Abstract
Water is one of the main sources of human exposure to microbiological hazards. Although legislation establishes regulatory standards in terms of fecal indicator bacteria to assess the microbiological quality of water, these do not necessarily predict the presence of pathogens such as parasites and viruses. Better surveillance and management strategies are needed to assess the risk of pathogens waterborne transmission. We established a baseline dataset to characterize river water quality, identify changes over time, and design a rational monitoring strategy. Data from a year-long monthly monitoring campaign of the polluted Arenales River (Argentina), were analyzed to statistically correlate physicochemical and microbiological variables, the seasonal and longitudinal variation of the water quality and determine the similarity between study sites. The measured variables (sixteen) reflected the deterioration in the river quality through the city. Different viruses and parasites found did not correlate with the concentration of total and thermotolerant coliforms. There was significant seasonal variation for temperature, turbidity, conductivity, dissolved oxygen, enterococci, and norovirus. Strong correlations between some variables were found; we selected eight variables (dissolved oxygen, conductivity, turbidity, total and thermotolerant coliforms, Enterococcus, and adenovirus and Microsporidium as viral and parasitological indicators, respectively) for future monitoring. There was similarity between the monitoring locations, which were grouped into four clusters validated by cophenetic correlation and supported by discriminant analysis. This allowed us to reduce the number of sites, from eleven down to five. Sixty seven percent of the total variance and the correlation structure between variables was explained using five principal components. All these analyses led to a new long-term systematic monitoring scheme A rational monitoring strategy based on the selection of the most suitable monitoring points and of the most significant variables to measure, will result in optimal use of the limited resources available to adequately protect public and environmental health.
Keywords: water quality, enteric virus, parasite, microbial indicator, surveillance strategy, PP7
1. Introduction
Water is the one of the main sources of human exposure to microbiological hazards. Pathogens are introduced into surface waters through different pathways such as industrial effluent, raw and treated sewage, stormwater and animal manure runoff, in addition to industrial and household solid waste disposal (van den Berg et al., 2005). Although Argentina has no specific legislation establishing limits for the presence of microbial organisms in surface water, International Standards from the Pan American Health Organization (PAHO) and from the European Community (EC) (Hederra, 1996; CEU-revised Bathing Water Directive, 2006; Kay et al., 2004; WHO, 2003) are used as models. In these regulations only bacterial indicators are designated (total and thermotolerant coliforms, enterococci and Escherichia coli) to assess the microbiological quality of water. However, these bacterial indicators do not accurately predict the presence of other pathogens such as protozoa, non-culturable pathogenic bacteria, and enteric viruses (Jiang et al., 2006; Noble and Fuhrman, 2001; Lipp et al., 2001). Therefore, specific monitoring for those pathogens should also be performed.
Over one hundred different species of viruses have been described in human and animal excreta (Melnick, 1984) and isolated from sewage water (Cukor and Blacklow, 1984). Between 105 and 1011 virus particles per gram of stool are excreted in the feces (Farthing, 1989). Enteric viruses are a major public health problem due to their low dose required for infection; the risk of infection is between 10- and 10,000-fold greater than a bacterial infection in similar situations of exposure (Haas et al., 1993). Although enteric virus infections are generally associated with gastroenteritis and diarrhea in vulnerable groups, these fecal-oral transmitted viruses may also cause other infections and diseases that have high mortality rates (Melnick, 1990; Kocwa-Haluch, 2001; Nwachuku and Gerba, 2006) and even chronic diseases (Peng and Hagopian, 2006). Emergence of waterborne outbreaks of enteric viruses is well documented in the literature (Gerba et al., 1996; Boccia et al., 2002; Mena and Gerba, 2009). Numerous investigations have associated virus polluted water-related recreational activities (swimming, canoeing, fishing, etc) with gastrointestinal disease (Hoebe et al., 2004; Sinclair et al., 2009), but only a small proportion of outbreaks were reported and investigated (Craun, 1991).
Some human viruses may be used as indicators of viral contamination (Pina et al., 1998; Albinana et al, 2009; Wyn-Jones et al., 2011). The strongest candidates seem to be adenovirus (AdV), norovirus (NV), and enterovirus (EV); polyomavirus was also suggested (McQuaig et al., 2009). Human AdV are commonly found in urban rivers and drinking water (Castignolles et al., 1998; Van Hereden et al., 2003). From the 51 serotypes known, only 40 and 41 have been recognized as a major cause of childhood diarrhea. AdVs have been identified by the Environmental Protection Agency (EPA, USA) as candidates for identifying human contamination in drinking water because they have shown to be more stable in water compared to other viruses and may be resistant to disinfection systems for drinking water (Thurston-Enriquez et al., 2003). On the other hand, NV are the most common cause of gastroenteritis in adults (Maunula et al., 2005) and also have a high incidence in children. They were reported to be responsible for approximately 10–30 million illnesses per year in the United States (Caro et al., 2001). NVs have recently been identified in aquatic environments such as wastewater effluents and rivers (Ueki et al., 2005; van den Berg et al., 2005). Several studies have documented their presence in recreational waters (Puig et al., 1994; Fuhrman et al. 2005; Donaldson et al., 2005). Despite extensive research, no unique universal indicator virus has been identified as yet and specific environmental characteristics may promote the existence of a particular indicator virus from the study site (Skraber et al., 2004).
Protozoan parasites are also responsible for various illnesses. Waterborne outbreaks of gastroenteritis have economic consequences related to the illnesses, but also produce a crisis in terms of confidence in the quality of the water sources and treatment. Many parasites that are transmitted by the fecal–oral route have been found in water (Dowd et al, 1998), but probably Cryptosporidium and Giardia are the most common ones (Gardner et al, 2001; Rose et al, 1996, Rose et al, 2002). They have been reported in wastewater (Mayer and Palmer, 1996), superficial water (Horman et al, 2004) and streambeds (Searcy et al, 2006), indoor pools (Schets et al, 2004), and drinking water (Hashimoto et al, 2002), living freely or in biofilms. Cryptosporidium caused the largest outbreak (400,000 people ill) ever documented in the U.S. when a drinking water treatment plant in Milwaukee (WI) malfunctioned (Mac Kenzie et al. 1994). Another very common protozoon, amoebae, present a double risk because they are pathogenic but can also transport amoeba-resistant strains of bacteria, viruses, and occasionally fungi, which can survive and grow after internalization and return safely to the environment (Greub and Raoult, 2004). When they, as well as other parasites, are confronted with severe environmental conditions that threaten their survival, they convert to cysts that are resistant to chlorination, adverse pH, osmotic pressure, and temperature.
Better surveillance and management strategies are needed to assess the risk of waterborne transmission of pathogens, and effective wastewater treatment is critical to protect the public health against epidemic enteric microbial infections. Oftentimes people have many questions regarding the quality of water resources like inland lakes, rivers, and streams. To find the correct answers it is necessary to measure some variables. Frequent issues at the moment of planning a monitoring scheme are the location and number of monitoring points to select, along a river for example, the number of samples to analyze to be representative of a place, the water volume needed to have meaningful results, whether the samples should be individually grabbed or be composite, the periodicity of sampling, and the number of variables necessary to measure in order to answer the questions without wasting scarce resources. Although different monitoring programs can be designed depending on the goals sought and the availability of resources, standardized procedures have already been developed and published (GWA, 2009; Eaton et al, 2005). The aims of this work were to establish a baseline dataset to characterize river water quality, to identify changes over time, and to design a better strategy for the rational monitoring in the future. The Arias-Arenales River (called simply the Arenales River subsequently), which crosses the city of Salta in Argentina, was selected as a model for this study. The results obtained from a year of monthly monitoring were analyzed to evaluate correlation between physicochemical and microbiological variables, the seasonal and longitudinal variation of the river quality, and the similarity between the studied sites. From that analysis we were able to identify the most relevant variables to be measured and the most suitable monitoring sites in order to design a rational scheme to check the river and/or discharges in the future to control (and eventually to correct) microbial pollution and to protect the public and environmental health.
2. Materials and Methods
2.1. Study area and sampling sites
The Arias-Arenales River belongs to the Juramento-Salado watershed in Salta, in the northwest of Argentina, and is subject to a seasonal fluctuation in water flow: very high flow during the rainy season in the summer (November to April) and very low during the dry season in the winter (May to October). At its source it runs west to east through a semi-rural area where the main uses are for water supply, agricultural irrigation, recreational activities, and livestock maintenance. Then it crosses the city of Salta in a south-east direction, discharging into the General Belgrano Reservoir located in the south, providing hydroelectric energy and water for different uses at downstream locations. When crossing the city, the river receives the impact of point and non-point source pollution (stormwater, illegal raw sewage, industrial effluents, and illegal solid waste disposal in Salta’s poorest areas, which developed unplanned through the years). Eleven points along the river as it crosses the city (12.5 Km), were initially identified as critical (Figure 1). Monthly monitoring was performed during 13 months (from February of 2009 to February of 2010). Five of the points (P) monitored were directly located on the river, while the other six corresponded to channels that discharge directly into the river. Briefly, P1 and P2 were selected as low-pollution controls on the Arias and Arenales Rivers, respectively, before entering the city; P3, P7, P8, and, P9 are stormwater sources flowing into the river; P4 is on the Isasmendi Creek at the confluence with the Arias River where a beef packing plant is located; P5 was a major discharge of untreated sewage; P6, on the river, is a recreational area called Parque Los Sauces (with picnic tables, grills, and a place for children to play); P10 and P11, on the river, are upstream and downstream of the wastewater treatment plant (WWTP) and municipal landfill.
Figure 1.
Geographical representation of the area under study: (a) Province of Salta in Argentina, (b) Capital Department in the Province of Salta, (c) Area of study in Salta City, main city of the Capital Department, (d) Monitoring points (P1-P11) along the Arias-Arenales River and wastewater treatment plant (WWTP). The ellipses correspond to points on the river while the rhombi correspond to points of discharges to the river.
2.2. Physicochemical variables and bacteriological analysis
Physicochemical variables including temperature, pH, turbidity, conductivity, salinity, and dissolved oxygen were measured in situ using a multiparametric equipment Horiba U-10 (Japan).
Three hundred milliliter water samples were collected in sterile bottles, kept refrigerated at 4 °C and taken to the laboratory for bacteriological analysis. Total and thermotolerant coliforms were determined by the multiple tubes method in MacConkey broth (48 h at 37 and 44 °C, respectively), applying the Most Probable Number Method (Eaton et al, 2005). E. coli (Method 1103.1) and enterococci (Method 1106.1) were determined using the Membrane Filtration Method (EPA, 2002).
2.3. Water concentration for parasitological and viral analysis
The number of parasites and viruses in environmental waters may be enough to produce illness but too low to reach the detection limit of the methods used, thus a concentration step is required. The concentration procedure described previously by Rajal et al. (2007a) was followed with small modifications. Briefly, 20 L-water samples were collected and spiked with PP7 (ATCC 15692-B2), bacteriophage of Pseudomonas aeruginosa, to a final concentration of 106 gene copies (gc) per mL. PP7 was used as an external control to calculate the efficiency of the concentration process and to assess for inhibition in PCR (Rajal et al, 2007a). The samples were filtered through two stainless-steel sieves (74 and 37 μm) to remove solids and received into the feed tank. From there, the water was pumped (using a peristaltic pump Apema BS6D) through the ultrafiltration unit with a 50,000 MW membrane cut-off (Microza AHP 1010, Pall Life Sciences, East Hills, NY) until the volume was reduced to about 30 mL. Two elution steps with 0.05 M glycine/NaOH (pH 7.0) and 0.1% Tween 80 were performed to recover material bound to the tubing in order to increase the recovery. The final concentrated sample, 50-70 mL, consisted of the mixture of the eluate from the ultrafiltration unit plus the final retentate.
2.4. Parasitological analysis
Twenty milliliters of the concentrated water sample were filtered through three layers of gauze to eliminate microscopic particles or remains that could be confused with parasites. After that the sample was split in two fractions, each of which was further concentrated by two methods: Sheather sucrose flotation (Sheather, 1923) and sedimentation by centrifugation using the Charles Barthelemy method, as modified by Bacigalupo and Rivero (Magaró, 2011). The pellet obtained after centrifugation (1000 rpm, 5 min) was resuspended in 10% sucrose solution and then preserved to avoid loss of material according to the following procedures: i) in 10% formol, ii) in sodium acetate-acetic acid-formalin (SAF) fixative (Yang and Scholten, 1977), and iii) in merthiolate-iodine-formaldehyde (MIF) (Sapero and Lawless, 1953).
The identification of trophozoites, cysts, oocysts, and larvae was performed by direct microscopic examination (1000x) of the preserved samples. Wet-mount preparations with Lugol’s solution were used to identify trophozoites and cysts from Entamoebas, Dientamoeba fragilis, Balantidium coli, Trichomonas, Giardia, helminths eggs or larvae like Enterobius vermicularis (formerly Oxyuris vermicularis), Ascaris lumbricoides, and oocysts from Isospora belli. Wet-mount preparations with eosin or methylene blue were used to identify Cyclospora oocysts and with modified safranin for Cryptosporidium and Microsporidium spores. Permanent trichrome stained (Weber et al, 1992) mounts were also prepared from the original concentrated sample or from the preserved samples in formol and SAF to detect protozoa and microsporidia spores.
Helminth eggs count was performed by the Stoll quantification method (Stoll and Hausheer, 1926). The detection of larva from Ancylostoma duodenale, Necator americanus, and Strongyloides stercorali was performed by culture on a strip of filter paper in an upright tube containing a shallow reservoir of water (Harada and Mori, 1955).
2.5. Virological analysis
The concentrated water samples were analyzed for three different viruses: adenovirus (AdV), enterovirus (EV), and Norovirus Genotype II (NV). Samples were also analyzed for the spiked PP7 as a process control.
Viral nucleic acids were extracted from 140 μL of each concentrated sample using the Qiagen Viral RNA Kit (Qiagen, Valencia, CA) according to the manufacturer’s directions. A final volume of 80 μL was obtained as eluate. Several dilutions of the nucleic acid extracted from each water samples were performed in order to check for inhibition.
To produce cDNA for the RNA viruses (EV, NV, and PP7) a commercial kit (Invitrogen Superscript III) was used. Ten microliters of the nucleic acids extracted were added to 30 μL of the reaction mixture, giving final concentrations of: 1 RT buffer, 500 μM dNTPs, 5 mM MgCl2, 2 U/μL RNaseOUT, 10 U/μL SuperScript III, and 2.5 ng/μL of random hexamers. cDNA was synthesized by incubating the mixture at 50 °C for 50 min, followed by another incubation step at 85 °C for 5 min to inactivate the RT enzyme. The detection and quantification of all the targets was performed by real-time PCR (ABI PRISM 5700 Sequence Detection System, Applied Biosystems). Each 25 μL of PCR reaction mixture contained 12.5 μL of commercially available 2 TaqMan Universal PCR Master Mix (Applied Biosystems), 5 μL of the DNA sample (for AdV) or cDNA (for EV, NV, and PP7), primers and probe, and water to complete the volume. The four sets of primers and probes used for the quantitative detection of each virus are compiled in Table 1. Standard amplification conditions were used: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 15 s at 95 °C, and 60 s at 60 °C.
Table 1.
Forward (FP) and reverse (RP) primers and TaqMan probes (TP) used for the detection of all the viral targets by real-time PCR: PP7, adenovirus (AdV), enterovirus (EV), and norovirus genotype II (NV).
| Virus | Oligonucleotide | Sequence (5′-3′)a | Final Concentration |
Reference |
|---|---|---|---|---|
| PP7 | PP7R-247f (FP) | GTTATGAACCAATGTGGCCGTTAT | 1000 nM | Rajal et al., 2007 |
| PP7R-320r (RP) | CGGGATGCCTCTGAAAAAAG | 1000 nM | ||
| PP7R-274p (TP) | FAM-CGGTGGTCAACGAGGAACTGGAAC-TAMRA | 300 nM | ||
| JTVXF (FP) | GGACGCCTCGGAGTACCTGAG | 250 nM | ||
| AdV | JTVXR (RP) | ACIGTGGGGTTTCTGAACTTGTT | 250 nM | Jothikumar et al., 2005 |
| JTVXP (TP) | FAM-CTGGTGCAGTTCGCCCGTGCCA-BHQ | 150 nM | ||
| EV | Ev1 (FP) | GATTGTCACCATAAGCAGC | 500 nM | Fuhrman et al., 2005 |
| Ev2 (RP) | CCCCTGAATGCGGCTAATC | 400 nM | ||
| Ev-probe (TP) | FAM-CGGAACCGACTACTTTGGGTGTCCGT-BHQ | 120 nM | ||
| NV-GIF (FP) | CGYTGGATGCGNTTYCATGA | 400 nM | ||
| NV | NV-GIR (RP) | CTTAGACGCCATCATCATTYAC | 400 nM | Kageyama et al., 2003 |
| NV-GIP(1) (TP) | FAM-AGATYGCGATCYCCTGTCCA-TAMRA | 300 nM | ||
| NV-GIP(2) (TP) | FAM-AGATCGCGGTCTCCTGTCCA-TAMRA | 100 nM |
FAM=6-carboxy fluorescein; BHQ= black hole quencher; TAMRA= 5-c arboxytetramethylrhodamine
A 10-fold serial dilution of plasmid DNA containing the target sequences was used to make a standard curve for each virus. The threshold cycles (Ct) were calculated using baseline values of 6 to 15 and a threshold of 0.05. The viral concentrations for each sample were calculated from the Cts of the qPCR reactions without inhibition taking into account the standard curve and the recovery for PP7.
2.6. Data Analysis to redesign the monitoring strategy
The abundant information obtained from the monitoring scheme followed was analyzed to assess for redundancy in order to reduce the number of variables to be measured and monitoring points required in a future systematic surveillance of the water quality. Kruskal-Wallis and Wilcoxon tests were performed to study the seasonal and spatial behavior of the variables measured, respectively.
The nonparametric Spearman test was applied in order to find associations between the physicochemical and microbiological evaluated variables. Moreover, to explain the variability and to study the structure of these associations between variables a principal component analysis/factor analysis (PCA/FA) was performed. The original variables were transformed into new uncorrelated ones called principal components (PCs). FA followed PCA and its goal was to reduce the contribution of the less significant variables obtained from PCA. This purpose was accomplished by rotating the axis defined by PCA and constructing new variables called varifactors (VFs). Varimax rotation distributes the PC loadings such that the dispersion is maximized by minimizing the number of large and small coefficients (Richman, 1986).
In addition, water quality data sets were subjected to two other multivariate statistical analyses: cluster analysis (CA), discriminant analysis (DA).
CA was performed including all the physicochemical and microbiological variables evaluated, excluding E. coli, which had lower number of records. A dendrogram was built using a hierarchic method, in order to gather the different monitoring points in clusters (Nnane et al., 2011). To validate the clusters obtained the cophenetic correlation coefficient was calculated (Sokal, 1986). DA was also applied to discriminate between naturally occurring groups (Wunderlin et al., 2001). In addition, DA possible to assess whether a small group of variables emerged from the correlation analysis, PCA/FA and descriptive statistics can describe satisfactorily the groups formed (Shrestha and Kazama, 2006). Statistical analyses were performed with InfoStat software (Di Rienzo et al., 2010).
3. Results and Discussion
3.1. Water quality variables and seasonal analysis
The dataset obtained from water quality variables was divided in two seasonal groups according to the records of rainfall for the period studied. The dry season (DS) spanned from April to October of 2009 with 31.9 mm rainfall, while the wet season (WS) included February and March 2009 and the period from November 2009 to February 2010 with 818.3 mm of rainfall. A total of 117 samples were analyzed (including the river and discharges), 62 during the dry season and 55 during the wet season. Some points could not be monitored during the DS because of lack of water in the river. The descriptive statistics for all the water quality variables, including physicochemical, bacteriological and viral levels variables were determined (Table 2). A Kruskal-Wallis test for two seasons was performed. Seasonal variations were statistically significant for temperature, turbidity, conductivity, dissolved oxygen, enterococci, and norovirus genogroup II (see p-values, Table 2).
Table 2.
Descriptive statistics of the physico-chemical and microbiological parameters for the Arias-Arenales River for dry (DS) and wet (WS) season: temperature (T), turbidity (Turb), conductivity (Cond), dissolved oxygen (DO), total coliforms (TC), thermotolerant coliforms (FC), Enterococci (ENT), Escherichia coli (EC), adenovirus (AdV), norovirus genogroup II (NV), and enterovirus (EV). Bold numbers show significant p-values.
| ParameterVariable | N | Mediana | Rangea | Percentile 25-75%a | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| DS | WS | DS | WS | DS | WS | DS | WS | ||
| T (°C) | 62 | 55 | 18.7 | 24 | 10.9-25.3 | 20.2-27.3 | 16.2-21 | 23.2-25.1 | <0.0001 |
| pH | 62 | 55 | 7.62 | 7.57 | 6.62-9.02 | 4.31-9.69 | 7.32-7.82 | 7.29-7.85 | 0.5389 |
| Turb (NTU) | 62 | 55 | 22 | 102 | 0-532 | 3-999 | 11-85 | 15-393 | 0.0020 |
| Cond (mS/cm) | 62 | 55 | 0.35 | 0.23 | 0.122-0.990 | 0.102-0.764 | 0.272-0.496 | 0.186-0.291 | 0.0001 |
| DO (mg O2/L) | 62 | 55 | 5.54 | 6.54 | 0.89-9.44 | 1.27-9.27 | 3.73-7.06 | 5.30-7.80 | 0.0154 |
| TC (log MPN/100 mL) | 62 | 55 | 5.36 | 4.97 | 2.36-10.97 | 2.63-10.36 | 4.36-6.63 | 4.08-5.97 | 0.0896 |
| FC (log MPN/100 mL) | 62 | 55 | 4.50 | 4.32 | 2.36-10.97 | 1.96-10.36 | 3.36-5.97 | 3.63-5.32 | 0.6131 |
| ENT (log cfu/100 mL) | 62 | 55 | 2.49 | 2.78 | 1.36-5.85 | 0.90-5.60 | 2.28-2.81 | 2.48-3.78 | 0.0272 |
| ECb (log cfu/100 mL) | 27 | 33 | 3.86 | 5.00 | 1.74-6.60 | 1.30-6.18 | 2.48-5.00 | 3.48-4.70 | 0.5132 |
| AdV (log gc/L) | 29 | 23 | 5.71 | 5.65 | 4.71-7.42 | 4.41-6.80 | 5.37-6.03 | 5.05-6.07 | 0.4533 |
| NV (log gc/L) | 11 | 0 | 5,51 | NA | 4.49-5.84 | NA | 4.85-5.69 | NA | 0.0011 |
| EV (log gc/L) | 6 | 4 | 5.47 | 5.13 | 4.71-5.99 | 4.76-6.26 | 5.22-5.92 | 4.76-5.33 | 0.6282 |
For the calculation of median, range, and percentile only the positive samples (N) have been taken.
Determination of Escherichia coli started in July 2009. All the samples analyzed were positive.
NA: does not apply
The WS includes spring and summer months with high temperatures and shallow water bodies. Rains were intense (high precipitation in a short time), leading to rapid increases of flow rate, resuspension and transport of sediments. High river flow turbulence (sample sites in the river) increased turbidity and showed a significant difference between seasons (p-value = 0.0003). Conversely, monitoring points located on the drainage channels did not show significant differences in turbidity values for different seasons (p-value = 0.3430) due to the lower sediment transport in concrete channels. The dilution of organic matter and pollutants due to precipitations, and the increasing turbulence, allowed a significant increment in dissolved oxygen concentration during the WS. Drainage channels had lower values of DO than points located in the river (p-value <0.0001) becoming potential sources of water contaminants; P5 was one of the sites that presented the lowest values of DO (mean = 1.58 mg O2/L; data not shown). The dilution effect mentioned before, also explains the statistically significant reduction of conductivity during WS at the points located on the river and at the drainage channels. These channels showed significantly higher values of conductivity compared to those obtained in the river (p-value= <0.0001) and P5 showed a higher conductivity (mean = 0.630 mS/cm). It is well known that microorganisms interact with sediments and that those that are adsorbed can be resuspended after a storm-surge (Marino and Gannon, 1991; Kebabijian, 1994) and during increased stream flow (Crabill et al., 1999). In some cases, these sediments can be reservoirs for coliforms (Doyle et al., 1992; Crabill et al., 1999) giving them longer persistence. Higher persistence of pathogens in the environment leads to enhancing the risk of infection. Despite of the studies cited before the increment of flow rate caused by rainfall, produced a significant increase only for enterococci concentration; however this microorganism was always found in lower concentration than the others, therefore, stochastic effects could be present.
The densities of total and thermotolerant coliforms were high, reaching in many points values that are commonly found in raw sewage discharges (Berg et al., 1978; Dudley et al, 1980). There are various legislations setting limits for microbiological contamination in water according to the uses. For recreational waters, Argentina has adopted guidelines levels according to international standards (SRHN, 2003). The European Union indicates for total coliform that they should not exceed 10,000 CT/100 mL but establishes a lower level guide (500 CT/100 mL) determined with a minimum of 15 days for primary contact (EC, 1975). Fecal coliforms should not exceed 2,000 FC/100 mL, although the level guide establishes the value of 100 FC/100 mL. In the case of Enterococcus and E. coli, the United States Environmental Protection Agency (USEPA) established for recreational waters a limit of 61 CFU/100 mL and 235 CFU/100 mL, respectively (USEPA, 1986). The concentration of AdV found in discharges and in the river water samples showed no significant difference between seasons, similar to what was observed by Pina et al. (1998). The reason of this could be that AdV groups can be eliminated in the feces for months or even years (Jiang, 2006). Only a few samples, not seasonally impacted, were positive for EV. Conversely, the NV were highly seasonal, and all positive cases (also few) were found during the dry season (autumn and winter), behavior also reported by other authors (Lopman et al, 2003; Sakon et al., 2007) and associated with the epidemiology of gastrointestinal illness due to this virus (Ike et al., 2006).
3.2. Water quality along the river
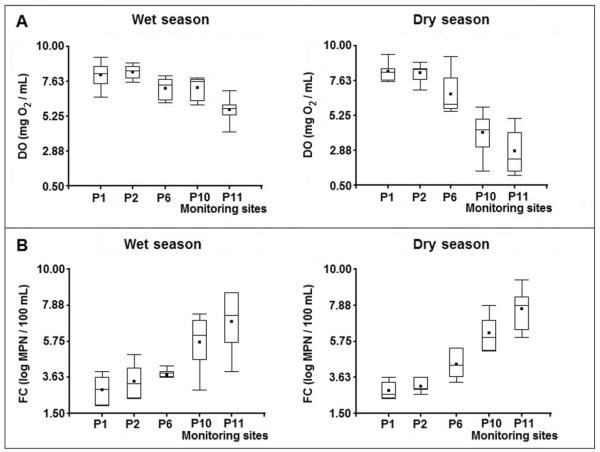
Previous studies performed on the river (in water and sediments) have shown increasing concentrations of some ions and trace elements, some of them in concentrations that affected the aquatic life, and the presence of various microorganisms including bacteria and parasites (Musso, 2000). The impact of these pollutants on the population (who uses the river for multiple purposes as hygiene, irrigation, and also recreation) is significant. Thus, high rates of diarrhea and parasitosis cases are present (Aramayo et al, 2009). The river showed an important deterioration in the water quality when passing through the city, which was observed with each variable studied. To illustrate this, the concentrations of dissolved oxygen (decreasing) and thermotolerant coliforms (increasing) along the river are presented in box plots (Figure 2). The strong DO drop during the dry season (Figure 2A) was probably caused by the concentration effect of organic matter and other pollutants due to lack of precipitation. Conversely, there was not significant difference between seasons for FC (Figure 2B).
Figure 2.
Monitoring points located on the river (P1, P2, P6, P10, and P11) for wet and dry seasons. A. Concentration of dissolved oxygen. B. Concentration of thermotolerant (or fecal) coliforms. The bottom and top of the box are the 25th and 75th percentiles. The whiskers represent 5 and 95 percentiles. The black bar indicates the median and the black square shows the mean.
The Wilcoxon test was applied for all of the variables studied to find critical points of contamination along the river (Table 3). P-values lower than 0.05 indicated significant differences for the variable measured between two consecutive monitoring points, suggesting the existence of events that may have caused disturbances located between them (i.e. between P6 and P10). The reduction of water quality was particularly remarkable between P10 and P11, due to the WWTP, which discharges most of the raw sewage directly into the river, when for operational failure it can not process them (Seghezzo, 2004) or when the capacity of the plant is exceeded, especially in the summer. Also, the global impact of passing through the city was evaluated by comparing a monitoring point before the entrance of the river into the city (P2) and the last point located after the WWTP (P11). The impact of pollution sources was stronger for the DS while it was attenuated for the WS due to dilution effect.
Table 3.
Wilcoxon (Mann-Whitney U) test used to assess longitudinal variation in physico-chemical and microbiological parameters of water quality from monitoring sites located in the Arias-Arenales River: temperature (T), turbidity (Turb), conductivity (Cond), dissolved oxygen (DO), total coliforms (TC), thermotolerant coliforms (FC), Enterococci (ENT), Escherichia coli (EC), (AdV), norovirus genogroup II (NV), and enterovirus (EV). The P-value between two monitoring points (upstream and downstream) resulted from comparing them in that particular parameter. Bold numbers show statistically significant differences, P-value<0.05.
| Parameter Variable | Wet Season |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P2 Mean | P-value | P6 Mean | P-value | P10 Mean | P-value | P11 Mean | P-value | P2 Mean | |
|
| |||||||||
| T (°C) | 23.93 | 0.738 | 24.17 | 0. 938 | 23.70 | 0.859 | 24.05 | 0.820 | 23.93 |
| pH | 8.03 | 0.093 | 7.57 | 0.240 | 6.65 | 0.818 | 6.71 | 0.026 | 8.03 |
| Turb (NTU) | 317.8 | 0.777 | 495.0 | 0.805 | 612.5 | 0.999 | 599.7 | 0.225 | 317.8 |
| Cond (mS/cm) | 0.22 | 0.999 | 0.23 | 0.699 | 0.19 | 0.017 | 0.24 | 0.258 | 0.22 |
| DO (mg O2/L) | 8.27 | 0.026 | 7.16 | 0.999 | 7.19 | 0.015 | 5.68 | 0.002 | 8.27 |
| TC (log MPN/100 mL) | 4.45 | 0.900 | 4.41 | 0.009 | 6.94 | 0.145 | 8.24 | 0.002 | 4.45 |
| FC (log MPN/100 mL) | 4.28 | 0.420 | 3.93 | 0.058 | 6.77 | 0.264 | 8.18 | 0.009 | 4.28 |
| ENT (log cfu/100 mL) | 3.41 | 0.818 | 3.22 | 0.180 | 4.41 | 0.420 | 4.87 | 0.093 | 3.41 |
| ECa (log cfu/100 mL) | 3.92 | 0.886 | 3.69 | 0.029 | 5.41 | 0.429 | 5.96 | 0.057 | 3.92 |
| AdV (log gc/mL) | 4.19 | 0.727 | 5.09 | 0.727 | 4.87 | 0.041 | 6.12 | 0.015 | 4.19 |
| NV (log gc/mL) | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 |
| EV (log gc/mL) | 0 | NA | 0 | NA | 0 | >0.999 | 5.48 | >0.999 | 0 |
|
| |||||||||
| Parameter Variable |
Dry Season
|
||||||||
| P2 Mean | P-value | P6 Mean | P-value | P10 Mean | P-value | P11 Mean | P-value | P2 Mean | |
|
| |||||||||
| T (°C) | 17.83 | 0.999 | 17.49 | 0.456 | 19.57 | 0.976 | 19.13 | 0.598 | 17.83 |
| pH | 7.85 | 0.999 | 7.79 | 0.163 | 7.57 | 0.381 | 7.40 | 0.073 | 7.85 |
| Turb (NTU) | 261.0 | 0.006 | 50.4 | 0.318 | 39.6 | 0.038 | 91.6 | 0.135 | 261.0 |
| Cond (mS/cm) | 0.26 | 0.773 | 0.27 | 0.010 | 0.43 | 0.097 | 0.53 | <0.001 | 0.26 |
| DO (mg O2/L) | 8.15 | 0.053 | 6.71 | 0.002 | 4.07 | 0.165 | 2.84 | <0.001 | 8.15 |
| TC (log MPN/100 mL) | 4.01 | 0.008 | 5.39 | 0.013 | 8.18 | 0.043 | 9.84 | <0.001 | 4.01 |
| FC (log MPN/100 mL) | 3.25 | 0.004 | 4.89 | 0.007 | 7.10 | 0.029 | 8.61 | <0.001 | 3.25 |
| ENT (log cfu/100 mL) | 2.34 | 0.259 | 2.93 | 0.456 | 3.86 | 0.029 | 5.23 | 0.018 | 2.34 |
| ECa (log cfu/100 mL) | 2.84 | 0.829 | 2.96 | 0.057 | 5.16 | 0.229 | 6.36 | 0.029 | 2.84 |
| AdV (log gc/mL) | 4.43 | 0.437 | 5.03 | 0.143 | 5.67 | 0.163 | 6.82 | 0.005 | 4.43 |
| NV (log gc/mL) | 0 | NA | 0 | 0.192 | 4.81 | 0.568 | 5.33 | 0.462 | 0 |
| EV (log gc/mL) | 0 | NA | 0 | >0.999 | 5.08 | >0.999 | 4.81 | 0.535 | 0 |
Determination of E.scherichia coli started in July 2009. All the samples analyzed were positive.
The same statistical test (Wilcoxon) was applied for monitoring points located in discharges and after them on the river to assess if the channels were potentially responsible of the pollution. Thus, P3, P4 and P5 were compared to P6, and P7, P8 and P9 were compared to P10. P5 proved to be the most relevant source of pollution in almost all of the variables exerting considerable influence on P6. Neither P7, P8 nor P9 seemed to be the cause of the increase in the conductivity and bacterial concentration, nor the reduction of dissolved oxygen between P6 and P10. As there was no additional point source of contamination these results suggested that the main pollution source was diffuse and spread along that section of the river. About eighty percent of the city of Salta (the main city of the province) has sanitation, and no more than fifty percent of the water used is transported and treated in the water treatment plant located in the south of the city. Numerous garbage dumps and small sewage effluent discharges from illegal urban settlements were identified on the sides of river.
3.3 Parasitological analysis
At least three different types of parasites were found in each of the 117 samples tested. The following genus and species were identified: Enterobius vermicularis, Dipylidium caninum, Giardia lamblia, Hymenolepsis nana, Microsporidium spp., Entamoeba histolytica, Entamoeba coli, Dientamoeba fragilis, Trichomonas spp., Trichostrongylus spp., Cyclospora, Cryptosporidium spp., Fasciola hepatica, Ascaris lumbricoides, Necator americanus, Amoebae spp., Trichiuris trichiura, Strongyloides stercoralis, Endolimax nana, Blastosistis hominis, and Balantidium. Yeast, fleas, and mites were also found although they were not the object of the study.
The most frequently parasites detected were Microsporidium spp. (82 positive, 70%), Entamoeba coli (79 positive, 68%), Ascaris lumbricoides (74 positive, 63%) and Enterobius vermicularis (71 positive, 61%). One of these four parasites was found in at least approximately 90% of the analyzed samples. Microsporidium spp., the most prevalent, showed significantly higher concentrations during the DS (p-value = 0.0008), for which it can be considered as a good parasitological indicator, and Enterobius vermicularis was significant during WS (p-value = 0.0040). Entamoeba coli and Ascaris lumbricoides did not show significant differences between seasons.
Microsporidium was reported for the first time in 1997 when Enterocytozoon bieneusi was found in the river Seine in France (Sparfel et al, 1997). After that Encephalitozoon intestinalis and Enterocytozoon bieneusi were detected in surface water, groundwater, and also wastewater (Dowd et al, 1998). That it was found in wastewater is not surprising since this pathogen, as most of the other parasites, is shed in human feces and urine (Dowd et al, 1998). Unfortunately, the Arenales River receives raw sewage at different points and effluents with insufficient treatment from the WWTP (Seghezzo, 2004; personal communication, 2009). In addition, spores of these organisms are potentially resistant to disinfection, similar to other protozoan parasites, such as Giardia spp. cysts and Cryptosporidium spp. Oocysts (John et al, 2005). On the other hand, the size suggests that they transport in sandy porous media governed by colloid-transport processes (Brusseau et al, 2005).
Other parasitic causal agents of waterborne zoonotic disease (Schuster and Visvesvara, 2004; Fayer, 2004; Ashbolt, 2004) found in the Arenales river have also been reported previously in water: Entamoeba coli, Entamoeba histolytica, and Dientamoeba fragilis in surface water and wastewater (Sukprasert et al, 2008; Ayed et al, 2009); Enterobius vermicularis, Ascaris spp. eggs, Entamoeba histolyca cysts and Giardia spp. cysts in irrigation water used for vegetables and fruits (Erdogrul and Sener, 2005; Ayed et al, 2009); cysts of Giardia lamblia, Entamoeba histolytica, adult stages of G. lamblia, Balantidium coli, Enterobius vermicularis, Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis and Enterobius vermicularis in water stored in overhead tanks for drinking and cooking (Jonnalagadda and Bhat, 1995); Giardia lamblia, Blastocystis hominis, Entamoeba coli, Cyclospora cayetanensis, Cryptosporidium spp., and Balantidium coli in ditches and wells and together with Endolimax nana in raw and cooked vegetables (Pérez-Cordón et al, 2008); Cryptosporidium in surface water (Stinear et al, 1996); Hymenolepsis nana in wastewater (Ayed et al, 2009). Fasciola hepatica has been detected in lettuce in Peru (Pérez-Cordón et al, 2008) and associated with drinking water through epidemiological studies (Esteban et al, 2002; Nouri et al, 2008).
According to Torgerson and Macpherson (2011) the global burden for most parasitic zoonoses is not yet known but probably has a similar human disease burden to any one of the big three human infectious diseases: malaria, tuberculosis or HIV. Most of the parasites found here are in general treated as waterborne parasites (Karanis et al, 2007) even though we did not find specific reports on the detection of Dipylidium caninum, Trichomonas, Necator americanus, and Trichostrongylus in water.
3.4 Virological analysis
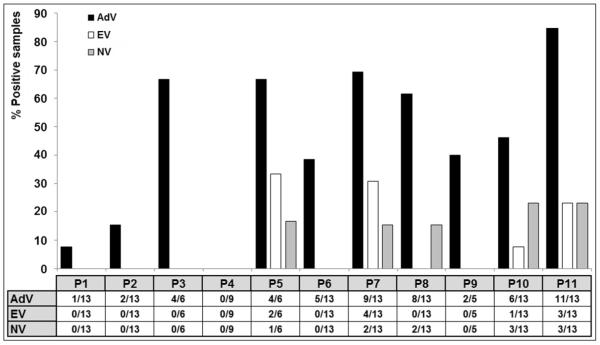
All the monitoring points, with the exception of P4, were positive at least once for the detection of any of the viral groups analyzed (Figure 3). About 45% of the samples were positive for AdV, found more often than NV (9%), and EV (8%). AdVs were detected almost ubiquitously in various monitoring points at high concentrations (2.6 104 – 2.6×107 gc/L) compared to other reports (Rajal et al., 2007b; Kishida et al., 2012), while the presence of NVs (3.1×104 – 6.9×105 gc/L) and EVs (5.1×104 – 1.8×106 gc/L) was restricted to channels P5, P7, and P8 (only NV) and to only two monitoring points in the river, P10 and P11, which presented the worse microbiological quality of the river section studied.
Figure 3.
Detection of adenovirus (AdV), enterovirus (EV), and norovirus genotype II (NV) in samples from the monitoring points (P1-P11) under study. Positive results are expressed as ratios (positive samples/total tested samples) and as a percentage.
AdV was uniformly detected all through the year, while all the positive samples for NV were found only during the dry season (fall, winter). Recent studies conducted in the European Community and in Brazil obtained similar results for the prevalence of AdV and NV in fresh and marine waters used for recreational activities (Wyn-Jones et al., 2010; Miagostovich et al., 2008). No significant seasonal difference was found in this study for EV, which may be due to the low number of positive samples. Conversely, the Center for Disease Control and Prevention (CDC, US) showed a marked seasonality for summer-autumn for this virus (Khetsuriani et al., 2006).
Besides its prevalence, ubiquity and lack of seasonality, other characteristics have been described that encourage the use of AdV (over other proposed viruses) as a viral indicator for environmental waters. Some AdVs showed the greater resistance to the traditional disinfection process by UV light and chlorination (Gerba et al. 2002; Thurston-Enriquez et al., 2003) and higher stability in various polluted waters including wastewater (Enriquez et al., 1995). As a DNA virus, AdV do have lower mutation rates than RNA viruses, thus allowing the use of highly specific molecular tools (e.g. PCR primers) to properly detect its presence and abundance by techniques such as qPCR, which is the most used methodology nowadays (Haramoto et al., 2010). Other advantages over RNA viruses are that the manipulation is easier and costs are lower. Additionally, the ability to specifically detect human AdV using qPCR allows tracking the human fecal contamination (Jothikumar et al., 2005).
3. 5 Correlation analysis, principal component analysis (PCA) and factor analysis (FA)
The dataset was divided into four subgroups, obtained by separation between data from monitoring points on the river or drainage channels and between data obtained during the WS or during the DS (Table 4). The nonparametric Spearman test was applied in order to find associations between evaluated variables. Such correlations would allow decreasing the number of variables required to measure by selecting some that could serve as indicators of others, therefore providing the same information. In this sense, measurement of total and thermotolerant coliforms, traditionally used as indicators of the microbiological quality of water, could not predict the presence of pathogens like viruses and parasites, which was also reported by other authors (Harwood et al., 2005; Bonadonna et al., 2002). In this study, they were correlated with other bacteria and with viruses only for the DS and for the river monitoring points, while there was not correlation at all with the parasites that were mostly found.
Table 4.
Significant Spearman rank correlations (p<0.01) between the variables for river (P1, P2, P6, P10, and P11) and channel (P3, P4, P5, P7, P8, and P9) points during dry (upper) and wet (lower, in parenthesis) season. The coefficients obtained from the river points are downward of the main diagonal, while those from the discharges are upward of the diagonal.
| Para- meter |
T | pH | Turb | Cond | DO | TC | FC | ENT | EC | AdV | NV | EV | Mic | Ever | Alu | Entc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | 1 | 0.62 | 0.73 | |||||||||||||
| pH | 1 | |||||||||||||||
| Turb 1 | 0.54 | 1 | 0.66 | |||||||||||||
| Cond | 1 | |||||||||||||||
| DO | 0.52 | (−0.82) | 1 | (−0.66) | (−0.77) | (−0.65) | ||||||||||
| TC | (−0.52) | (0.73) | −0.55 (−0.85) |
1 | 0.82 (0.85) |
(0.56) | ||||||||||
| FC | (−0.57) | 0.54 | (0.81) | −0.54 (−0.84) |
0.82 (0.93) |
1 | (0.72) | (0.56) | ||||||||
| ENT | (0.70) | (−0.60) | (0.56) | (0.65) | 1 | 0.96 | ||||||||||
| EC | 0.72 | (0.82) | −0.67 (−0.68) |
0.66 (0.78) |
(0.79) | 0.93 (0.79) |
1 | 0.84 | ||||||||
| AdV | 0.40 | 0.66 (0.63) |
(−0.49) | 0.57 (0.68) |
0.61 (0.73) |
(0.61) | 0.53 (0.69) |
1 | ||||||||
| NV | (0.50) | (0.58) | (0.61) | 0.65 (0.69) |
1 | 0.84 (0.78) |
||||||||||
| EV | (0.50) | 0.54 (0.59) |
(0.56) | (0.57) | 0.42 (0.49) |
0.50 | 0.69 (0.70) |
0.95 (0.81) |
1 | |||||||
| Mic | (0.51) | 0.52 | 1 | |||||||||||||
| Ever | (0.61) | 0.55 | 1 | 0.62 | ||||||||||||
| Alu | (0.78) | 0.55 | 0.54 | 1 | 0.60 | |||||||||||
| Entc | 0.55 | (0.57) | (0.58) | 1 |
Temperature (T), turbidity (Turb), conductivity (Cond), dissolved oxygen (DO), total coliforms (TC), thermotolerant coliforms (FC), Enterococcus (ENT), Escherichia coli (EC), adenovirus (AdV), norovirus genogroup II (NV), enterovirus (EV), Microsporidium sp (Mic), Enterobius vermicularis (Ever), Ascaris lumbricoides (Alu), Entamoeba coli (Entc)
From the main correlations found it is interesting to mention that the decrease of DO is related to increasing bacterial contamination in the river for both seasons (WS and DS), while the conductivity seems to be associated with viral contamination. Variables expressing the concentration of bacteria showed a close relationship between the various measurements made; TC and FC were associated with the presence of virus, especially AdV, in the river but not in the discharges. Thus, TC and FC can not be used to predict viral contamination. The three viral groups were strongly related. The parasites did not show any significant relationship to predict their presence.
The largest number of significant relationships appear in the subgroup of data from monitoring points located on the river. In the case of drainage channels, only a small number of significant relationships were observed, probably due to the varied and fluctuating nature of discharges carried (later attenuated by the river). A higher number and stronger correlations appeared during the DS (low flow rate).
In order to explain the variability and to study the correlation structure between variables a PCA/FA was performed on the standardized data set (15 variables, E. coli was excluded) to eliminate the effect of data measurement scales. According to Kim and Muller (1987), only PCs having eigenvalues > 1 were considered significant. PCA yielded five PCs with eigenvalues > 1, explaining 67% of the total variance in water quality data set. Five VFs were obtained by FA performed on the PCs.
Following the criteria of Liu et al. (2003) the factor loadings were classified as “strong”, and “moderate”, corresponding to absolute loading values of >0.75 and 0.75-0.50, respectively. VF1 (eigenvalue 3.33), explaining 22% of total variance, had strong negative loading on dissolved oxygen, strong positive loadings on total and thermotolerant coliforms, and moderate positive loadings on conductivity. VF1 represented the impact of contamination from human activities on river. The inverse relationship between dissolved oxygen and microbiological variables was caused by discharges and disposition of organic waste on the Arenales River. This organic waste is decomposed by aerobic bacteria in a process that consumes oxygen and produces proliferation of bacterial groups. Increased conductivity values also stem from discharges and disposition of pollutants on the river. VF2 (eigenvalue 2.47), explaining 16% of the total variance, represented the impact of parasites in the river water quality. VF3 (eigenvalue 1.75), explaining 12% of the total variance, had strong negative loading on turbidity, moderate negative loading on temperature, and moderate positive loading on pH. This factor explains seasonal relations, during the WS coinciding with the spring and summer seasons, intense rains led to rapid raise of flow rate, resuspension and transport of sediments increasing turbidity in the river.
VF4 (eigenvalue 1.40), explaining 9% of total variance, had strong negative loadings on norovirus and moderate negative loadings on enterococci. Finally, VF5 (eigenvalue 1.15) explaining the lowest variance (8%), had moderate positive loadings on enterovirus.
3.6 Cluster analysis (CA) and discriminant analysis (DA)
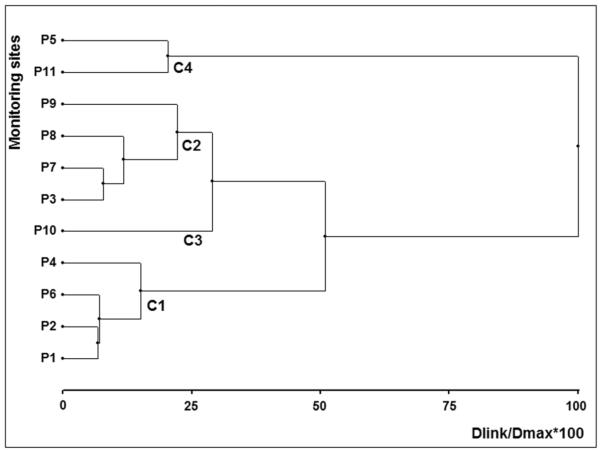
A dendrogram was built using the Ward method, and squared Euclidean distances were calculated as a measure of similarity (Nnane et al., 2011). Four major clusters were built (C1, C2, C3, and C4) obtaining a cophenetic correlation coefficient of 0.851. A high cophenetic correlation (>0.85) indicates that the classification obtained by the clustering method is a reasonably faithful representation (Sokal, 1986).
Cluster 1 (C1) consists of four monitoring points, three of them located in the river (P1, P2, and P6) and the remaining (P4) belonging to a stream that flows into the River. C1 includes the three points on the river that showed less deterioration of water quality variables. From this group, P6 is a good candidate to be monitored since it is a recreational area.
Cluster 2 (C2) included only discharges that do not seem to be the most important sources of contamination. However, P7 was selected to be a representative monitoring point of the cluster because it showed the higher number of positive detects for viruses and also because it has permanent flow. Cluster 3 (C3) only includes P10, which is a potential candidate to be monitored because it is located at the end of the city and before the discharge from the WWTP. Cluster 4 (C4) was constituted only for the monitoring points that showed the worse quality variables. P5 was a raw sewage discharge, while P11 is on the river after the discharge of the WWTP. Monitoring P11 would allow us to obtain information about the quality of the river at that point and also to control the impact of the WWTP upon the quality of water.
The group of clusters with strong homology allows the introduction of reliable criteria to choose a sampling site that represents a group and, therefore, the establishment of monitoring strategies to reduce the amount of work that has to be done. According to this analysis, the eleven monitoring points could be reduced to only the proposed four: P6, P10, P7, and P11. In addition, we suggest including also either P1 or P2 as a control of the water condition before it enters the city. By the application of this analysis the monitoring points (and the resources involved) can be reduced to half without losing significant information.
Discriminant analysis was implemented in standard and reduced mode after grouping into four clusters (C1, C2, C3, C4) as obtained through cluster analysis (Table 5). Standard mode using 15 discriminant variables (E. coli was excluded) yielded the cross-classification matrix assigning more than 87% observations correctly, supporting the natural structure of the clusters generated by the CA. Similar level of correct observations were obtained in a Japanese river basin (Shrestha and Kazama, 2006). In the reduced mode, eight variables were included based on the correlation analysis, established regulations for microbiological water quality, seasonality, frequency, and ubiquity results for viruses and parasites (conductivity, dissolved oxygen, turbidity, enterococci, total coliforms, thermotolerant coliforms, adenovirus, and Microsporidium sp.). The cross-classification matrix showed 82% correct assignations. Thus, the proposed variables were adequate to discriminate between the clusters allowing that few of them explain large variations in water quality.
Table 5.
Classification matrix for discriminant analysis of clusters C1 (P1, P2, P4, P6), C2 (P3, P7, P8, P9), C3 (P10), and C4 (P5, P11). Two modes were analyzed, the standard including 15 variables (E. coli was excluded) and the reduced one including only selected variables (conductivity, dissolved oxygen, turbidity, enterococci, total coliforms, thermotolerant coliforms, adenovirus, and Microsporidium sp.).
| Monitoring clusters | % Correct | Clusters assigned by DA |
|||
|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | ||
| Standard DA mode | |||||
| C1 | 91.7 | 44 | 4 | 0 | 0 |
| C2 | 86.5 | 4 | 32 | 1 | 0 |
| C3 | 84.6 | 1 | 0 | 11 | 1 |
| C4 | 78.9 | 1 | 1 | 2 | 15 |
| Total | 87.2 | 50 | 37 | 14 | 16 |
| Reduced DA mode | |||||
| C1 | 93.8 | 45 | 3 | 0 | 0 |
| C2 | 75.7 | 6 | 28 | 2 | 1 |
| C3 | 61.5 | 2 | 1 | 8 | 2 |
| C4 | 78.9 | 1 | 1 | 2 | 15 |
| Total | 82.1 | 54 | 33 | 12 | 18 |
4. Conclusions
The following general strategy applied in this work was successful; therefore we recommend it to develop a long-term systematic monitoring scheme: 1) To identify the potential critical points to monitor; 2) To periodically monitor the selected points measuring physicochemical and microbiological variables; 3) To analyze the seasonal and spatial variability; 4) To assess the correlation between variables; and 5) To analyze the similarity between the different monitoring points.
The experimental results obtained during monthly monitoring of the river for a full year allowed us to construct a comprehensive database on the characteristics and behaviour of the Arenales River. Statistical analysis performed, led us to conclude that:
The studied variables reflected the deterioration in the water quality when passing through the city. The comparison of two adjacent (upstream and downstream) monitoring points allowed the detection of significant differences between variables that could indicate a particular type of contaminant being discharged at the river between those two points. Identifying the location of such sources facilitates their mitigation or correction.
There was significant seasonal variation for temperature, turbidity, conductivity, dissolved oxygen, enterococci, and norovirus genogroup II and the reasons related were discussed above.
Different viruses and parasites were found and they did not correlate with the concentration of total and thermotolerant coliforms. Adenovirus was ubiquitous and was selected for the future as viral indicator, while Microsporidium was selected as parasitological indicator.
Strong correlations between some variables were found mainly for the points located on the river and during the dry season. This allowed us to select some variables as indicators of others, reducing the number of them (from sixteen to only eight) that need to be measured in future regular monitoring. The most relevant and sensitive of the physicochemical characteristics were dissolved oxygen, conductivity, and turbidity (especially during the wet season). The most relevant and sensitive of the microbiological quality indexes were the detection of total and thermotolerant coliforms (the latter especially important during the dry season), enterococci, adenovirus, and Microsporidium.
There was similarity between the monitoring points, which were grouped in four clusters that were validated by the cophenetic correlation and also supported by discriminant analysis. One point per cluster was selected as representative, thus reducing the number of sites that need to be analyzed in future regular monitoring. For the particular case of the Arenales River we suggest to include only the points P2 (as quality control for the river water entering the city), P6 (a recreational site), P7 (as a representative discharge), P10 and P11 (as a control of the impact of the WWTP and to know the quality of the river water leaving the city). The eight selected variables were adequate to discriminate between the clusters allowing that few of them explain large variations in water quality.
By the principal component analysis and factor analysis we were able to explain 67% of the total variance and to study the correlation structure between variables that were involved in five principal components.
Summarizing, the statistical analysis of all the information led us to develop a new monitoring scheme completely different than the protocol used to initiate the study. This new scheme (including only five points instead of eleven and measuring only eight variables instead of sixteen) is a rational monitoring strategy since it is based on the selection of the most suitable monitoring points and also of the most significant variables to measure, which will result in an optimal use of the limited resources available.
Highlights.
The seasonal and longitudinal variations of the river water quality were analyzed
Correlation analyses allowed identify the most relevant variables to be measured.
From similarity analysis the most suitable monitoring sites were identified.
A general strategy for long-term systematic monitoring is suggested.
The new monitoring scheme involves a more rational use of the resources involved.
Figure 4.
Cluster analysis (Ward’s hierarchical method) for the eleven monitoring sites based on microbial and physicochemical variables. Four mayor clusters (C1, C2, C3, and C4) were formed.
Acknowledgments
This research was part of project PICT-Red 276/06 funded by the Agencia Nacional de Promoción de Ciencia y Técnica in Argentina (ANPCyT). This project was partially supported by NIH Grant # D43 TW005718 funded by the Fogarty International Center from the University of California at Davis and the National Institute of Environmental Health Sciences. Hugo Ramiro Poma and Dolores Gutierrez Cacciabue are recipients of graduate fellowships from CONICET. The authors would like to thank Dr. Jerold Last for his kind help with the English corrections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albinana-Gimenez N, Miagostovich MP, Calgua B, Huguet JM, Matia L, Girones R. Analysis of adenovirus and polyomaviruses quantified by a PCR as indicators of water quality in source and drinking-water treatment plants. Water Res. 2009;43:2011–2019. doi: 10.1016/j.watres.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Aramayo CF, Gil JF, Cruz MC, Poma HR, Last MS, Rajal VB. Diarrhea and parasitosis in Salta, Argentina. J Infect Devel Countries. 2009;3(2):105–111. doi: 10.3855/jidc.57. [DOI] [PubMed] [Google Scholar]
- Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayed LB, Schijven J, Alouini Z, Jemli M, Sabbahi S. Presence of parasitic protozoa and helminth in sewage and efficiency of sewage treatment in Tunisia. Parasitol Res. 2009;105:393–406. doi: 10.1007/s00436-009-1396-y. [DOI] [PubMed] [Google Scholar]
- Berg G, Dahling DR, Brown GA, Berman D. Validity of fecal coliforms, total coliforms, and fecal streptococci as indicators of viruses in chlorinated primary sewage effluents. Appl Environ Microbiol. 1978;36:880–884. doi: 10.1128/aem.36.6.880-884.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia D, Tozzi AE, Cotter B, Rizzo C, Russo T, Buttinelli G, Caprioli A, Marziano ML, Ruggeri FM. Waterborne outbreak of Norwalk-like virus gastroenteritis at a tourist resort, Italy. Emerging Infect Dis. 2002;8(6):563–568. doi: 10.3201/eid0806.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol. 2000;66:238–245. doi: 10.1128/aem.66.1.238-245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna L, Briancesco R, Ottaviani M, Veschetti E. Occurrence of Cryptosporidium oocysts in sewage effluents and correlation with microbial, chemical and physical water variables. Environ Monit Assess. 2002;75:241–252. doi: 10.1023/a:1014852201424. [DOI] [PubMed] [Google Scholar]
- Brusseau ML, Oleen JK, Santamaria J, Cheng L, Orosz-Coghlan P, Chetochine AS, Blanford WJ, Rykwalder P, Gerba CP. Transport of microsporidium Encephalitozoon intestinales spores in sandy porous media. Water Res. 2005;39:3636–3642. doi: 10.1016/j.watres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Caro V, Guillot S, Delpeyroux F, Crainic R. Molecular strategy for “serotyping” of human enteroviruses. J Gen Virol. 2001;82:79–91. doi: 10.1099/0022-1317-82-1-79. [DOI] [PubMed] [Google Scholar]
- Castignolles N, Petit F, Mendel L, Simon L, Cattolico L, Buffet-Janvresse C. Detection of adenovirus in the waters of the Seine River estuary by nested-PCR. Molecular and Cell Probes. 1998;12:175–180. doi: 10.1006/mcpr.1998.0166. [DOI] [PubMed] [Google Scholar]
- CEU Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC.
- Crabill C, Donald R, Snelling J, Foust R, Southam G. The impact of sediment faecal coliform reservoirs on seasonal water quality in Oak Creek, Arizona. Water Res. 1999;9:2163–2171. [Google Scholar]
- Craun GF. Causes of waterborne outbreaks in the United States. Water Sci Technol. 1991;24:17–20. [Google Scholar]
- Cukor G, Blacklow NR. Human viral gastroenteritis. Microbiol Rev. 1984;48:157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW. InfoStat versión 2010. Grupo InfoStat, FCA, Universidad Nacional de Córdoba; Argentina: URL http://www.infostat.com.ar. [Google Scholar]
- Donaldson KA, Griffin DW, Paul JH. Detection, quantitation and identification of enteroviruses from surface waters and sponge tissue from the Florida Keys using real-time RT–PCR. Water Res. 2002;36:2505–2514. doi: 10.1016/s0043-1354(01)00479-1. [DOI] [PubMed] [Google Scholar]
- Dowd SE, Gerba CP, Pepper IL. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol. 1998;64(9):3332–3335. doi: 10.1128/aem.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JD, Tunnicliff B, Kramer R, Kuehl R, Brickler SK. Instability of fecal coliform populations in waters and bottom sediments at recreational beaches in Arizona. Water Res. 1992;26:979–988. [Google Scholar]
- Dudley DJ, Guentzel MN, Ibarra MJ, Moore BE, Sagik BP. Enumeration of potentially pathogenic bacteria from sewage sludges. Appl Environ Microbiol. 1980;39:118–126. doi: 10.1128/aem.39.1.118-126.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton AD, Clesceri LS, Riceand EW, Greenberg AE. Standard methods for the examination of water and wastewater. 21st ed American Public Health Association; Washington DC: 2005. [Google Scholar]
- [Last access: 01/12/2012];EC. COUNCIL DIRECTIVE of 16 June 1975 concerning the quality required of surface water intended for the abstraction of drinking water in the Member States - 75/440/EEC (OJ No L 194, 25. 7. 1975, p. 26.) Available at: www.nomosphysis.org.gr/attachments/39/75-440.pdf.
- Enriquez CE, Hurst CJ, Gerba CP. Survival of the enteric adenoviruses 40 and 41 in tap, sea, and waste water. Water Res. 1995;29:2548–2553. [Google Scholar]
- EPA, Environmental Protection Agency Method 1103.1: Escherichia coli in water by membrane filtration using membrane-thermo tolerant Escherichia coli agar (mTEC) 2002. EPA-821-R-02-020.
- EPA, Environmental Protection Agency Method 1106.1: Enterococci in water by the Membrane Filter Procedure. 2002. EPA 600-4-85-076.
- Erdogrul Z, Sener H. The contamination of various fruit and vegetable with Enterobius vermicularis, Ascaris eggs, Entamoeba histolyca cysts and Giardia cysts. Food Control. 2005;16:559–562. [Google Scholar]
- Esteban JG, Gonzalez C, Bargues MD, Angles R, Sanchez C, Naquira C, Mas-Coma S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop Med Int Health. 2002;7(4):339–348. doi: 10.1046/j.1365-3156.2002.00870.x. [DOI] [PubMed] [Google Scholar]
- Farthing MJG. Smith Kline and French. Walwyn Garden City; Hertfordshire: 1989. Viruses and the gut. [Google Scholar]
- Fayer R. Cryptosporidium: a water-borne zoonotic parasite. Vet Parasitol. 2004;126:37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Fuhrman JA, Liang X, Noble RT. Rapid detection of enteroviruses in small volumes of natural waters by real-time quantitative reverse transcriptase PCR. Appl Environ Microbiol. 2005;71(8):4523–4530. doi: 10.1128/AEM.71.8.4523-4530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev. 2001;14:114–128. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba CP, Rose JB, Haas CN, Crabtree KD. Waterborne rotavirus: a risk assessment. Water Res. 1996;30(12):2929–2940. [Google Scholar]
- Gerba CP, Gramos DM, Nwachuku N. Comparative Inactivation of Enteroviruses and Adenovirus 2 by UV Light. Appl Environ Microbiol. 2002;68:5167–5169. doi: 10.1128/AEM.68.10.5167-5169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G, Raoult D. Microorganisms Resistant to Free-Living Amoebae. Clin Microbiol Rev. 2004;17(2):413–433. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GWA, Government of Western Australia [Last access: 01/12/2012];Surface water sampling methods and analysis – Technical appendices. Standard operating procedures for water sampling. Methods and analysis. 2009 Available at: http://www.geology.wmich.edu/Koretsky/EnvironmentalGeochemistry/Surface%20water%20sampling%20methods%20and%20analysis.pdf.
- Haas CN, Rose JB, Gerba CP, Regli R. Risk assessment of viruses in drinking water. Risk Anal. 1993;13:545–552. doi: 10.1111/j.1539-6924.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Harada Y, Mori O. A new method for culturing hookworm. Yonago Acta Medica. 1955;1:177–179. [Google Scholar]
- Haramoto E, Kitajima M, Katayama H, Ohgaki S. Real-time PCR detection of adenoviruses, polyomaviruses, and torque teno viruses in river water in Japan. Water Res. 2010;44:1747–1752. doi: 10.1016/j.watres.2009.11.043. [DOI] [PubMed] [Google Scholar]
- Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR, Rose JB. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl Environ Microbiol. 2005;71:3163–3170. doi: 10.1128/AEM.71.6.3163-3170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A, Kunikane S, Hirata T. Prevalence of Cryptosporidium oocysts and Giardia cysts in the drinking water supply in Japan. Water Res. 2002;36:519–526. doi: 10.1016/s0043-1354(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Hederra R, Novaes H, Cuneo C, Porras F Zepeda, Forero R Säenz. Manual for Public Health Surveillance (Spanish) Organización Panamericana de la Salud; Washington DC: 1996. [Google Scholar]
- Hoebe CJ, Vennema H, Husman AM, van Duynhoven YT. Norovirus outbreak among primary school children who had played in a recreational water fountain. J Infect Dis. 2004;189:699–705. doi: 10.1086/381534. [DOI] [PubMed] [Google Scholar]
- Horman A, Rimhanen-Finne R, Maunula L, Von Bonsdorff C, Torvela N, Heikinheimo A, Hanninen ML. Campylobacter spp., Giardia spp., Cryptosporidium spp., Noroviruses, and indicator organisms in surface water in southwestern Finland, 2000-2001. Appl Environ Microbiol. 2004;70(1):87–95. doi: 10.1128/AEM.70.1.87-95.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike AC, Brockmann SO, Hartelt K, Marschang RE, Contzen M, Oehme RM. Molecular epidemiology of norovirus in outbreaks of gastroenteritis in southwest Germany from 2001 to 2004. J Clin Microbiol. 2006;44:1262–1267. doi: 10.1128/JCM.44.4.1262-1267.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SC. Human Adenoviruses in Water: Occurrence and Health Implications: A Critical Review. Environ Sci Technol. 2006;40:7132–7140. doi: 10.1021/es060892o. [DOI] [PubMed] [Google Scholar]
- John DE, Haas CN, Nwachuku N, Gerba CP. Chlorine and ozone disinfection of Encephalitozoon intestinalis spores. Water Res. 2005;39:2369–2375. doi: 10.1016/j.watres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Jonnalagadda PR, Bhat RV. Parasitic contamination of stored water used for drinking/cooking in Hyderabad. The Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26(4):789–94. [PubMed] [Google Scholar]
- Jothikumar N, Cromeans TL, Hill VR, Lu X, Sobsey MD, Erdman DD. Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Appl Environ Microbiol. 2005;71:3131–3136. doi: 10.1128/AEM.71.6.3131-3136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5(1):1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- Kay D, Bartram J, Prüss A, Ashbolt N, Wyer MD, Fleisher JM, Fewtrell L, Rogers A, Rees G. Derivation of numerical values for the World Health Organization guidelines for recreational waters. Water Res. 2004;38(5):1296–1304. doi: 10.1016/j.watres.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Kebabijian R. Monitoring the effects of urban run-off on recreational waters. J Environ Health. 1994;56:15–18. [Google Scholar]
- Khetsuriani N, LaMonte-Fowlkes A, Oberste MS, Pallansch MA. MMWR 55 (SS08) 2006. Enterovirus surveillance – United States, 1970-2005. Center for Disease Control and Prevention; pp. 1–20. [PubMed] [Google Scholar]
- Kim JO, Mueller CW. Introduction to factor analysis: what it is and how to do it. Quantitative Applications in the Social Sciences Series. Sage University Press; Newbury Park: 1987. [Google Scholar]
- Kishida N, Morita H, Haramoto E, Asami M, Akiba M. One-year weekly survey of noroviruses and enteric adenoviruses in the Tone River water in Tokyo metropolitan area, Japan. Water Res. 2012 doi: 10.1016/j.watres.2012.03.010. doi: 10.1016/j.watres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Kocwa-Haluch R. Waterborne enteroviruses as a hazard for human health. Pol J Environ Studies. 2001;10:485–487. [Google Scholar]
- Lipp EK, Kurz R, Vincent R, Rodriguez-Palacios C, Farrah SR, Rose JB. The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries. 2001;24:266–276. [Google Scholar]
- Liu CW, Lin KH, Kuo YM. Application of factor analysis in the assessment of groundwater quality in a Blackfoot disease area in Taiwan. Sci Total Environ. 2003;313:77–89. doi: 10.1016/S0048-9697(02)00683-6. [DOI] [PubMed] [Google Scholar]
- Lopman BA, Adak GK, Reacher MH, Brown DW. Two epidemiologic patterns of norovirus outbreaks: surveillance in England and Wales, 1992–2000. Emerging Infect Dis. 2003;9:71–7. doi: 10.3201/eid0901.020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB, Davis JP. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331(3):161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Magaró HM. Topics of Parasitology. Parasites of the Human Intestinal Tract (Spanish) 1st Ed Editorial Académica Española; Saarbrücken: 2011. [Google Scholar]
- Marino RP, Gannon JJ. Survival of fecal coliforms and fecal streptococci in storm drain sediment. Water Res. 1991;25:1089–1098. [Google Scholar]
- Maunula L, Miettinen IT, von Bonsdorff C. Norovirus outbreaks from drinking water. Emerging Infect Dis. 2005;11:1716–1721. doi: 10.3201/eid1111.050487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaig SM, Scott TM, Lukasik JO, Paul JH, Harwood VJ. Quantification of human polyomaviruses JC virus and BK virus by TaqMan quantitative PCR and comparison to other water quality indicators in water and fecal samples. Appl Environ Microbiol. 2009;75:3379–3388. doi: 10.1128/AEM.02302-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CL, Palmer CJ. Evaluation of PCR, nested PCR, and fluorescent antibodies for detection of Giardia and Cryptosporidium species in wastewater. Appl Environ Microbiol. 1996;62(6):2081–2085. doi: 10.1128/aem.62.6.2081-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick JL. Enteric viruses in water. Vol. 15. Basel; Karger: 1984. Etiologic agents and their potential for causing waterborne virus diseases; pp. 1–16. [Google Scholar]
- Melnick JL. Enteroviruses. In: Fields BN, Knipe DM, editors. Virology. Raven Press; New York: 1990. pp. 549–605. [Google Scholar]
- Mena KD, Gerba CP. Waterborne adenovirus. Reviews on Environmental Contamination and Toxicology. 2009;198:133–167. doi: 10.1007/978-0-387-09647-6_4. [DOI] [PubMed] [Google Scholar]
- Miagostovich MP, Ferreira FF, Guimarães FR, Fumian TM, Diniz-Mendes L, Luz SL, Silva LA, Leite JP. Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, central Amazonia, Brazil. Appl Environ Microbiol. 2008;74:375–382. doi: 10.1128/AEM.00944-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso HE. MSc Thesis. Universidad Nacional de Tucuman; Argentina: San Miguel de Tucuman: 2000. Impact of the Salta City on the pollution of the Arenales River (Spanish) [Google Scholar]
- Nnane DE, Ebdon JE, Tatlor HD. Integrated analysis of water quality parameters for cost-effective faecal pollution management in river catchments. Water Res. 2011;45:2235–2246. doi: 10.1016/j.watres.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Noble RT, Fuhrman JA. Enteroviruses detected by reverse transcriptase polymerase chain reaction from the coastal waters of Santa Monica Bay, California: low correlation to bacterial indicator levels. Hydrobiologia. 2001;460:175–184. [Google Scholar]
- Nouri J, Mahvi AH, Saeedi R, Dindarloo K, Rafiee M, Dobaradaran S. Purification and removal of Ascaris and Fasciola hepatica eggs from drinking water using roughing filters. Korean J Chem Eng. 2008;25(3):501–504. [Google Scholar]
- Nwachuku N, Gerba CP. Health risks of enteric viral infections in children. Reviews of Environmental Contamination and Toxicology. 2006;186:1–56. doi: 10.1007/0-387-32883-1_1. [DOI] [PubMed] [Google Scholar]
- Peng H, Hagopian W. Environmental factors in the development of Type 1 diabetes. Reviews in Endocrine & Metabolic Disorders. 2006;7(3):149–162. doi: 10.1007/s11154-006-9024-y. [DOI] [PubMed] [Google Scholar]
- Perez-Cordon G, Rosales MJ, Valdez RA, Vargas-Väsquez F, Cordova O. Detection of intestinal parasites in food and water, Trujillo, Peru (Spanish) Revista Peruana de Medicina Experimental y Salud Püblica. 2008;25(1):144–148. [Google Scholar]
- Pina S, Puig M, Lucena F, Jofre J, Girones R. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol. 1998;64:3376–3382. doi: 10.1128/aem.64.9.3376-3382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60(8):2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajal VB, McSwain BS, Thompson DE, Leutenegger CM, Kildare BJ, Wuertz S. Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Res. 2007a;41:1411–1422. doi: 10.1016/j.watres.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Rajal VB, McSwain BS, Thompson DE, Leutenegger CM, Wuertz S. Molecular quantitative analysis of human viruses in California stormwater. Water Res. 2007b;41:4287–4298. doi: 10.1016/j.watres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Richman MB. Rotation of principal components. J Climatol. 1986;6:293–335. [Google Scholar]
- Rose JB, Dickson LJ, Farrah SR, Carnahan RP. Removal of pathogenic and indicator microorganisms by full-scale water reclamation facility. Water Res. 1996;30(11):2785–2797. [Google Scholar]
- Rose JB, Huffman DE, Gennaccaro A. Risk and control of waterborne cryptosporidiosis. FEMS Microbiol Rev. 2002;26:113–123. doi: 10.1111/j.1574-6976.2002.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Sakon N, Yamazaki K, Yoda T, Tsukamoto T, Kase T, Taniguchi K, Takahashi K, Otake T. Norovirus Storm in Osaka, Japan, Last Winter (2006/2007) Jap J Infect Dis. 2007;60:409–410. [PubMed] [Google Scholar]
- Sapero JJ, Lawless DK. The MIF strain preservation technique for the identification of intestinal protozoa. Am J Tropical Med Hyg. 1953;2:613–619. doi: 10.4269/ajtmh.1953.2.613. [DOI] [PubMed] [Google Scholar]
- Schets FM, Engels GB, Evers EG. Cryptosporidium and Giardia in swimming pools in the Netherlands. Water Health. 2004;2(3):191–200. [PubMed] [Google Scholar]
- Schuster FL, Visvesvara GS. Amebae and ciliated protozoa as causal agents of waterborne zoonotic disease. Vet Parasit. 2004;126:91–120. doi: 10.1016/j.vetpar.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Searcy KE, Packman AI, Atwill ER, Harter T. Deposition of Cryptosporidium oocysts in streambeds. Appl Environ Microbiol. 2006;72(3):1810–1816. doi: 10.1128/AEM.72.3.1810-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzo L. PhD Thesis. University of Wageningen; The Netherlands: 2004. Anaerobic treatment of domestic wastewater in subtropical regions. ISBN: 90-8504-029-9. [Google Scholar]
- Sheather AT. The detection of intestinal protozoa and mange parasites by a flotation technique. J Comp Pathol. 1923;36:266–275. [Google Scholar]
- Shrestha S, Kazama F. Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji river basin, Japan. Environ mod soft. 2007;22:464–475. [Google Scholar]
- Sinclair RG, Jones EL, Gerba CP. Viruses in recreational water-borne disease outbreaks: a review. J Appl Microbiol. 2009;107(6):1769–1780. doi: 10.1111/j.1365-2672.2009.04367.x. [DOI] [PubMed] [Google Scholar]
- Skraber S, Gassilloud B, Gantzer C. Comparison of coliforms and coliphages as tools for assessment of viral contamination in river water. Appl Environ Microbiol. 2004;70:3644–3649. doi: 10.1128/AEM.70.6.3644-3649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR. Phenetic taxonomy: theory and methods. Ann Rev Ecol Syst. 1986;17:423–442. [Google Scholar]
- Sparfel JM, Sarfat IC, Liguory O, Caroff B, Dumoutier N, Gueglio B, Billaud E, Raffi F, Molina JM, Miegeville M, Derouin F. Detection of microsporidia and identification of Enterocytozoon bieneusi in surface water by filtration followed by specific PCR. The Journal of Eukaryotic Microbiology. 1997;44(6S):78S. doi: 10.1111/j.1550-7408.1997.tb05792.x. [DOI] [PubMed] [Google Scholar]
- SRHN . Subsecretaría de Recursos Hídricos de la Nación. Repüblica Argentina: 2003. Development of national guidelines for quality levels of environmental water for Escherichia coli / Enterococci (Spanish) [Google Scholar]
- Stinear T, Matusan A, Hines K, Sandery M. Detection of a single viable Cryptosporidium parvum oocyst in environmental water concentrates by reverse transcription-PCR. Appl Environ Microbiol. 1996;62(9):3385–3390. doi: 10.1128/aem.62.9.3385-3390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll NR, Hausheer WC. Concerning two options in dilution egg counting: small drop and displacement. Am J Hyg. 1926;6:134–145. [Google Scholar]
- Sukprasert S, Rattaprasert P, Hamzah Z, Shipin OV, Chavalitshewinkoon-Petmitr P. PCR detection of Entamoeba spp from surface and waste water samples using genus-specific primers. The Southeast Asian Journal of Tropical Medicine and Public Health. 2008;39(1):6–9. [Google Scholar]
- Thurston-Enriquez J, Haas C, Jacangelo J, Gerba C. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl Environ Microbiol. 2003;69:3979–3985. doi: 10.1128/AEM.69.7.3979-3985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Macpherson CNL. The socioeconomic burden of parasitic zoonoses: global trends. Vet Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Sano D, Watanabe T, Akiyama K, Omura T. Norovirus pathway in water environment estimated by genetic analysis of strains from patients of gastroenteritis, sewage, treated wastewater, river water and oysters. Water Res. 2005;39:4271–4280. doi: 10.1016/j.watres.2005.06.035. [DOI] [PubMed] [Google Scholar]
- USEPA . Ambient water quality criteria for bacteria. EPA/440/5–84/002. Office of Water. Environmental Protection Agency; U.S. Washington, D.C.: 1986. [Google Scholar]
- Van den Berg H, Lodder W, Van der Poel W, Vennema H, de Roda Husman AM. Genetic diversity of noroviruses in raw and treated sewage water. Res Microbiol. 2005;156:532–540. doi: 10.1016/j.resmic.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Van Hereden J, Ehlers M, Van Zyl W, Grabow W. Incidence of adenoviruses in raw and treated water. Water Res. 2003;37:3704–3708. doi: 10.1016/S0043-1354(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- WHO . Guidelines for Safe Recreational Water Environments. Volume 1: Coastal and Freshwaters. World Health Organization; Geneva, Switzerland: 2003. p. 219. 2003. [Google Scholar]
- Wunderlin DA, Diaz MP, Ame MV, Pesce SF, Hued AC, Bistoni MA. Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia river basin (Cordoba, Argentina) Water Res. 2001;35:2881–2894. doi: 10.1016/s0043-1354(00)00592-3. [DOI] [PubMed] [Google Scholar]
- Wyn-Jones A, Carducci A, Cook N, D’Agostino M, Divizia M, et al. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res. 2011;45:1025–1038. doi: 10.1016/j.watres.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Scholten T. A fixative for intestinal parasites permitting the use of concentration and permanent staining procedures. American Journal of Clinical Pathology. 1977;67:300–304. doi: 10.1093/ajcp/67.3.300. [DOI] [PubMed] [Google Scholar]