Abstract
Background
Bacillary angiomatosis (BA) is a rare manifestation of infection caused by Bartonella species, which leads to vasoproliferative lesions of skin and other organs. Bacillary angiomatosis affects individuals with advanced HIV disease or other immunocompromised individuals. In sub-Saharan Africa, despite the high prevalence of HIV infection and documentation of the causative Bartonella species in humans, mammalian hosts, and arthropod vectors, BA has only rarely been described.
Methods
Three adult patients from Uganda and Kenya with deep purple dome-shaped papules or nodules of the skin underwent punch biopsies for histopathologic diagnosis. The biopsies of all 3 patients were sent to a local pathologist as well as to a dermatopathologist at the University of California, San Francisco.
Results
All 3 patients were clinically suspected to have Kaposi’s sarcoma (KS), and local pathologists had interpreted the lesions as KS in 2 of the cases and nonspecific inflammation in the third. Histologic examination by dermatopathologists in the United States revealed nodular dermal proliferations of irregular capillaries lined by spindled to epithelioid endothelial cells. The surrounding stroma contained a mixed inflammatory infiltrate with lymphocytes, eosinophils, and neutrophils. Extracellular deposits of pale amphophilic granular material were noted in the surrounding stroma. A Warthin-Starry stain highlighted clumps of bacilli, confirming the diagnosis of BA.
Conclusions
These 3 cases, to our knowledge, are the first reports of BA in East Africa in the biomedical literature. Each had been originally incorrectly diagnosed as KS. We speculate BA is underdiagnosed and underreported in resource-poor regions, such as sub- Saharan Africa, that have high endemic rates of HIV infection.
Keywords: bacillary angiomatosis, Kaposi’s sarcoma, HIV, dermatology, Africa
Introduction
Bacillary angiomatosis (BA), first described by Stoler et al in 1983, represents a Bartonella henselae or B quintana infection causing vasoproliferative lesions in immunocompromised persons, including those with advanced HIV disease.1–3 Bartonella is a genus of fastidious gram-negative bacteria found most commonly in humans, cats, dogs, rodents, and cattle, although a range of mammalian hosts have been documented.4,5 Infection is spread to mammals by arthropod vectors, including ticks, biting flies, fleas, and body lice.6–8 In addition to BA, B henselae and B quintana cause a variety of other diseases, including endocarditis, cat scratch fever, trench fever, and peliosis hepatis.9–14
The typical cutaneous lesions of BA appear as single or multiple bright red to deep purple dome-shaped papules, nodules, or plaques.15,16 Bacillary angiomatosis is often clinically indistinguishable from Kaposi’s sarcoma (KS), which similarly presents as red to purple papules, nodules, or plaques in immunocompromised patients. Differentiating BA from KS is largely reliant on skin biopsy with histopathologic examination, which, for BA, demonstrates protuberant endothelial cells surrounded by clumps of bacilli that are visible with Warthin-Starry staining.17
Despite some of the highest rates of HIV infection in the world, BA has only rarely been reported in sub-Saharan Africa. Only 5 cases of BA from the African continent have been published in the English literature to date, affecting patients from Zimbabwe, South Africa, and the Ivory Coast.18–21 In addition to an abundance of immunocompromised hosts, there is convincing evidence that the causative Bartonella species is present on the continent. Bartonella bacteremia was found by polymerase chain reaction in 10% of HIV-infected outpatients in Johannesburg, South Africa.9 There have been reports of greater than 20% sero-positivity in dogs and cats in South Africa and Zimbabwe.12,13 Finally, a study of body lice from Rwanda and Burundi frequently isolated B quintana by PCR.22
Following the introduction of skin biopsy and supportive dermatopathology services to several medical centers in East Africa, we describe 3 cases of biopsy-proven BA.
Case Reports
Case 1
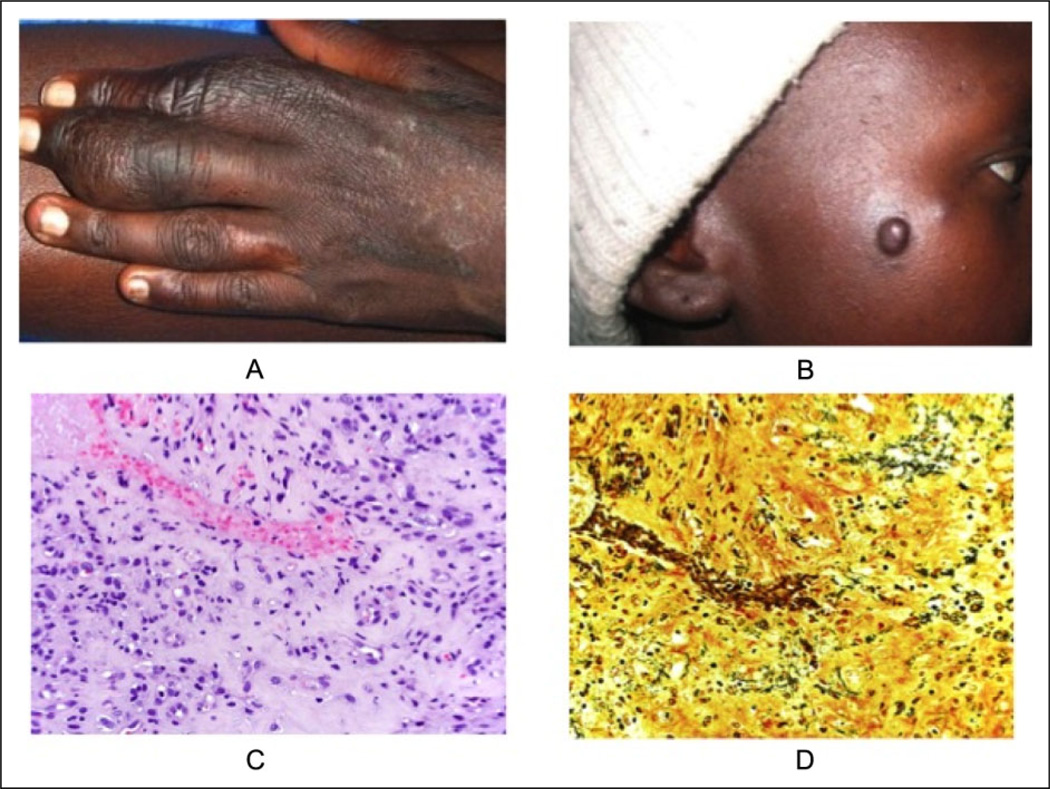
In January 2009, a 25-year-old HIV-infected Kenyan woman presented with red-purple dome-shaped papules on the face and swelling of her left third finger (Figure 1A and B). Her most recent CD4 count, performed in July 2008, was 93 cells/mm3. In January 2009, she began antiretroviral treatment and underwent a punch biopsy that was interpreted by a local pathologist as KS. A second biopsy, performed in February 2009, was also read as KS. Chemotherapy with adriamycin, vincristine, and bleomycin was initiated in March 2009. The patient received a total of 4 cycles of chemotherapy without clinical improvement. A third biopsy was obtained in May 2009 and was sent, along with the initial biopsies, for a second opinion to the University of California, San Francisco (UCSF). Conventional microscopy revealed a diffuse nodular vascular proliferation throughout the entire dermis. The epidermis was hyperplastic with an overlying hemorrhagic crust. A Warthin-Starry stain was performed and highlighted many small bacilli within the stroma of the vascular proliferation (Figure 1C and D). Doxycycline 100 mg twice per day was initiated. Within 2 weeks of beginning doxycycline, the patient’s vascular papules and the finger swelling had resolved. As of February 2012, the patient continued to do well, with no recurrence of her skin lesions.
Figure 1.
Case 1: a 25-year-old woman from Eldoret, Kenya. A) Swelling of left finger; B) Papule on right cheek; C) Vascular proliferation surrounded by inflamed, fibrotic stroma; D) Warthin-Starry stain highlights stubby bacilli within the stroma of vascular proliferation.
Case 2
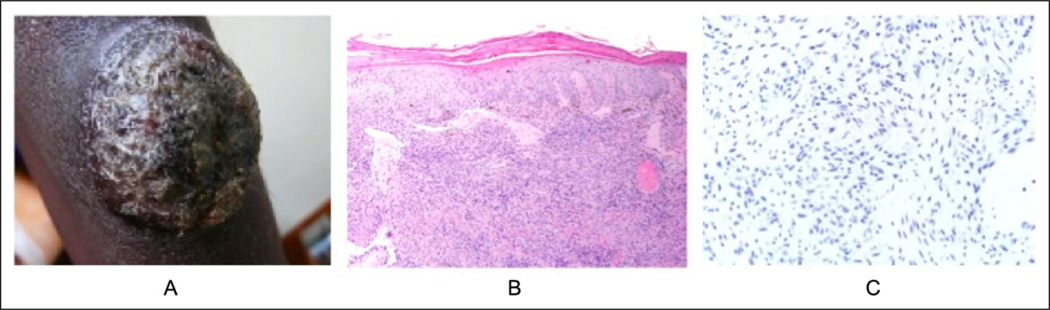
In January 2009, a 29-year-old Ugandan woman with unknown HIV status presented with a 10-cm dome-shaped crusted deep red lesion on the right upper arm (Figure 2A). The clinical differential diagnosis included KS and keratoacanthoma-type squamous cell carcinoma. The patient was sent for HIV testing, and biopsy material was sent to both UCSF and a local pathologist who interpreted the findings as KS. At UCSF, the histopathologic examination revealed an irregular epidermal hyperplasia with parakeratosis (Figure 2B and C). Scattered melanophages were noted below this. Nearly the entire sampled dermis was involved, with a diffuse vascular proliferation composed of spindled to plump endothelial cells. The surrounding stroma had areas that had an amphophilic granular quality. Warthin-Starry stain was performed and showed black-staining bacilli within the stroma of the proliferation, confirming the diagnosis of BA. Immunostaining for HHV-8 was negative. Following the biopsy, the patient was lost to follow-up, and her clinical course remains unknown.
Figure 2.
Case 2: a 29-year-old female from Mbarara, Uganda. A) 10cm crusted dome-shaped tumor on the upper arm; B) Irregular epidermal hyperplasia with parakeratosis overlying and inflamed vascular dermal proliferation with fibrotic stroma; C) An HHV-8 stain for latent nuclear antigen of human herpesvirus-8 shows lack of nuclear immunopositivity.
Case 3
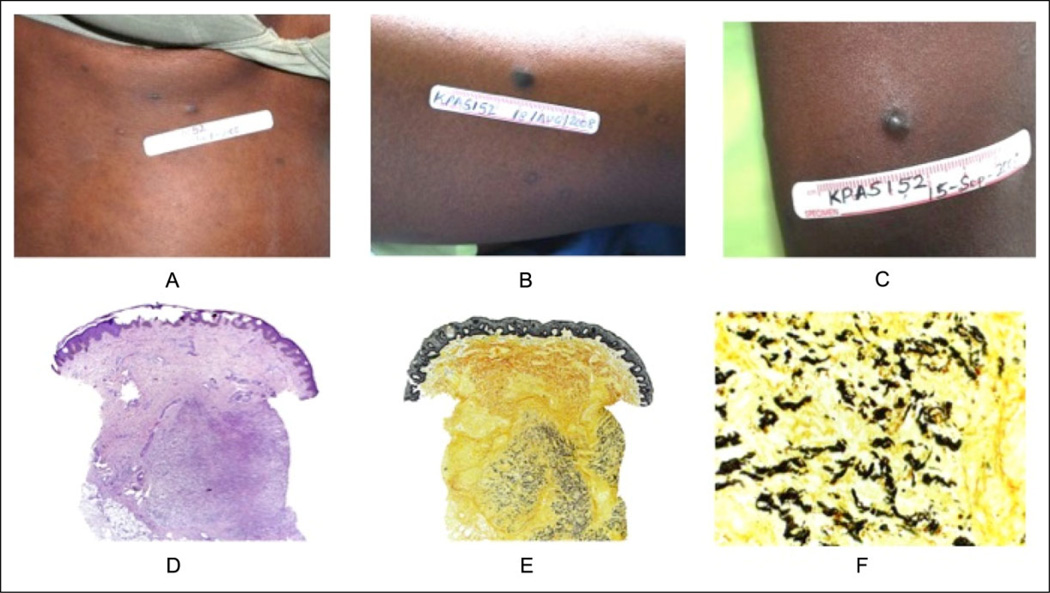
A 22-year-old HIV-infected woman was referred to a clinical trial for KS in Uganda in July 2008 with multiple deep-purple papules and plaques on her face, trunk, and extremities (Figure 3A–C). An initial biopsy from the chest was consistent with KS. Her CD4 count at the time of presentation was 160 cells/mm3, and she began antiretroviral treatment. After 1 month, her CD4 count had risen to 252 cells/mm3 and new similar-appearing papules had developed. Another biopsy was performed, this time from the left thigh, and was read by the local pathologist as “chronic nonspecific inflammation, with no evidence of Kaposi sarcoma.” A third biopsy from the left upper arm was performed a month later and was also read as KS. The patient discontinued her antiretroviral therapy in September 2008 and died in October 2008. After the patient’s death, the second biopsy was later interpreted by a dermatopathologist at UCSF and demonstrated a nodular vascular proliferation within the deep reticular dermis (Figure 3D–F). Warthin-Starry stain highlighted filamentous organisms in the interstitium of an inflamed vascular proliferation, confirming the diagnosis of BA. The first and third biopsies were consistent with KS.
Figure 3.
Case 3: a 22 year-old female from Kampala, Uganda. A) Site of first biopsy, papule on chest; B) Site of second biopsy, papule on left thigh, called KS by local pathologist, later BA by UCSF dermatopathologists; C) Site of 3rd biopsy, papule on left upper arm; D) from lesion B: Nodular vascular proliferation within deep reticular dermis; E) From lesion B: Nodular vascular proliferation within deep reticular dermis; F) From lesion B: High-power view of Warthin-Starry stain.
Discussion
Bacillary angiomatosis presents as vasoproliferative lesions that most frequently involve the skin but can also affect other organs such as the respiratory tract, bone, lymph nodes, gastrointestinal tract, and brain. Cutaneous lesions have a variety of morphologies, including papular, nodular, pedunculated, or verrucous forms, which often appear as small red-purple papules before enlarging. The lesions may bleed profusely with trauma and are usually singular or several in number but may rarely appear in disseminated form.14,15 The clinical differential diagnosis for a lesion of cutaneous BA may include KS, pyogenic granuloma, cherry angioma, dermatofibroma, hemangioma, mycobacterial infection such as tuberculosis, coccidioidomycosis, cryptococcosis, and histoplasmosis.23
Where resources permit, skin biopsy is the preferred method of diagnosing BA. Culture and serology are insensitive.24,25 Although PCR is sensitive and specific,26 it is expensive and is used primarily for research or in the rare clinical setting when no skin lesion is present to biopsy. Differentiating BA histologically from other benign and malignant vascular proliferations, including KS, is facilitated by the use of histochemical (ie, Warthin-Starry staining to detect B bacilli) and immunohistochemical (ie, anti- HHV-8) stains. In resource-limited settings, where these stains may not be available, distinguishing between BA and KS can be challenging, as the pathologist must rely on routine histopathologic poorly clues. Both BA and KS may have nodular architecture on low power and are composed of thin-walled vessels. Bacillary angiomatosis lesions have well-developed capillaries and inflamed stroma that harbors clumps of basophilic bacilli and acute inflammatory cells. On the other hand, KS is composed of ill-developed vasculature with slit-like spaces, prominent spindle cells, many extravasated erythrocytes, and siderophages. These features are less commonly seen in BA. Additionally, KS often has an abundance of plasma cells, in contrast to the mixed infiltrate, including neutrophils, that serve as an additional clue to the diagnosis of BA.
In the African context, even at many academic medical centers, skin biopsy is not routinely performed when KS is suspected clinically. In the cases we describe here, it was the presence of various research studies that made skin biopsy available in these medical centers. However, even when biopsies are performed, lack of training in dermatopathology and lack of access to histochemical and immunohistochemical stains may lead to misdiagnosis by pathologists, which occurred in all 3 of our cases.
These cases of BA in Eastern Africa illustrate the likely underdiagnosis of BA as a whole, and more specifically, the clinical misdiagnosis of BA as KS. Bacillary angiomatosis misdiagnosed as KS can have 2 dire consequences: failure to treat BA, which can affect bone and viscera and may be life threatening, and unnecessary administration of highly toxic chemotherapeutic agents intended to treat KS. Treatment with appropriate antibiotic therapy (current recommendations favor either doxycycline or erythromycin for 3 months) typically leads to complete resolution.25 Failure to treat often results in death from hepatic or pulmonary failure.27
In summary, BA is likely underdiagnosed and underreported in resource-limited countries heavily affected by the HIV pandemic. Notably, BA can be clinically and histopathologically confused with KS. Our 3 cases from East Africa underscore the need for a comprehensive strategy aimed at improving the diagnosis of KS and its clinical mimickers in sub-Saharan Africa and emphasize the need to increase the availability of biopsies, augment training of pathologists in dermatopathology, and enhance access to special stains.
Acknowledgments
We would like to thank Dr Gerold Jaeger for providing the clinical photograph of patient 2.
Dr Amerson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Amerson, Maurer, and Martin contributed to study concept and design. Naujokas and McCalmont contributed to data analysis and interpretation. Forrestel and Naujokas contributed to drafting of the manuscript. Amerson, Martin, McCalmont, Maurer, Laker-Oketta, Mulyowa, and Busakhala contributed to critical revision of the manuscript for important intellectual content. Statistical analysis is not applicable for this article. Amerson, Maurer, and Martin obtained funding for this article. Administrative, technical, or material supports are not applicable.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Dermatology Foundation, the NIH-funded International Epidemiologic Databases to Evaluate AIDS Consortium (U01 AI069911), the Antiretroviral Therapy for Kaposi Sarcoma (R01 CA119903) study, and the UCSF-Uganda Training Program in HIV-associated Malignancies (D43 CA153717).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Stoler MH, Bonfiglio TA, Steigbigel RT, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983;80(5):714–718. doi: 10.1093/ajcp/80.5.714. [DOI] [PubMed] [Google Scholar]
- 2.Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis. an approach to the identification of uncultured pathogens. N Engl J Med. 1990;323(23):1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 3.Slater LN, Welch DF, Min KW. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch Intern Med. 1992;152(3):602–606. [PubMed] [Google Scholar]
- 4.Chomel BB, Boulouis HJ. Zoonotic diseases caused by bacteria of the genus Bartonella genus: new reservoirs? new vectors? Bull Acad Natl Med. 2005;189(3):465–477. [PubMed] [Google Scholar]
- 5.Pitassi LHU, Magalhães RF, Barjas-Castro ML, de Paula EV, Ferreira MR, Velho PE. Bartonella henselae infects human erythrocytes. Ultrastruct Pathol. 2007;31(6):369–372. doi: 10.1080/01913120701696510. [DOI] [PubMed] [Google Scholar]
- 6.Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008;22(1):1–15. doi: 10.1111/j.1365-2915.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- 7.Cotté V, Bonnet S, Le Rhun D, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008;14(7):1074–1080. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Chomel BB, Kasten RW, Romano V, Tietze N. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol. 2001;39(4):1221–1226. doi: 10.1128/JCM.39.4.1221-1226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frean J, Arndt S, Spencer D. High rate of Bartonella henselae infection in HIV-positive outpatients in Johannesburg, South Africa. Trans R Soc Trop Med Hyg. 2002;96(5):549–550. doi: 10.1016/s0035-9203(02)90437-2. [DOI] [PubMed] [Google Scholar]
- 10.Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerging Infect Dis. 2005;11(2):216–224. doi: 10.3201/eid1102.040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Znazen A, Rolain JM, Hammami N, Kammoun S, Hammami A, Raoult D. High prevalence of Bartonella quintana endocarditis in Sfax, Tunisia. Am J Trop Med Hyg. 2005;72(5):503–507. [PubMed] [Google Scholar]
- 12.Kelly PJ, Rooney JJ, Marston EL, Jones DC, Regnery RL. Bartonella henselae isolated from cats in Zimbabwe. Lancet. 1998;351(9117):1706. doi: 10.1016/s0140-6736(05)77744-8. [DOI] [PubMed] [Google Scholar]
- 13.Kelly PJ, Eoghain GN, Raoult D. Antibodies reactive with Bartonella henselae and Ehrlichia canis in dogs from the communal lands of Zimbabwe. J S Afr Vet Assoc. 2004;75(3):116–120. doi: 10.4102/jsava.v75i3.465. [DOI] [PubMed] [Google Scholar]
- 14.Koehler JE, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1993;17(4):612–624. doi: 10.1093/clinids/17.4.612. [DOI] [PubMed] [Google Scholar]
- 15.Tappero JW, Perkins BA, Wenger JD, Berger TG. Cutaneous manifestations of opportunistic infections in patients infected with human immunodeficiency virus. Clin Microbiol Rev. 1995;8(3):440–450. doi: 10.1128/cmr.8.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohle-Boetani JC, Koehler JE, Berger TG, et al. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus: clinical characteristics in a case-control study. Clin Infect Dis. 1996;22(5):794–800. doi: 10.1093/clinids/22.5.794. [DOI] [PubMed] [Google Scholar]
- 17.LeBoit PE, Berger TG, Egbert BM, Beckstead JH, Yen TS, Stoler MH. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am J Surg Pathol. 1989;13(11):909–920. [PubMed] [Google Scholar]
- 18.Chitsike I, Muronda C. Bacillary angiomatosis in an HIV positive child. first case report in Zimbabwe. Cent Afr J Med. 1997;43(8):238–239. [PubMed] [Google Scholar]
- 19.Burgin S, Spencer DC. Bacillary angiomatosis–another South African case. S Afr Med J. 1996;86(1):95–96. [PubMed] [Google Scholar]
- 20.Levy GR, Nayler S. Bacillary angiomatosis. the first case reported in South Africa. S Afr Med J. 1993;83(11):855–856. [PubMed] [Google Scholar]
- 21.Ramdial PK, Sing Y, Ramburan A, Dlova NC, Bagratee JS, Calonje E. Bartonella quintana-induced vulval bacillary angiomatosis. Int J Gynecol Pathol. 2012;31(4):390–394. doi: 10.1097/PGP.0b013e31823f8463. [DOI] [PubMed] [Google Scholar]
- 22.Fournier PE, Ndihokubwayo JB, Guidran J, Kelly PJ, Raoult D. Human pathogens in body and head lice. Emerg Infect Dis. 2002;8(12):1515–1518. doi: 10.3201/eid0812.020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortes EE, Saraceni V, Medeiros D, Ribeiro I. Bacillary angiomatosis and Kaposi’s sarcoma in AIDS. AIDS Patient Care and STDS. 2000;14(4):179–182. doi: 10.1089/108729100317777. [DOI] [PubMed] [Google Scholar]
- 24.Comer JA, Flynn C, Regnery RL, Vlahov D, Childs JE. Antibodies to Bartonella species in inner-city intravenous drug users in Baltimore. Md Arch Intern Med. 1996;156(21):2491–2495. [PubMed] [Google Scholar]
- 25.Koehler JE, Sanchez MA, Tye S, et al. Prevalence of Bartonella infection among human immunodeficiency virus-infected patients with fever. Clin Infect Dis. 2003;37(4):559–566. doi: 10.1086/375586. [DOI] [PubMed] [Google Scholar]
- 26.Sander A, Penno S. Semiquantitative species-specific detection of Bartonella henselae and Bartonella quintana by PCR-enzyme immunoassay. J Clin Microbiol. 1999;37(10):3097–3101. doi: 10.1128/jcm.37.10.3097-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray MC, Gately LE., III Dermatologic manifestations of HIV infection and AIDS. Infect Dis Clin North Am. 1994;8(3):583–605. [PubMed] [Google Scholar]