Abstract
Background
Mortality is a main driver in zooplankton population biology but it is poorly constrained in models that describe zooplankton population dynamics, food web interactions and nutrient dynamics. Mortality due to non-predation factors is often ignored even though anecdotal evidence of non-predation mass mortality of zooplankton has been reported repeatedly. One way to estimate non-predation mortality rate is to measure the removal rate of carcasses, for which sinking is the primary removal mechanism especially in quiescent shallow water bodies.
Objectives and Results
We used sediment traps to quantify in situ carcass sinking velocity and non-predation mortality rate on eight consecutive days in 2013 for the cladoceran Bosmina longirostris in the oligo-mesotrophic Lake Stechlin; the outcomes were compared against estimates derived from in vitro carcass sinking velocity measurements and an empirical model correcting in vitro sinking velocity for turbulence resuspension and microbial decomposition of carcasses. Our results show that the latter two approaches produced unrealistically high mortality rates of 0.58-1.04 d-1, whereas the sediment trap approach, when used properly, yielded a mortality rate estimate of 0.015 d-1, which is more consistent with concurrent population abundance data and comparable to physiological death rate from the literature.
Ecological implications
Zooplankton carcasses may be exposed to water column microbes for days before entering the benthos; therefore, non-predation mortality affects not only zooplankton population dynamics but also microbial and benthic food webs. This would be particularly important for carbon and nitrogen cycles in systems where recurring mid-summer decline of zooplankton population due to non-predation mortality is observed.
Introduction
Importance of non-predation mortality
Zooplankton are the conduit of matter and energy from primary producers to higher trophic levels; consequently population dynamics of zooplankton often determine the functioning of pelagic food webs [1, 2]. Full description of zooplankton population dynamics requires knowledge of growth, reproduction and mortality; of these, mortality is the most poorly constrained [3, 4]. Mortality estimation is commonly derived from imbalance between recruitment rate and observed abundance changes, and the so-estimated mortality rate reflects (but not equals) the sum of predation and non-predation mortalities [5, 6], where non-predation mortality is defined as mortality not due to predation, hence it could have countless possible causes [4]. Predation is often assumed to be the major, if not the only, cause of mortality. In reality, however, non-predation mortality is also important in regulating zooplankton populations [6] and may contribute greatly to the detrital pool in various aquatic systems [7].
A meta-analysis suggested that predation can only account for two-thirds to three-quarters of the total mortality among epipelagic marine copepods [8]. In some lakes and reservoirs, periodic declines of zooplankton populations are caused almost entirely by non-predation factors that leave behind intact carcasses [9–11]. The fate of zooplankton carcasses is to a large extent hinged on their sinking velocity: Slow sinking carcasses provide high quality substrates for microbes and favor nutrient retention within the water column [12], whereas fast sinking carcasses increase the organic matter flux to the benthos [13, 14].
Measuring non-predation mortality with sediment traps
Non-predation mortality can be estimated on a basis of carcass abundance [9, 15, 16]. For example, Gries and Güde [9] calculated the non-predation mortality of Daphnia in Lake Constance as daily loss due to sedimentation of intact and presumed dead individuals into non-poisoned sediment traps to be 0.002–0.18 d-1 (0.2% and 18% of the standing stock). Likewise, Frangoulis et al. [16] estimated depth-average non-predation mortality rate of copepods in the Western Mediterranean as <0.01–0.05 day-1 by applying the method of Gries and Güde [9] to ‘swimmer-excluding’ sediment traps with all settled animals considered as dead.
An original method of direct in situ estimation of non-predatory mortality based on sediment trap data, proposed by Gladyshev and Gubanov [17], was applied to Daphnia longispina and Cyclops vicinus populations in a small Siberian reservoir (e.g. [11, 18, 19]). The method utilizes the equation of the vertical transport of the carcasses with a source term in the following form:
| (1) |
where m (d-1) is the specific non-predation mortality, F is the vertical flux of carcasses, N and y are abundances of live individuals and carcasses (ind m-3), respectively. Integration of this equation over the layer 0 < z < h above the sediment trap yields:
| (2) |
where the vertical flux F* of carcasses at the trap exposure depth h (ind m-2 d-1) is directly measured by sediment traps as
| (3) |
Here, Y is number of carcasses accumulated in a sediment trap per day (ind d-1), S is the input area of the trap (m2). Using the condition of zero flux of the carcasses across the air-water boundary F(0) = 0, and applying the mean value theorem, one arrives at
| (4) |
where the overbar denotes vertical averaging over the layer 0 < z < h (m). Assuming the mortality rate to be approximately constant within the integration layer, the expression for the non-predatory specific mortality m becomes
| (5) |
Sinking velocity of carcasses v* (m d-1) at the depth of traps exposure can be found from the concentration of the carcasses at the exposure depth y* (ind m-3) and vertical flux F* (Eq 3) as
| (6) |
Using the definition for the specific rate of elimination of carcasses from the sampling layer, G (d-1):
| (7) |
and applying forward finite differences for time integration of the differential Eq (5), one arrives at the equation for non-predatory specific mortality:
| (8) |
or, assuming nearly homogeneous vertical distribution of the carcasses (y i* = y i), as originally proposed by Gladyshev and Gubanov [17]:
| (8A) |
where ∆y = y i+1−y i is the difference in mean carcass abundances in the layer 0—h (ind m-3) over the period ∆t = t i+1 − t i of the trap exposure and zooplankton sampling (day); i = 1, 2, … n; n being the total number of samples taken at the location over the study period.
Eq 8A was originally applied to the shallow (0–2 m), well-mixed layer of small reservoirs (e.g. [11, 17, 19]), while Eq 8 may perform better in deep-water layer studies. Note that, strictly speaking, elimination of carcasses (Eq 7) includes sedimentation, decomposition and ingestion. Sedimentation is however assumed the primary (fastest) component of the elimination because carcasses in general sink faster than they decompose [9, 20–22]. Nonetheless, such an assumption can be properly evaluated by comparing the so-estimated non-predation mortality rate with concurrent population abundance data.
In this study, we applied Eqs 6, 7 and 8A to estimate non-predation mortality rates. Carcass sinking velocity—a key variable in the equations—was obtained by three approaches: a) settling column method, b) settling column method corrected for turbulence resuspension and microbial degradation, and c) in situ sediment trap data (Eq 6). Settling column method is commonly used to measure in vitro sinking velocity of detrital particles, including zooplankton carcasses, under constant temperature and viscosity in the absence of convective or turbulent motions [23], and sometimes these measurements are extrapolated to in situ conditions without sufficient consideration of the effects of turbulence or decomposition (e.g. [16, 24]). Kirillin et al. [21] introduced a model to correct settling column measurements for turbulence resuspension and microbial degradation of zooplankton carcasses, but it has not been verified by field observations. Our goal is to compare the mortality estimates by the different approaches, discuss probable causes for discrepancies, and highlight the importance of non-predation zooplankton mortality for food web dynamics and biogeochemical cycling in aquatic systems.
Materials and Methods
Study site
Lake Stechlin (53°10' N, 13°02' E), a dimictic oligo-mesotrophic lake in Germany [25], has been intensively studied for the last 4 decades [26]. It is a site member of the Global Lake Ecological Observatory Network (GLEON), and recently its zooplankton live/dead composition [27] and microbial carcass decomposition [12] have been investigated. Our study was conducted on 3rd-11th July, 2013 at close to the deepest point (ca. 70 m) of the lake, ca. 100 m from a moored autonomous monitoring station LakeESP. Field permit was granted to the Leibniz institute by the Stechlin natural park authorities on a permanent basis.
Sediment traps
Three pairs of sediment traps were deployed at 12 m (± 1 m) in the lower part of the thermocline each day for 6 consecutive days. Each trap, after the design of Håkanson [28], consisted of a pair of cylindrical collectors with a closing mechanism (Fig 1). Each collector had the dimensions of 0.077 m dia. × 0.485 m height; the height-to-diameter ratio therefore satisfied the recommended value to prevent resuspension [29]. Before deploying, the cylinders were filled with water from 12 m pre-screened through a 90 μm mesh; no poison was used. The cable was anchored, stretched by a submerged buoy and marked by a surface buoy. Trap exposure time was 1 day, except on 10th July when the exposure time was 20 hours. After traps retrieval, zooplankton samples from the paired collectors were pooled, concentrated on a 90 μm mesh and processed to obtain Y in Eq 6.
Fig 1. Photo of the sediment trap.

Water column zooplankton samples
Zooplankton at trap depth were sampled daily at around 10 a.m. using a Schindler-Patalas sampler (volume 14 L, collector mesh size 90 μm) to obtain y* in Eq 6. Samples were pooled from triplicate runs (total 42 L); on 4th July an additional sample was taken after 6 hours of trap exposure. In addition, to obtain depth-averaged N i, y i and y i+1, pooled samples (total 56 L) were taken at 0, 3, 6 and 9 m on 3rd, 5th and 10th July.
Staining and counting of zooplankton
To distinguish between live and dead zooplankton, samples from the Schindler-Patalas sampler and sediment traps were stained within an hour after collection with aniline blue [27, 30] using a staining device [19, 20], and fixed in 10% formalin. Live (unstained) and dead (stained blue) zooplankton were identified and counted, and their body length measured under a microscope [20]. Entire samples were counted, but for samples with >500 individuals, random subsamples were examined until at least 100 individuals of the same species were counted.
In situ sinking velocity
In situ carcass sinking velocity was calculated according to Eq 6. Because samples were pooled from each pair of collectors, trap input area was calculated as: S = (0.077/2)2π ∙2 = 0.0093 m2. y* was calculated as the average of samples taken at the beginning and at the end of the daily trap exposure. For reliable calculations, only the most abundant species in each trap was used, in this case Bosmina longirostris (O.F. Müller).
In vitro carcass sinking velocity and carcass density
Experiments were conducted to measure carcass density and in vitro carcass sinking velocity. Carcass density was measured by the density gradient method: A saline solution (0.1 g NaCl ml-1) was mixed with distilled water in 50-ml centrifuge tubes to create various densities (1.055, 1.060, 1.065, 1.070, 1.075 and 1.080 g ml-1). Live B. longirostris collected from the lake was killed by brief exposure to the saline solution, then briefly rinsed in lake water and examined under the microscope to ensure no air bubbles were trapped underneath the carapace. The carcasses were then gently added individually with a thin pipette into the centrifuge tubes, and their sinking patterns were noted. If the carcass sank or floated quickly, it was removed and transferred to the next higher or lower density. This process continued until the carcass was at or near neutral buoyancy, at which point its density was assumed to be equal to the solution density. If the carcass sank in one solution density but floated in the next higher solution density, its density was assumed to be the median between the two densities.
In vitro carcass sinking velocity under constant temperature and absence of turbulence was measured in a 1-L graduated cylinder filled with lake water and equilibrated to room temperature (~20°C) to minimize convection. Fresh carcasses produced as described before were gently released individually just below the surface. The time required for the carcasses to sink at least 20 cm was recorded; if the trajectory clearly deviated from a vertical line the trial was discarded.
Mortality calculations
Non-predation specific mortality (m) was calculated for 0–12 m according to Eq 8A. The specific rate of elimination (G) was calculated from Eq 7 using h = 12 m and sinking velocity v* determined from Eq 6. Steps were taken to minimize potential errors in estimation of zooplankton and carcass abundances [20]: 1) traps were exposed for ≤24 h to increase accuracy of Y; 2) three replicate traps were exposed simultaneously to increase accuracy of Y by increasing the total value of S and to increase precision of Y; 3) large volume plankton samples (42 L) were collected at trap depth to increase accuracy of y*; 4) sampling at trap depth was done 2–3 times per day to increase precision of y* and to integrate daily variations of y*.
Environmental data
The autonomous environmental station LakeESP measured major meteorological variables (air temperature and humidity, wind characteristics, incoming solar radiation) as well as underwater light conditions, temperature and oxygen profiles. Data were recorded at 10 min interval.
Turbulence estimation
A free-falling shear microstructure profiler MSS-60 (Wassermesstechnik Prandke) equipped with two airfoil velocity shear sensors was used to measure the dissipation rate of turbulence kinetic energy (ε) [31]. The instrument was allowed to fall freely at a speed of 0.5 m s-1 taking measurements at 1024 Hz. Series of 10–15 profiles with intervals of 10–15 min were taken at noon on each day. The vertical turbulent diffusivity (K Z) was determined from the shear microstructure profiles [21] and was subsequently used to add turbulence effect to the in vitro sinking velocities.
Carcass sinking and decomposition model
In vitro carcass sinking velocity in non-turbulent environment (U S) was estimated as:
| (9) |
where L (m) is the equivalent spherical diameter of the carcass, υ (m2 s-1) is the kinematic viscosity of water, B = g∆ρ/ρ w (m s-2) is carcass buoyancy, g (m s-2) is the gravity acceleration, ∆ρ = (ρ p −ρ w) is the difference between carcass density (ρ p) and water density (ρ w), C 1 = 24 and C D = 0.75 are empirical constants [32].
The turbulence effect is introduced into the model by adopting the following equation for carcass trajectory in a spatially heterogeneous turbulence field [33]:
| (10) |
where Z is the vertical coordinate of a carcass, R is a normally distributed random number with zero mean and variance of 1.
The effect of microbial degradation on carcasses density was parameterized as a function of time and temperature as [34]:
| (11) |
where ρ pi is the initial carcass density (kg m-3), T is temperature (°C) and t is time in hours (t = 0 at 0.25 h after death).
Statistical analysis
Standard deviation (SD), coefficient of variation (CV), standard error (SE) and the Kolmogorov–Smirnov test for normality (DK-S) were calculated conventionally using STATISTICA software, 9.0 (StatSoft Inc., Tulsa, OK, U.S.A.). Relative error was calculated as %SE relative to the mean.
Results
Environmental conditions, thermal stratification and turbulence
The weather was calm during the study with daily mean wind speed <2 m s-1; surface water temperature increased from 19.5°C on 3rd July to 23°C on 10th July (Fig 2A). A strong thermocline began at 8 m, and temperature at the sediment trap depth (12 m) was ca. 7°C (Fig 2B). ε decreased from 5×10−9 m2 s-3 at 8 m to 5×10−10 m2 s-3 at 20 m and remained close to the detection limit below (Fig 3). In the epilimnion, ε increased rapidly from ~10−8 m2 s-3 at 5 m to ~10−5 m2 s-3 at 1.5 m, characteristic of turbulence produced by wind shear and surface wave breaking [21]. K Z was close to kinematic molecular viscosity (~10−6 m2 s-1) in the metalimnion, increased slightly in the deeper water, and was high (~10−4 m2 s-1) across the surface mixed layer (Fig 3 ). Overall, the water column was characterized by a shallow epilimnion exposed to wind mixing, a strong thermocline, and a cold hypolimnion with low mixing intensity between ~15 m and 65 m. Therefore, the bulk of the water column was a nearly homogeneous non-turbulent environment, close to the classical Stokes’ condition.
Fig 2.
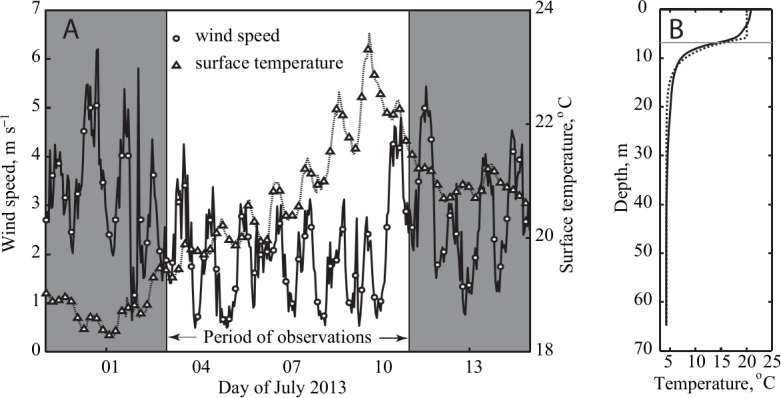
(A) Lake surface temperature and wind speed before, during and after the field experiment. (B) The vertical temperature profile averaged over the observations period. Horizontal dash line marks the bottom of the epilimnion determined from location of the maximum vertical temperature gradient.
Fig 3. Turbulent mixing within the water column during the field experiment.
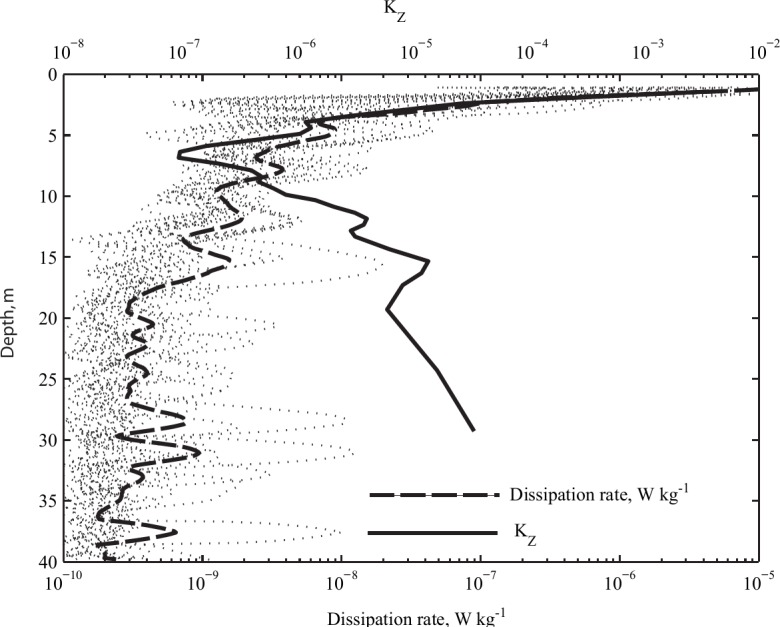
Turbulent diffusion coefficient K Z averaged for the study period (thick solid line) and the dissipation rate of the turbulent kinetic energy (thin dash lines are individual profiles; thick dash line is the average for the whole study).
Trap and water column samples
Number of B. longirostris carcasses accumulated in the traps per day varied from 20 to 104 (Table 1), and the data showed a normal distribution (Kolmogorov–Smirnov test DK-S = 0.164, p > 0.20). Relative errors of carcass abundance among the 3 traps were low (12.4–27.6%; Table 1), indicating good trap precision. Carcasses of other zooplankton species were too rare to be included in the analysis. Relative errors of estimation of carcass abundance at trap depth ranged from 1.3 to 66.2% (Table 1). At that depth, dead B. longirostris comprised 2.4–8.2% of the total abundance, averaging 6.0 ± 0.9% (Table 2). Percentage of dead B. longirostris was much higher inside the traps averaging 48.0 ± 4.8% (range 37.3–65.1%, estimated only on 9th-11th July).
Table 1. Number of Bosmina longirostris carcasses accumulated in sediment traps per day (Y in Eq 6) and carcass abundances at trap depth (12 m, average of 2–3 samples taken at the beginning and at the end of daily trap exposure; y* in Eq 6) in Lake Stechlin.
| Carcasses in sediment trap (ind) | Carcasses at trap depth (ind m-3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Date in July | Trap 1 | Trap 2 | Trap 3 | CV (%) | Relative error (%) | CV (%) | Relative error (%) | |
| 3–4 | 44 | 43 | 20 | 38.1 | 22.0 | 3095 | 43.5 | 30.8 |
| 4–5 | 78 | 48 | 42 | 34.4 | 19.9 | 2493 | 18.5 | 10.7 |
| 5–6 | 63 | 41 | 50 | 21.5 | 12.4 | 3056 | 1.8 | 1.3 |
| 6–7 | 86 | 75 | 52 | 24.4 | 14.1 | 3553 | 18.2 | 12.9 |
| 9–10 | 63 | 103 | 87 | 23.9 | 13.8 | 6576 | 93.6 | 66.2 |
| 10–11 | 35 | 104 | 90 | 47.7 | 27.6 | 7965 | 52.7 | 37.2 |
| Mean | 62.4 | 39.4 | 9.3 | 4087 | 67.1 | 22.4 | ||
CV = Coefficient of variation.
Table 2. Abundance and % dead of Bosmina longirostris at trap depth (12 m) in Lake Stechlin.
| Date in July | Live (ind m-3) | Dead (ind m-3) | % dead |
|---|---|---|---|
| 3 | 46190 | 4048 | 8.06 |
| 4 | 37857 | 2143 | 5.4 |
| 4* | nd | 2321 | nd |
| 5 | 80167 | 3016 | 3.63 |
| 6 | nd | 3095 | nd |
| 7 | 48512 | 4010 | 7.6 |
| 9 | 89286 | 2222 | 2.4 |
| 10 | 122619 | 10931 | 8.18 |
| 11 | 67857 | 5000 | 6.86 |
| Mean ± SE | 70356 ± 11244 | 4087 ± 914 | 6.0 ± 0.9 |
nd = no data.
*additional sample taken 6 hr after trap exposure.
Live B. longirostris appeared to congregate in the metalimnion (Table 2). By 10th July, only a few dead individuals remained in the 0–9 m layer, whereas all live ones were found deeper (Table 3). We calculated the mean abundances (N i, y i and y i+1 for Eq 8A) for the 0–12 m layer by extrapolating the abundances at 12 m to that in the 9–12 m layer, i.e. weighted mean for 0–12 m = [N(0–9)×9m + N12×(12-9m)]/12m, where N(0–9) and N12 are abundances in the 0–9 m layer and at 12 m, respectively (Table 3).
Table 3. Abundances and % dead of Bosmina longirostris in water column above the traps (0–9 m) and weighted mean for 0–12 m (N i and y i) in Lake Stechlin.
| Date in July | Layer (m) | Live (ind m-3) | Dead (ind m-3) | Er (ind m-3) | % dead |
|---|---|---|---|---|---|
| 3 | 0–9 | 20982 | 2035 | 748.4 | 8.84 |
| 0–12 | 27284 | 2538 | 883.2 | 8.51 | |
| 5 | 0–9 | 23725 | 520 | 269.0 | 2.15 |
| 0–12 | 37835 | 1144 | 485.9 | 2.95 | |
| 10 | 0–9 | 0 | 232 | 146.8 | 100 |
| 0–12 | 30655 | 2907 | 977.9 | 8.66 |
Er = Error of carcass abundance estimation (see text for explanation).
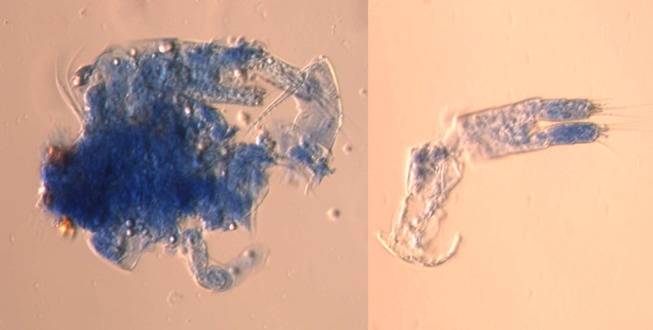
Active ‘swimmers’ of the large copepod Megacyclops (Acanthocyclops) gigas (Claus) appeared in the traps, which might cause errors in our calculation if they removed some of the carcasses. However, M. gigas consumes mainly live animals by sucking out the body content of their prey [35], the remains of which would appear very differently than carcasses from non-predation mortality [20]. We did not find sucked carcasses or partial remains of B. longirostris, and we saw only remains of copepods in extracted gut content of M. gigas (Fig 4).
Fig 4. Gut content extracted from Megacyclops gigas showing remains of cyclopoid copepods.
Carcass sinking velocity
In situ sinking velocity of B. longirostris carcasses was calculated for each trap and each day, and it varied from 0.47 to 3.36 m d-1 (Table 4). The data were normally distributed (DK-S = 0.116, p > 0.20). The mean ± SD of 10 replicate in vitro carcass sinking velocity measurements was 134.26 ± 32.31 m d-1 (Table 5). The mean ± SD of 10 replicate measurements of carcass density was 1.070 ± 0.008 g ml-1 (Table 6).
Table 4. In situ sinking velocity of Bosmina longirostris carcasses calculated from Eq 6 using data from Table 1 for Lake Stechlin.
| Sinking velocity (m d-1) | |||
|---|---|---|---|
| Date in July | Trap 1 | Trap 2 | Trap 3 |
| 3–4 | 1.53 | 1.49 | 0.69 |
| 4–5 | 3.36 | 2.07 | 1.81 |
| 5–6 | 2.21 | 1.44 | 1.76 |
| 6–7 | 2.60 | 2.27 | 1.57 |
| 9–10 | 1.23 | 2.02 | 1.70 |
| 10–11 | 0.47 | 1.40 | 1.21 |
| Mean ± SE | 1.71 ± 0.16 | ||
Table 5. In vitro sinking velocity of Bosmina longirostris carcasses measured by settling column method.
| Replicate | Sinking velocity (m d-1) |
|---|---|
| 1 | 136.06 |
| 2 | 87.27 |
| 3 | 138.24 |
| 4 | 121.69 |
| 5 | 140.49 |
| 6 | 205.71 |
| 7 | 115.97 |
| 8 | 161.50 |
| 9 | 128.96 |
| 10 | 106.67 |
| Mean ± SE | 134.26 ± 10.22 |
Table 6. Density of Bosmina longirostris carcasses measured with the density gradient method.
| Replicate | Density (g ml-1) |
|---|---|
| 1 | 1.073 |
| 2 | 1.060 |
| 3 | 1.058 |
| 4 | 1.078 |
| 5 | 1.080 |
| 6 | 1.080 |
| 7 | 1.073 |
| 8 | 1.068 |
| 9 | 1.063 |
| 10 | 1.070 |
| Mean ± SE | 1.070 ± 0.0025 |
The equivalent spherical diameter of B. longirostris was estimated as 243.3 μm by substituting the in vitro sinking velocity into Eq 9. This value is close to that determined by microscopy (body length 300 × height 230 × width 120 = 0.008 μm3; equivalent spherical diameter ~250 μm). Modeled sinking velocity varied from ~140 m d-1 in the epilimnion to ~80 m d-1 in the bulk water column. Adding turbulence and decomposition effects decreased the sinking velocity in the hypolimnion to ~75 m d-1, which was still much higher than the in situ estimate from sediment trap data.
Mortality rate estimations
Error of carcass abundance estimation (Er) can be calculated as Er = 2.47 y 0.75 [36]. This allows us to compare carcass abundances in 0–12 m (y i, Table 3) and in the trap exposure layer (y*, Table 1): on 3rd July, y i = 2538 ind m-3 (Er = 883) and y* = 3095 (Er = 1025) (Student’s t-test = 0.41, p>0.05); on 10th July, y i = 2907 (Er = 978) and y* = 6576 (Er = 1804) (Student’s t-test = 1.79, p>0.05). Thus, mean carcass abundances between the two layers were not significantly different, i.e. y i ≈ y*, that is a pre-requisite for mortality calculation using Eq 8A. Abundance of live B. longirostris in 0–12 m (N i in Eq 8A) was 27284 ind m-3 on 3rd July (Table 3); the corresponding y i was 2538 ind m-3 on 3rd July and y i+1 was 2907 ind m-3 on 10th July. Thus, ∆y = 369 ind m-3. G = v*/h = 1.71 m d-1/12 m = 0.14 d-1. m calculated using Eq 8A for the interval ∆t = 7 days was 0.015 d-1. As y i ≈ y*, this is not significantly different from mortality rate estimated from Eq 8 (m = 0.018 d-1).
Carcass sinking velocity from settling column measurements was much higher (average v = 134.26 m d-1), yielding a much higher m (1.043 d-1). The average v from our hydrodynamic model was 75 m d-1 and the corresponding m was 0.583 d-1.
Discussion
Non-predation mortality has the potential to control zooplankton dynamics and therefore it represents an important factor in population biology and organic matter cycling in aquatic systems. We compared different ways to derive non-predation mortality and evaluate them against observed population abundance data in order to arrive at a more reliable estimate. Carcass sinking velocity of B. longirostris calculated from our sediment trap data was comparable to that measured by sediment traps (0.70–1.40 m d-1) in the small non-stratified reservoir Bugach (Siberia, Russia) [37]. Similar approach has been used to measure sinking velocities of marine snow and fecal pellets [38, 39]. Carcass sinking velocities obtained from lab experiments and model calculations were both much higher, and the almost two orders of magnitude difference led to very different non-predation mortality rates for B. longirostris in Lake Stechlin: 1.043 d-1 (lab), 0.583 d-1 (model) and 0.015 d-1 (sediment trap). Below we consider probable causes for this discrepancy and the ecological implications of our results.
Overestimation by settling column method
The settling column method measures in vitro carcass sinking velocity under constant temperature and viscosity and in the absence of water motions. Such conditions are rare in natural water bodies; consequently, this method gives the maximum sinking velocities that may not be transferrable to natural conditions [40]. For example, small krill fecal strings in the surface ocean could potentially sink 100–200 m d-1 based on settling column measurements, but they were absent in 150 m traps, suggesting that the sinking was greatly decreased by turbulence, stratification or coprophagy [23]. Likewise, the settling column method likely overestimated the in situ carcass sinking velocity in our study.
Possible bias in model calculations
Our hydrodynamic model showed that stratification, turbulence and microbial degradation lowered carcass sinking velocity to ~75 m d-1. Applying room temperature in the model produced sinking velocities observed in vitro, showing that the model correctly reproduced temperature effects on sinking velocities. It, however, did not account for nighttime convection in the lake. Although the net heat budget for the lake was positive and the average surface temperature increased during our study, air temperatures dropped down to 6°C below the water surface temperature at night, suggesting nighttime surface cooling at a rate of 6–12 W m-2. This would produce vertical convective water motions, whose root mean square velocity can be estimated from the surface cooling rate to be ~3–4 mm s-1 [41], which is 2–3 times higher than in vitro carcass sinking velocity. Hence, nighttime convection might significantly slow down carcass sinking in the epilimnion. In an asymptotic case, if all carcasses were produced in the epilimnion and if carcass sinking was stopped by nighttime convection, the daily mean in situ carcass sinking velocity would be decreased by half. Convection will be absent if the atmosphere is warmer than the lake surface. Still, the retention time of carcasses in the epilimnion can be increased by vertical wave currents or Langmuir circulations, resolution of which will require information on fine-scale structure of the velocity fields [21]. Nevertheless, their effects on sinking of carcasses in our study were likely minor given the practically windless conditions during the field experiments.
The Stokes’ equation may overestimate the sinking velocity of irregularly shaped objects. For instance, the sinking velocity of undisturbed marine snow aggregates (2.4–75 mm in length) measured by SCUBA diving was lower than that derived from Stokes’ formula for spherical and ellipsoid objects [42], but similar to those calculated from sediment trap and particle abundance data ([38]; similar to our Eq 6). The modified Stokes formula (Eq 9) takes into account the effect of irregular shape on carcasses sinking that is confirmed by the good agreement of the equivalent spherical diameters provided by Eq 9 and carcasses size determined by microscopy.
Porosity and density of zooplankton carcasses may change due to decomposition and subsequently affect sinking velocity [43]. Zelezinskaya [44] reported that laboratory sinking velocity of Labidocera brunescens (Copepoda) carcass one hour after death was 355 m d-1, but in 5 hours it decreased to 309 m d-1 (-13%) and in 30 hours to 244 m d-1 (-31%) under constant salinity and temperature. Carcass sinking velocity of copepodite IV-VI in the York River estuary decreased from 107.1 to 67.4 m d-1 in the first 4 hour after death (recalculated from Table 3 in [34]). Sinking velocity of decapod zoea carcasses decreased by 2.2 times in 20 hours and 6.7 times in 70 hours after death [44]. Decomposition of carcasses may also result in formation of interstitial microbubbles of gases, which further increase buoyancy and as a result, carcasses may remain suspended or even float [44]. This “anti-rain” of carcasses may partly explain their high percentage occasionally observed in zooplankton and neuston samples and in surface foam [44, 45].
Possible bias in trap collection
Appropriate choice of sediment trap dimensions is important for avoiding under-trapping [46]. We used cylinders with a height-to-diameter (H/D) ratio of 6.3, which is sufficient to prevent resuspension even without baffles [47]. According to Lau [48], for cylinder traps with a H/D ratio of 6, Reynolds number needs to be ca. 10 000 to cause resuspension from the trap. Given our trap diameter (7.7 cm) and ambient water temperature (ca. 7°C; corresponding viscosity = 0.0145 cm2 s-1), such a high Reynolds number would require a current velocity of 18.8 cm s-1, much higher than the observed current velocity (5 cm s-1) at trap depth. Thus, resuspension from our traps was unlikely.
Another possible error is tilting of the traps [49]. Our trap design maintained the cylinders in upright position independent of cable angle [28]. The traps were moored to a small surface marker buoy and a large subsurface one. Occasionally the subsurface buoys got close to surface causing vibration of the cables and possibly the traps. However, considering the low variability among replicate traps (Table 1) and the calm weather, possible error due to these occasional cable motions was likely negligible.
The coefficients of variance and relative errors of total and carcass abundances were low to moderate (Table 1), which fall within the limits of error of zooplankton abundance estimation of 25–66% for similar sampling method [36]. The one exception was 9-10th July when both live and dead B. longirostris abundances were increasing. Given the large sample volumes and replications, we believe that we estimated carcass abundances (in water and in traps) with good precision despite known high spatial heterogeneity and patchiness of Bosmina spp. [50, 51].
Bosmina longicornis has been shown to perform diel vertical migration (DVM) [52]. Even if it occurred in Lake Stechlin, it would not affect y or ∆y in Eq 8A, nor would it affect our sediment trap data because the traps were not poisoned, they were set out for 20–24 hours, and only carcasses in the traps were used for the calculations. DVM, however, could lead to an underestimation of N i in Eq 8A and subsequently an overestimation of non-predation mortality rate. Nevertheless, the estimated non-predation mortality rates from Eq 8A were consistent with the water column abundance data, suggesting that any error due to DVM was likely small.
Potential effects of swimmers
Despite the presence of M. gigas in the traps, they are not known to feed on carcasses [35] and Bosmina (our gut content data) and therefore did not affect our calculations. Nevertheless, if we assume they did, we can estimate the associated errors based on their daily energy expenditure. Krylov [35] estimated a respiration rate of 0.29 μl O2 ind-1 h-1 at 19°C for M. gigas. Assuming a body wet weight of 0.348 mg for M. gigas [53] and an ambient temperature of 7°C, we estimated the daily energy expenditure as R = [(0.00029 × 24 × 4.86) / 2.9] / 0.6, where 4.86 cal ml-1 O2 is oxycalorific coefficient, 2.9 is temperature correction for Q10 = 2.3 [54], and 0.6 cal mg-1 is caloric content of wet mass [35]. The so-estimated respiratory cost was 0.019 mg wet weight ind-1 d-1. Assuming an assimilation efficiency of 0.8, the required prey consumption would be 0.024 mg ind-1 d-1. Given a wet weight of 0.0049 mg for Bosmina [53], this translates to a consumption of ~5 carcasses ind-1 d-1. The average number of M. gigas in our traps was 41±6 ind, which could consume 205 Bosmina carcasses. The corrected carcass abundance in the traps would be ~267 and the in situ carcass sinking velocity would increase to 7.0 m d-1. The corresponding m would be 0.056 d-1, which still would not explain the discrepancy with the other two estimates.
Reality check of non-predation mortality estimates
Based on in vitro carcass sinking velocity, we obtained a non-predation mortality rate of 1.04 d-1 for B. longirostris, which is unrealistically high. If we used a sinking velocity of 75 m d-1 from model calculations, the mortality rate would be 0.58 d-1, and the B. longirostris population would have completely collapsed in a few days, which obviously was not the case in our study (cf. [11]). The non-predation mortality rate derived from sediment trap data (0.015 d-1) is comparable to physiological death rate for marine and freshwater zooplankton (0.01–0.05 d-1; [3, 22]). This value also falls within the non-predation mortality rates of Daphnia measured by sediment traps in Lake Constance (0.002–0.18 d-1; [9]) and of mesozooplankton in coastal Mediterranean measured by ‘swimmer-excluding’ sediment traps (<0.01–0.05 d-1; [16]).
At a velocity of 1.71 m d-1 a carcass would take ~6 days to sink 12 m, during which time an unknown amount of the carcass materials could be lost to decomposition and detritivory in the upper water column. Although the copepod M. gigas was unlikely to feed on the carcasses, we could not rule out the possibility that other planktivores (including fish) may have consumed some of the carcasses. For example, B. longirostris and B. coregoni are major prey for the fish Coregonus albula in the summer in Lake Stechlin [55]. Although it is uncertain whether the fish feed on carcasses, we expect the true non-predation mortality rate to be somewhat higher than 0.015 d-1. Elliott and Tang [6] showed that non-predation mortality accounted for 12% of the total Acartia tonsa copepodite mortality over 2 years. Following their work, we estimate that the total (predation + non-predation) mortality of B. longirostris in Lake Stechlin would be ca. 0.13 d-1. Assuming a spherical diameter of 0.25 mm, we estimated a biovolume of 0.008 mm3 for B. longirostris. Applying this value to the algorithm of Hansen et al. [56] gives us an estimated maximum specific growth rate of ca. 0.24 d-1. These crude approximations show that the total mortality rate was on par with the growth rate, meaning that the population size should be rather stable. Judging from Tables 2 and 3, the abundance of live B. longirostris did not show any consistent increase or decrease, suggesting that the population was at close to equilibrium during our study. Similar findings were reported by Ivanova [54]: seasonal average total mortality rate of B. longirostris in a Russian lake was 0.13 d-1, measured juvenile growth rate 0.20 d-1 and generative growth rate of adults 0.23 d-1; overall for planktonic crustaceans the average specific production CB could be related to the average specific total mortality rate m as CB = (1.86 ±0.60) m [54].
Conclusion and implications for future research
Estimation of non-predation mortality of B. longirostris in Lake Stechlin was sensitive to carcass sinking velocity, which varied widely among the measurement methods. Mortality rates derived from in vitro sinking velocity and its model correction were both unreasonably high; only the mortality rate from in situ sediment trap measurements was realistic and consistent with concurrent population abundance data. Nevertheless, the actual mortality rate was expected to be slightly higher than 0.015 d-1 because of likely loss of some carcasses within the water column and underestimation of carcass abundance at trap depth, but these errors will be smaller for sediment traps set at shallower depth as long as the ambient current is below the resuspension threshold. Shallower depth would also minimize differences in carcass concentrations within the studied layer (y i) and at the trap depth (y*) because physical and ecological conditions tend to be more homogenous in thinner strata. The thinner the strata being studied, the greater the role of sedimentation in carcass elimination and the greater the accuracy in using Eqs 8 and 8A.
Despite the lack of evidence of ingestion of carcasses by M. gigas, hypothetical calculations of the swimmers’ consumption in the traps would potentially increase carcass sinking rate and the corresponding mortality rate by ~4 times. Thus, in cases when swimmers occur in traps, their gut contents and feeding biology need to be taken into careful consideration.
As we demonstrated, consecutive days of trap deployment along with water column sampling are needed to perform a reality check of the results; i.e. doing only one or the other and doing snap-shot sampling could give a wrong picture of the population dynamics. To further improve the sediment trap approach, imaging device may be used concurrently to observe and quantify sinking carcasses in situ [39, 57].
Our field data suggest that zooplankton carcasses might remain in the water column for days, during which time they could function as microbial hotspots supporting elevated bacterial production and accelerating nutrient recycling in the water column ([22] and reference therein). The labile organics from zooplankton carcasses may even provide a priming effect to facilitate degradation of recalcitrant allocthonous organic matter [58, 59]. In lakes and reservoirs where zooplankton experience recurring mass mortality due to non-predation factors [10, 18, 19], sinking carcasses can also provide a significant pulse of nutrients to the benthos [7, 60]. Therefore, the study of non-predation mortality is relevant to understanding not only zooplankton population dynamics but also biogeochemical cycles and the microbial and benthic food webs.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by joint projects of Russian Foundation for Basic Research and Belarusian Republican Foundation for Fundamental Research (No 14-05-90005-Bel-a to O.P.D and A.P.T., and No B14R-066 to Zh.B.). K.W.T. was supported by a Humboldt Fellowship for Experienced Researchers (Germany), M.I.G. was supported by Russian Federal Tasks of Fundamental Research (project No. 51.1.1), H.P.G. and G.K. were supported by grants from the German Science Foundation (GR 1540/20-1 and KI-853/8-1).
References
- 1. McQueen DJ, Post JR, Mills EL. Trophic relationships in freshwater pelagic ecosystems. Can J Fish Aquat Sci 1986;43: 1571–1581. [Google Scholar]
- 2. Kiørboe T. Population regulation and role of mesozooplankton in shaping marine pelagic food webs. Hydrobiologia 1998;363: 13–27. [Google Scholar]
- 3. Dubovskaya OP. Non-predatory mortality of the crustacean zooplankton, and its possible causes (a review). Zhurnal Obshchei Biologii 2009;70: 168–192. (in Russian) [PubMed] [Google Scholar]
- 4. Tang KW, Elliott DT. (2013) Copepod carcasses: Occurrence, fate and ecological importance In: Seuront L, editor. Copepods: Diversity, habitat and behavior. Hauppauge: Nova Science Publishers; 2013. pp. 255–278. [Google Scholar]
- 5. Aksnes DL, Miller CB, Ohman MD, Wood SN. Estimation techniques used in studies of copepod population dynamics—a review of underlying assumptions. Sarsia 1997;82: 279–296. [Google Scholar]
- 6. Elliott DT, Tang KW. Influence of carcass abundance on estimates of mortality and assessment of population dynamics in Acartia tonsa . Mar Ecol Prog Ser 2011;427: 1–12. [Google Scholar]
- 7. Simon M, Grossart HP, Schweitzer B, Ploug H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat Microb Ecol 2002;28: 175–211. [Google Scholar]
- 8. Hirst AG, Kiørboe T. Mortality of marine planktonic copepods: global rates and patterns. Mar Ecol Prog Ser 2002;230: 195–209. [Google Scholar]
- 9. Gries T, Güde H. Estimates of the nonconsumptive mortality of mesozooplankton by measurement of sedimentation losses. Limnol Oceanogr 1999;44: 459–465. [Google Scholar]
- 10. Hülsmann S., Voigt H. Life history of Daphnia galeata in a hypertrophic reservoir and consequences of non-consumptive mortality for the initiation of a midsummer decline. Freshwater Biol 2002;47: 2313–2324. [Google Scholar]
- 11. Dubovskaya OP, Gladyshev MI, Gubanov VG, Makhutova ON. Study of non-consumptive mortality of crustacean zooplankton in a Siberian reservoir using staining for live/dead sorting and sediment traps. Hydrobiologia 2003;504: 223–227. [Google Scholar]
- 12. Tang KW, Bickel SL, Dziallas C, Grossart H-P. Microbial activities accompanying decomposition of cladoceran and copepod carcasses under different environmental conditions. Aquat Microb Ecol 2009;57: 89–100. [Google Scholar]
- 13. Sampei M, Sasaki H, Hattori H, Forest A, Fortier L. Significant contribution of passively sinking copepods to the downward export flux in Arctic waters. Limnol Oceanogr 2009;54: 1894–1900. [Google Scholar]
- 14. Ivory JA, Tang KW, Takahashi K. Use of Neutral Red in short-term sediment traps to distinguish between zooplankton swimmers and carcasses. Mar Ecol Prog Ser 2014;505: 107–117. [Google Scholar]
- 15. Tang KW, Freund CS, Schweitzer CL. Occurrence of copepod carcasses in the lower Chesapeake Bay and their decomposition by ambient microbes. Estuar Coastal Shelf Sci 2006; 68: 499–508. [Google Scholar]
- 16. Frangoulis C, Skliris N, Lepoint G, Elkalay K, Goffart A, Pinnegar JK, et al. Importance of copepod carcasses versus faecal pellets in the upper water column of an oligotrophic area. Estuar Coastal Shelf Sci 2011;92: 456–463. [Google Scholar]
- 17. Gladyshev MI, Gubanov VG. Seasonal dynamics of specific mortality of Bosmina longirostris in a forest pond determined on the basis of counting of dead individuals. Doklady Biological Sciences 1996; 348: 244–245 (in English) [Google Scholar]
- 18. Dubovskaya OP, Gladyshev MI, Gubanov VG. Seasonal dynamics of number of alive and dead zooplankton in a small pond and some variants of mortality estimation. Zhurnal Obshchei Biologii 1999;60: 543–555. (in Russian) [Google Scholar]
- 19. Gladyshev MI, Dubovskaya OP, Gubanov VG, Makhutova ON. Evaluation of non-predatory mortality of two Daphnia species in a Siberian reservoir. J Plankton Res 2003;25: 999–1003. [Google Scholar]
- 20. Dubovskaya OP. Evaluation of abundance of dead crustacean zooplankton in a water body using staining of the samples by aniline blue technique: methodological aspects. J Siberian Federal Univ Biol 2008;1: 145–161. (in Russian) [Google Scholar]
- 21. Kirillin G, Grossart H-P, Tang KW. Modeling sinking rate of zooplankton carcasses: Effects of stratification and mixing. Limnol Oceanogr 2012;57: 881–894. [Google Scholar]
- 22. Tang KW, Gladyshev MI, Dubovskaya OP, Kirillin G, Grossart H-P. Zooplankton carcasses and non-predatory mortality in freshwater and inland sea environments. J Plankton Res 2014;36: 597–612. [Google Scholar]
- 23. Cadée GC, González H, Schnack-Schiel SB. Krill diet affects faecal string settling. Polar Biol 1992;12: 75–80. [Google Scholar]
- 24. Lebrato M, Mendes P, Steinberg DK, Cartes JE, Jones B, Birsa LM, et al. Jelly biomass sinking speed reveals a fast carbon export mechanism. Limnol Oceanogr 2013;58: 1113–1122. [Google Scholar]
- 25. Casper SJ. Lake Stechlin: A temperate oligotrophic lake Dordrecht: Dr W Junk Publishers; 1985. [Google Scholar]
- 26. Koschel R, Adams DD. Lake Stechlin: an approach to understand an oligotrophic lowland lake. Arch Hydrobiol Spec Iss Adv Limnol 2003;58: 1–311. [Google Scholar]
- 27. Bickel SL, Tang KW, Grossart H-P. Use of aniline blue to distinguish live and dead crustacean zooplankton composition in freshwaters. Freshwater Biol 2009;54: 971–981. [Google Scholar]
- 28. Håkanson L. Suspension and calibration of a sediment trap. Schweiz Z Hydrol 1984;46: 171–175. [Google Scholar]
- 29. Håkanson L, Floderus S, Wallin M. Sediment trap assemblages—a methodological description. Hydrobiologia 1989;176/177: 481–490. [Google Scholar]
- 30. Seepersad B, Crippen RW. Use of aniline blue for distinguishing between live and dead freshwater zooplankton. J Fish Res Bd Canada 1978;35: 1363–1366. [Google Scholar]
- 31. Prandke H, Stips A. Test measurements with an operational microstructure-turbulence profiler: Detection limit of dissipation rates. Aquatic Sci 1998;60: 191–209. [Google Scholar]
- 32. Ferguson RI, Church M. A simple universal equation for grain settling velocity. J Sed Res 2004;74: 933–937.31. [Google Scholar]
- 33. Hunter JR, Craig PD, Phillips HE. On the use of random walk models with spatially variable diffusivity. J Comput Phys 1993;106: 366–376. [Google Scholar]
- 34. Elliott DT, Harris CK, Tang KW. Dead in the water: the fate of copepod carcasses in the York River estuary, Virginia. Limnol Oceanogr 2010;55: 1821–1834. [Google Scholar]
- 35. Krylov PI. Predation of the freshwater cyclopoid copepod Megacyclops gigas on lake zooplankton: functional response and prey selection. Archiv für Hydrobiologie 1988;113: 231–250. [Google Scholar]
- 36. Gladyshev MI. Assessment of the statistical significance of differences in zooplankton abundance on single samples. Gidrobiologicheskii Zhurnal 1985;5: 65–70. (Translated into English as Hydrobiological Journal) [Google Scholar]
- 37.Dubovskaya OP. Natural mortality of zooplankton in reservoirs of Yenisei basin. PhD thesis, Zool Inst Russian Acad Sci St. Petersburg; 2006. (In Russian)
- 38. Asper VL. Measuring the flux and sinking speed of marine snow aggregates. Deep-Sea Res 1987;34: 1–17. [Google Scholar]
- 39. McDonnell MP, Buesseler KO. Variability in the average sinking velocity of marine particles. Limnol Oceanogr 2010;55: 2085–2096. [Google Scholar]
- 40. Parsons TR, Takahashi M, Hargrave B. Biological oceanographic processes Oxford: Pergamon Press; 1988. [Google Scholar]
- 41. Deardorff JW. Convective velocity and temperature scales for the unstable planetary boundary layer and for Rayleigh convection. J Atm Sci 1970;27: 1211–1213. [Google Scholar]
- 42. Alldredge AL, Gotschalk C. In situ settling behavior of marine snow. Limnol Oceanogr 1988;33: 339–351. [Google Scholar]
- 43. Stepanov VN, Svetlichnyyi LS. Research of hydromechanical characteristics of plankton copepods Naukova Dumka, Kiev; 1981. (in Russian) [Google Scholar]
- 44.Zelezinskaya LM. Natural mortality of some forms of ichthyo- and zooplankton of the Black Sea. PhD thesis, IBSS UAS, Odessa; 1966. (in Russian)
- 45. Baumann HI, Jüttner F. Inter-annual stability of oligopeptide patterns of Planktothrix rubescens blooms and mass mortality of Daphnia in Lake Hallwilersee. Limnologica 2008;38: 350–359. [Google Scholar]
- 46. Buesseler KO, Antia AN, Chen M, Fowler SW, Gardner WD, Gustafsson O, et al. An assessment of the use of sediment traps for estimating upper ocean particle fluxes. J Mar Res 2007;65: 345–41. [Google Scholar]
- 47. Bloesch J, Burns NM. A critical review of sediment trap technique. Schweiz Z Hydrol 1980;42: 15–55. [Google Scholar]
- 48. Lau YL. Laboratory study of cylindrical sedimentation traps. J Fish Res Bd Canada 1979;36: 1288–1291. [Google Scholar]
- 49. Gardner WD. The effect of tilt on sediment trap efficiency. Deep-Sea Res 1985; 32: 349–361. [Google Scholar]
- 50. Pinel-Alloul B. Spatial heterogeneity as a multiscale characteristic of zooplankton community. Hydrobiologia 1995;300/301: 17–42. [Google Scholar]
- 51. Cerbin S, Balayla DJ, Van de Bund WJ. Small-scale distribution and diel vertical migration of zooplankton in a shallow lake (Lake Naardermeer, the Netherlands). Hydrobiologia 2003;491: 111–117. [Google Scholar]
- 52. Staker RD. A diurnal zooplankton migration study in Lake Mead. J Arizona Acad Sci 1974;9: 85–88. [Google Scholar]
- 53. Balushkina EV, Winberg GG. Relation between mass and body size of plankton animals In: Winberg GG, editor. General backgrounds for study of aquatic ecosystems. Leningrad: Nauka; 1979. pp. 169–172. (in Russian) [Google Scholar]
- 54. Ivanova MB. Production of planktonic crustaceans in fresh water Zool Inst USSR Acad Sci; Leningrad: 1985. (in Russian) [Google Scholar]
- 55. Schulz M, Mehner T, Kasprzak P, Anwand K. Diet and consumption of vendace (Coregonus albula L.) in response to seasonal zooplankton succession in Lake Stechlin. Archiv für Hydrobiologie 2003;58: 215–226. [Google Scholar]
- 56. Hansen PJ, Bjørnsen PK, Hansen BW. Zooplankton grazing and growth: Scaling within the 2–2,000-μm body size range. Limnol Oceanogr 1997;42: 687–704. [Google Scholar]
- 57. Zadereev ES, Tolomeyev AP, Drobotov AV, Emeliyanova AY, Gubanov MV. The vertical distribution and abundance of Gammarus lacustris in the pelagic zone of the meromictic lakes Shira and Shunet (Khakassia, Russia). Aquatic Ecol 2010;44: 531–539. [Google Scholar]
- 58. Wetzel RG. Gradient-dominated ecosystems: sources and regulatory functions of dissolved organic matter in freshwater ecosystems. Hydrobiologia 1992;229: 181–198. [Google Scholar]
- 59. Guenet B, Danger M, Abbadie L, Lacroix G. Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 2010;91: 2850–2861. [DOI] [PubMed] [Google Scholar]
- 60. Grossart HP, Simon M. Limnetic macroscopic organic aggregates (lake snow): abundance, characteristics, and bacterial dynamics in Lake Constance. Limnol Oceanogr 1993;38: 532–546. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


