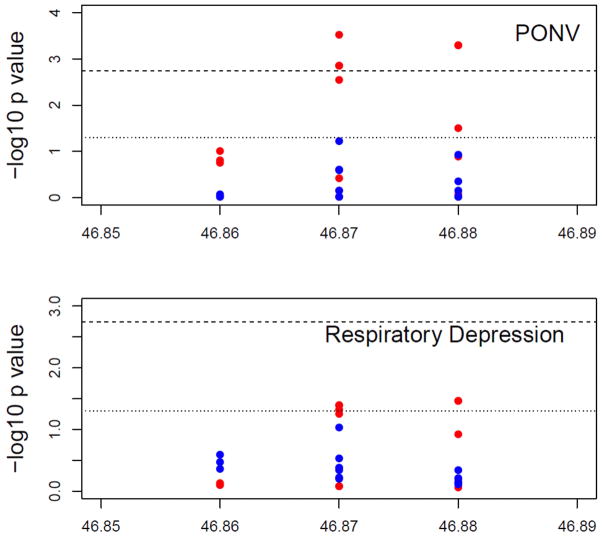
Figure 2. FAAH genotypes associated with Respiratory Depression, PONV and Morphine requirement.
Figure 2a. They axis shows the −log10 P values and the x axis shows the chromosomal positions of the FAAH SNPs. Results are shown for whites (red dots) and blacks (blue dots) separately. P values of the genetic association of the 39 FAAH SNPs with PONV (top) and RD (bottom). The reference lines represent the thresholds of p=0.0018 (shot dash line) and p=0.05 (dotted line), respectively. PACU = Post Anesthesia Care Unit; RD = respiratory depression; PONV = Postoperative Nausea and Vomiting; FAAH = Fatty Acid Amide Hydrolase.
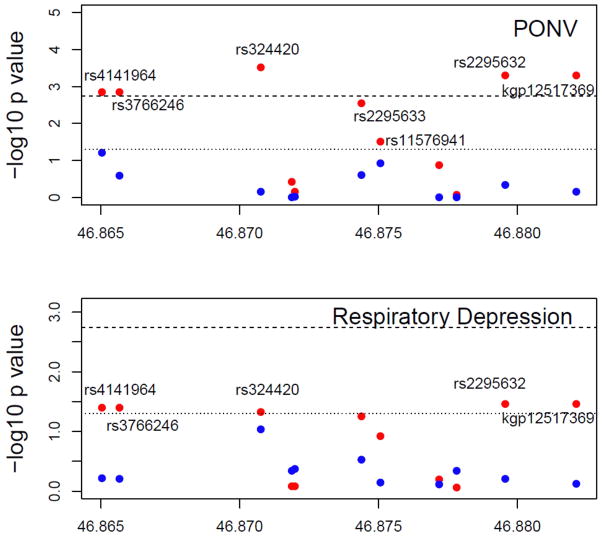
Figure 2b. The y axis shows the −log10 P values and the x axis shows the chromosomal positions of the of the 11 FAAH SNPs between 46.86 to 46.89 Mb of Chromosome 1 with PONV (top panel) and RD (bottom panel). Results are shown for whites (red dots) and blacks (blue dots) separately. The reference lines represent the thresholds of p=0.0018 (shot dash line) and p=0.05 (dotted line), respectively. PACU = Post Anesthesia Care Unit; RD = respiratory depression; PONV = Postoperative Nausea and Vomiting; FAAH = Fatty Acid Amide Hydrolase.
Genetic association with PONV
In white patients, statistically significant association was detected between PONV and five SNPs (rs4141964, rs3766246, rs324420, rs2295632 and kgp12517369). One additional copy of the minor allele of rs4141964, rs3766246, rs324420, rs2295632 and kgp12517369 increased the odds of PONV by 2.42, 2.42, 2.73, 2.61 and 2.61 fold, respectively (Table 2). In addition, two nominal associations were observed (Table 2). However, in black children, no association was detected with any of the SNPs (Table 2). Prolonged PACU stay due to refractory PONV represent a severe form of PONV and is a subset of children with PONV. When tested genetic association with prolonged stay due to PONV were tested in white children only (since no black child had prolonged PACU stay due to refractory PONV), we observed significant associations with rs4141964 (p=0.0097), rs3766246 (p=0.0097), rs324420 (p=0.0089), rs2295633 (p=0.0373), rs11576941 (p=0.0404), rs2295632 (p=0.0016), and kgp12517369 (p=0.0016).
Genetic association with Respiratory Depression
We detected nominal association of respiratory depression with rs324420, rs4141964, rs2295632, rs3766246, and kgp12517369 in white children (Table 2). No association was detected for any of the SNPs in black children. When genetic association with prolonged stay in PACU due to respiratory depression was tested, no genetic association was detected with prolonged stay in PACU due to respiratory depression in white or black children.
Genetic association with perioperative morphine requirement
No genetic association was detected with total intraoperative and postoperative morphine use in white or black children.
Linkage Disequilibrium analysis
Based on single SNP association tests, an interesting region was identified in FAAH gene ranging from 46865040 bp to 46882118 bp of chromosome 1 (Human Genome, version HG37.5), with 11 SNPs harbored in this region. Out of these 11 SNPs, 7 were associated with PONV and 5 associated with RD in whites (Figure 2A and 2B) with high linkage disequilibrium between these 11 SNPs (Supplemental Figure 2).
Sex specific SNP effect
For significant associations between rs4141964, rs3766246, rs324420, rs2295632 and kgp12517369 and PONV, there was no sex-specific SNP effects (p>0.05).
Genomic inflation
We detected statistically significant genetic association with PONV in white. To evaluate the effect of population structure on the association, we assessed the genomic inflation factor (λ) using all FAAH SNPs and ancestry informative markers genome wide. We found that λ is 1, suggesting no strong confounding by population stratification exists on the SNP association with PONV. When adjusted with up to 10 PCs, the genetic association with PONV in whites remained unchanged (data not shown).
Reliability of FAAH genetic association with PONV
Because of biological and significant statistical significant associations between FAAH SNP, rs324420 and PONV, in order to validate associations, we reanalyzed associations with additional 66 white children who had tonsillectomy with similar protocol. The bigger cohort (original cohort of 216 white children plus 66 additional white children) reproduced following consistent and significant associations. FAAH SNP, rs324420 was significantly associated with PONV (p=0.0053) with addition of one copy of minor allele (A) increasing OR by 2.0 folds; and it was also associated with PONV leading to prolonged PACU stay (p=0.0209) with addition of one copy of minor allele (A) increasing OR by 2.2 folds. Though not statistically significant, rs324420 AA genotype children overall stayed in PACU longer [97.9 (84.3 –113.6) minutes] than CC and CA genotype children [83.9 (79.9–88.2 minutes, p=0.072), which is clinically and economically relevant following a common outpatient surgery.
Discussion
Our study showed significant associations between FAAH polymorphisms and refractory PONV following a common outpatient surgery, tonsillectomy. In addition, nominal associations with opioid-induced respiratory depression, and prolonged recovery room stays due to PONV with specific FAAH SNPs were identified in a group of white children. Specifically, in white children, addition of one copy of the minor allele of rs4141964, rs3766246, rs324420, rs2295632 and kgp12517369 increased the odds of PONV by 2.42, 2.42, 2.73, 2.61 and 2.61 fold, respectively (p<0.0018, Table 2). These 5 FAAH SNPs including a missense polymorphism, rs324420, had nominal associations with opioid related respiratory depression and prolonged stays in PACU due to refractory PONV, highlighting possible biological synergistic interactions between opioid and endocannabinoid pathway.
The FAAH-1 gene, located on chromosome 1, codes for FAAH which degrades anandamide. After sequencing all 15 exons of the FAAH gene, a human study identified, FAAH SNP, rs324420 as a significant polymorphism; the minor allele of this relatively common missense mutation (385C>A) converts a conserved proline to threonine, resulting in a FAAH variant that has an enhanced sensitivity to proteolytic degradation and reduced cellular stability,27 potentially resulting in high anandamide levels. As can be seen from Supplemental Figure 3, P129 is located in an exposed and highly variable loop, away from the active site and FAAH dimerization interface. This position and the lack of evolutionary conservation make direct functional effect on enzymatic activity unlikely, supporting the alternative hypothesis of a lower number of active copies for the mutant protein with higher sensitivity to proteolytic degradation. In our study population, this particular SNP is in strong linkage disequilibrium with other SNPs of a haploblock of in FAAH gene region (Supplemental Figure 2) and is significant associated with both PONV and respiratory depression (Figure 3). Two different central opioid adverse effects, PONV and respiratory depression, are associated with multiple FAAH SNPs with high linkage; 7 SNPs were associated with PONV and 5 SNPs were associated with RD (Table 2). When sequential modeling (type 1 analysis) in whites was performed to test whether other significantly associated FAAH SNPs would explain PONV and RD in addition to rs324420, no other FAAH provided additional information to the association between rs324420 and PONV or RD.
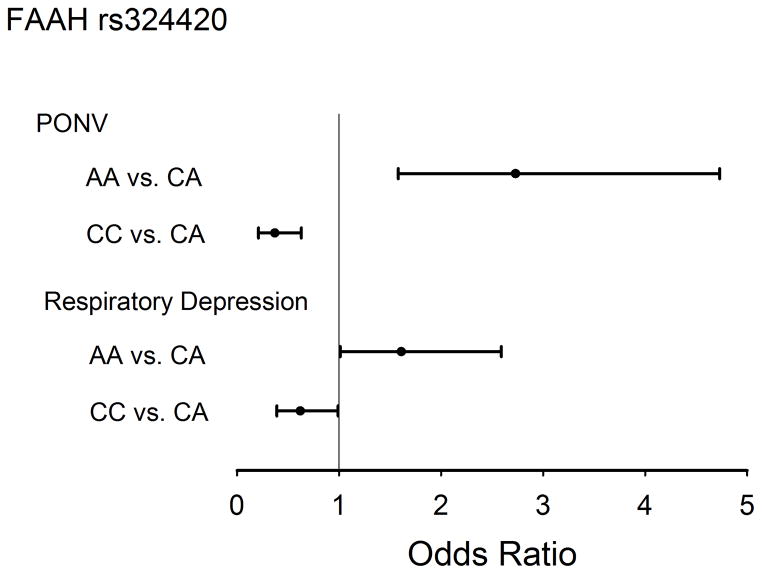
Figure 3. Missense FAAH SNP, rs324420 and risk of Respiratory Depression and PONV.
Compared to CA genotype of FAAH polymorphism, rs324420, children with AA genotype had higher risk of PONV [Odds ratio of 2.73 (1.58–4.73), p=0.0003] and RD [Odds ratio of 1.61 (1.01, 2.59), p=0.0473]; on the other hand relatively children with CC genotype had less risk of PONV and RD than children with CA and AA genotypes. PONV = Postoperative Nausea and Vomiting; FAAH = Fatty Acid Amide Hydrolase.
Interestingly, this particular missense FAAH SNP, rs324420 is strongly associated with both street drug and alcohol abuse and dependence.27–32 In the USA, opioid overdose/respiratory depression related deaths are more frequent than motor vehicle related injury deaths.33 Our finding of association of respiratory depression with FAAH gene (especially AA genotype of rs324420) may have potential significance for millions of patients prescribed and individuals abusing opioid agonists and/or cannabinoids (e.g. marijuana, synthetic cannabinoids)22, 34–39 every year, for both the abuse potential and the potential for life-threatening respiratory depression.
Respiratory depression related to opioids is a serious, potentially life threatening, however preventable complication. When we used a clinically relevant, widely accepted definition for respiratory depression, we found associations with FAAH SNPs (Table 2). Genetic risk factors (e.g. codeine in ultrarapid metabolizers) can increase the risk of respiratory depression and death.40, 41 Proactive risk identification and prevention are important in minimizing the negative impact of central opioid adverse effects.
Another central opioid adverse effect following surgery is PONV, often referred to as “the big little problem” after anesthesia.42 In humans, stress and motion sickness are associated with impaired endocannabinoid activity.43 Anandamide transport inhibition has been shown to attenuate vomiting in animals.44 General anesthesia influences anandamide levels in a drug-dependent way, which may explain high incidence of PONV with inhalational anesthetics.45 Since PONV remains as a big problem despite prophylactic anti-emetics and is often associated with opioids, we associated FAAH genetic variants with refractory PONV and prolonged PACU stay due to PONV. In white children, statistically significant higher risk for refractory PONV was detected with FAAH SNPs (Table 2). Though protection against nausea is anticipated with expected higher levels of endocannabinoid with these genotypes, the emetogenic effect of morphine was more pronounced than endocannabinoid effects in these patients. Same polymorphisms were also independently associated with respiratory depression highlighting higher risk for morphine’s central adverse effects. In our study, we observed a relatively lower incidence of PONV compared to respiratory depression; this could be due to intraoperative prophylactic use of dexamethasone and ondansetron, and possibly due to antiemetic properties of expected high endocannabinoid levels (with low expected FAAH function) with associated FAAH SNPs.
Though the exact molecular mechanisms behind central opioid adverse effects and genetic variations in endocannabinoid system are not well known, we found significant associations. Opioid and cannabinoid systems reciprocally and synergistically modulate functions at multiple levels including co-localization of opioid and cannabinoid receptors, direct receptor associations, altered release of endogenous peptide, shared signal transduction pathways, mutual potentiation, receptor cross-talk and cross-tolerance.46 In rhesus monkeys, cannabinoid agonists such as tetrahydrocannabinol and anandamide produce antinociception and respiratory depression; these effects were reversed with a specific cannabinoid receptor antagonist but not the opioid antagonist, revealing a cannabinoid mechanism.47 Though opioids and cannabinoids can independently cause analgesia and respiratory depression that could be reversed by respective antagonists in monkeys47 and FAAH inhibition can attenuate morphine withdrawal effects in mice,48 in humans synergism between opioid and endocannabinoid systems are not well studied. Our study provides early evidence of synergistic postoperative effects between opioids and endocannabinoid system.
Our earlier study demonstrated that white children had higher incidence of opioid related adverse effects than black children following surgery.26 Though black children required higher total morphine dose than white children in this current study, they tended to have lower incidence of refractory PONV (7% versus 17%, p=0.159). In this study, we have found some of the allelic frequencies of FAAH polymorphisms associated with opioid related PONV and RD are significantly different between Caucasian and African-American children (Table 2) consistent with previous human studies15 and could potentially explain and contribute to racial differences in clinical outcomes.
Though an adult volunteer study found associations between cold pain sensitivity and variations in FAAH in a gender dependent manner,15 in our pediatric study, we did not find any association between FAAH SNPs and morphine requirement. Two small studies that specifically examined sex differences showed greater in morphine-induced respiratory depression in women than men.49, 50 In our current study, girls had higher incidences of opioid-induced respiratory depression and PONV with higher doses of morphine than boys (data not shown). However, frequencies of FAAH variants in boys and girls did not explain the sex differences in postoperative RD and PONV in our study; furthermore, no sex specific FAAH associations with clinical outcomes were observed.
There are a few limitations in our study. Although our study found an association between FAAH genetic variants and postoperative opioid-induced respiratory depression, it is not possible to say whether these differences are related to specific FAAH genetic variants per se or to some unknown or not measurable variable that are highly linked to FAAH polymorphisms studied; sequencing of the entire FAAH gene might provide additional information. Secondly, due to local demographics, we enrolled mainly African-American and Caucasian children. Our current study does not address other races. We did not explore interactions between FAAH and other genes in study (which is relatively small for testing multiple gene-gene interactions) that might affect the incidence of respiratory depression. Despite these limitations, the results of the study has novel, clinically and economically important findings as it demonstrates that FAAH variants are associated with central opioid adverse effects and prolonged stays in PACU in children, which were reproduced in an extended cohort.
In conclusion, we found novel associations between FAAH polymorphisms and postoperative outcomes, PONV and respiratory depression in children undergoing tonsillectomy. Though our study demonstrates clinically and economically important associations between genetic variants of FAAH and central opioid related adverse effects and there are biological functional evidences to support our associations, causality for these adverse effects needs to be further studied. In future, when managing pain with possible proactive genotyping to personalize care, potentially higher incidences of opioid-induced respiratory depression and PONV in children with certain FAAH genetic variants need to be anticipated. To advance personalized pain management, more studies are needed to validate our findings in diverse population and understand the mechanistic pathways behind the genetic associations with opioid related adverse effects.
Supplementary Material
This figure illustrates human endocannabinoid system with key endocannabinoid, anandamide and its regulating enzyme, Fatty Acid Amide Hydrolase (FAAH). Key physiological effects of anandamide and potential interaction with opioids are described.
The LD plot for SNPs of within 20–70 Mb of chromosome 1 in white children. Red color indicates high linkage and blue color indicates low linkage. Total of 14 SNPs found in FAAH gene and 5 Kb upstream and downstream of FAAH were highly linked with a mean D prime of 0.976 in white children. FAAH missense SNP rs324420 is highlighted orange color. Legend represents heat map colors. LD = Linkage Disequilibrium; FAAH = Fatty Acid Amide Hydrolase.
Side and top views of Crystal structure of a humanized FAAH are shown in panels A and B, respectively.
Supplemental Figure 3a. Panel A: Mapping P129T variant encoded by rs324420 into FAAH structure. Crystal structure of a humanized FAAH in complex with an inhibitor (PDB code 2VYA) is used. Two chains of FAAH homodimer are shown in green and blue, respectively, with active sites (AS) in each monomer indicated by red circles. P129 is shown in magenta; the location of membrane with respect to the FAAH complex is indicated by yellow rectangle. Side and top views are shown in panels A and B, respectively. The location of P129 into an exposed and evolutionary variable loop is highlighted in the bottom part of panel B. FAAH = Fatty Acid Amide Hydrolase.
Supplemental Figure 3b. Panel B: Local structural environment around P129 is shown in the bottom part of panel B. First row: conserved and variable positions are indicated by red and blue background, respectively; second row: helices, beta strands and loops are shown as red braids, green arrow and blue lines, respectively; third row: secondary structure propensities; fourth row: buried and exposed positions are indicated by black and grey/white boxes, respectively.
Acknowledgments
This work was supported in part by USPHS Grant #UL1 RR026314 from the National Center for Research Resources, NIH and with the Place Outcomes Research Award (PI: SS) and Translational Research Award (PIs: JMA and SS), Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA. Additional research funding support was provided by the Department of Anesthesia, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA. No financial support except departmental salary support for the authors. All authors listed in this manuscript have no conflicts of interest relevant to this article to disclose. This pharmacogenetic study was designed and undertaken by the authors. The sponsor of this study, the Cincinnati Children’s Hospital Medical Center (CCHMC) provided funding support for the genetic analyses and supported salary of the research team. The authors directed and had access to all the analyses and the full clinical and genetic database, wrote all drafts of the report, decided to publish the results, and attest for the accuracy and completeness of the data.
Footnotes
Trial Registration: This clinical trial reflects a portion of a larger study, Personalizing Perioperative Morphine Analgesia in Children, NCT01140724, registered at clinicaltrials.gov.
Conflict of Interests
None of the authors have any conflicts of interest to disclose.
References
- 1.Angst MS, Lazzeroni LC, Phillips NG, Drover DR, Tingle M, Ray A, et al. Aversive and reinforcing opioid effects: a pharmacogenomic twin study. Anesthesiology. 2012;117(1):22–37. doi: 10.1097/ALN.0b013e31825a2a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angst MS, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, et al. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153(7):1397–1409. doi: 10.1016/j.pain.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohmann AG, Suplita RL., 2nd Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8(4):E693–708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. The Journal of pharmacology and experimental therapeutics. 1997;282(1):243–247. [PubMed] [Google Scholar]
- 6.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(16):9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlosburg JE, Kinsey SG, Lichtman AH. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. AAPS J. 2009;11(1):39–44. doi: 10.1208/s12248-008-9075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suplita RL, 2nd, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49(8):1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 10.Hama AT, Germano P, Varghese MS, Cravatt BF, Milne GT, Pearson JP, et al. Fatty acid amide hydrolase (FAAH) inhibitors exert pharmacological effects, but lack antinociceptive efficacy in rats with neuropathic spinal cord injury pain. PloS one. 2014;9(5):e96396. doi: 10.1371/journal.pone.0096396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichna J, Salaga M, Stuart J, Saur D, Sobczak M, Zatorski H, et al. Selective inhibition of FAAH produces antidiarrheal and antinociceptive effect mediated by endocannabinoids and cannabinoid-like fatty acid amides. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2014;26(4):470–481. doi: 10.1111/nmo.12272. [DOI] [PubMed] [Google Scholar]
- 12.Caprioli A, Coccurello R, Rapino C, Di Serio S, Di Tommaso M, Vertechy M, et al. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. The Journal of pharmacology and experimental therapeutics. 2012;342(1):188–195. doi: 10.1124/jpet.111.191403. [DOI] [PubMed] [Google Scholar]
- 13.Bisogno T, Maccarrone M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert opinion on drug discovery. 2013;8(5):509–522. doi: 10.1517/17460441.2013.780021. [DOI] [PubMed] [Google Scholar]
- 14.Otrubova K, Ezzili C, Boger DL. The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH) Bioorg Med Chem Lett. 2011;21(16):4674–4685. doi: 10.1016/j.bmcl.2011.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Mittal DP, Iadarola MJ, Dionne RA. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet. 2006;43(8):e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller LL, Picker MJ, Umberger MD, Schmidt KT, Dykstra LA. Effects of alterations in cannabinoid signaling, alone and in combination with morphine, on pain-elicited and pain-suppressed behavior in mice. The Journal of pharmacology and experimental therapeutics. 2012;342(1):177–187. doi: 10.1124/jpet.112.191478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Pacheco DF, Klein A, Perez AC, Pacheco CM, de Francischi JN, Reis GM, et al. Central antinociception induced by mu-opioid receptor agonist morphine, but not delta- or kappa-, is mediated by cannabinoid CB1 receptor. British journal of pharmacology. 2009;158(1):225–231. doi: 10.1111/j.1476-5381.2009.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haller VL, Stevens DL, Welch SP. Modulation of opioids via protection of anandamide degradation by fatty acid amide hydrolase. Eur J Pharmacol. 2008;600(1–3):50–58. doi: 10.1016/j.ejphar.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Tree K, Caravagna C, Hilaire G, Peyronnet J, Cayetanot F. Anandamide centrally depresses the respiratory rhythm generator of neonatal mice. Neuroscience. 2010;170(4):1098–1109. doi: 10.1016/j.neuroscience.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Ostrea EM, Jr, Ostrea AR, Simpson PM. Mortality within the first 2 years in infants exposed to cocaine, opiate, or cannabinoid during gestation. Pediatrics. 1997;100(1):79–83. doi: 10.1542/peds.100.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix I, Cabou C, Montastruc JL, Damase-Michel C. Adverse drug reactions in pregnant women. Therapie. 2007;62(5):455–460. doi: 10.2515/therapie:2007067. [DOI] [PubMed] [Google Scholar]
- 22.Ali K, Wolff K, Peacock JL, Hannam S, Rafferty GF, Bhat R, et al. Ventilatory response to hypercarbia in newborns of smoking and substance-misusing mothers. Annals of the American Thoracic Society. 2014;11(6):933–938. doi: 10.1513/AnnalsATS.201403-124OC. [DOI] [PubMed] [Google Scholar]
- 23.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 24.Chervin RD, Weatherly RA, Garetz SL, Ruzicka DL, Giordani BJ, Hodges EK, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216–222. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 25.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297. [PubMed] [Google Scholar]
- 26.Sadhasivam S, Chidambaran V, Ngamprasertwong P, Esslinger HR, Prows C, Zhang X, et al. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics. 2012;129(5):832–838. doi: 10.1542/peds.2011-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13(18):2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- 28.Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(12):8394–8399. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flanagan JM, Gerber AL, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Human genetics. 2006;120(4):581–588. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- 30.Dlugos AM, Hamidovic A, Hodgkinson CA, Goldman D, Palmer AA, de Wit H. More aroused, less fatigued: fatty acid amide hydrolase gene polymorphisms influence acute response to amphetamine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(3):613–622. doi: 10.1038/npp.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(4):967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Moreno JA, Echeverry-Alzate V, Buhler KM. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacol. 2012;26(1):133–143. doi: 10.1177/0269881111416689. [DOI] [PubMed] [Google Scholar]
- 33.CDC. Centers for Disease Prevention and Control Grand Rounds: Prescription Drug Overdoses—a US Epidemic. 2013 http://wwwcdcgov/mmwr/preview/mmwrhtml/mm6101a3htm.
- 34.Behonick G, Shanks KG, Firchau DJ, Mathur G, Lynch CF, Nashelsky M, et al. Four Postmortem Case Reports with Quantitative Detection of the Synthetic Cannabinoid, 5F-PB-22. Journal of analytical toxicology. 2014;38(8):559–562. doi: 10.1093/jat/bku048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrows DL, Hagardorn AN, Harlan GC, Wallen ED, Ferslew KE. A fatal drug interaction between oxycodone and clonazepam. Journal of forensic sciences. 2003;48(3):683–686. [PubMed] [Google Scholar]
- 36.Eiden C, Cathala P, Mathieu-Daude JC, Marson B, Baccino E, Leglise Y, et al. Methadone-related deaths in Montpellier and Region, from 2000 to 2010. Therapie. 2012;67(6):515–522. doi: 10.2515/therapie/2012072. [DOI] [PubMed] [Google Scholar]
- 37.Havis S, Best D, Carter J. Concealment of drugs by police detainees: lessons learned from adverse incidents and from ‘routine’ clinical practice. Journal of clinical forensic medicine. 2005;12(5):237–241. doi: 10.1016/j.jcfm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Kunsdorf-Wnuk A, Musiol E, Karpel E, Arct-Danielak D. Rhabdomyolysis, disseminated intravascular coagulation and acute renal failure after severe narcotics intoxication (MDMA, THC, amphetamine) Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2005;18(106):436–439. [PubMed] [Google Scholar]
- 39.Lemos NP, Ingle EA. Cannabinoids in postmortem toxicology. Journal of analytical toxicology. 2011;35(7):394–401. doi: 10.1093/anatox/35.7.394. [DOI] [PubMed] [Google Scholar]
- 40.Kelly LE, Rieder M, van den Anker J, Malkin B, Ross C, Neely MN, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129(5):e1343–1347. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- 41.Sadhasivam S, Myer CM., 3rd Preventing opioid-related deaths in children undergoing surgery. Pain Med. 2012;13(7):982–983. doi: 10.1111/j.1526-4637.2012.01419.x. author reply 984. [DOI] [PubMed] [Google Scholar]
- 42.Kapur PA. The big “little problem”. Anesthesia and analgesia. 1991;73(3):243–245. doi: 10.1213/00000539-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Chouker A, Kaufmann I, Kreth S, Hauer D, Feuerecker M, Thieme D, et al. Motion sickness, stress and the endocannabinoid system. PloS one. 2010;5(5):e10752. doi: 10.1371/journal.pone.0010752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien LD, Limebeer CL, Rock EM, Bottegoni G, Piomelli D, Parker LA. Anandamide transport inhibition by ARN272 attenuates nausea-induced behaviour in rats, and vomiting in shrews (Suncus murinus) British journal of pharmacology. 2013 doi: 10.1111/bph.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schelling G, Hauer D, Azad SC, Schmoelz M, Chouker A, Schmidt M, et al. Effects of general anesthesia on anandamide blood levels in humans. Anesthesiology. 2006;104(2):273–277. doi: 10.1097/00000542-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–654. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. The Journal of pharmacology and experimental therapeutics. 1998;286(2):697–703. [PubMed] [Google Scholar]
- 48.Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ, et al. Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38(6):1039–1049. doi: 10.1038/npp.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahan A, Sarton E, Teppema L, Olievier C. Sex-related differences in the influence of morphine on ventilatory control in humans. Anesthesiology. 1998;88(4):903–913. doi: 10.1097/00000542-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Sarton E, Teppema L, Dahan A. Sex differences in morphine-induced ventilatory depression reside within the peripheral chemoreflex loop. Anesthesiology. 1999;90(5):1329–1338. doi: 10.1097/00000542-199905000-00017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This figure illustrates human endocannabinoid system with key endocannabinoid, anandamide and its regulating enzyme, Fatty Acid Amide Hydrolase (FAAH). Key physiological effects of anandamide and potential interaction with opioids are described.
The LD plot for SNPs of within 20–70 Mb of chromosome 1 in white children. Red color indicates high linkage and blue color indicates low linkage. Total of 14 SNPs found in FAAH gene and 5 Kb upstream and downstream of FAAH were highly linked with a mean D prime of 0.976 in white children. FAAH missense SNP rs324420 is highlighted orange color. Legend represents heat map colors. LD = Linkage Disequilibrium; FAAH = Fatty Acid Amide Hydrolase.
Side and top views of Crystal structure of a humanized FAAH are shown in panels A and B, respectively.
Supplemental Figure 3a. Panel A: Mapping P129T variant encoded by rs324420 into FAAH structure. Crystal structure of a humanized FAAH in complex with an inhibitor (PDB code 2VYA) is used. Two chains of FAAH homodimer are shown in green and blue, respectively, with active sites (AS) in each monomer indicated by red circles. P129 is shown in magenta; the location of membrane with respect to the FAAH complex is indicated by yellow rectangle. Side and top views are shown in panels A and B, respectively. The location of P129 into an exposed and evolutionary variable loop is highlighted in the bottom part of panel B. FAAH = Fatty Acid Amide Hydrolase.
Supplemental Figure 3b. Panel B: Local structural environment around P129 is shown in the bottom part of panel B. First row: conserved and variable positions are indicated by red and blue background, respectively; second row: helices, beta strands and loops are shown as red braids, green arrow and blue lines, respectively; third row: secondary structure propensities; fourth row: buried and exposed positions are indicated by black and grey/white boxes, respectively.