Abstract
Identifying the mechanisms of natural control of HIV-1 infection could lead to novel approaches to prevent or cure HIV infection. Several studies have associated natural control of HIV-1 infection with IgG antibodies against HIV-1 Gag proteins (e.g. p24) and/or production of IgG2 antibodies against HIV-1 proteins. These antibodies likely exert their effect by activating anti-viral effector cell responses rather than virus neutralization. We hypothesized that an opsonophagocytic IgG antibody response against HIV-1 p24 that activates plasmacytoid dendritic cells (pDCs) through FcγRIIa would be associated with control of HIV and that this would be enhanced by antibody isotype diversification. Using the Gen2.2 pDC cell line, we demonstrated that pDC-reactive opsonophagocytic IgG antibody responses against HIV-1 p24 were higher in HIV controllers (HIV RNA <2000 copies/mL) than non-controllers (HIV RNA >10,000 copies/mL) particularly in controllers with low but detectable viremia (HIV RNA 75–2000 copies/mL). Opsonophagocytic antibody responses correlated with plasma levels of IgG1 and IgG2 anti-HIV-1 p24 and notably, correlated inversely with plasma HIV RNA levels in viremic HIV patients. Phagocytosis of these antibodies was mediated via FcγRIIa. Isotype diversification (towards IgG2) was greatest in HIV controllers and depletion of IgG2 from immunoglobulin preparations indicated that IgG2 antibodies to HIV-1 p24 do not enhance phagocytosis, suggesting that they enhance other aspects of antibody function, such as antigen opsonization. Our findings emulate those for pDC-reactive opsonophagocytic antibody responses against coxsackie, picorna and influenza viruses and demonstrate a previously undefined immune correlate of HIV-1 control that may be relevant to HIV vaccine development.
Introduction
Combination antiretroviral therapy (ART) is extremely effective in controlling HIV replication but cannot eradicate the infection. HIV genomes integrate into DNA of long-lived cells, such as central memory CD4+ T cells, and form a latent reservoir of infection that reactivates if ART is ceased. Furthermore, individuals with HIV infection treated with ART may experience low-level viral replication, which contributes to immune activation, inflammation and activation of the coagulation system that are associated with an increased risk of atherosclerotic vascular disease, osteoporosis and non-AIDS cancers (1). A large international research effort is currently focused on ways to decrease the size of latent HIV reservoirs and potentially eradicate the infection (2). It is generally accepted that the initial step should be to activate the reservoir of HIV proviral DNA from latency with latency inhibitors such histone deacetylase inhibitors (3). However, inhibiting HIV latency alone is unlikely to decrease the size of the HIV reservoir and other measures, such as enhancement of endogenous retroviral restriction factors and/or ‘protective’ immune responses against HIV antigens by therapeutic vaccines, are likely to be required to eliminate HIV-infected cells (4). It is therefore important to elucidate ‘protective’ immune responses against HIV that have the potential to be enhanced by a therapeutic vaccine.
Data from numerous studies of individuals who can naturally control HIV infection (HIV controllers), indicate that the strongest correlate of immune control is CD8+ T cell responses against proteins encoded by the Gag gene of HIV that are restricted by particular ‘protective’ HLA-B alleles, especially HLA-B*57 (5). Peptides of HIV Gag proteins are expressed by class I major histocompatibility complex molecules of T cells latently infected by HIV (6, 7) and are potential targets for vaccine-induced immune responses. However, vaccines that induce T cell responses against HIV Gag proteins have been ineffective in preventing or controlling HIV infection (8). Research efforts are therefore being focused on enhancing other ‘protective’ immune responses. Studies in simian-human immunodeficiency virus (SHIV)-infected macaques have shown that human monoclonal antibodies against HIV-1 Env antigens suppress replication of SHIV and are capable of inducing long-term suppression of SHIV infection in a subset of animals (9, 10). Numerous studies have also demonstrated that IgG antibodies against HIV-1 Gag proteins are associated with slower progression of HIV disease (reviewed in (11)) but it is unclear what role, if any, these antibodies play in controlling HIV-1 replication.
Studies of acute SIV infection in macaques have shown that IFN-α suppresses SIV replication, though prolonged exposure to IFN-α has deleterious effects (12). In addition, administration of IFN-α therapy to HIV patients receiving ART may decrease the size of the HIV DNA reservoir (13, 14). Natural control of HIV-1 replication is associated with higher activity of IFN-α-stimulated NK cells (15) and of plasmacytoid dendritic cells (pDC) (16, 17), which are the major producers of IFN-α. Plasmacytoid dendritic cells can be activated to produce IFN-α by opsonophagocytic antibody responses against coxsackieviruses (18) and picornaviruses (19), both of which are non-enveloped RNA viruses. Therefore, although a mechanism for control of HIV replication by IgG antibodies against Gag proteins remains to be established, it is possible that they exert opsonophagocytic antibody activity that activates pDCs to produce IFN-α.
Opsonophagocytic antibody responses against encapsulated bacteria are enriched for IgG2 antibodies against capsular polysaccharides (20–22). This is likely to reflect structural and functional characteristics of IgG2 antibodies that promote opsonization of multivalent antigens and preferential binding to FcRs that facilitate phagocytosis. Thus, IgG2 antibodies can exist as multiple structural isoforms consisting of different heavy chain/light chain/hinge covalent complexes that result from differences in disulfide bonding between the hinge and Fab regions of the molecule (23–25) and also form covalent dimers at the hinge region (26, 27). In addition, while IgG2 antibodies bind to FcRs with lower affinity than other IgG subclasses, binding is predominantly to the 131H genotype of FcγRIIa (28), which is carried by 75% of individuals from all racial groups and is the major FcγR mediating phagocytosis (29). Furthermore, IgG2 and IgG1 antibodies are more effective than IgG3 antibodies in activating intracellular pathways of dendritic cells following phagocytosis via FcγRIIa (30). FcγRIIa is expressed on multiple cell-types that undertake antibody-dependent phagocytosis, including pDCs on which FcγRIIa is the most abundant activatory FcγR (31). Of note, the 131H genotype of FcγRIIa is associated with slower progression of HIV disease (32).
An IgG2 antibody response against HIV proteins has been associated with control of HIV replication or slower HIV disease progression (33, 34) and we have provided evidence that antibodies against HIV Gag proteins that include IgG2 antibodies, may contribute to HIV immune control mechanisms (35). There is also evidence that IgG2 antibodies may be beneficial in controlling other viruses. For example, IgG2 antibodies against L1 capsids of human papillomavirus (HPV)-16 have been associated with regression of HPV16-positive cervical intraepithelial neoplasia (36). Production of IgG2 antibodies during the maturation of an antibody response occurs through class switch recombination of immunoglobulin heavy chain (IGH) genes, in which the ‘upstream’ Cγ3 (IgG3) and Cγ1 (IgG1) genes of the IGH locus are switched with the Cγ2 (IgG2) and Cγ4 (IgG4) genes located further downstream (37, 38). Thus, switching to ‘downstream isotypes’, to include IgG2, leads to isotype diversification of the IgG antibody response and broadens antibody function.
Here, we have examined IgG antibody responses against HIV-1 p24 in HIV controllers and non-controllers and demonstrated that both pDC-reactive opsonophagocytic antibody responses and greater isotype diversification are associated with control of HIV-1 infection in patients with viremic HIV infection.
Methods
Study groups
Cryopreserved plasma samples were obtained from two groups of HIV patients: (A) 34 ART-naive adult HIV patients recruited in Perth, consisting of 14 HIV controllers (plasma HIV RNA levels <2000 copies/mL for at least one year) and 20 HIV non-controllers (plasma HIV RNA levels >5000 copies/mL) (Perth cohort), (B) 89 ART-naive adult HIV patients from the University of California, San Francisco SCOPE cohort, consisting of 30 elite HIV controllers (HIV RNA levels <75 copies/mL for at least one year), 29 viremic HIV controllers (HIV RNA levels 75–2000 copies/mL for at least one year) and 30 HIV non-controllers (HIV RNA levels >10,000 copies/mL). Demographic characteristics for the two study groups are summarized in Table I. Informed consent was obtained from all subjects and the study was approved by the ethics committees of Royal Perth Hospital and UCSF.
TABLE I.
Demographic data of study participants.
| HIV patients recruited in Perth, Australia (n=34) | SCOPE (n=89) | ||||
|---|---|---|---|---|---|
|
| |||||
| Controller (n=14) | Non-controller (n=20) | Elite controller (n=30) | Viremic controller (n=29) | Non-controller (n=30) | |
| CD4+ T cell counta (cells/μL of blood) | 672b (504–1302) | 58 (4–196) | 1029c (363–2199) | 519 (359–988) | 466 (356–1287) |
| HIV-1 viral loada (copies/mL of blood) | 40 (40–513) | 120 534 (6 310 – 602 560) | <75d | 313 (75–1658) | 52 850 (10 619 – 334 034) |
| Gender (M/F/Trans/Intersex) | 7/7/0/0 | 17/3/0/0 | 18/11/1/0 | 24/3/2/0 | 25/2/2/1 |
| Agea | 43 (23–65) | 49 (25–67) | 50e (32–77) | 49 (28–60) | 46 (27–71) |
The data in this table are presented as median (range)
CD4+ T cell counts are significantly higher than non-controllers of the Perth cohort (p<0.0001)
CD4+ T cell counts are significantly higher than viremic controllers (p=0.0001) and non-controllers of the SCOPE cohort (p<0.0001)
Viral loads shown here are below the limit of detection as assessed by different diagnostic platforms (Roche COBAS Ampliprep/COBAS TaqMan >20, Abbott real-time HIV-1 PCR >40, Roche PCR >50, branched DNA >75)
Age significantly higher than non-controllers of the SCOPE cohort (p=0.03)
Propagation and culture of cell lines
Three cell lines were utilized for the assays of opsonophagocytic antibodies: (A) The Gen2.2 (pDC) cell line, obtained from the French Collection of National Microorganism Cultures (Institut Pasteur, Paris, France) and described previously by Chaperot et al. (39). Gen2.2 cells were grown in a 75cm2 culture flask (Cellstar®, Monroe, NC, USA) with an 80% confluent MS-5 feeder monolayer, 20mL RPMI medium 1640 supplemented with GlutaMAX™ (Gibco®, Mount Waverely, VIC, Australia) and 10% heat-inactivated FBS (HIFBS; Gibco®). 4×106 Gen2.2 cells were passaged every third day into a new 75cm2 culture flask, containing a fresh 80% confluent MS-5 feeder monolayer and 20mL of Gen2.2 growth media. (B) The irradiated murine stromal cell line MS-5, obtained from the German Collection of Microorganisms and Cell Cultures (Leibniz Institute Deustsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany). Adherent MS-5 feeder cells were grown in 20mL of α-MEM (Gibco®) supplemented with 10% HIFBS in a 75cm2 culture flask. MS-5 cells were detached with 0.25% trypsin/EDTA (Gibco®) and passaged 1:3 every third day into a new 75cm2 culture flask containing 20mL fresh MS5-growth media. (C) The THP-1 (monocyte) cell line, obtained from laboratory stocks. THP-1 cells were grown in 30mL RPMI 1640 supplemented with 2mM L-glutamine (Gibco®) and 10% HIFBS. 4×106 THP-1 cells were passaged into a 75cm2 culture flask containing 30mL of THP-1 growth media, every third day. Cell lines were cultured at 37°C, 5% CO2.
Assessment of pDC-reactive opsonophagocytic antibody responses against HIV-1 p24
Opsonophagocytic antibody responses against HIV-1 p24 were assessed using the method of Ackerman et al. (40) with modifications to assess phagocytosis by a pDC cell line (Gen2.2). IgG was isolated from plasma using Melon Gel IgG Purification kits (Pierce®, Rockford, IL, USA). Plasma was diluted 1:10 with Melon Gel purification buffer and subsequently mixed-end-over-end with Melon Gel purification support in a Pierce® spin column for 5 mins. The Pierce® spin column was then centrifuged (6010 × g for 1 min), and the eluate (containing purified IgG) was collected. The concentration of IgG in the eluate was measured using a Nanodrop spectrophotometer (Thermo Scientific, Wilimington, DE, USA) and adjusted to 1mg/mL. Purified IgG (50μL, 100μg/mL) was incubated with 50μL of a master mix containing biotinylated, recombinant (baculovirus-expressed) HIV-1 p24 (20μg/mL; Protein Sciences, Meriden, CT, USA) and NeutrAvidin®-labeled Fluospheres (1.8×109 particles/mL; 0.1μm diameter; Molecular Probes, Eugene, OR, USA) for 2 hrs (37°C, 5% CO2), in a sterile 96-well tissue culture plate. Gen2.2 cells (100μL, 2×105 cells/mL) were added to each well and cultured for 16 hrs (37°C, 5% CO2). Gen2.2 cells were transferred to 5mL polystyrene round-bottom tubes (BD Biosciences, San José, CA, USA), washed with 1% BSA (AusgeneX, Loganholme, QLD, Australia)/PBS (Sigma, Castle Hill, NSW, Australia) and resuspended in the residual volume for acquisition. Data were acquired on a FACSCanto™ II using Diva software (BD Biosciences) with an acquisition stopping gate set at 10,000 Gen2.2 cells as characterized by their FSC and SSC profiles. Data files were analyzed using FlowJo software (v7.6, TreeStar, Ashland, OR, USA).
The degree of opsonophagocytosis (phagocytic index) was calculated by multiplying the percentage of fluosphere+ Gen2.2 cells with the mean fluorescence intensity of the fluosphere+ Gen2.2 cells (40). Phagocytic indices were further corrected for the phagocytic index obtained when Gen2.2 cells or THP-1 cells were incubated with HIV-1 p24-conjugated beads in the absence of IgG (corrected phagocytic index). In addition, to account for inter-assay variability in the results of the SCOPE cohort, each patient’s corrected phagocytic index was divided by the phagocytic index of fluospheres conjugated with IgG alone to produce a relative phagocytic index.
Assessment of FcγR expression on Gen2.2 and THP-1 cell lines
2×105 Gen2.2 and THP-1 cells were transferred to 5mL polystyrene tubes, washed with 1% BSA/PBS and stained with the following mAbs: CD64-APC (10.1; Invitrogen; Carlsbad, CA, USA), mouse anti-human CD32a (2C3B11B8; Sino Biological Inc., Beijing, China) and rabbit anti-human CD32b (112; Sino Biological Inc.) for 15 min. Cells were washed with 1% BSA/PBS and the secondary polyclonal antibodies: goat anti-mouse IgG-Fc-PE (Ab5881; Abcam, Cambridge, MA, USA) and goat anti-rabbit IgG-Fc-FITC (Ab98484; Abcam) were added for 30 min. Cells were washed twice with 1% BSA/PBS and resuspended in residual volume for acquisition.
ELISA of HIV-1 p24- and gp140-specific IgG1 and IgG2 antibodies
96-well microtitre plates were coated overnight at 4°C with (A) 0.1μg/mL HIV-1 (IIIB) p24 purified native protein (Advanced Biotechnologies Inc., Columbia, MS, USA) or (B) 0.1μg/mL HIV-1 (clade B) gp140 (Immune Technology Corp., New York, NY, USA). Plates were washed three times with 0.05% Tween/PBS and blocked with 5% BSA/PBS (1 hr). Plates were washed three times and plasma serially diluted in 2% BSA/PBS was added (2 hrs). Plasma from an individual with high absorbance readings was used as a standard and an arbitrary unit of 1000 U/mL was assigned to the top standard. Plates were washed three times and horseradish peroxidase conjugated anti-human IgG1 (Invitrogen) was diluted (A) 1:2000 for p24, or (B) 1:1000 for gp140 in 2% BSA/PBS, and added to the plate (1 hr). For both p24- and gp140-specific IgG2 ELISAs, biotinylated anti-human IgG2 (Southern Biotech, Birmingham, AL, USA) diluted 1:1000 in 2% BSA/PBS was added (1 hr). In addition, for IgG2, plates were further washed three times and streptavidin-conjugated HRP (BD Pharmigen, San Diego, CA, USA) diluted 1:5000 in 2% BSA/PBS was added (1 hr). Plates were washed five times and color development followed the addition of tetramethylbenzidine substrate (Sigma). Reactions were stopped with 1M sulfuric acid (AnalaR grade; BDH, Poole, Dorset, UK) and absorbance measured at 450nm.
Magnetic bead depletion of IgG2 from purified IgG preparations
The method described by Chung et al. (41) for the depletion of IgG3 and IgG4 was modified to deplete IgG2 from purified IgG preparations. Streptavidin-conjugated M-270 Dynabeads® (Invitrogen) were washed three times in PBS and incubated with biotin-conjugated mouse anti-human IgG2 (Southern Biotech) at a mass ratio of 100:1 for 30 mins (room temperature). Beads were then washed three times in 0.1% BSA/PBS and incubated with Melon-Gel purified IgG samples (80:1) for 24 hrs (4°C). Magnetic beads were removed from the suspension using a Dynal MPC®-E magnet (Dynal A.S., Oslo, Norway) and the IgG2-depleted plasma collected. To obtain maximal depletions, this procedure was undertaken twice for each sample. The extent of IgG1 and IgG2 depletion was assessed by ELISA using a similar protocol to that used for detection of HIV p24- and HIV gp140-specific IgG1 and IgG2 antibodies, but plates were coated with 1μg/mL recombinant protein G (Pierce®) instead of antigens and antibodies were detected with either biotinylated anti-human IgG1 (Invitrogen) diluted 1:2000 or biotinylated anti-human IgG2 (Southern Biotech) diluted 1:4000.
Statistics
Statistical testing was performed using GraphPad Prism software (v5.0, GraphPad software, San Diego, CA, USA). Mann-Whitney t-tests were performed for between group comparisons. Wilcoxon signed rank tests were performed for comparisons between paired data sets. Correlations were assessed using Spearman’s rank correlation.
To assess isotype diversification of IgG antibodies to HIV-1 p24, serum levels of HIV-1 p24-specific IgG1 and IgG2 antibodies in SCOPE patients were transformed using the natural log, to produce a linear relationship. Robust regression was then used to test if the relationship between log p24-specific IgG1 and log p24-specific IgG2 varied between groups (elite or viremic controllers versus non-controllers) using an interaction between group and log p24-specific IgG2.
Results
Opsonophagocytic antibody responses against HIV-1 p24 were higher in HIV controllers than non-controllers with both Gen2.2 and THP-1 cell lines but were more uniform with the former cell line
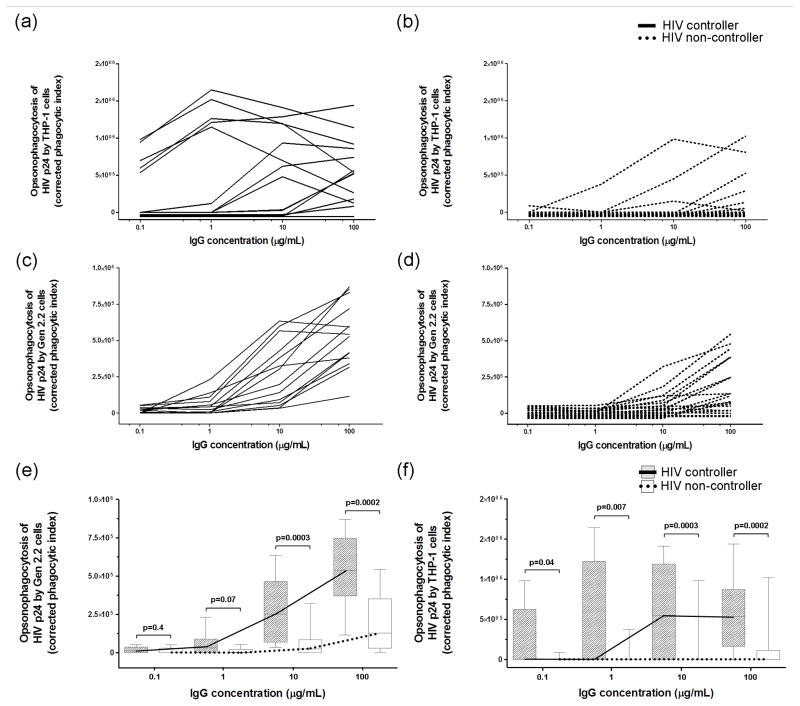
Initially, IgG was purified from plasma of 14 HIV controllers and 20 HIV non-controllers (Perth cohort) and the opsonization of HIV-1 p24 conjugated to fluorescent beads and phagocytosis by the Gen2.2 or THP-1 cell lines was assessed. IgG was analyzed at four concentrations (100, 10, 1 and 0.1μg/mL). Although opsonophagocytic antibody responses were higher with the THP-1 cell line (Figure 1a and b), the dose response curves were more uniform with Gen2.2 cells (Figure 1c and d). To investigate these differences between Gen2.2 and THP-1 cells, FcγR expression was assessed by flow cytometry. While FcγRIIa and IIb expression was similar on the two cell lines, THP-1 cells expressed markedly higher levels of the ‘high-affinity’ FcγRI compared to Gen2.2 cells (Supplementary Figure 1). FcγRIIIa was not detected on Gen2.2 cells.
Figure 1. HIV controllers exhibited higher opsonophagocytosis of HIV-1 p24 in comparison to non-controllers when assessed with either Gen2.2 or THP-1 cells, with opsonophagocytosis of HIV-1 p24 being more uniform with the former cell line.
IgG antibodies were purified from HIV controllers and non-controllers of the Perth cohort and incubated (100, 10, 1, 0.1μg/mL) with HIV-1 p24-conjugated beads (0.1μg/mL) in the presence of 20,000 THP-1 (a,b) or Gen2.2 cells (c,d) to assess opsonophagocytosis of HIV-1 p24. Opsonophagocytic scores for controllers and non-controllers are shown as box and whisker plots for the assay with (e) Gen2.2 cells and (f) with THP-1 cells.
HIV controllers exhibited higher corrected phagocytic indices than non-controllers with Gen2.2 cells when IgG was incubated at a concentration of 100μg/mL (p=0.0002) and 10μg/mL (p=0.0003), but this difference was lost at 1μg/mL (p=0.07) and 0.1μg/mL (p=0.4) of IgG (Figure 1e). Similarly, when the assay was performed with THP-1 cells, HIV controllers exhibited higher corrected phagocytic indices when IgG was incubated at a concentration of 100μg/mL (p=0.0002), 10μg/mL (p=0.0003), 1μg/mL (p=0.007) and 0.1μg/mL (p=0.04) (Figure 1f). Subsequent studies of opsonophagocytic antibody responses against HIV-1 p24 were undertaken with Gen2.2 cells alone and with IgG preparations at a concentration of 25μg/mL.
pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 were higher in HIV controllers than non-controllers, particularly viremic controllers
We next attempted to replicate the findings of the prior experiment for Gen2.2 cells, but this time in a larger cohort of patients consisting of 30 elite HIV controllers, 29 viremic HIV controllers and 30 HIV non-controllers (SCOPE cohort). Viremic controllers exhibited higher opsonophagocytic antibody responses against HIV-1 p24 than non-controllers (p=0.0001) and also elite controllers (p=0.01). Similarly, elite controllers exhibited higher responses than non-controllers (p=0.05; Figure 2).
Figure 2. Viremic HIV controllers exhibited higher pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 than non-controllers and elite controllers.
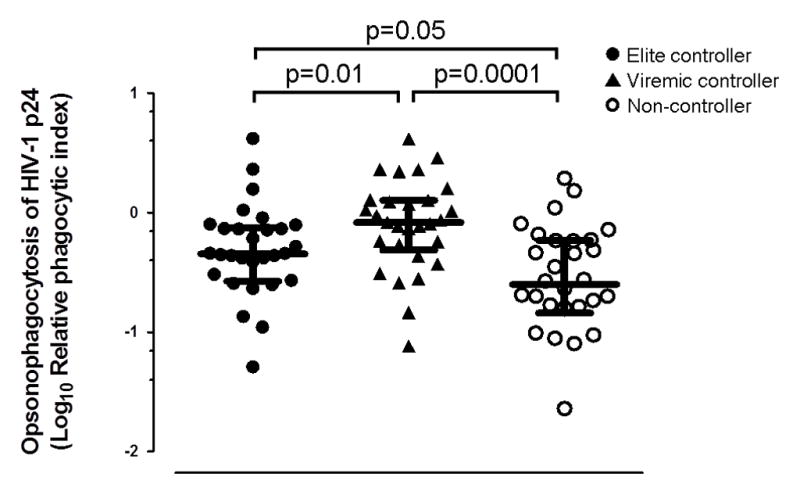
IgG antibodies were isolated from elite-, viremic- and non-controller patients of the SCOPE cohort and incubated (25μg/mL) with HIV-1 p24-conjugated beads (0.1μg/mL) and 20,000 Gen2.2 cells. Median (IQR) opsonophagocytic scores are shown.
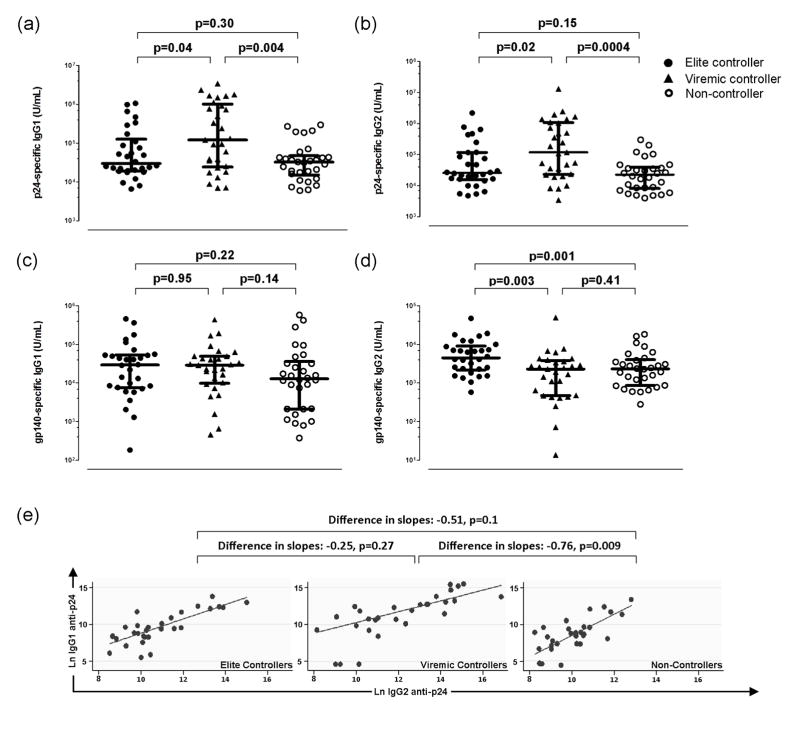
The magnitude and isotype diversification of IgG antibodies against HIV-1 p24 were higher in HIV controllers, particularly viremic controllers
We next compared plasma levels of IgG1 and IgG2 antibodies to HIV-1 p24 in controllers and non-controllers from the SCOPE cohort. IgG1 antibodies to HIV-1 p24 were higher in viremic controllers than in non-controllers (p=0.004) and elite controllers (p=0.04) but there was no difference between elite controllers and non-controllers (p=0.30) (Figure 3a). Similarly, IgG2 antibodies to HIV-1 p24 were higher in viremic controllers than non-controllers (p=0.0004) and elite controllers (p=0.02) but there was no difference between elite controllers and non-controllers (p=0.15) (Figure 3b).
Figure 3. Viremic HIV controllers exhibited higher IgG antibodies to HIV-1 p24 associated with greater isotype diversification.
(a) anti-p24 IgG1, (b) anti-p24 IgG2, (c) anti-gp140 IgG1 and (d) anti-gp140 IgG2 (median±IQR) antibody titers of SCOPE patients were measured using in-house indirect ELISAs. (e) Isotype diversification of HIV-1 p24-specific IgG antibodies were assessed by comparing the slopes of the regression line generated by plotting plasma levels of Ln IgG1 anti-p24 versus Ln IgG2 anti-p24, with slopes skewed towards Ln IgG2 anti-p24 indicating higher isotype diversification.
To further examine the significance of higher IgG1 and IgG2 antibodies to HIV-1 p24 in viremic controllers, we also examined plasma levels of IgG1 and IgG2 antibodies to HIV-1 gp140. As shown in Figure 3c, there was no difference in plasma levels of IgG1 antibodies to HIV-1 gp140 amongst the three groups of patients. However, plasma levels of IgG2 antibodies to gp140 were higher in elite controllers compared with both viremic controllers (p=0.003) and non-controllers (p=0.001; Figure 3d).
We next sought to assess isotype diversification of HIV-1 p24-specific IgG antibodies. As a relatively higher production of ‘downstream’ IgG2 antibodies is indicative of greater isotype diversification of an IgG antibody response, we constructed linear regression lines by plotting plasma levels of HIV-1 p24-specific IgG1 versus those of HIV-1 p24-specific IgG2 and compared the slope of these lines between patient groups. A skewing of the slope towards IgG2 indicates a greater degree of isotype diversification. Viremic controllers exhibited greater isotype diversification of IgG antibodies to HIV-1 p24 compared to non-controllers (difference in slopes = −0.76, p=0.009). While the slope of the lines was also different, and in the same direction, for the comparison of elite controllers with non-controllers, the difference was not statistically significant (difference in slopes = −0.51, p=0.1). Similarly, isotype diversification was not different between viremic controllers and elite controllers (difference in slopes = −0.25, p=0.27).
pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 correlated positively with plasma levels of HIV-1 p24-specific IgG1 and IgG2 antibodies but negatively with HIV-1 viral load
To examine functional aspects of IgG antibodies to HIV-1 p24, pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 were correlated with plasma levels of HIV-1 p24-specific IgG1 and IgG2 antibodies and plasma HIV RNA levels in the SCOPE cohort. There were strong positive correlations of pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 with both IgG1 and IgG2 antibodies to HIV-1 p24 (r=0.75, p<0.0001 and r=0.61, p<0.0001, respectively, data not shown). In contrast, there was a highly significant inverse correlation of opsonophagocytic IgG antibody responses against HIV-1 p24 with plasma HIV RNA levels in all viremic patients (r= −0.51, p<0.0001; Figure 4a). There were also weaker inverse correlations between IgG1 and IgG2 antibodies to HIV-1 p24 and plasma HIV RNA levels, moreso for IgG2 antibodies (r= −0.29, p=0.02 and r= −0.38, p=0.0003, respectively; Figures 4b and c).
Figure 4. IgG antibody responses against HIV-1 p24 correlated inversely with plasma HIV viral load in all viremic patients.
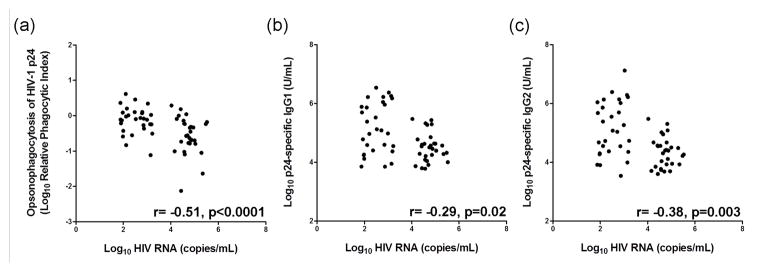
The correlation between plasma HIV RNA levels and (a) pDC-reactive opsonophagocytic antibody responses against HIV-1 p24, (b) IgG1 anti-p24, and (c) IgG2 anti-p24 was examined in viremic controllers and non-controllers from the SCOPE study group using Spearman’s rank correlation method. Elite controllers were excluded from the analysis because HIV RNA was not detected in plasma.
IgG antibodies to HIV-1 p24 did not correlate with CD4+ T cell counts
To examine the possible determinants of higher opsonophagocytic antibody responses against HIV-1 p24 in HIV controllers, we correlated plasma levels of opsonophagocytic antibodies and IgG1 and IgG2 antibodies to HIV-1 p24 with CD4+ T cell counts in the entire SCOPE cohort (n=89). We also examined the correlation of IgG2 antibodies to HIV-1 gp140 with CD4+ T cell counts. Opsonophagocytic antibody responses against HIV-1 p24 did not correlate with CD4+ T cell counts (r=−0.08, p=0.44; data not shown). Similarly, there was no correlation between IgG1 or IgG2 anti-HIV-1 p24 and CD4+ T cell counts (r= −0.16, p=0.13; r= −0.15, p=0.16, respectively; data not shown). However, there was a weak correlation of IgG2 anti-HIV-1 gp140 with CD4+ T cell counts (r=0.23, p=0.03; data not shown).
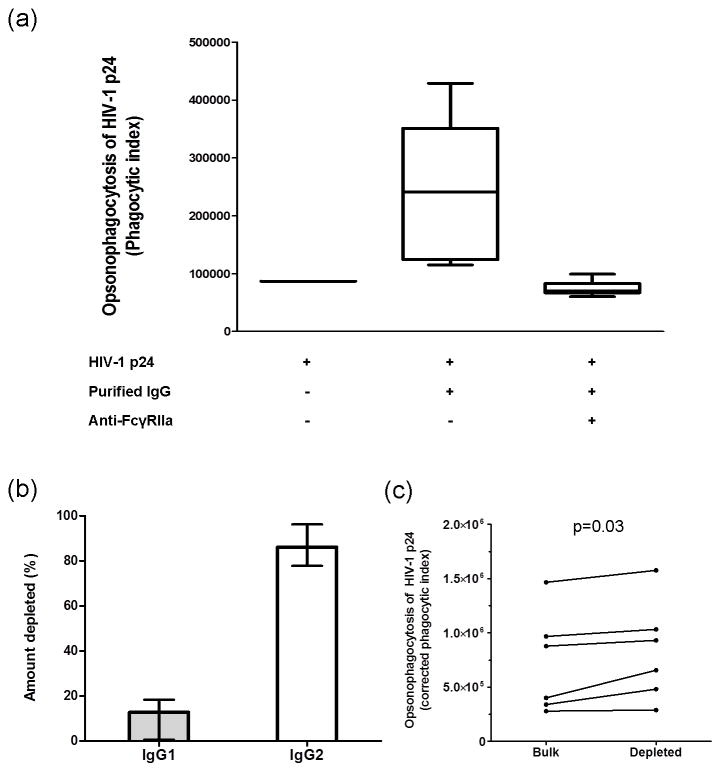
Phagocytosis of HIV-1 p24 opsonized with IgG antibodies by Gen2.2 cells is mediated through FcγRIIa and not enhanced by IgG2 antibodies
To determine if phagocytosis of HIV-1 p24 opsonized by IgG antibodies was mediated through FcγRIIa, Gen2.2 cells were pre-treated with a monoclonal antibody to FcγRIIa (AF1875, R&D systems, Minneapolis, MN, USA) at a concentration of 50μg/mL or with PBS. Purified IgG (25μg/mL) from two elite-, viremic- and non-controllers from the SCOPE cohort who had demonstrated the highest pDC-reactive opsonophagocytic antibody responses against HIV-1 p24, were examined. Compared to PBS pre-treatment, the phagocytic indices of all samples were reduced to the level of the negative control (Gen2.2 cells incubated with HIV p24-conjugated beads in the absence of IgG) when cells were pre-treated with anti-FcγRIIa (Figure 5a).
Figure 5. pDC reactive opsonophagocytic antibody responses against HIV-1 p24 are mediated via FcγRIIa and not decreased by depletion of IgG2 from immunoglobulin preparations.
Purified plasma IgG from 2 elite-, 2 viremic- and 2 non-controllers, who had previously exhibited the highest phagocytic scores, were used to determine whether: phagocytosis of antibodies to HIV-1 p24 by Gen2.2 cells was mediated through FcγRIIa and also to examine the effect of IgG2 depletion on pDC reactive opsonophagocytic antibody responses against HIV-1 p24. (a) Gen2.2 cells were pre-treated with anti-FcγRIIa at a concentration of 50μg/mL or with PBS. Subsequently, pre-treated cells were cultured with purified plasma IgG (25μg/mL). (b) The percentage of IgG1 and IgG2 that was depleted from immunoglobulin preparations relative to non-depleted samples (median ± range). (c) The effect of IgG2 depletion on the pDC-reactive opsonophagocytic antibody response against HIV-1 p24.
One explanation for our observation that isotype diversification (skewing towards IgG2) of IgG antibodies to HIV-1 p24 was greater in viremic controllers than elite controllers and non-controllers is that IgG2 antibodies enhance opsonization of HIV-1 p24 or phagocytosis of IgG antibodies to HIV-1 p24 through FcγRIIa. We therefore depleted the immunoglobulin preparations, referred to above, of IgG2 and compared corrected phagocytic indices for those samples with the indices of samples that had not been depleted of IgG2. Following depletion of IgG2 from immunoglobulin samples by a median (range) of 86% (83–95%) (Figure 5b) there was a small increase in phagocytosis of HIV-1 p24 opsonized by IgG antibodies (p=0.03, Figure 5c), suggesting that IgG2 antibodies did not enhance phagocytosis of opsonized HIV-1 p24 by pDC (Gen2.2 cells).
Discussion
We have demonstrated that HIV controllers, when compared to non-controllers, exhibit an IgG antibody response against HIV-1 p24 that is characterized by higher pDC-reactive opsonophagocytic antibody activity and greater isotype diversification. Furthermore, these characteristics were higher in viremic controllers than elite controllers. In addition, pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 inversely correlated with plasma HIV viral load in all viremic patients. We also demonstrated that IgG2 antibodies to HIV-1 gp140 were higher in elite controllers than non-controllers and viremic controllers, supporting the findings of previous studies, which showed that slower progression of HIV disease in long-term non-progressors was associated with IgG2 antibodies against HIV Env antigens (33, 34).
Numerous studies have demonstrated that higher serum/plasma levels or avidity of IgG antibodies against HIV-1 Gag proteins are associated with slower progression of HIV disease (reviewed in (11)). However, to our knowledge, this is the first study to show an association between the functional activity of an IgG antibody response against HIV Gag proteins and natural control of HIV infection. It has been proposed that IgG antibodies against HIV-1 p24 are markers of CD4+ T cell numbers and/or responses against Gag proteins (42). However, we did not demonstrate a correlation between CD4+ T cell counts and either opsonophagocytic antibody responses against HIV-1 p24 or the plasma level of IgG1 and IgG2 antibodies to HIV-1 p24. Furthermore, our observation that pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 were negatively correlated with plasma HIV RNA levels in all viremic patients provides evidence that these antibodies might contribute to control of HIV replication.
A notable finding of our study was that IgG antibody responses against HIV-1 p24 were higher in viremic controllers than in elite controllers as well as non-controllers. This finding was demonstrated by independent assays (ELISAs and pDC-reactive opsonophagocytic antibodies) and corroborated by the finding that isotype diversification of IgG antibodies against HIV-1 p24 was higher in viremic controllers than elite controllers, who had a greater diversification than non-controllers (Figure 3e). Possible explanations for these findings are, on the one hand that higher antibody responses are a consequence of greater HIV replication in viremic controllers compared to elite controllers, and on the other hand that IgG antibodies to HIV-1 p24 contribute to control of HIV replication in patients who cannot exert elite control of the infection. We posit two arguments against the former explanation. Firstly, patients with the highest amount of HIV replication (non-controllers) had the lowest magnitude and isotype diversification of IgG antibody responses against HIV-1 p24, which is unlikely to simply reflect more severe immunodeficiency in this group because there was no relationship between IgG antibody responses against HIV-1 p24 and CD4+ T cell counts in the whole SCOPE cohort, which was selected for patients with CD4+ T cell counts >350/μL. Secondly, IgG2 antibodies to HIV gp140 were highest in elite controllers who, by definition, had the lowest amount of HIV replication.
Our findings for opsonophagocytic antibody responses, and plasma levels of IgG1 and IgG2 antibodies against HIV-1 p24 are similar to those for CD4+ T cell responses against HIV-1 Gag proteins in HIV patients with persistent low-level HIV replication on ART, who exhibited higher CD4+ T cell responses against HIV-1 Gag proteins than ART-treated patients with optimally suppressed HIV replication or ART-naïve patients (43, 44). In those studies, the highest responses were also demonstrated in individuals with low but detectable HIV viremia. Theoretically, in states in which the immune system is functional and contributing to the control of a pathogen (as in HIV controllers), a higher steady-state level of that pathogen (HIV RNA in this case) will be associated with higher host responses aimed at controlling that pathogen (IgG antibody responses) (43, 44). This proposal would provide an explanation for why other investigators have not shown an association between IgG1 or IgG2 antibodies to HIV-1 Gag proteins and control of HIV infection in unselected HIV controllers (45) or HIV controllers who did not carry ‘protective’ HLA-B alleles (46), because those studies examined predominantly, or only, elite HIV controllers.
Our hypothesis that pDC-reactive opsonophagocytic antibody responses against HIV Gag proteins contribute to control of HIV infection is supported by the findings of studies on influenza virus infection in mice, which demonstrated that nucleoprotein-specific IgG antibodies mediated clearance of, and heterotypic immunity against, influenza viruses (47). Clearance of influenza virus infection was dependent on class-switched IgG antibodies against influenza virus nucleoproteins that mediated downstream IFNα/β production by a mechanism involving the cooperation of FcγR and Toll-like receptor 7 (TLR7) (48). We propose that a similar control mechanism, involving an opsonophagocytic antibody response against complexes of HIV-1 Gag proteins and HIV RNA, their uptake by pDC and subsequent downstream activation of TLR7 and IFNα-mediated restriction of viral replication is a potential control mechanism for HIV-1 infection. While it is possible that production of IgG antibodies to HIV-1 Gag proteins is a consequence of CD8+ T-cell- or NK cell-mediated killing of HIV-1-infected cells and subsequent release of HIV Gag/RNA complexes contained in their cytosol (49, 50), a pDC-reactive opsonophagocytic antibody response against HIV-1 Gag/RNA complexes may be a complementary immune control mechanism to CD8+ T cell responses, as suggested by studies of influenza virus infection in mice (47).
There is a growing awareness that the function of IgG antibodies determined by the hinge and Fc regions of IgG molecules is crucial to the activity of some IgG antibody responses against HIV-1 antigens, and that this reflects the IgG subclass composition of antibodies and affects antibody-mediated activation of effector cell responses, such as those mediated by NK cells via FcγRIIIa and/or FcγRIIc (51) and phagocytic cells via FcγRIIa (52). Antibody-dependent NK cell responses against HIV Env are higher in elite HIV controllers than non-controllers (53) and prevention of HIV-1 infection in subjects enrolled into the RV144 HIV vaccine study has been associated with both antibody-dependent NK cell responses and production of IgG3 antibodies against Env (41). Our study, which addressed the role of antibodies in the control rather than prevention of HIV infection, demonstrated that opsonophagocytic antibody responses against HIV-1 p24 mediated by pDC (Gen2.2 cells) occurred through FcγRIIa (Figure 5a), which is the major activating FcγR on human pDCs (31). Possession of the FcγRIIaH-131 genotype, which encodes for an FcγRIIa variant with high affinity binding to IgG antibodies (28), is associated with a slower rate of CD4+ T cell decline in HIV-1 infected men (54). In addition, we have shown that HIV-infected patients who possessed both the FcγRIIaH-131 genotype and produced IgG2 antibodies against HIV-1 p24 after vaccination with a DNA vaccine encoding HIV Gag-Pol and IFNγ, maintained lower viral loads than those patients who had only one of these characteristics (55).
We also demonstrated that isotype diversification (skewing towards IgG2) of IgG antibodies to HIV-1 p24 was greater in viremic HIV controllers than elite controllers and non-controllers. Furthermore, while plasma levels of IgG1 or IgG2 antibodies to HIV-1 p24 exhibited weaker negative correlations with plasma HIV RNA than pDC-reactive opsonophagocytic antibody responses against HIV-1 p24 in all viremic HIV patients, the correlation for IgG2 anti-HIV-1 p24 was stronger than for IgG1 anti-HIV-1 p24 (Figure 4). These findings suggest that enrichment of the IgG antibody response against HIV-1 p24 for IgG2 antibodies is a component of the IgG antibody response against HIV-1 Gag that is associated with control of HIV infection. We therefore examined the effect of IgG2 antibodies in the assay of pDC-reactive opsonophagocytic antibody responses against HIV-1 p24. Depletion of IgG2 from immunoglobulin preparations slightly increased phagocytosis of HIV-1 p24-coated beads complexed with antibodies. These findings support those of Forthal et al. (56), who demonstrated that IgG2 antibodies inhibited phagocytosis of antibody-opsonized HIV-1 virus-like particles by monocytes, and are in accord with observations that IgG2 has a lower affinity for all Fcγ receptors than other IgG subclasses (28). Therefore, while the function of the Fc region of IgG2 antibodies is more restricted to phagocytosis than other IgG subclasses, by virtue of dominant binding to FcγRIIaH-131 (28), our findings suggest that IgG2 antibodies are likely to exert their effect on opsonophagocytic antibody responses against antigens of the HIV core by affecting opsonization and/or immune complex formation more than Fc receptor binding. This may reflect the characteristics of IgG2 molecules that facilitate the binding of the Fab region to multivalent antigens (23–27). Furthermore, plasma IgG/IgM complexes containing IgG2 predominate in healthy individuals (57) and we have shown that FcγRIIa-binding immune complexes in the plasma of HIV controllers are enriched for IgG2 when compared with non-controllers (35).
As with all studies of immune correlates of HIV-1 control, we acknowledge that our study is unable to discern cause and effect. However, our data presents evidence that IgG antibody responses against HIV-1 p24 may contribute to control of HIV replication and of a mechanism by which this may occur. We also acknowledge that by using the Gen2.2 cell line to assay opsonophagocytic antibody responses we have not addressed the possible contribution of the patient’s pDC. However, in this study, we sought to analyze the opsonophagocytic function of IgG antibodies against HIV-1 p24 independent of cellular factors.
In summary, we provide novel data indicating that natural control of HIV-1 infection is associated with higher pDC-reactive opsonophagocytic antibody responses and greater isotype diversification of IgG antibodies against HIV-1 p24 compared with non-controllers. Furthermore, this effect was most notable in viremic controllers and was independent of CD4+ T cell counts. Further studies are required to elucidate the properties of IgG2 antibodies against HIV proteins that are associated with natural control of HIV-1 infection.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (grant 510448) and the Medical Research Foundation of Royal Perth Hospital (grant 2011/027). The UCSF SCOPE cohort was supported by the Delaney AIDS Research Enterprise (DARE; AI096109), the UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), and the CFAR Network of Integrated Systems (R24 AI067039). S.F. was supported by a priming grant from the Raine Medical Research Foundation.
We thank Drs J. Plumas and L. Chaperot for kindly providing the Gen2.2 cell line.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archin N, Liberty A, Kashuba A, Choudhary S, Kuruc J, Crooks A, Parker D, Anderson E, Kearney M, Strain M. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emu B, Sinclair E, Hatano H, Ferre A, Shacklett B, Martin JN, McCune J, Deeks SG. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008;82:5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace MJ, Graf EH, Agosto LM, Mexas AM, Male F, Brady T, Bushman FD, O’Doherty U. Directly infected resting CD4+ T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Path. 2012;8:e1002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graf EH, Pace MJ, Peterson BA, Lynch LJ, Chukwulebe SB, Mexas AM, Shaheen F, Martin JN, Deeks SG, Connors M. Gag-Positive Reservoir Cells Are Susceptible to HIV-Specific Cytotoxic T Lymphocyte Mediated Clearance In Vitro and Can Be Detected In Vivo. PLoS One. 2013;8:e71879. doi: 10.1371/journal.pone.0071879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J. More woes for struggling HIV vaccine field. Science. 2013;340:667–667. doi: 10.1126/science.340.6133.667. [DOI] [PubMed] [Google Scholar]
- 9.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:227–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French MA, Abudulai LN, Fernandez S. Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection. Vaccines. 2013;1:328–342. doi: 10.3390/vaccines1030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H, Buzon MJ, Shaw A, Berg RK, Xu GY, Ferrando-Martinez S, Leal M, Ruiz-Mateos E, Lichterfeld M. Hepatitis C Therapy With Interferon-α and Ribavirin Reduces CD4 T-Cell–Associated HIV-1 DNA in HIV-1/Hepatitis C Virus–Coinfected Patients. J Infect Dis. 2014;209:1315–1320. doi: 10.1093/infdis/jit628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomescu C, Duh FM, Hoh R, Viviani A, Harvill K, Martin MP, Carrington M, Deeks SG, Montaner LJ. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. AIDS. 2012;26:1869–1878. doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barblu L, Machmach K, Gras C, Delfraissy JF, Boufassa F, Leal M, Ruiz-Mateos E, Lambotte O, Herbeuval JP. Plasmacytoid Dendritic Cells (pDCs) From HIV Controllers Produce Interferon-α and Differentiate Into Functional Killer pDCs Under HIV Activation. J Infect Dis. 2012;206:790–801. doi: 10.1093/infdis/jis384. [DOI] [PubMed] [Google Scholar]
- 17.Machmach K, Leal M, Gras C, Viciana P, Genebat M, Franco E, Boufassa F, Lambotte O, Herbeuval J, Ruiz-Mateos E. Plasmacytoid dendritic cells reduce HIV production in elite controllers. J Virol. 2012;86:4245–4252. doi: 10.1128/JVI.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 19.Lannes N, Python S, Summerfield A. Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses. Vet Res. 2012;43:64. doi: 10.1186/1297-9716-43-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikolajczyk MG, Concepcion NF, Wang T, Frazier D, Golding B, Frasch CE, Scott DE. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin Diagn Lab Immunol. 2004;11:1158–1164. doi: 10.1128/CDLI.11.6.1158-1164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soininen A, Seppälä I, Nieminen T, Eskola J, Käyhty H. IgG subclass distribution of antibodies after vaccination of adults with pneumococcal conjugate vaccines. Vaccine. 1999;17:1889–1897. doi: 10.1016/s0264-410x(98)00475-7. [DOI] [PubMed] [Google Scholar]
- 22.Jung DJ, An JH, Kurokawa K, Jung YC, Kim MJ, Aoyagi Y, Matsushita M, Takahashi S, Lee HS, Takahashi K. Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. J Immunol. 2012;189:4951–4959. doi: 10.4049/jimmunol.1201294. [DOI] [PubMed] [Google Scholar]
- 23.Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, Plant M, Henkle B, Li Y, Deechongkit S. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 2008;283:16206–16215. doi: 10.1074/jbc.M709988200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wypych J, Li M, Guo A, Zhang Z, Martinez T, Allen MJ, Fodor S, Kelner DN, Flynn GC, Liu YD. Human IgG2 antibodies display disulfide-mediated structural isoforms. J Biol Chem. 2008;283:16194–16205. doi: 10.1074/jbc.M709987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez T, Guo A, Allen MJ, Han M, Pace D, Jones J, Gillespie R, Ketchem RR, Zhang Y, Balland A. Disulfide connectivity of human immunoglobulin G2 structural isoforms. Biochemistry. 2008;47:7496–7508. doi: 10.1021/bi800576c. [DOI] [PubMed] [Google Scholar]
- 26.Yoo EM, Wims LA, Chan LA, Morrison SL. Human IgG2 can form covalent dimers. J Immunol. 2003;170:3134–3138. doi: 10.4049/jimmunol.170.6.3134. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Goetze AM, Flynn GC. Assessment of naturally occurring covalent and total dimer levels in human IgG1 and IgG2. Mol Immunol. 2014;58:108–115. doi: 10.1016/j.molimm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 29.Syam S, Mero P, Pham T, McIntosh CA, Bruhns P, Booth JW. Differential recruitment of activating and inhibitory FcγRII during phagocytosis. J Immunol. 2010;184:2966–2973. doi: 10.4049/jimmunol.0900016. [DOI] [PubMed] [Google Scholar]
- 30.den Dunnen J, Vogelpoel LT, Wypych T, Muller FJ, de Boer L, Kuijpers TW, Zaat SA, Kapsenberg ML, de Jong EC. IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcγRIIa in human dendritic cells. Blood. 2012;120:112–121. doi: 10.1182/blood-2011-12-399931. [DOI] [PubMed] [Google Scholar]
- 31.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcγ receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 32.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 33.Ngo-Giang-Huong N, Candotti D, Goubar A, Autran B, Maynart M, Sicard D, Clauvel J-P, Agut H, Costagliola D, Rouzioux C. HIV type 1-specific IgG2 antibodies: markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res Hum Retroviruses. 2001;17:1435–1446. doi: 10.1089/088922201753197105. [DOI] [PubMed] [Google Scholar]
- 34.Martinez V, Costagliola D, Bonduelle O, N’go N, Schnuriger A, Théodorou I, Clauvel JP, Sicard D, Agut H, Debré P. Combination of HIV-1-specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term nonprogression. J Infect Dis. 2005;191:2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- 35.French MA, Center RJ, Wilson KM, Fleyfel I, Fernandez S, Schorcht A, Stratov I, Kramski M, Kent SJ, Kelleher AD. Isotype-switched immunoglobulin G antibodies to HIV Gag proteins may provide alternative or additional immune responses to ‘protective’ human leukocyte antigen-B alleles in HIV controllers. AIDS. 2013;27:519–528. doi: 10.1097/QAD.0b013e32835cb720. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Yoshikawa H, Yasugi T, Nakagawa S, Kawana K, Nozawa S, Hoshiai H, Shiromizu K, Kanda T, Taketani Y. Balance of IgG subclasses toward human papillomavirus type 16 (HPV16) L1-capsids is a possible predictor for the regression of HPV16-positive cervical intraepithelial neoplasia. Biochem Biophys Res Commun. 1999;258:128–131. doi: 10.1006/bbrc.1999.0588. [DOI] [PubMed] [Google Scholar]
- 37.Jackson KJ, Wang Y, Collins AM. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol Cell Biol. 2014;92:729–733. doi: 10.1038/icb.2014.44. [DOI] [PubMed] [Google Scholar]
- 38.Pan-Hammarström Q, Zhao Y, Hammarström L. Class switch recombination: a comparison between mouse and human. Adv Immunol. 2007;93:1–61. doi: 10.1016/S0065-2776(06)93001-6. [DOI] [PubMed] [Google Scholar]
- 39.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 40.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Sci Transl Med. 2014;6:228ra238–228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 42.Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, Moore JP. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeks SG, Martin JN, Sinclair E, Harris J, Neilands TB, Maecker HT, Hagos E, Wrin T, Petropoulos CJ, Bredt B. Strong Cell-Mediated Immune Responses Are Associated with the Maintenance of Low-Level Viremia in Antiretroviral–Treated Individuals with Drug-Resistant Human Immunodeficiency Virus Type 1. J Infect Dis. 2004;189:312–321. doi: 10.1086/380098. [DOI] [PubMed] [Google Scholar]
- 44.Alatrakchi N, Duvivier C, Costagliola D, Samri A, Marcelin A, Kamkamidze G, Astriti M, Agher R, Calvez V, Autran B. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS. 2005;19:25–33. doi: 10.1097/00002030-200501030-00003. [DOI] [PubMed] [Google Scholar]
- 45.Banerjee K, Klasse P, Sanders RW, Pereyra F, Michael E, Lu M, Walker BD, Moore JP. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses. 2010;26:445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai JI, Licht AF, Dugast AS, Suscovich T, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. Divergent antibody subclass and specificity profiles but not protective HLA-B alleles are associated with variable antibody effector function among HIV-1 controllers. J Virol. 2014;88:2799–2809. doi: 10.1128/JVI.03130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaMere MW, Moquin A, Lee FEH, Misra RS, Blair PJ, Haynes L, Randall TD, Lund FE, Kaminski DA. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J Virol. 2011;85:5027–5035. doi: 10.1128/JVI.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaMere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, Kaminski DA. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. 2011;186:4331–4339. doi: 10.4049/jimmunol.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proceedings of the National Academy of Sciences. 2009;106:19114–19119. doi: 10.1073/pnas.0907364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Grunwald D, Sardo L, Galli A, Plisov S, Nikolaitchik OA, Chen D, Lockett S, Larson DR, Pathak VK. Cytoplasmic HIV-1 RNA is mainly transported by diffusion in the presence or absence of Gag protein. Proceedings of the National Academy of Sciences. 2014;111:E5205–E5213. doi: 10.1073/pnas.1413169111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, Pyo CW, Zolla-Pazner S, Montefiori D, Liao HX. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J Clin Invest. 2014;124:3879–3890. doi: 10.1172/JCI75539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J Virol. 2013;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, Delfraissy JF, Saez-Cirion A, Ferrari G. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS One. 2013;8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcγRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- 55.French MA, Tanaskovic S, Law MG, Lim A, Fernandez S, Ward LD, Kelleher AD, Emery S. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a ‘high-affinity’ FcγRIIa genotype. AIDS. 2010;24:1983–1990. doi: 10.1097/QAD.0b013e32833c1ce0. [DOI] [PubMed] [Google Scholar]
- 56.Forthal DN, Landucci G, Ding H, Kappes JC, Wang A, Thung I, Phan T. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS. 2011;25:2099–2104. doi: 10.1097/QAD.0b013e32834b64bd. [DOI] [PubMed] [Google Scholar]
- 57.Stahl D, Sibrowski W. IgG2 containing IgM–IgG immune complexes predominate in normal human plasma, but not in plasma of patients with warm autoimmune haemolytic anaemia. Eur J Haematol. 2006;77:191–202. doi: 10.1111/j.1600-0609.2006.00691.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.