Abstract
Cognitive performance has been shown to be enhanced when performance-based rewards are at stake. On the other hand, task-irrelevant threat processing has been shown to have detrimental effects during several cognitive tasks. Crucially, the impact of reward and threat on cognition has been studied largely independently of one another. Hence, our understanding of how reward and threat simultaneously contribute to performance is incomplete. To fill in this gap, the present study investigated how reward and threat interact with one another during a cognitive task. We found that threat of shock counteracted the beneficial effect of reward during a working memory task. Furthermore, individual differences in self-reported reward-sensitivity and anxiety were linked to the extent to which reward and threat interacted during behavior. Together, the current findings contribute to a limited but growing literature unraveling how positive and negative information processing jointly influence cognition.
Keywords: reward, emotion, threat, cognition, working memory
INTRODUCTION
Cognitive performance across a diverse set of tasks has shown to be enhanced when performance-based rewards are at stake (Pessoa, 2013). For instance, rewards boosted performance during working memory (Savine et al., 2010) and response conflict (Krebs et al., 2010) tasks. On the other hand, task-irrelevant threat processing has been shown to have detrimental effects on performance during some cognitive tasks (Robinson et al., 2013). For instance, during conditions that involved anticipation of mild aversive shocks, response interference scores were increased in a Stroop-like paradigm (Choi et al., 2012) and performance was reduced during working memory task (Vytal et al., 2012).
Crucially, the impacts of reward and threat on cognition have been studied largely independent of one another. In other words, our current understanding about how reward and threat processes simultaneously contribute to cognitive performance is incomplete. To fill in this gap, the present study investigated how reward and threat interact with one another during a cognitive task. Understanding these interactions is important because many real-life situations involve both positive and negative dimensions. In particular, organisms are faced with situations that simultaneously offer potential rewards (obtaining a food item) while posing actual dangers (being eaten). Accordingly, solving cognitive problems involves the joint processing of positive and negative signals. Moreover, understanding interactions between reward and threat during cognition might be of potential relevance in some clinical disorders such as depression (Dillon et al., 2014).
As previously noted, rewards boosted performance during working memory tasks (Savine et al., 2010) but threat of shock reduced performance (Vytal et al., 2012). In this study, we investigated the interaction between reward and threat during a delayed match-to-sample version of the working memory task using a factorial design (Fig. 1). Based on previous behavioral findings where threat of shock reduced the reward responsiveness in a probabilistic learning task (Bogdan and Pizzagalli, 2006) and our recent fMRI findings (Choi et al., 2014), where we observed that threat of shock reduced reward anticipation related responses in regions that are associated with reward processing (e.g., midbrain and striatum) and working-memory (e.g., lateral prefrontal cortex), we expected that threat would counteract the beneficial effect of reward on task performance.
Figure 1.
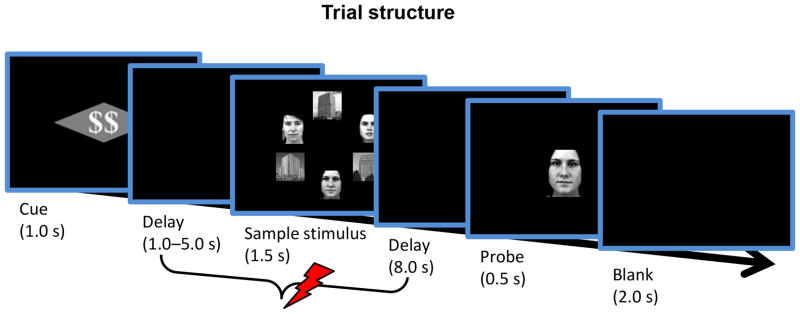
Experimental Design. On each trial, an initial cue indicated both the reward condition (dollar vs. pound sign) and the threat condition (rectangle vs. diamond). After a 1–5 s interval, a sample stimulus was shown containing faces and scenes, which had to be remembered during the delay period. When the final probe stimulus was shown, participants had to indicate whether or not it was part of the sample display. During the reward condition, participants could earn extra cash for correct performance. During the threat condition, a mild electrical stimulus could be administered anytime between the cue offset and onset of the subsequent probe stimulus.
In past studies that investigated how threat affects cognition, individual differences in self-reported anxiety were related to the impact of threat on behavior (e.g., Choi et al., 2012). Similarly, in studies that investigated how rewards affect cognition, individual differences in self-reported reward sensitivity were related to the effect of reward on cognitive performance (e.g., van Steenbergen et al., 2010). But it is currently unknown how these self-reported measures relate to the behavior during tasks that involve simultaneous reward and threat manipulations. Thus, in the current study, we investigated how individual differences in self-reported reward-sensitivity and anxiety relate to the reward-by-threat interactions in behavior.
METHODS
Subjects
Twenty-six participants (8 males; age range: 18–36 years old) took part in the study and provided informed consent, as approved by the Institutional Review Board of Indiana University, Bloomington, IN. Subjects were free from psychiatric or neurological disease or related past history, as indicated via self-report.
Personality questionnaires
Before the start of the experiment, participants completed the state-trait anxiety inventory (Spielberger, 1983) and the Behavioral Activation System (BAS) scale, which assesses multiple personality characteristics related to reward sensitivity (Carver and White, 1994). As done in previous studies (van Steenbergen et al., 2010), we employed scores on BAS-drive subscale as an index of reward sensitivity, as it has been shown to have the highest internal reliability (Carver and White, 1994) and has been proposed to provide a clear measure of reward-driven behavior (Dawe et al., 2004).
Stimuli and task
Each trial (Fig. 1) started with the presentation (1000 ms) of a rectangle- or diamond-shaped cue stimulus overlaid with a double-dollar ($$) or double-pound sign (##). The pound and dollar signs indicated the Reward condition (no-reward and reward, respectively) and the geometric shape cue indicated the Threat condition (safe or threat, counterbalanced across participants). Thus the cue stimulus simultaneously specified Reward and Threat conditions. The threat cue indicated that a mild electric shock could be delivered anytime between the cue offset and onset of the subsequent probe (see below). Physical shocks were administered in 33% of the threat trials and those trials were discarded from additional analyses. Participants were not informed about the probability of shock, but they were told that shock would never occur during safe cue trials. The reward cue indicated that a monetary bonus (20 cents) would be given if the response was made correctly. Fast performance was not emphasized, although responses had to be made within 2000 ms for them to be considered (well below mean RTs, which were less than 1000 ms for all conditions).
The cue was followed by a delay period that lasted for 1–5 seconds, and then a sample stimulus was presented (1500 ms). The sample stimulus consisted of either three building or house pictures and either three male or female faces arranged in a circular fashion centered on the middle of screen (3° radius). The face pictures (2.6° × 2.6°) were presented in either a “V” or an “inverted-V” arrangement (see Fig. 1 for an example). A second delay period (8000 ms) and a probe stimulus (500 ms) followed the sample stimulus. The participant’s task was to maintain images of the sample faces in their working memory for the duration of the subsequent delay period and to indicate whether or not it matched a face stimulus shown during the final probe phase. The probe stimulus was either one of the faces from the sample set or a novel face (50% probability). Responses were made on the keyboard using index and middle fingers of the right hand and were counterbalanced across participants in terms of “yes” and “no” responses. A final blank display (2000 ms) ended the trial.
Each participant performed 9 experimental runs, each consisting of 24 trials, resulting in a total of 216 trials and 54 trials per condition. After eliminating the trials that actually had physical shocks, there were 36 trials in the threat condition. All experimental conditions were intermixed randomly with the constraint that each possible trial combination occurred an equal number of times in terms of the Reward and Threat conditions. To avoid any cues that may have indicated the likelihood of shock, trials containing physical shocks were equally split between those containing males and females at the sample stimulus; likewise for houses and buildings.
To calibrate the intensity of the electric shock, each participant was asked to choose his/her own stimulation level immediately prior to the beginning of the experiment, such that the stimulus would be “highly unpleasant but not painful”. After each run, participants were asked about the unpleasantness of the stimulus and were asked to, if needed, re-calibrate it so that the shock would still be “highly unpleasant but not painful”. Shocks were administered with an electrical stimulator (Coulbourn Instruments, PA, USA) on the fourth (“ring”) and fifth (“pinky”) fingers of the left hand. Skin conductance response (SCR) data were also collected using the MP-150 system (BIOPAC Systems, Inc., CA, USA) with 10 Hz low-pass and 0.05 Hz high-pass hardware filters at a sampling rate of 250 Hz by using electrodes attached to the index and middle fingers of the left hand.
For the presentation of visual stimuli and recording of participant’s responses, Presentation software (Neurobehavioral Systems, Albany, CA, USA) was used.
SCR data analysis
Raw SCR data from each participant was initially smoothed with a median-filter over 50 samples (200 ms) to reduce noise and resampled at 1 Hz. The pre-processed SCR data were analyzed using multiple regression framework in AFNI (Cox, 1996); for related approaches, please see Hu et al.(2013). There were a total of four main event types in the design matrix: no-reward and reward correct trials, separately for the safe and threat conditions. Trials involving physical shocks and errors (pooled over all four conditions) were modeled as two additional events of no interest. No assumptions were made about the shape of the SCR function. The average response to each trial type was estimated via deconvolution. Responses were estimated starting from cue onset to 15 s post onset using cubic spline basis functions. This method is closely related to the use of finite impulses (“stick functions”), the commonly employed technique that can be considered the simplest form of basis expansion. Cubic splines allow a smoother approximation of the underlying responses, instead of the discrete approximation obtained by finite impulses. Constant and linear terms were included for each run separately (as covariates of no interest) to model baseline and drifts of the SCR. As done previously (Hu et al., 2013), we used the estimated responses at 5 sec post cue onset as an index of response strength for each event type. Finally, in order to help with equalizing variance, response-strength indices were transformed using a logarithm function [log10(1+SCR)]. Then, a 2 × 2 repeated-measures ANOVA was run to investigate interactions between Reward (no-reward, reward) and Threat (safe, threat).
Behavioral data analysis
Threat trials containing a physical shock were excluded from the analysis. Working memory performance is typically evaluated in terms of accuracy because RT is less informative given that correct performance entails bridging the delay period. So we mainly focused on accuracy data, but additional analyses of RT data were also conducted. For the RT analysis, error trials and trials with an RT exceeding three standard deviations from the condition-specific mean (0.9% of the trials) were excluded in each participant. For each participant, mean accuracy rate and RT data were determined as a function of Reward (no-reward, reward) and Threat (safe, threat) and repeated-measures ANOVAs were conducted. We used an alpha-level of 0.05 for all statistical tests.
Individual difference analysis
To investigate the relationship between individual differences in personality scores and the interaction pattern observed in accuracy data (see RESULTS), we ran a robust linear regression because it is less sensitive to influential outlier data points compared to standard regression (Wilcox, 2005). The state-anxiety and BAS-drive scores were used as independent variables as well as the interaction between BAS-drive and state-anxiety. The reward-by-threat interaction score in accuracy data was dependent variable. The resulting regression model was as follows:
where “Behav_interact” is the accuracy rate interaction score calculated as [(reward − no-reward)safe − (reward − no-reward)threat], “BAS_drive” is the score on BAS-drive sub-scale of BAS inventory, “State_Anx” is the score on the state-anxiety portion of STAI and i is a subject index. Both the state-anxiety and BAS-drive scores were standardized (i.e., transformed to z-scores) before entering them into the regression model. In our sample, state-anxiety scores and BAS-drive scores are minimally correlated (r = −0.08). Further, in our sample, state- and trait-anxiety scores are highly correlated (r = 0.94) and so a separate analysis with trait-anxiety scores instead of state-anxiety scores yielded almost identical results.
RESULTS
SCR data
Skin conductance responses (SCRs) were evaluated according to a 2 Reward (no-reward, reward) × 2 Threat (safe, threat) repeated-measures ANOVA. The main effects of Threat was significant (F1, 25 = 4.41, p = .046, ηp2 = .150). SCR was greater during threat compared to safe trials, providing evidence for successful threat manipulation that resulted in increased arousal. The main effect of Reward (F1, 25 = 0.45, p = .507, ηp2 =.018) and Reward x Threat interaction (F1, 25 = 0, p = .975, ηp2 < .001) were not detected.
Behavioral data
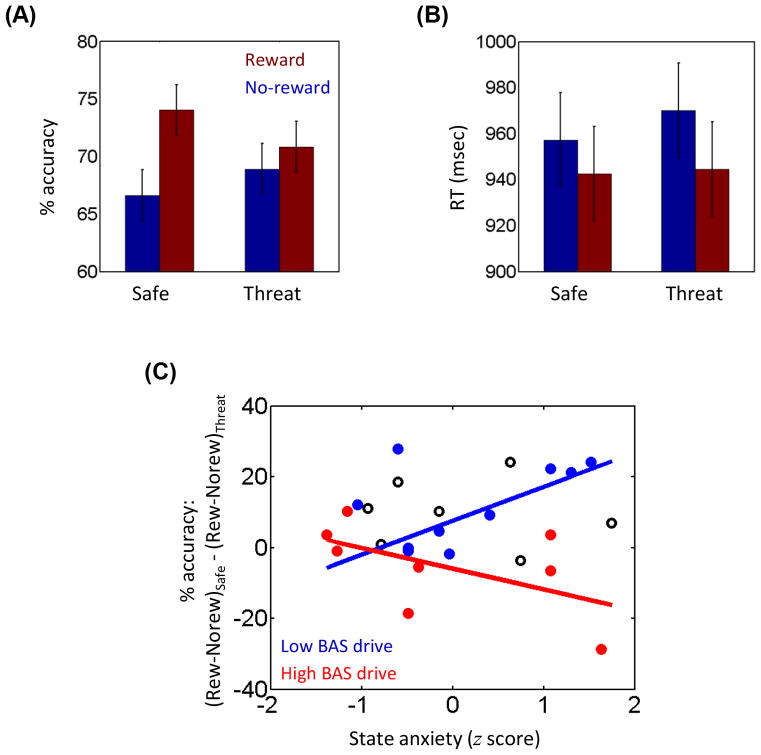
We investigated reward-threat interactions during a working memory task by employing a 2 Reward (no-reward, reward) × 2 Threat (safe, threat) factorial design. Accuracy data were evaluated according to a 2 Reward (no-reward, reward) × 2 Threat (safe, threat) repeated-measures ANOVA. The main effect of Reward was significant (F1,25 = 11.93, p = .002, ηp2 = 0.323; Fig. 2A). Mean accuracy increased during reward (72.45%) relative to no-reward trials (67.77%), replicating previously reported effects of reward on working memory performance (Savine et al., 2010). The main effect of Threat was not significant (F1,25 = 0.21, p = .651, ηp2 = 0.008). Critically, a significant Reward by Threat interaction was detected (F1,25 = 4.52, p = .044, ηp2 = 0.153). For completeness, we performed pairwise comparisons to test the effect of reward during safe and threat trials separately: reward increased accuracy during the safe condition (t25 = 4.58, p < .001, Cohen’s d = 0.90), but the beneficial effect of reward was not detected during the threat condition (t25 = 0.92, p = .365, Cohen’s d = 0.18). In addition, we ran two pairwise comparisons to evaluate the effect of threat during reward and no-reward trials separately: threat decreased accuracy during the reward condition (t25 = 2.26, p < .05, Cohen’s d = 0.44), but the effect of threat was not detected during the no-reward condition (t25 = 1.25, p = .221, Cohen’s d = 0.25).
Figure 2.
Results. (A) During safe trials, accuracy rate improved with reward. This beneficial effect of reward was eliminated during threat trials. (B) During both safe and threat trials, RT was faster in reward compared to no-reward trials. (C) For participants with low reward-sensitivity scores (i.e., below the median and shown in blue), the relationship between state-anxiety scores and interaction pattern in accuracy rate was positive, whereas for participants with high reward-sensitivity scores (i.e., above the median and shown in red), the relationship was flat (or even negative). The data from participants who were exactly at the median value of reward-sensitivity scores are shown in black. Error bars in panels A and B denote the standard within-subject error term for interaction effects (Loftus and Masson, 1994). RT = reaction time; Rew = reward condition; Norew = no-reward condition.
We also analyzed RT data (Fig. 2B) with a 2 Reward (no-reward, reward) × 2 Threat (safe, threat) repeated-measures ANOVA. The main effect of Reward revealed a trend towards significance (F1,25 = 3.59, p = .070, ηp2 = .126), such that RT was faster during reward (944 msec) compared to no-reward (964 msec) condition. Significant effects were not detected in either the main effect of Threat (F1,25= 0.82, p = .373, ηp2 = .032) or Reward x Threat interaction (F1,25 = 0.21, p = .652, ηp2 = .008).
Relationship between personality scores and behavioral data
We employed robust linear regression analysis to investigate how individual differences in self-reported reward-sensitivity and anxiety were related to the accuracy data (see METHODS). We observed a significant relationship between the BAS-drive x state-anxiety interaction and the interaction pattern in behavioral accuracy (b3 = −6.63; p = .042; a similar analysis with BAS-reward responsiveness scores instead of BAS-drive scores did not reveal a significant relationship: b3 = −3.02; p = .299). To illustrate this, we plotted the relationship between state-anxiety and behavior at two different levels of reward-sensitivity scores (Fig. 2C): participants with low BAS-drive scores (i.e., below the median) had a positive relationship between state-anxiety and behavioral interaction pattern, but participants with high BAS-drive scores (i.e., above the median) had a flat (or even slightly negative) relationship between state-anxiety and the behavioral interaction. Therefore, the effect of state-anxiety on behavioral interaction depended on the level of reward-sensitivity. The regression coefficient corresponding to BAS-drive alone was also significant (b1 = −6.44; p = .034), but the regression coefficient corresponding to state-anxiety alone was not (b2 = 1.44; p = .571).
DISCUSSION
In this study, we investigated interactions between reward and threat during cognition. We observed that threat counteracted the beneficial effect of reward during a working memory task. Further, the interaction between self-reported reward-sensitivity and anxiety measures exhibited a significant linear relationship with the reward-by-threat interaction pattern observed in behavior.
Traditionally, the impact of reward on cognition was thought to be relatively unspecific. Whereas reward has generalized, energizing contributions to behavior (Robbins and Everitt, 2007), recent work underscores the ability of reward to influence behavior in specific manners during diverse cognitive tasks, including reduction of response conflict, reduction of task-switching costs, and selective effects on working memory (Krebs et al., 2010, Savine et al., 2010). In this study, we tested whether a strong aversive manipulation such as threat anticipation would counteract the beneficial effect of reward on task performance in the context of a cognitive task. Indeed, threat eliminated working memory performance differences between reward and no-reward conditions. Electrophysiology studies in non-human primates have shown that neural firing rates sustained during the delay period of a working memory task was modulated by reward (Watanabe, 1996, Leon and Shadlen, 1999). It is thus possible that, under threat, maintenance-related processes were unable to capitalize on the beneficial effects of potential reward. This is in line with findings from our recent fMRI study (Choi et al., 2014), where we observed that reward anticipation responses in regions of lateral prefrontal cortex that are believed to be critical for working memory maintenance (Curtis and D’Esposito, 2003) were reduced when participants were simultaneously anticipating the threat of shock.
A limitation of our design is that it does not allow us to isolate the effect of threat on reward during specific phases of working memory. This is because shock could occur at any time, from prior the sample stimulus to the end of the delay period. In addition, the prospect of future reward signaled by the cue could affect multiple task phases. For instance, a previous fMRI study suggested that reward-related improvements in working memory could be related to enhanced processing at the initial encoding step (Krawczyk et al., 2007). In our study, given the possibility of shock during the initial sample stimulus period, threat might have prevented enhancement by reward. It is also possible that the impact of threat on reward was tied to the processing of the probe stimulus. Clearly, future studies are required to delineate reward-threat interactions during specific phases of a working memory task.
Somewhat unexpectedly, the effect of threat during the no-reward condition was modest (i.e., compare the first and third bars of Fig. 2A). However, the impact of emotion on cognition is relatively modest or absent at times in a laboratory setting, even when mild shock is administered (Robinson et al., 2011, Hu et al., 2012). One recent study found that stress induction prior to an N-back working memory task was associated with changes in activation in frontal cortex, but did not result in a behavioral effect (Qin et al., 2009). One potential reason behind the weak effect of threat during the no-reward condition in this study could be due to cognitive load. In a behavioral study with N-back verbal working memory task (Vytal et al., 2012), Vytal and colleagues have observed that threat of shock reduced the working memory performance during low-load conditions (1- and 2-back task blocks), but not during high-load conditions (3-back task blocks). The average accuracy during the baseline condition of the current study (i.e., no-reward and safe) was below 70%, which suggests that the task was cognitively demanding and this could have led to modest threat effect during no-reward condition. Critically, the impact of reward on cognitive performance was very robust, as required for the evaluation of a counteracting relationship between reward and threat processing.
We have recently reported interactions between reward and negative emotion during perception. In one study (Hu et al., 2013), participants performed a discrimination task on two stimulus types that were overlaid on a background color that was previously paired (CS+) or unpaired (CS−) with shock. One of the foreground stimulus types was associated with performance-based reward and the other with no-reward. In another study (Padmala and Pessoa, 2014), participants performed an orientation discrimination task on peripheral bars while ignoring centrally presented task-irrelevant neutral or negative pictures. As in the current study, each trial started with an initial reward or no-reward cue before the task phase, which informed participants about the chance of earning additional money based on performance. In both studies, we observed that reward reduced the interference effect of task-irrelevant negative pictures. The results of the current study reveal a complementary pattern of competitive interactions where we observed that negative emotion induced by threat of shock reduced the beneficial effect of reward during a cognitive task. These results are broadly consistent with the behavioral findings where threat of shock reduced the reward responsiveness in a probabilistic learning task (Bogdan and Pizzagalli, 2006).
Individual differences in self-reported reward-sensitivity and anxiety previously have been shown to be related to the effects of reward and threat on cognition, respectively (van Steenbergen et al., 2010, Choi et al., 2012). In the present study, we investigated how these self-reported measures were related to reward-by-threat interactions in behavior. We employed the following behavioral interaction score: [(reward − no-reward)safe − (reward − no-reward)threat]. Thus, higher interaction scores index stronger deleterious effects of threat on reward, whereas lower interaction scores index weaker effects of threat on reward. Here, we observed a significant linear relationship between interactions involving individual-difference scores and the interaction scores based on accuracy data (Fig. 2C). This implies that the relationship between one individual-differences score (say anxiety) and behavior depended on the level of other individual-differences score (reward sensitivity). For participants with low reward-sensitivity scores, the relationship between anxiety and behavior was positive, such that behavioral interaction scores were higher with increasing anxiety – which suggests that threat had a stronger counteracting effect on reward. For participants with high reward-sensitivity scores, the relationship between anxiety and behavior was flat – which suggests that the effect of threat on reward was weaker, supporting the notion that stronger reward sensitivity could offset the deleterious effect of threat. Overall, the current pattern of results reveals that during cognitive tasks that involve simultaneous reward and threat, individual-differences measures along both positive and negative dimensions influence behavior.
In conclusion, we investigated the effects of threat on reward during a cognitive task. We found that threat counteracted reward enhancements during working memory. We also showed that the interaction between individual-differences linked to reward and threat was related to reward-by-threat interactions in behavior. Our findings contribute to a limited but growing literature about how positive and negative information processing simultaneously influence cognition.
Acknowledgments
Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589). We would like to thank Brenton McMenamin and Mihai Sirbu for discussions and feedback on the manuscript.
References
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biological Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuro Image. 2012;59:1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Spechler P, Pessoa L. Pervasive competition between threat and reward in the brain. Social cognitive and affective neuroscience. 2014;9:737–750. doi: 10.1093/scan/nst053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in cognitive sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dawe S, Gullo MJ, Loxton NJ. Reward drive and rash impulsiveness as dimensions of impulsivity: implications for substance misuse. Addictive Behaviors. 2004;29:1389–1405. doi: 10.1016/j.addbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, Killgore WD, Rauch SL, Pizzagalli DA. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Bauer A, Padmala S, Pessoa L. Threat of bodily harm has opposing effects on cognition. Emotion. 2012;12:28–32. doi: 10.1037/a0024345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Padmala S, Pessoa L. Interactions between reward and threat during visual processing. Neuropsychologia. 2013;51:1763–1772. doi: 10.1016/j.neuropsychologia.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D’Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Research. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Boehler CN, Woldorff MG. The influence of reward associations on conflict processing in the Stroop task. Cognition. 2010;117:341–347. doi: 10.1016/j.cognition.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson ME. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Motivation versus aversive processing during perception. Emotion. 2014;14:450–454. doi: 10.1037/a0036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. The Cognitive-Emotional Brain: From Interactions to Integration. Cambridge: MIT Press; 2013. [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cognitive, Affective, & Behavioral Neuroscience. 2011;11:217–227. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savine AC, Beck SM, Edwards BG, Chiew KS, Braver TS. Enhancement of cognitive control by approach and avoidance motivational states. Cognition & Emotion. 2010;24:338–356. doi: 10.1080/02699930903381564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (form Y)(“self-evaluation questionnaire”) 1983. [Google Scholar]
- van Steenbergen H, Band GPH, Hommel B. In the Mood for Adaptation: How Affect Regulates Conflict-Driven Control. Psychological Science. 2010;21:1629–1634. doi: 10.1177/0956797610385951. [DOI] [PubMed] [Google Scholar]
- Vytal K, Cornwell B, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: from impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology. 2012;49:842–852. doi: 10.1111/j.1469-8986.2012.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Encyclopedia of statistics in behavioral science. 2005. Outlier detection. [Google Scholar]