Abstract
Hetero-oligomers of G-protein-coupled receptors have become the subject of intense investigation because their purported potential to manifest signaling and pharmacological properties that differ from the component receptors makes them highly attractive for the development of more selective pharmacological treatments. In particular, dopamine D1 and D2 receptors have been proposed to form hetero-oligomers that couple to Gαq proteins, and SKF83959 has been proposed to act as a biased agonist that selectively engages these receptor complexes to activate Gαq and thus phospholipase C. D1/D2 heteromers have been proposed as relevant to the pathophysiology and treatment of depression and schizophrenia. We used in vitro bioluminescence resonance energy transfer (BRET), ex vivo analyses of receptor localization and proximity in brain slices, and behavioral assays in mice to characterize signaling from these putative dimers/oligomers. We were unable to detect Gαq or Gα11 protein coupling to homomers or heteromers of D1 or D2 receptors using a variety of biosensors. SKF83959-induced locomotor and grooming behaviors were eliminated in D1 receptor knockout mice, verifying a key role for D1-like receptor activation. In contrast, SKF83959-induced motor responses were intact in D2 receptor and Gαq knockout mice, as well as in knock-in mice expressing a mutant Ala286-CaMKIIα, that cannot autophosphorylate to become active. Moreover, we found that in the shell of the nucleus accumbens, even in neurons in which D1 and D2 receptor promoters are both active, the receptor proteins are segregated and do not form complexes. These data are not compatible with SKF83959 signaling through Gαq or through a D1–D2 heteromer and challenge the existence of such a signaling complex in the adult animals that we used for our studies.
Keywords: dopamine, Gαq, biased agonism, hetero-oligomer, D1, D2, D5, BRET, proximity ligation assay, striatum
INTRODUCTION
Increasing evidence suggests that G protein-coupled receptors (GPCR) can form dimers and/or oligomers with properties distinct from those of the component receptors. These altered properties include allosteric modulation between protomers, altered affinity for ligands, differential coupling to downstream signaling pathways, and cross-regulation of receptor trafficking1, 2. These findings are of great interest since they suggest new routes to the development of selective ligands that could selectively target specific GPCR complexes with reduced off-target effects. However, most of the evidence supporting the formation of GPCR dimers and oligomers has come from heterologous systems, and both the existence of such signaling complexes in the native context and their biological significance remain controversial3. In particular, it is extremely difficult to dissociate downstream crosstalk from the actual physical interaction of two receptors in a signaling complex4, 5.
Heteromers putatively formed by dopamine (DA) D1 and D2 receptors have been the subject of intense research because of their unusual signaling properties and potential implication in various pathologies. Although D1 and D2 receptors are largely segregated in neurons of the striatal direct and indirect pathways, respectively6, 7, they have been reported to be colocalized in a subset of ventral striatal neurons, where they have been proposed to form a third striatal output pathway8–13.
Classically, these receptors are thought to mediate cyclic-AMP-dependent signaling through the activation of Gαs/olf or Gαi/o/z by D1 and D2 receptors, respectively14. It has also been reported that specific D1-like receptor ligands can activate alternative signal transduction systems resulting in phosphatidylinositol (PI) hydrolysis and accumulation of inositol triphosphate in the amygdala, hippocampus, striatum and frontal cortex15–18. Notably, SKF83959, a D1-like receptor partial agonist has been reported to activate D1-like receptors linked to stimulation of PI hydrolysis19–21 but to only have minimal or even antagonistic effects on the activation of adenylyl cyclase19, 20, 22 (but see23, 24). This compound has been inferred to selectively mediate phospholipase C (PLC) activation through Gαq recruitment to D1/D2 receptor heteromers25. The resulting intracellular calcium mobilization has been proposed to mediate D1/D2 heteromer-specific activation of Ca2+/calmodulin-dependent protein kinase II (CaMKIIα)10, 25, 26.
Although the biological significance of signaling mediated by the proposed D1/D2 receptor heteromer remains largely unknown, the complex has fascinated the field because of its potential involvement in pathological conditions including schizophrenia9 and depression27, as well as its unique pharmacological properties that could allow for the development of highly selective compounds. D1/D2 heteromers have been implicated in a variety of physiological processes such as BDNF expression28, neuronal growth28, modulation of fibroblast growth factor (FGF)-2 in astrocytes29, inhibition of high-voltage Ca2+ currents in striatal cultures30, facilitation of long-term depression in the hippocampus31 and spontaneous glutamate release in the cortex32.
Despite these exciting findings, there are inconsistencies in the literature that are difficult to reconcile with these proposed functions of D1/D2 heteromers and necessitate further investigation. In particular, using global knockout mice, D1-like receptor-dependent stimulation of PI hydrolysis has been shown to be independent of D1 receptors but rather to depend on D5 receptors33, 34, and a requirement for D1/D2 heteromers in SKF83959-induced calcium mobilization has recently been challenged23, 35. Here, we assessed the ability of DA receptors to recruit Gαq signaling both in vitro and in vivo. First, using bioluminescence resonance energy transfer (BRET) and complemented donor acceptor-RET (CODA-RET), we systematically investigated DA receptor activation of specific G-proteins in vitro. Next, we used behavioral pharmacologic approaches in mutant mouse models to assess the roles of functional DA receptors, Gαq, and CaMKII in the behavioral responses induced by SKF83959. Finally, we analyzed the extent of colocalization of D1 and D2 receptors ex vivo by immunohistochemistry coupled with the identification of cells co-expressing both receptors in Drd1a-tdTomato/Drd2-eGFP double BAC transgenic mice. We also measured ex vivo the level of interaction of endogenous D1 and D2 receptors using a proximity-ligation assay (PLA). Our findings challenge the existence of an atypical Gαq signaling pathway activated by D1/D2 heteromers in vitro and in vivo, as well as the existence of such complexes in vivo at endogenous receptor expression levels.
MATERIALS AND METHODS
HEK Cell Culture
The cDNAs for the human DA D1 receptor, D5 receptor, muscarinic M1 receptor, 5HT2A receptor, and Gαs short were obtained from www.cdna.org. The D1, D5, and M1 receptors were tagged at their amino termini with a signal peptide36 followed by a Myc epitope tag using standard molecular biology procedures. The cDNAs encoding full length Renilla Luciferase 8 (RLuc8) or fragments for the Luciferase 1 (L1: a.a. 1–229) or Luciferase 2 (L2: a.a. 230–311) were fused in frame to the C-terminus of D1R, D5R, M1R, or 5HT2AR in the pcDNA3.1 vector. The sensors for the human D2 receptor short isoform (D2SR) have been previously reported37 and constructs for the D2 receptor long isoform (D2LR) were made accordingly. These D2 receptor constructs were N-terminally fused to a signal peptide followed by a flag epitope.
The following human G-protein constructs were used: Gαq-mVenus with mVenus inserted at position 97, Gαi-RLuc8 with RLuc8 inserted at position 91, Gαs short-RLuc8 with RLuc8 inserted at position 67, Gαq-RLuc8 with RLuc8 inserted at position 97, Gα11-Rluc8 with RLuc8 inserted at position 92, untagged Gβ1, untagged Gγ2 and Gγ2 fused to full-length mVenus or GFP10 at its N-terminus. All the constructs were confirmed by sequencing analysis. A constant amount of plasmid cDNA (15 µg) was transfected into HEK-293T cells using polyethylenimine (PEI) (Polysciences Inc, Warrington, PA) in a 1 to 3 ratio in 10 cm dishes. Cells were maintained in culture with DMEM supplemented with 10% FBS. The transfected amount and ratio among the receptor-L1, receptor-L2, Gα, Gβ1, Gγ2 for complemented donor acceptor RET (CODA-RET) or BRET between Gα and Gγ subunits was optimized for maximal dynamic range for drug-induced BRET. Experiments were performed approximately 48 hours post-transfection.
BRET
BRET1 uses a yellow fluorescent protein variant (mVenus) as an acceptor for energy transfer from luciferase and was measured as described37. BRET2 uses a green fluorescent protein variant (GFP10) as an acceptor for energy transfer from luciferase and was performed as described38. Briefly, cells were harvested, washed and resuspended in PBS. Approximately 200,000 cells/well were distributed in 96-well plates and 5 µM coelenterazine H (substrate for luciferase in BRET1) or 5 µM deep blue c (substrate for RLuciferase8 in BRET2) was added to each well. Luciferase substrates were added to each well 5 minutes prior to detection. Typically antagonist was added 15 min before the addition of agonist. The fluorescence (excitation at 500 nm and emission at 540 nm for 1 sec recording in BRET1 or excitation at 410 nm and emission at 510 nm for 1s recording in BRET2) and luminescence (no filters, 1 sec recording) were detected 2.5 min after a ligand was added (Polarstar, Pherastar, or Pherastar FS BMG). In parallel, the BRET signal from the same batch of cells was determined by quantifying and calculating the ratio of the light emitted by mVenus (510–540 nm) over that emitted by RLuc8 or RLuc (485 nm) for BRET1 and the ratio of the light emitted by GFP10 (515 nm) over that emitted by RLuc8 (370–450 nm) for BRET2. The net BRET values were obtained by subtracting the background determined in cells expressing RLuc8 or RLuc alone. Results are expressed as the BRET change produced by the corresponding drug. As shown in the cartoons, bimolecular complementation of donor was incorporated into the BRET assay. RET took place between complemented luciferase complex and Gα-mVenus.
Measurement of Calcium Flux in Stably Expressed Cell Lines
Stable cell lines expressing D1, D2S, or D1 + D2S were generated as previously described4. Surface expression of HA-tagged D1 and FLAG-tagged D2S receptors was confirmed by fluorescence-activated cell sorting (data not shown). Calcium flux was measured using the Flipr Calcium 5 Assay kit (Molecular Devices, Sunnyvale, CA) according to the manufacturer’s protocol. Briefly, cells were resuspended in HBSS buffer containing 20 mM HEPES and 2.5 mM probenecid and distributed in 40 µl volumes in 96 well plates (500,000 cells/well). Fifty µl Flipr5 dye (Molecular devices) was added to each well and plates were incubated in 37°C 5% CO2 for 1 hour. Plates containing 10x concentrated ligand were prepared in HBSS with 20 mM HEPES. During the calcium reading, performed on a Flexstation 3 (Molecular Devices), 10 µl ligand was injected to the well at the indicated times. Intracellular calcium levels were measured every 2 sec over the course of ~110 sec. Data was analyzed by Softmax Pro 5.4 (Molecular Devices) and Prism 5.0 (Graphpad, La Jolla, CA).
Animals
C57Bl/6J mice (Jackson, 9–12 weeks old) were utilized for dose-response experiments, which examined the effects of dopaminergic antagonists on SKF83959-mediated behaviors. For behavioral experiments, D139, D240 and D5 receptor41 knockouts, Gαq knockouts and CaMKIIα-Thr286Ala knockin mice were bred at Vanderbilt University using strategies previously described for generation of mixed litters and assignment of genotypes42. All lines were fully backcrossed (>10 generations) to a C57Bl/6J background.
Twelve-week old D240 and D139 receptor knockout (KO) and their C57Bl/6J wildtype (WT) littermates were used for immunohistochemical experiments. Twelve to sixteen week old C57Bl/6J mice (Jackson) were used for viral injections (see below).
Male mice were housed under standard housing conditions on a 12 h light/dark cycle with conditions previously described42. Mice were extensively handled prior to testing and were habituated to the testing rooms for ~30 min prior to beginning of every experiment. All procedures were approved by the Institutional Animal Care and Use Committees at Vanderbilt University or Columbia University.
For visualization of D1- and D2- expressing neurons, Drd1a-tdTomato (Tg(Drd1a-tdTomato)5Calak)43 and GENSAT Drd2-eGFP (Tg(Drd2-EGFP)S118Gsat/Mmnc) mice44 were used. The lines were intercrossed to report the gene expression patterns of the D1 and D2 receptors in the same animal. Male and female breeders, each hemizygous for one of the aforementioned transgenes, were bred to attain mice that were singly hemizygous for both Drd1a-tdTomato and Drd2-eGFP. Genotypes were confirmed via PCR43 (see MMRRC web site for detailed PCR conditions). Similar results were obtained when a brighter Drd1a-tdTomato line, the Tg(Drd1a-tdTomato)6Calak)45, was crossed with the Drd2-eGFP mice. This latter double-transgenic line was used for counting of tdTomato/eGFP-positive cells.
Locomotor Behavior and Grooming
Motor responses were measured using commercial open field activity chambers (Med Associates, 29 × 29 × 20.5 cm) that were contained within light- and air-controlled environmental chambers (Med Associates, St. Albans, VT; 64 × 45 × 42 cm). Location and movement were detected by the interruption of infrared beams. A three-day protocol was employed where the mice received injections of 0.9% saline for two days and SKF83959 on the third day as previously described42. Following the SKF83959 injection, the mice were placed in the activity chambers for 60 min. Data presented are from this third session, following repeated habituation to the chambers. For bar graph displays “Pre-injection” refers to the average ambulatory distance during 5 min epochs from min 10–30 (that is, just prior to injection) and “Post-injection” to the same measure from min 40–90 (10–60 min after the injection).
During the open field testing, mice were additionally monitored by an overhead camera for analyses of grooming. Grooming was analyzed from the video recordings by assessing the number of grooming events for a 5 min period during the baseline session and 5 min from the post-injection period. The rater was blinded to genotype.
Surgeries/viral injections
Viral injections were performed as described previously46, 47. Adeno-associated viruses 1/2 expressing the D2L receptor fused to mVenus46, 47 and lentiviruses expressing an untagged rat D1 receptor under the synapsin promoter were used48. The nucleus accumbens was targeted with a single injection site bilaterally (2 sites total, 0.5 µl for AAV1/2, 2 µl for lentiviruses injected in each site): A–P, 1.7 mm; M–L, ±1.1 mm relative to bregma; and both 3.85 mm ventral to brain surface. Animals were sacrificed 1 month after surgery.
Drugs
For the cell culture assays, the following pharmacologic reagents were used: 5-HT (Sigma, St. Louis, MO), acetylcholine (Sigma, St. Louis, MO), DA (Sigma, St. Louis, MO), the 5-HT2A/C receptor antagonist ketanserin (Sigma, St. Louis, MO), the M1 muscarinic receptor antagonist pirenzepine (Sigma, St. Louis, MO), the D2-like receptor agonist quinpirole (Tocris Biosciences, Minneapolis, MN) and the D1-like receptor agonist SKF83959 (Tocris Biosciences, Minneapolis, MN).
For the behavioral studies, SKF83959 (Tocris Biosciences, Minneapolis, MN) was dissolved in 0.9% saline solution at 0.2 mg/cc (1 mg/kg) and injected intraperitoneally (i.p.). For the dose response experiments, additional doses of SKF83959 (0.05 and 0.25 mg/kg) were also used. The D2-like receptor antagonist raclopride (Sigma, St. Louis, MO) was used at 0.5 mg/kg and the D1-like receptor antagonist SCH23390 (Sigma, St. Louis, MO) was used at 0.01 and 0.25 mg/kg.
Immunohistochemistry
Brain tissue preparation, immunohistochemistry, confocal microscopy and image acquisition were performed as described previously46, 47. The following primary antibodies were used: rat anti-D1 receptor antibody (1:200–1:500, Sigma Aldrich, St. Louis, MO; cat# D2944), rabbit anti-D2 receptor antibody (1:100–1:300, see46, 47), anti-GFP antibody (1:500, Abcam, Cambridge, MA; cat# ab13970) and rabbit anti-dsRED antibody (1:300, Clontech, Mountain View, CA; cat# 632496). For double labeling of D1 and D2 in double BAC transgenic mice, the slices were first incubated with rabbit anti-D2 receptor, rat anti-D1 receptor and chicken anti-GFP antibodies followed by appropriate secondary antibodies. The tomato signal was then enhanced using rabbit anti-DsRed antibody that was directly labeled with Alexa 568 using APEX Alexa Fluor 568 Antibody Labeling Kit (Invitrogen; A10494). The following secondary goat antibodies from Invitrogen were used: anti-rabbit Alexa 568 or 405, anti-rat Alexa 647, anti-chicken 488, all at a concentration of 1/1000.
Proximity-Ligation Assay (PLA)
PLA was performed using the Duolink in situ kit (Olink Bioscience) according to the manufacturer’s instructions with the following modifications: Incubation with PLA probes was for 2 hours at 37°C; ligation step was performed for 45 min at 37°C; amplification step was extended to 2 hours at 37°C with a concentration of polymerase of 1/60. Anti-rat PLA probes were generated according to the manufacturer’s instructions using the Duolink Probemaker (Olink Bioscience) and goat-anti rat IgGs (Santa Cruz).
Image Processing and Quantification of PLA signal
To process and quantify the PLA signal we used a method described in detail in Biezonski et al. (under review). All images were loaded into MATLAB (The MathWorks, Inc.) and converted from 12-bit into double precision intensity values ranging from 0 to 1. The resolution of all images was 210 nm/pixel. To develop a database for representative PLA signal for our images, individual signal was first selected from three positive control images (D1D2 overexpression). Approximately 70 such areas that ranged in size from 5 pixels to 45 pixels were chosen from each of three images. Similarly, a number of background regions were chosen from negative control images (D1 and D2 KO). To achieve the greatest separation between the DuoLink signal and the background, we standard deviation filtered all the images and analyzed the standard deviation values within our pre-selected regions. The DuoLink signal and background were conservatively separable at 0.08 units of standard deviation. This threshold captured 95% of all identified signal pixels and only 40% of identified background pixels. Of these background pixels derived from the negative control image, 85% belonged to connected objects that were below 5 pixels in size. Thus, we applied a size threshold of 5 pixels for all DuoLink signal and this way excluded approximately 50% of the background pixels. In parallel, to treat the confounding areas of high fluorescence intensity within nuclei, we entropy filtered all of the intensity images with a filter size of our minimum particle size (5 pixels). Within the entropy image, nuclei contained uniformly high values while smaller signals receded into the background. This allowed us to exclude any fluorescence originating within nuclei from the ultimate analysis of the DuoLink signal. All standard deviation filtered images were then thresholded at 0.08 units of standard deviation and the surviving pixels formed masks. Any mask was excluded if its size was below 5 pixels. The masks created from these thresholded images represented the final DuoLink signal.
Data Analysis and Statistics
Data and statistical analysis were performed with Prism 5.01 (GraphPad Software, San Diego, CA) or with SPSS version 22 (IBM, Armonk, NY). For BRET studies, dose response curves of agonist response and non-responsive counterpart (i.e. antagonist treatment or non-responsive G protein coupling) were examined with an F-test analysis. Behavioral data were subjected to one- or-two way analysis of variance (ANOVA) with genotype as a between-group factor. Post-hoc Tukey’s multiple comparison tests or Bonferroni comparisons were used to compare groups to each other. PLA data were analyzed using unpaired t-tests. Graphs are marked with asterisks (*p<0.05, **p<0.01, ***p<0.001) to denote statistical significance.
RESULTS
D1 and D2 receptors expressed individually activate their cognate G proteins but do not activate Gαq
Activation of G-proteins by specific DA receptor populations was systematically assessed using the BRET biosensor technique (Figure 1a). This approach allows measurement of G-protein activation based on a conformational change between the α and γ subunits (Figure 1a). The conformational changes assessed by the sensors have been previously demonstrated to accurately reflect activation of the G proteins38, 49.
Figure 1.
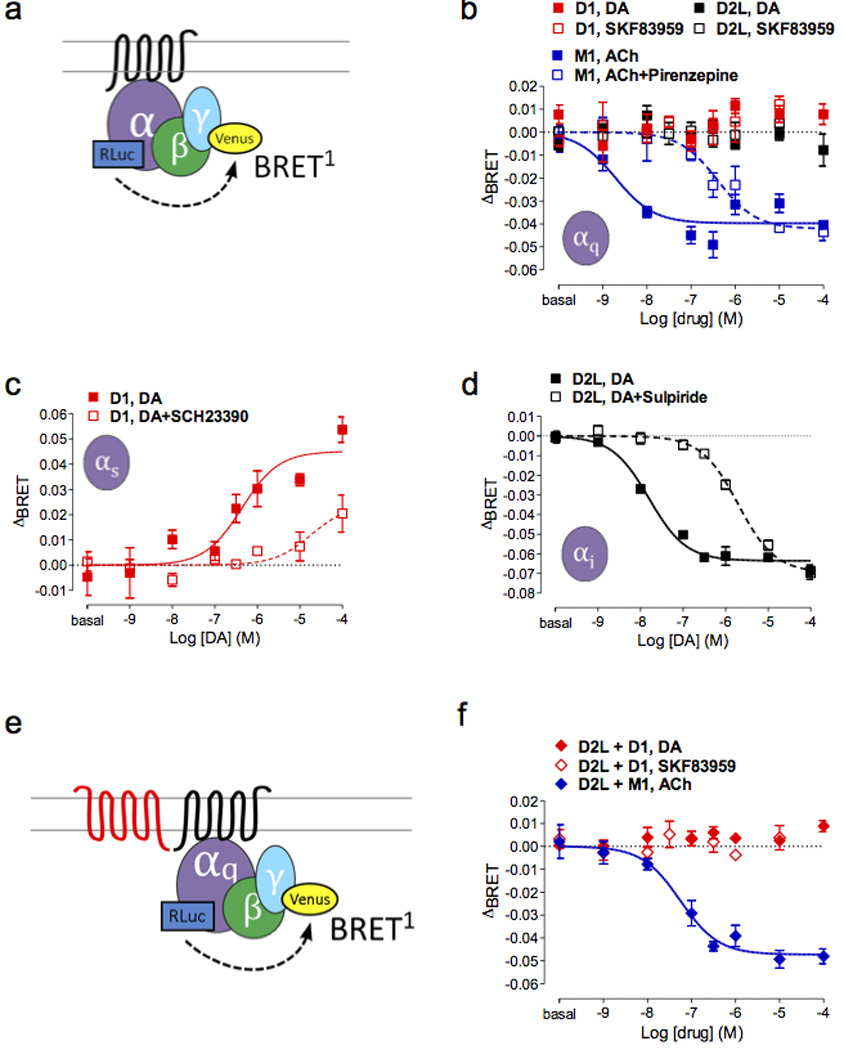
Drug-induced conformational change of Gαq is not detected in D1 and/or D2L-expressing cells. (a) Drug-induced BRET change shown as ΔBRET (= BRET ratio with drug applied - basal BRET ratio) is detected between Gα and Gγ subunits. Note that due to the different positions of the donor sensors within the Gα subunits, activation of the heterotrimer is manifested as a decrease in BRET between the Gα donor and the Gγ acceptor for the Gαq and Gαι1 sensors but as an increase in BRET for the Gαs sensor. (b) M1R is shown as a positive control for Gαq activation (blue solid = ACh, blue open = ACh + pirenzepine, p < 0.001) whereas Gαq activation was not observed for either DA or SKF83959 in the D1R (red) or D2R (black). Conformational change of cognate G proteins by DA was induced and blocked by an agonist and an antagonist for (c) D1R (red, p < 0.001), (d) D2LR (black, p < 0.001). (e) Diagram showing the BRET configuration in (f). (f) Co-expression of D1 and D2L did not lead to Gαq activation after drug stimulation (red solid = DA, red open = SKF83959) whereas robust Gαq coupling to M1R was still seen after acetylcholine addition in the presence of D2LR (blue, p < 0.001).
DA receptor activation of Gαq was assessed with either DA or the agonist SKF83959. We failed to detect any drug-induced BRET changes in the Gαq biosensor in response to DA or SKF83959 stimulation of D1 or D2long (D2L) (Figure 1b) or from D5 or D2short (D2S) receptors (Supplementary Figure 1b). To demonstrate the functionality of the Gαq biosensor50, the M1 muscarinic receptor, a Gαq-coupled receptor, was expressed and stimulated by acetylcholine (Figure 1b), which resulted in a robust concentration-dependent activation of BRET. As expected, acetylcholine-induced activation was inhibited by the selective antagonist pirenzepine (Figure 1b).
In contrast, robust cognate G-protein activation (i.e. D1-Gαs, D5-Gαs, D2S-Gαi1 and D2L-Gαi1) was detected by a change in BRET between the α and γ subunits after stimulation with DA (Figure 1c and d for D1-Gαs and D2L-Gαi1, Supplementary Figure 1c and d for D5-Gαs and D2S-Gαii1). These signals were inhibited, as expected, by the appropriate antagonists (Figure 1c and d, Supplementary Figure 1c and d).
D1 and D2 receptors expressed together fail to activate Gαq
Both D1/D2 receptor25 and D5/D2 receptor51 heteromers have been reported to couple to Gαq activation, and D1 and D2 receptors when coexpressed led to mobilization of calcium in response to DA (but not SKF83959)35. Therefore, Gαq activation was tested after co-expression of the unfused DA receptors with the Gαq biosensor proteins (Figure 1e and Supplementary Figure 1e). Neither stimulation by DA nor SKF83959 led to Gαq activation in cells co-expressing non-fused receptors – D1+D2L receptors (Figure 1f), D5+D2L (Supplementary Figure 1f), D1+D2S receptors (Supplementary Table 1), or D5+D2S receptors (Supplementary Figure 1g). As a positive control, the M1 receptor was co-expressed with DA receptors. Drug-induced BRET mediated by the M1 receptor was not altered in the presence of D2L or D2S, indicating that the co-expression did not impair the function of the Gαq biosensor (Figure 1f and Supplementary Figure 1f and g, blue plot).
When two receptors are co-expressed, it is not possible to differentiate signaling between individual protomers, homomers, or heteromers. Therefore we used the CODA-RET configuration5 (Figure 2a), to measure Gαq-coupling specifically from the defined heterodimer. In this method, split luciferase is fused to the C terminus of two receptors, and luminescence only results from complementation of the luciferase due to association of the receptors bearing the halves. Thus, BRET between receptor and an acceptor-tagged Gα is indicative of signaling of the defined dimer through a defined G protein5. Homodimer complementation of the prototypical Gαq-coupled M1 receptor (Figure 2b) or 5HT2A receptor (Figure 2c) showed efficient agonist-stimulated Gαq-coupling and antagonist blockade. This pair of positive controls showed that complemented luciferase does not interfere with Gαq-coupling, at least in the case of these homodimers. In contrast, DA stimulation alone or SKF83959 and quinpirole co-stimulation failed to induce Gαq-coupling of complemented D1-D2S receptors (Figure 2d), D2S-D5 receptors (Figure 2e), D1-D2L receptors (Figure 2f), or D2L-D5 receptors (Figure 2g). Despite its better sensitivity, the BRET2 configuration also failed to detect drug-induced recruitment of Gαq to either individual (Supplementary Table 1) or complemented DA receptors in any combination (Supplementary Table 1). We showed previously using CODA-RET that D1 and D2 receptors can interact in HEK cells and that signaling of the D1/D2 heteromer to Gαi is differentially altered for the agonist NPA relative to DA5. Thus, although these receptors can interact and modulate one another in HEK cells, our new data demonstrate that in these cells D1/D2 receptor heteromers are unable to recruit Gαq in response to DA, SKF83959, or combined quinpirole and SKF83959.
Figure 2.
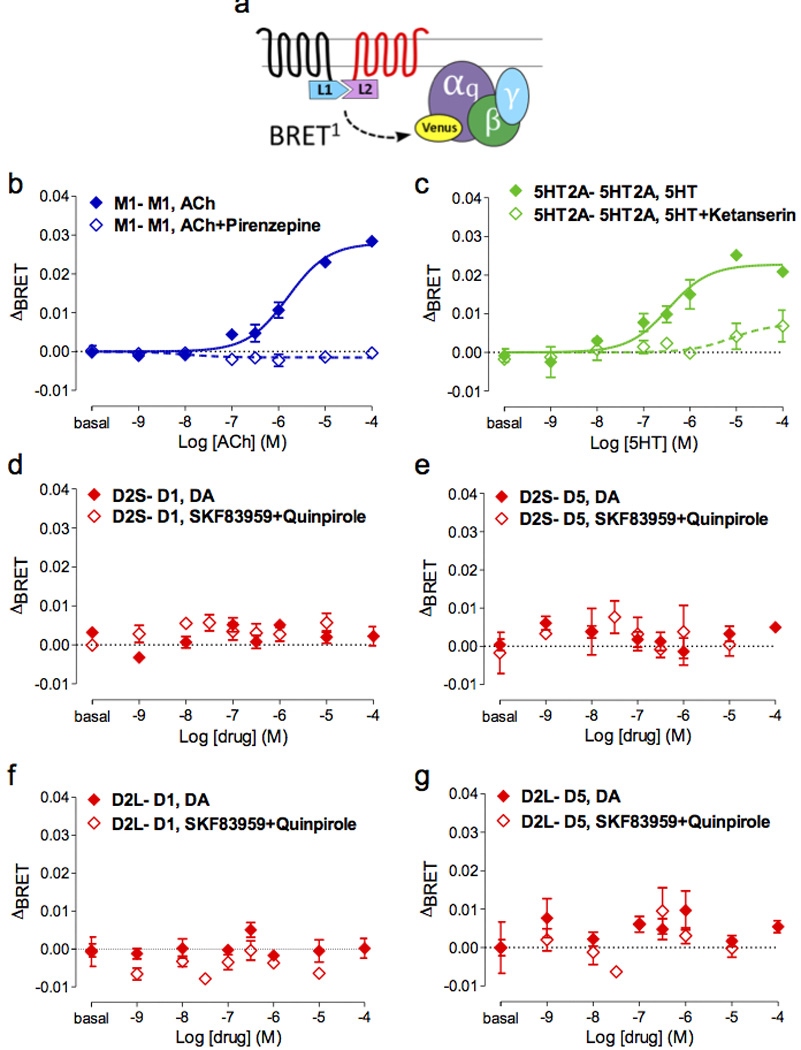
Defined receptor dimer pairs do not recruit Gαq after drug stimulation. Using the CODA-RET approach, a receptor dimer pair is defined by complementation of the luminescent protein Rluc8. (a) Diagram showing the BRET configuration. (b, c) The complemented M1RL1-M1RL2 and 5HT2ARL1-5HT2ARL2 pairs are shown as positive controls for Gαq coupling (solid = agonist, open = agonist + antagonist, p < 0.001). In contrast, the complemented DA receptor dimer pairs shown (d) D2SR-D1R, (e) D2SR-D5R, (f) D2LR-D1R, (g) D2LR-D5R] failed to reveal drug-induced Gαq recruitment (red solid = DA, red open = SKF83959 + quinpirole).
D1 and D2 receptors expressed individually or together also fail to activate Gα11
Gα11 shares significant structural and functional homology with Gαq; they both couple to PLC and are expressed in the striatum. Therefore, it is possible that effects ascribed to Gαq might instead result from Gα11. We demonstrated the lack of recruitment of Gα11, in response to activation of D1, D2S, D2L, or D5 receptors (Supplementary Figure 2c and d). Gα11 activation was also tested after co-expression of the unfused DA receptors with the Gα11 biosensor proteins, and as was the case with Gαq, neither stimulation by DA nor SKF83959 led to Gα11 activation in cells co-expressing non-fused receptors: D1+D2L receptors (Supplementary Figure 2f), D5+D2L (Supplementary Figure 2g), D1+D2S receptors Supplementary Figure 2f), or D5+D2S receptors (Supplementary Figure 2g). Once again, positive control experiments using M1 receptor activation verified the integrity of our Gα11 activation assay (Supplementary Figure 2b).
D1 and D2 receptors fail to induce intracellular calcium responses
At the functional level, D1/D2 heteromer-dependent activation of Gαq/11 has been proposed to lead to the recruitment of calcium signaling28, 52. We therefore tested whether, despite the absence of Gαq or Gα11 recruitment, co-expression of D1 and D2 receptors leads to calcium mobilization (Figure 3). Neither D1 (Figure 3a) nor D2S activation (Figure 3b) in the respective stably transfected cells caused calcium mobilization. We next created dual D1/D2S stable cells but still did not observe achange in intracellular calcium in response to D1 and D2 co-stimulation with either DA or SKF83959 (Figure 3c). In contrast, acetylcholine potently stimulated calcium via endogenously expressed muscarinic M3 receptors (Figure 3a, b, c).
Figure 3.
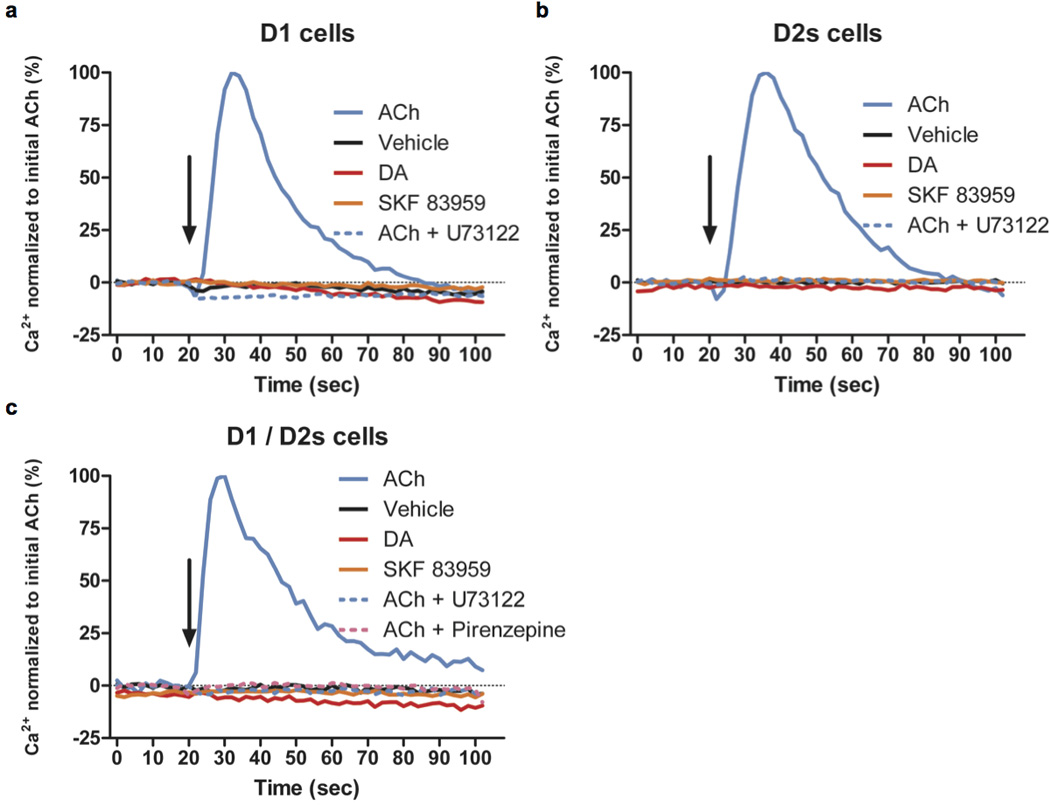
Neither DA nor SKF 83959 induces calcium mobilization in stable cell lines expressing D1 and/or D2 receptors. Using the FLIPR5 calcium assay system, intracellular calcium levels were measured every 2 seconds and plotted against time. Ligands (ACh = 10 µM acetylcholine, vehicle, DA = 10 µM dopamine, 10 µM SKF83959, 10 µM U73122, or 10 µM pirenzepine) were added at 20 secs, indicated by an arrow, in (a) D1R, (b) D2R, and (c) D1R/D2R stable cells. Calcium level is shown in percentage normalized to 10 µM acetylcholine (ACh). Traces are representatives of n = 3 experiments.
The behavioral effects of SKF83959 in vivo depend on activation of D1 receptors but not of D2, Gαq or CaMKII phosphorylation on Thr286
In order to extend our in vitro findings to the in vivo setting, we evaluated SKF83959-induced behavioral effects in mutant mice disrupted for the putative signaling mediators, D1, D5, D2, Gαq or CAMKII. In WT mice, a peripheral injection of SKF83959 (0.05–1 mg/kg) produced a dose-dependent increase in locomotor activity (Supplementary Figure 3a and b; F(3,20)=39.9, p<0.001) and a specific motor stereotypy involving orofacial grooming (Supplementary Figure 3c; F(3,20)=39.9, p<0.05), as previously described53, 54. The grooming response was already maximal at 0.05 mg/kg (Supplementary Figure 3c; F(3,23)=12.8, p<0.001).
That pharmacological blockade of D1 or D2 receptors blunted the SKF83959-induced behaviors (data not shown) does not establish that the drug necessarily acts directly on these receptors. For example, D2 antagonism is well known to reduce locomotor activity, and it may prevent SKF83959-induced effects indirectly. In order to assess more directly which DA receptors are necessary for mediating SKF83959-induced signaling and behavior, we investigated the SKF83959-induced grooming and locomotor activity in a panel of DA receptor knockout mice. SKF83959 elicited a significant orofacial grooming response (Figure 4a; F(1,69)=23.6, p<0.001) in WT mice but not in D1 receptor knockout mice (post-hoc Bonferroni multiple comparison test; p<0.001). We also assessed SKF83959-induced locomotion (Figure 4b; F(1,34)=135.0, p<0.001). D1 receptor knockout mice were initially hyperactive compared to their WT littermates during the pre-injection baseline session (Figure 4b and Supplementary Figure 4). During the post-injection period, WT mice increased their locomotor activity in response to SKF83959 (Figure 4b, post-hoc Bonferroni multiple comparison test, p<0.001). The D1 null mice, in contrast, did not respond to SKF83959 but continued to habituate to the chambers (Figure 4b and Supplementary Figure 4), resulting in a significant decrease in locomotor activity, as compared to pre-injection baseline (post-hoc Bonferroni multiple comparison test, p<0.001).
Figure 4.
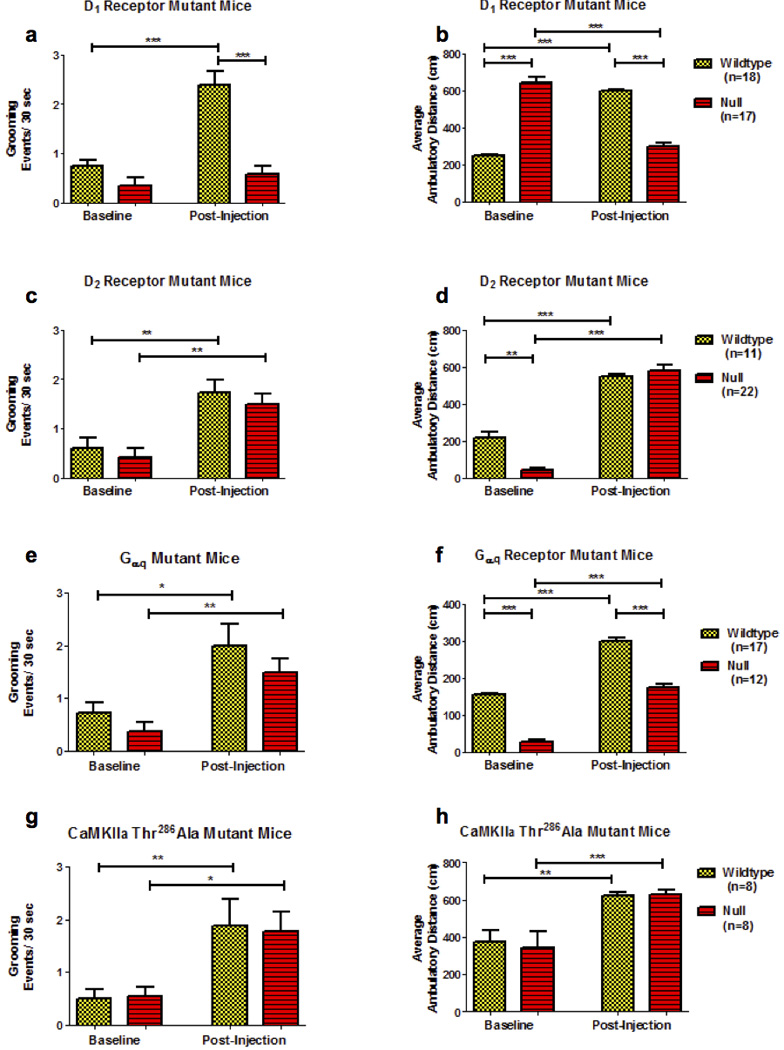
SKF83959-induced orofacial grooming (a, c, e, g) and locomotor activation (b, d, f, h) is absent in mice lacking the D1 receptor (a, b) but present in D2 receptor knockouts (c, d), Gαq null (e, f) and CaMKII (g, h) mutant mice. Asterisks denote significance (*p<0.05, **p<0.01, ***p<0.001) based on post-hoc Bonferroni multiple comparison tests following repeated measures ANOVA. The data contained in panel f from the Gαq null mice are a modified presentation of those published in42. Yellow bars = WT, Red bars = Mutant.
We next examined the effects of SKF83959 in mice lacking D2 receptors and observed significant increases in grooming in both WT and D2 null mice (Figure 4c; F(1,33)=8.32, p<0.001; post-hoc Bonferroni multiple comparison tests, p<0.01 for each). D2 receptor null mice expressed sharply reduced locomotor activity during the pre-injection period (Figure 4d, post-hoc Bonferroni multiple comparison test, p<0.01), but both the WT controls and D2 receptor −/− mice responded significantly to SKF83959 (Figure 4d; F(1,33)=72.3, p<0.001; post-hoc Bonferroni multiple comparison tests, p<0.001). Given the reduced locomotion in the D2 receptor null mice, these animals expressed an even more robust response to SKF83959 when considering the percent change from pre-injection baseline (data not shown).
We next tested a potential role for D5 receptors in the effects of SKF83959. SKF83959 significantly increased grooming (F(3,40)=12.3, p<0.001) and locomotor activity (F(1,40)=108.6, p<0.001) in D5 receptor knockout mice, although the locomotor responses were slightly reduced compared to WT (Supplementary Figure 5, post-hoc Bonferroni multiple comparison test, p<0.05).
In addition to evaluating the contribution of the DA receptors to SKF83959-mediated actions, we also assessed the contribution of the G-protein Gαq (Figure 4e, f). Similar to the D2 receptor knockouts, Gαq knockout mice were hypoactive at pre-injection baseline, but still responded robustly to SKF83959. SKF83959 significantly increased grooming in WT and Gαq knockout mice (Figure 3e, F(1,28)=5.90, p<0.01; post-hoc Bonferroni multiple comparison tests, p<0.05). Similar results were observed for locomotor activity (Figure 4f, F(1,28)=4.99, p<0.01; post-hoc Bonferroni multiple comparison tests, p<0.001).
Next we assessed the role of CaMKIIα in mediating the behavioral responses to SKF83959, as phosphorylation of CaMKIIα on Thr286 has been suggested to be a critical downstream component in the signaling of SKF8395925, 26, 55. To address this question we evaluated CaMKIIα – Thr286Ala knockin mice, in which CaMKII is incapable of phosphorylation at the Thr286 residue56, 57. Both SKF83959-induced grooming (Figure 4g, F(3,15)=4.99, p<0.01; post-hoc Bonferroni multiple comparison tests, p<0.05) and locomotor activity (Figure 4h, F(3,15)=12.2, p<0.01; post-hoc Bonferroni multiple comparison tests, p<0.001) were fully intact in CaMKIIα – Thr286Ala knockin mice.
We additionally normalized the SKF83959-induced grooming data to the pre-injection baseline observed for each mutant genotype to provide more direct comparisons within each line. As can be seen in Supplementary Figure 6, D1 receptor knockout mice expressed significantly reduced SKF83959-induced grooming as compared to controls (p=.0126 by unpaired t-test). However, no other mutant mice (D2 receptor null, Gαq null, CaMKIIα – Thr286Ala knockin, D5 receptor null) differed from WT littermate controls.
D1 and D2 receptors do not form complexes in the striatum
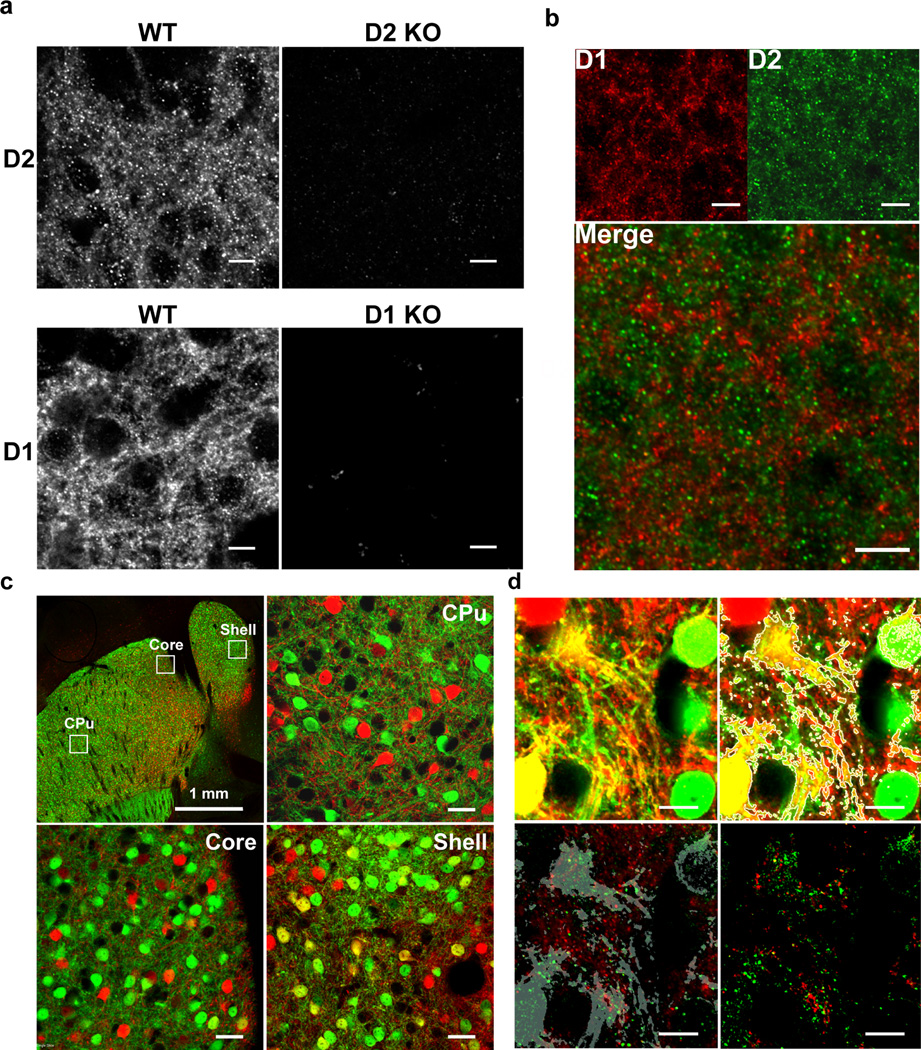
Even if the behavioral effects of SKF83959 are not mediated by D1/D2 receptor dimers, it is conceivable that such a complex nonetheless mediates differential signaling and represents a novel target for therapeutics. In fact, despite our inability to detect the activation of Gαq by D1/D2 receptors in HEK293 cells, previously using CODA-RET we did obtain support for functional selectivity in the Gαi pathway of a defined D1/D2 receptor heteromer5. This piqued our interest in this potential heteromer as a drug target and motivated us to pursue studies in ventral striatal brain slices where the receptors have been reported to be co-expressed. Coexpression of D1 and D2 receptors in the striatum and nucleus accumbens has been a contentious topic6. In situ hybridization and electron microscopy studies have generally supported the segregation of these receptors in distinct subpopulations of neurons7, 58, 59. However, modest colocalization as well as fluorescence resonance energy transfer (FRET) between D1 and D2 receptors have been observed in the shell of the nucleus accumbens using antibodies9, 10. Indirect measures using BAC transgenic GFP mice support co-expression of D1 and D2 receptor genes in a sub-population of neurons in the shell of the nucleus accumbens13, 60. In order to clarify whether D1 and D2 receptors colocalize in the shell of the nucleus accumbens, we took advantage of an anti-D2 receptor antibody that we previously generated and that shows high selectivity as confirmed by the lack of staining in D2 receptor-KO mice46, 47 (Figure 5a). We detected very limited colocalization of D1 and D2 receptors in the shell of the nucleus accumbens in WT mice (Figure 5b) or rats (Supplementary Figure 7), or in the ventral striatum of rhesus monkeys (Supplementary Figure 7). Because D1 and D2 receptor subcellular localization is mostly in the neuropil11, 46, 47, 59, 61, it is virtually impossible to visualize cell bodies that express – or not – specific dopamine receptors. In order to better identify cells that co-express both receptors we crossed two transgenic reporter lines, Drd1a-tdTomato45 and Drd2-eGFP44. As previously reported based on comparing expression in the individual reporter lines60, in the crossed lines we observed co-expression of both genes in a small fraction of cells restricted to the shell of the nucleus accumbens, but in only a handful of neurons within the NAc core or the dorsal striatum (Figure 5c).
Figure 5.
D1 and D2 receptors are co-expressed but do not colocalize in the shell of the nucleus accumbens. (a) Immunohistochemical detection of D2 (top) and D1 (bottom) in the shell of the nucleus accumbens in WT mice. Staining was virtually absent in D2 or D1 KO mice. (b) Co-staining of D1 and D2 receptors in the shell of the nucleus accumbens in mice revealed extremely limited colocalization. (c) Top-left: Low magnification confocal image of a horizontal section going through the striatum and nucleus accumbens in a double BAC Drd1a-tdTomato/Drd2-eGFP transgenic mouse. The Ds-Red (red) and eGFP (green) signals were enhanced using specific antibodies. Cells co-expressing active Drd1 and Drd2 promoters (yellow) were detectable in the shell (bottom-right) but not in the core (bottom-left) of the nucleus accumbens or in the dorsal striatum (CPu: top-right). (d) Zoom on tomato+ (D1) and eGFP+ (D2) dendritic areas in the shell of the nucleus accumbens after quadruple staining of DsRed, eGFP, D1 and D2. Top-left: tomato and eGFP signals; Top-right: yellow areas were detected using ImageJ software and identified with a mask (grey outline); Bottom-left: D1 (green) and D2 (red) receptors signals merged with the mask (grey). Bottom-right: D1 (red) and D2 (green) signals extracted within the mask area only showed no colocalization of D1 and D2 receptors. Scale bars=10 µm unless otherwise indicated.
We performed cell counts of D1-tomato+ and D2-eGFP+ cells at multiple levels throughout the rostral to caudal extent of the NAc and dorsal striatum (Supplementary Figure 8). The vast majority of cells in all regions were positive for only D1-tomato or D2-eGFP, but colocalized reporter proteins were observed in 5–7% of cells within the NAc shell (Supplementary Figure 8a). Within the NAc core, only 2–3% of reporter-expressing cells expressed both D1-tomato and D2-eGFP (Supplementary Figure 8b). The vast majority of the dorsal striatum (Supplementary Figure 8c) resembled the NAc core with only ~2% colocalization; however in the caudal tail of the striatum, colocalization increased to ~7%. We next analyzed D1 and D2 receptor expression with high resolution, selectively in those neurons that co-expressed Tdtomato and eGFP in the shell of the nucleus accumbens. Staining of D1 and D2 receptor signals in those neurons revealed complete segregation of the receptors even on dendritic segments that co-express both reporters (Figure 5d). These data suggest that even in the few neurons in which both D1 and D2 receptor promoters are active, there is little if any colocalization of the receptor proteins.
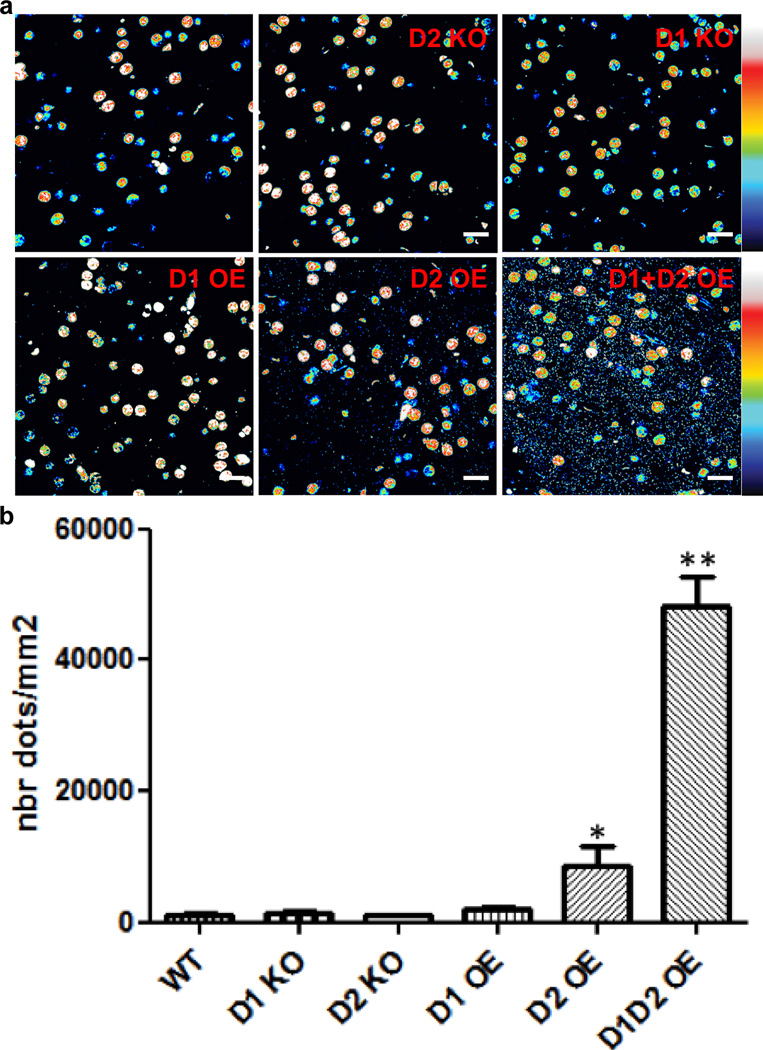
We confirmed the lack of interaction of D1 and D2 receptors using a proximity-ligation assay (PLA) that allows for the detection of receptor complexes ex vivo47. PLA signals for this heteromer were virtually absent in the nucleus accumbens of WT, D1 receptor KO and D2 receptor KO mice (Figure 6). Overexpression of either D1 or D2 receptors by viral gene transfer led to a small increase in PLA signal, whereas overexpression of both receptors resulted in a very large increase in PLA signal (Figure 6). These data suggest that even in the small fraction of neurons that co-express D1 and D2 receptors, the receptors are segregated and do not physically interact. In contrast, when both receptors are dramatically overexpressed - either in HEK cells or in vivo - the receptors have the ability to interact.
Figure 6.
Proximity ligation assay (PLA) shows that endogenous D1 and D2 receptors do not physically interact in the shell of the nucleus accumbens. (a) Whereas the non-specific nuclear background did not vary between conditions, the PLA signal (small dots) indicative of molecular proximity between D1 and D2 receptors was virtually absent in WT mice, as well as in D1 and D2 KO mice. Virally-mediated overexpression of D1 (D1OE) did not significantly increase PLA signal, whereas D2 (D2OE) receptor overexpression led to a small, but significant increase in PLA signal. Simultaneous overexpression of both receptors (D1D2OE) led to a very strong PLA signal, suggesting that the 2 receptors have the ability to interact if highly expressed in close proximity. (b) Quantification of the PLA signals was performed as described in Methods. N=4–8 (3–4 animals). * p<0.05; ** p<0.01. Scale bars=10 µm.
DISCUSSION
We were unable to detect Gαq/11-coupling to monomers or heteromers of DA receptors using multiple sensitive biosensors expressed in HEK cells. SKF83959-induced locomotor and grooming behaviors were eliminated or reduced in D1 receptor knockout mice but were intact in D2 receptor and Gαq knockouts as well as in non-autophosphorylatable Ala286-CaMKIIα knockin mice. These data are thus incongruent with a dependence of SKF83959-induced behavior on D1/D2 heteromers or Gαq/11 signaling. Moreover, we found that D1 and D2 receptors are segregated at the subcellular level even in medium spiny neurons in the shell of the nucleus accumbens that co-express both receptors, suggesting that these receptors do not form heteromers in vivo.
In our BRET studies, we considered the possibility that the fused complemented luciferase might interfere with Gαq/11 coupling to the heteromer. However, Gαs, Gαi and Gαo coupling to the complemented receptors was as robust and consistent as with the individual protomers that were not complemented5, and Gαq/11 were robustly recruited to complemented receptors known to couple to these G proteins. Nonetheless, we also pursued studies with Gαq/11 biosensors with DA receptors with unmodified cytoplasmic tails to rule out any potential disruption of Gαq/11 recruitment due to the RLuc8 fusions. Although we observed robust activation of the Gαs, Gαi, and Gαo sensors, we again failed to observe evidence for Gαq or Gα11 activation by D1 and D2 receptors, individually or together, despite the fact that the Gαq and Gα11 sensors worked as expected for the M1 or 5HT2A receptors, both of which are known to couple to Gαq. We conclude that DA receptors in any combination are unable to activate Gαq/11 in HEK293 cells.
It is important to note that most of the published findings that led to the inference of Gαq as an intermediary mechanism for calcium mobilization did not study Gαq directly, but instead relied on calcium measurements or IP3 production. While both of these are consistent with PLC activation, they do not necessarily require Gαq/11 activation. Reports have shown GTPγS binding to Gαq upon activation of DA receptors but these methods depend on selective immunoprecipitation of endogenous Gαq by antibodies to differentiate the signal from that from other Gα isoforms20, 25, 62, 63. Using much more robust biosensors, we have failed to find evidence that D1 or D1/D2 receptors can activate Gαq/11. Consistent with our results, Mailman and colleague also failed to detect IP3 release by D1, D2 or coexpressed receptors23, 24. Chun et al.35 have recently shown in HEK cells that activation of coexpressed D1 and D2 receptors by dopamine leads to calcium mobilization, but this was blocked by a scavenger of Gβγ as well as by pertussis toxin or cholera toxin, suggesting that the calcium signal results from downstream crosstalk and/or Gβγ-enhanced PLC signaling and not from direct activation of Gαq by DA receptors. We failed to observe calcium mobilization in response to 10 µM DA or SKF83959 in cells expressing D1, D2 or D1/D2 receptors (Fig. 3). We did observe a small calcium signal in response to both DA and SKF81297 at 100 µM (data not shown) in cells expressing D1 as well as both D1/D2 receptors. This suggests a minimal ability to engage PLC at extremely high concentrations of agonist, but this is clearly not heteromer-mediated since it occurs in cells expressing only D1, and is also not mediated by Gαq/11, as demonstrated above.
SKF83959-induced behavioral responses were absent in D1 receptor knockout mice, confirming the role of the DA D1 receptor in mediating the motor effects of SKF83959. Additionally, we were able to block the effects of SKF83959 with the D1-like antagonist SCH23390 (data not shown). Since SKF83959 also has affinity for D5 receptors35, we also assessed the SKF83959-induced behaviors in D5 receptor null mice. SKF83959-induced locomotor activity and grooming were largely intact in D5 receptor knockouts, further confirming the role of the D1 receptor as the primary D1-like receptor target for the motor-activating effect of SKF83959. A small but statistically significant reduction of locomotion but not grooming responses in D5 receptor knockouts, however, suggests a possible accessory role for the D5 receptor in the actions of SKF83959.
Interestingly, we observed pronounced effects of SKF83959 in both D2 receptor and Gαq knockout mice, demonstrating that these proteins are not necessary for SKF83959-induced behavioral responses. These results, together with our signaling studies, contradict the current hypothesis in the literature regarding the signaling mechanism of SKF8395910, 25. In this model, SKF83959 acts through a D1/D2 receptor oligomer coupled to Gαq and a downstream signaling system involving PI hydrolysis and intracellular calcium release. If SKF83959 signaled through such a mechanism, then we would have expected to observe a loss of SKF83959-induced locomotor and grooming responses in the D2 receptor and Gαq null mice, as we did with the D1 receptor knockout mice. Contrary to this hypothesis, however, D2 receptor and Gαq knockout mice appear to be more sensitive to SKF83959; both exhibiting greater percentage change from baseline in the locomotor assay compared to WT mice (data not shown). Previous findings have demonstrated that SKF83959-induced behaviors can be blocked by the D2-like receptor antagonist raclopride25, 26, a finding we have replicated (data not shown). However, these results are confounded by the fact that the doses required to block SKF83959-induced behaviors induces significant catalepsy9, 64, suggesting, in light of our results with the D2 receptor knockout mice, that the inhibition of locomotion by raclopride is not mediated at a D1/D2 receptor heteromer but rather is indirect through blockade of D2 receptor signaling.
Previous reports have also suggested that Thr286 phosphorylation of CaMKIIα is a critical downstream component in the signaling of SKF8395925, 26, 55. However, we observed intact SKF83959-induced locomotor and grooming responses in CaMKIIα–Thr286Ala knockin mice that lack the major regulatory autophosphorylation site, suggesting again that CaMKIIα signaling is not an essential component of SKF83959-induced signaling.
Our data also directly challenge the idea that D1 and D2 receptors form oligomers in medium spiny neurons (MSNs) in adult animals. Co-expression of D1 and D2 receptors in MSNs has been a matter of long debate6. Whereas in situ hybridization7 supports an almost complete separation of D1- and D2 receptor-expressing MSNs, single-cell PCR65 and immunohistochemical8–12 methods showed a larger degree of colocalization. The use of BAC transgenic reporter mice confirmed that the segregation of the two receptors is not complete6, 66, particularly in the shell of the nucleus accumbens60. Our data using the Drd1a-tdTomato/Drd2-eGFP double BAC transgenic mice directly confirm that a small number of neurons do express both D1 and D2 receptors, especially within the shell of the nucleus accumbens and the caudal tail of the neostriatum.
However, using traditional immunohistochemistry or PLA, we were unable to detect colocalization or molecular proximity of D1 and D2 receptors in the adult ventral striatum, in contrast to previous reports8–10, 28. This discrepancy is not readily explained by differences in anti-D2 receptor primary antibodies or immunostaining protocols used, since we also failed to detect colocalization using antibodies and conditions identical to those published (data not shown). The lack of PLA signal is unlikely to be related to a lack of sensitivity of the assay, since we have succeeded in detecting endogenous receptor complexes in the striatum, including D2/A2A receptors47 or D1/NR1 receptors67 (see also Biezonski et al., in revision). Moreover, we failed to detect colocalization of D1 and D2 receptors by immunohistochemistry in 3 different mammalian species (i.e. mouse, rat and monkey). It is important to note that when colocalization and interaction between D1 and D2 receptors was described previously, it was most evident within cell bodies8–10, 28. However, DA receptors have generally been shown to be mainly expressed in the neuropil11, 46, 47, 59, 61, even when overexpressed46, 47. Moreover, we were able to show that, even when one or the other receptor was overexpressed, the level of colocalization/molecular proximity of D1 and D2 receptors was extremely low, suggesting that an active mechanism may keep the receptors segregated at the subcellular level. Importantly, our data are in accordance with the observation using electron microscopy that D1 and D2 receptors do not colocalize at the subcellular level even when expressed in the same cellular compartments59. The mechanisms that support segregation of those two receptors are unknown but could rely on distinct properties of the individual receptors, such as interaction with specific scaffolding proteins or localization in different membrane microdomains. Nonetheless, our data in HEK cells (5; current study) as well as ex vivo with overexpression of both D1 and D2 suggest that the receptors have the ability to interact under conditions in which the segregating mechanism is either not present or is overwhelmed by receptor excess.
Despite the fact that our data do not support the existence of D1/D2 receptor heteromers in adult brain, the presence of cells within the shell of the nucleus accumbens that co-express both receptors does suggest the existence of a third neuronal circuit within the basal ganglia, distinct from the classical direct and indirect pathways of the striatum, that could exhibit atypical signaling and physiological properties10. It is conceivable that the extent of D1 and D2 receptor coexpression and/or heteromerization in these cells varies during different developmental periods (although heteromerization of these receptors was reported to be less, not more, in juvenile brain68), or even in pathological states that might alter the segregation process that normally keeps the receptors apart. Regardless, a systematic characterization of the anatomical, physiological and molecular properties of the small subset of neurons that coexpress D1 and D2 receptors will be necessary to understand the role of these cells in basal ganglia physiology and pathophysiology6, 60.
Our current data call into question the interpretations of several previous studies suggesting the presence of a D1/D2 heteromer-containing neurons as a target for drug discovery (for review, see10, 69). In fact, dysregulation of D1/D2 receptor heteromers has been specifically postulated in both schizophrenia9 and depression27. The main findings relevant to schizophrenia have been increases in the high affinity state of D2 receptors that can be preferentially competed with SKF83959 in the striatum following repeated amphetamine and in the post-mortem globus pallidus of schizophrenia patients9. For major depression, a peptide that disrupts D1/D2 co-immunoprecipitation produced anti-depressant effects in an animal model27. While our findings cannot rule out the possibility of increased D1/D2 interaction in pathological states, our complete inability to support this interaction in WT mice, rats and monkeys suggests that a role for D1/D2 heteromers in pathological conditions must be reconsidered. Moreover, our findings challenge the idea that D1/D2 heteromers are promising targets for the development of highly selective ligands for treatment of psychiatric symptoms. Rather than focusing on D1/D2 heteromer ligands, basic and clinical research programs might gain more traction by instead performing mechanistic evaluation of D1 receptor agonists, such as DAR-0100A, which was recently shown to attenuate working memory impairments in patients with schizotypal personality disorder70.
Taken together, these data suggest that current models of DA receptor functional selectivity based on D1/D2 heteromerization are incorrect, and that D1/D2 heteromers do not play a significant role in the normal adult brain. The field needs to reconsider previous data interpreted with this hypothesis in mind.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grants RO1MH086629 (GDS), F31DA029499 (ALF), TL1 RR024158-04 (HY), K05DA022413 and R01MH54137 (JAJ), RO1MH093672 (CK), R01NS078291 (RJC), a Research scientist award from the Research Foundation for Mental Hygiene (PT), and the Lieber Center for Schizophrenia Research and Treatment. Behavioral work was performed at the Vanderbilt Mouse Neurobehavioral Core, which is supported in part by P30HD15052. We thank Dr. Celine Gales (Institut National de la Santé et de la Recherche Médicale, Toulouse, France) and Dr. Nevin Lambert (Georgia Health Sciences University, Augusta, Georgia) for kindly sharing Rluc and Venus fusion G protein constructs, Dr. Stefan Offermanns (University of Heidelberg, Germany) for supplying the Gαq mutant line, and Dr. Nicole Calakos (Duke University) for the Drd1a-tdTomato reporter line. We also thank Matt Buendia, Heather Durai, and Dr. John Allison for excellent technical assistance.
Footnotes
Supplementary information is available at Molecular Psychiatry’s website.
REFERENCES
- 1.Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5(3):131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends Neurosci. 2008;31(2):74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert NA, Javitch JA. CrossTalk opposing view: Weighing the evidence for class A GPCR dimers, the jury is still out. J Physiol. 2014;592(Pt 12):2443–2445. doi: 10.1113/jphysiol.2014.272997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5(9):688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urizar E, Yano H, Kolster R, Gales C, Lambert N, Javitch JA. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol. 2011;7(9):624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertran-Gonzalez J, Herve D, Girault JA, Valjent E. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanatomy. 2010;4:136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355(3):418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 8.Perreault ML, Fan T, Alijaniaram M, O'Dowd BF, George SR. Dopamine D1–D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: regulation of BDNF, GAD67 and VGLUT1/2. PloS One. 2012;7(3):e33348. doi: 10.1371/journal.pone.0033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, et al. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285(47):36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perreault ML, Hasbi A, O'Dowd BF, George SR. The dopamine D1–D2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanatomy. 2011;5:31. doi: 10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanatomy. 2006;32(2–4):101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, et al. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3(3):226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- 13.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 15.Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253(3):987–992. [PubMed] [Google Scholar]
- 16.Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J Neurochem. 1994;62(5):2045–2048. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- 17.Undie AS, Berki AC, Beardsley K. Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology. 2000;39(1):75–87. doi: 10.1016/s0028-3908(99)00106-9. [DOI] [PubMed] [Google Scholar]
- 18.Mahan LC, Burch RM, Monsma FJ, Jr, Sibley DR. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc Natl Acad Sci USA. 1990;87(6):2196–2200. doi: 10.1073/pnas.87.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnt J, Hyttel J, Sanchez C. Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioural effects and stimulation of adenylate cyclase activity in vitro. Eur J Pharmacol. 1992;213(2):259–267. doi: 10.1016/0014-2999(92)90690-6. [DOI] [PubMed] [Google Scholar]
- 20.Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85(2):378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 21.Panchalingam S, Undie AS. SKF83959 exhibits biochemical agonism by stimulating [(35)S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology. 2001;40(6):826–837. doi: 10.1016/s0028-3908(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 22.Andringa G, Drukarch B, Leysen JE, Cools AR, Stoof JC. The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur J Pharmacol. 1999;364(1):33–41. doi: 10.1016/s0014-2999(98)00825-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee SM, Kant A, Blake D, Murthy V, Boyd K, Wyrick SJ, et al. SKF-83959 is not a highly-biased functionally selective D dopamine receptor ligand with activity at phospholipase C. Neuropharmacology. 2014;86:145–154. doi: 10.1016/j.neuropharm.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryman-Rasmussen JP, Nichols DE, Mailman RB. Differential activation of adenylate cyclase and receptor internalization by novel dopamine D1 receptor agonists. Mol Pharmacol. 2005;68(4):1039–1048. doi: 10.1124/mol.105.012153. [DOI] [PubMed] [Google Scholar]
- 25.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, et al. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104(2):654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng J, Rashid AJ, So CH, O'Dowd BF, George SR. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1–D2 dopamine receptor complex. Neuroscience. 2010;165(2):535–541. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, et al. Uncoupling the dopamine D1–D2 receptor complex exerts antidepressant-like effects. Nat Med. 2010;16(12):1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- 28.Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, et al. Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci USA. 2009;106(50):21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Zhou Z, Wang D, Li A, Yin Y, Gu X, et al. Activation of phosphatidylinositol-linked D1-like receptor modulates FGF-2 expression in astrocytes via IP3-dependent Ca2+ signaling. J Neurosci. 2009;29(24):7766–7775. doi: 10.1523/JNEUROSCI.0389-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma LQ, Liu C, Wang F, Xie N, Gu J, Fu H, et al. Activation of phosphatidylinositol-linked novel D1 dopamine receptors inhibits high-voltage-activated Ca2+ currents in primary cultured striatal neurons. J Neurophysiol. 2009;101(5):2230–2238. doi: 10.1152/jn.90345.2008. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Wang W, Wang F, Cai F, Hu ZL, Yang YJ, et al. Phosphatidylinositol-linked novel D(1) dopamine receptor facilitates long-term depression in rat hippocampal CA1 synapses. Neuropharmacology. 2009;57(2):164–171. doi: 10.1016/j.neuropharm.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Chu HY, Yang Z, Zhao B, Jin GZ, Hu GY, Zhen X. Activation of phosphatidylinositol-linked D1-like receptors increases spontaneous glutamate release in rat somatosensory cortical neurons in vitro. Brain Res. 2010;1343:20–27. doi: 10.1016/j.brainres.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 33.Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, et al. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51(1):6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- 34.Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75(3):447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, Sibley DR. D1–D2 dopamine receptor synergy Promotes calcium signaling via multiple mechanisms. Mol Pharmacol. 2013;84(2):190–200. doi: 10.1124/mol.113.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278(7):4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 37.Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27(17):2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gales C, Rebois RV, Hogue M, Trieu P, Breit A, Hebert TE, et al. Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods. 2005;2(3):177–184. doi: 10.1038/nmeth743. [DOI] [PubMed] [Google Scholar]
- 39.Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91(26):12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, et al. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91(3):911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 41.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, et al. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22(24):10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frederick AL, Saborido TP, Stanwood GD. Neurobehavioral phenotyping of G(alphaq) knockout mice reveals impairments in motor functions and spatial working memory without changes in anxiety or behavioral despair. Front Behav Neurosci. 2012;6:29. doi: 10.3389/fnbeh.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28(11):2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425(6961):917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 45.Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An Improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Systems Neurosci. 2011;5:32. doi: 10.3389/fnsys.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry. 2013;18(9):1025–1033. doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, et al. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. BioTechniques. 2011;51(2):111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology. 2014;231(8):1615–1626. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13(9):778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 50.Sauliere A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, et al. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol. 2012;8(7):622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 51.So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O'Dowd BF, et al. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1–D2 receptor hetero-oligomers. Mol Pharmacol. 2009;75(4):843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasbi A, O'Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol. 2010;10(1):93–99. doi: 10.1016/j.coph.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downes RP, Waddington JL. Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine 'D1-like' receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur J Pharmacol. 1993;234(1):135–136. doi: 10.1016/0014-2999(93)90718-w. [DOI] [PubMed] [Google Scholar]
- 54.Deveney AM, Waddington JL. Pharmacological characterization of behavioural responses to SK&F 83959 in relation to 'D1-like' dopamine receptors not linked to adenylyl cyclase. Br J Pharmacol. 1995;116(3):2120–2126. doi: 10.1111/j.1476-5381.1995.tb16420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhen X, Goswami S, Abdali SA, Gil M, Bakshi K, Friedman E. Regulation of cyclin-dependent kinase 5 and calcium/calmodulin-dependent protein kinase II by phosphatidylinositol-linked dopamine receptor in rat brain. Mol Pharmacol. 2004;66(6):1500–1507. doi: 10.1124/mol.104.002279. [DOI] [PubMed] [Google Scholar]
- 56.Gustin RM, Shonesy BC, Robinson SL, Rentz TJ, Baucum AJ, 2nd, Jalan-Sakrikar N, et al. Loss of Thr286 phosphorylation disrupts synaptic CaMKIIalpha targeting, NMDAR activity and behavior in pre-adolescent mice. Mol Cell Neurosci. 2011;47(4):286–292. doi: 10.1016/j.mcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279(5352):870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 58.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 59.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15(7 Pt 2):5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gangarossa G, Espallergues J, de Kerchove d'Exaerde A, El Mestikawy S, Gerfen CR, Herve D, et al. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65(3):709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- 62.Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol Pharmacol. 1995;48(6):988–994. [PubMed] [Google Scholar]
- 63.Mannoury la Cour C, Vidal S, Pasteau V, Cussac D, Millan MJ. Dopamine D1 receptor coupling to Gs/olf and Gq in rat striatum and cortex: a scintillation proximity assay (SPA)/antibody-capture characterization of benzazepine agonists. Neuropharmacology. 2007;52(3):1003–1014. doi: 10.1016/j.neuropharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Wadenberg ML, Kapur S, Soliman A, Jones C, Vaccarino F. Dopamine D2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology. 2000;150(4):422–429. doi: 10.1007/s002130000466. [DOI] [PubMed] [Google Scholar]
- 65.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16(20):6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, et al. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PloS One. 2009;4(3):e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cahill E, Pascoli V, Trifilieff P, Savoldi D, Kappes V, Luscher C, et al. D1R/GluN1 complexes in the striatum integrate dopamine and glutamate signalling to control synaptic plasticity and cocaine-induced responses. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.73. E-pub ahead of print 29 July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perreault ML, Hasbi A, Alijaniaram M, O'Dowd BF, George SR. Reduced striatal dopamine D1–D2 receptor heteromer expression and behavioural subsensitivity in juvenile rats. Neuroscience. 2012;225:130–139. doi: 10.1016/j.neuroscience.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perreault ML, Hasbi A, O'Dowd BF, George SR. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology. 2014;39(1):156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosell DR, Zaluda LC, McClure MM, Perez-Rodriguez MM, Strike KS, Barch DM, et al. Effects of the D Dopamine Receptor Agonist Dihydrexidine (DAR-0100A) on Working Memory in Schizotypal Personality Disorder. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.192. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.