Abstract
Oxidant production from DUOX1 has been proposed to lead to neutrophil recruitment into the airways when lung homeostasis is compromised. The objective of this study was to determine whether DUOX-derived hydrogen peroxide is required for LPS-induced neutrophil recruitment, using a functional DUOX knock out mouse model. We found that LPS induced profound neutrophilic lung inflammation in both Duoxa+/+ and Duoxa-/- mice between 3h and 24h. Duoxa-/- mice had significantly higher neutrophil influx 24h after LPS instillation despite similar cytokine levels (KC, MIP-2, or TGF-α) between the two groups. These findings suggest that LPS-TLR-4-induced KC or MIP-2 cytokine induction and subsequent neutrophil recruitment in the airway does not require DUOX-derived hydrogen peroxide from airway epithelium.
Introduction
Dual oxidases (DUOX1 and DUOX2) are NADPH oxidases located at the epithelial surface of airway cells, and locally produce hydrogen peroxide (H2O2)[1]. The function of DUOX-mediated hydrogen peroxide production in the airway has been shown to provide direct lung host defense functions against Gram-negative[2] or Gram positive bacteria[3]. Additionally, increasing data suggest that DUOX-mediated H2O2 is responsible for oxidant-mediated signaling important for airway host defense.
Previously, several model systems have shown that H2O2 directly causes neutrophil chemotaxis[4–8] and several recent reports suggest that DUOX plays an important role in neutrophil chemotaxis in the airway in response to a variety of stimuli[9–11]. In vitro studies suggest that DUOX-derived H2O2 is essential for TGF-α signaling that subsequently leads to increased production of the neutrophil chemokine IL-8[10,11]. An in vivo murine model demonstrated that secretion of a mouse homolog of IL-8, MIP-2, is dependent upon DUOX2 activity[12]. And, we recently reported that DUOX is required for neutrophil recruitment in a mouse model of allergic asthma[9].
Collectively, these studies strongly implicate that DUOX regulates neutrophil chemotaxis through the canonical lipopolysaccharide-induced TLR4 signaling pathway, subsequently upregulating IL-8. Lipopolysaccharide (LPS) found on gram negative bacteria such as Pseudomonas aeruginosa was effective in stimulating DUOX activity, which potentially leads to neutrophil chemotaxis and wound repair[2,10,11,13]. Recently, an in vivo study by Li et al. found that diphenyleneiodonium chloride (DPI), a nonspecific NADPH oxidase inhibitor, suppressed neutrophil localization in bronchoalveolar lavage fluid after LPS exposure implicating DUOX as the key regulator of this recruitment[14].
It is firmly established that TLR4 is expressed on airway epithelium. However, the relative contribution of the airway epithelium versus hematopoietic cells in recruiting neutrophils after LPS challenge is less clear[15]. To better characterize the role of the airway epithelium, through DUOX-derived H2O2, to activate LPS-mediated neutrophil chemotaxis, we utilized a Duoxa -/- knockout mouse model that does not express functional DUOX1 or DUOX2[9,16]. DUOX1 and DUOX2 have both been implicated as having active roles in LPS-generated inflammatory signaling, thus a model system that is deficient in both DUOX isoforms was an important first step to determine the specific isoform mediating LPS-dependent signaling. Because DUOX isoforms are not expressed in hematopoietic cells, this model allowed us to specifically characterize the role of DUOX expressed in airway epithelial cells. In addition, this model excludes the possibility of one DUOX isoform compensating for the loss of the other.
We hypothesized that DUOX-derived hydrogen peroxide is necessary to signal neutrophil migration into the lungs following LPS exposure, and that lack of functional DUOX will result in reduced neutrophil chemotaxis into the lung.
Materials and Methods
Knockout mouse model
Duoxa -/- knockout mice were generated as described previously[16] and mice were obtained as a generous gift from Dr. Helmut Grasberger. All in vivo experiments were performed in accordance with the University of California at Davis Institutional Animal Care and Use Committee (IACUC). Mice utilized for our experiments were acquired through subsequent breeding of these breeding pairs at the UC Davis facility. Male mice, of 129Sv6 background, were maintained in HEPA-filtered laminar flow cage racks with a 12-hour light/dark cycle and allowed free access to food (Purina Rodent Chow) and water. Mice were housed and cared for by the veterinary staff of the UC Davis Animal Resource in AALAC-accredited facilities. Because Duoxa -/- mice are severely hypothyroid without hormone replacement[16], we supplemented mice with L-T4 hormone replacement as described previously[9]. Anesthesia and euthanasia procedures were performed according to UC Davis IACUC-approved protocols. All in vivo experiments were performed in accordance with the University of California at Davis Institutional Animal Care and Use Committee (IACUC) and specifically approved this study.
LPS Exposure
LPS from Pseudomonas aeruginosa 10, source strain ATCC 27316 (Sigma-Aldrich L8643) was diluted with phosphate-buffered saline (PBS). Mice were anesthetized with isoflurane and 40uL of either PBS (control), or 1μg or 10μg LPS dissolved in PBS was administered via intratracheal instillation. LPS-exposed animals and PBS controls were necropsied at 3h, 6h, 12h, 24h, and 7 days after instillation.
Bronchoalveolar lavage sample collection and processing
Mice were euthanized at specified timepoints with an intraperitioneal (IP) overdose of pentobarbital. The lungs were then lavaged two times with 1mL sterile PBS (pH = 7.4) to collect bronchoalveolar lavage fluid (BALF). BALF was centrifuged at 2000 rpm for 10 minutes and supernatant was collected and stored at -80°C. The resulting BALF cell pellet was resuspended in ACK/RBC lysis buffer and the pellet was resuspended in PBS. Live cell concentrations were estimated by counting trypan-blue-excluding cells on a hemacytometer. To determine BALF cell differentials, cytocentrifuge preparations were stained with a Hema3 kit as described in the manufacturer's instructions (Fisher Scientific, Kalamazoo, MI), and sealed using Cytoseal 60 (Richard-Allen Scientific, Kalamazoo, MI). Cell percent differentials were calculated by counting 10 fields at 400× magnification and classifying cell types as alveolar macrophage, neutrophil, eosinophil, lymphocyte, or “other” based upon standard morphological characteristics and staining profiles. Absolute cell counts were calculated by multiplying live cell counts by the cell type percent.
Enzyme-Linked Immunosorbant Assay (ELISA) analyses
The supernatant fraction of the BALF was thawed on ice and used in enzyme-linked immunosorbant assays (ELISA). The mouse homologs of human interleukin (IL)-8, Keratinocyte-Derived Cytokine (KC) and Macrophage Inflammatory Protein (MIP)-2, were detected using ELISA (R&D Systems, Product Number MKC00B and MM200, respectively). TGF-α was also analyzed similarly (R&D Systems, Product number DTGA00). BALF cytokine concentrations were determined by comparison to standard curves for each cytokine provided by the supplier.
Statistics
All data was processed using Prism 5 software (GraphPad Software, Inc., San Diego, California). Data was analyzed using 2-Way ANOVA followed by Bonferroni correction when appropriate. Data was deemed statistically significant at p ≤0.05.
Results
LPS Dose Response and Time Course
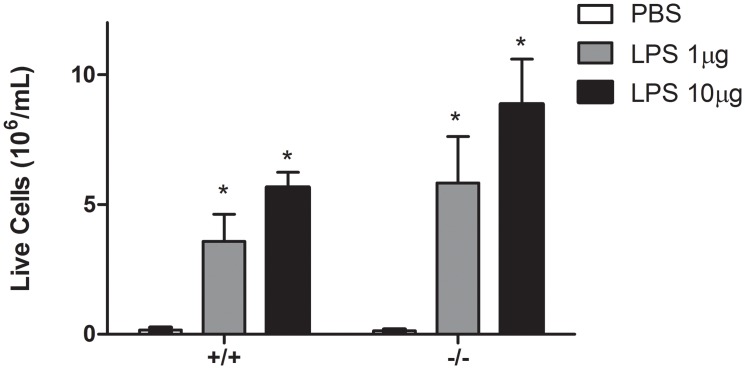
We evaluated live cell count dose responses to 1μg or 10μg LPS between Duoxa -/- and Duoxa +/+ mice (Fig 1). Both Duoxa -/- and Duoxa +/+ mice had robust increases in live cell counts after LPS instillation compared to PBS control. However, there appeared to be no dose response between the two doses of LPS we utilized. Given the lack of statistical significance in cell counts between the two doses, we utilized the 1μg dose of LPS for the remainder of our experiments.
Fig 1. Dose response to LPS in Duoxa +/+ and Duoxa -/- mice.
Leukocytes were collected from the airway compartment by BAL 24 hours after intratrachael instillation of LPS (1μg or 10μg). The number of live cells was determined by trypan blue exclusion. Live cell counts are displayed for PBS control (open box), 1μg LPS (gray box), or 10μg LPS for both Duoxa -/- (-/-) and Duoxa +/+ (+/+) mice. Data represent mean ± SEM from six animals in each group; * = p<0.05 compared to PBS control.
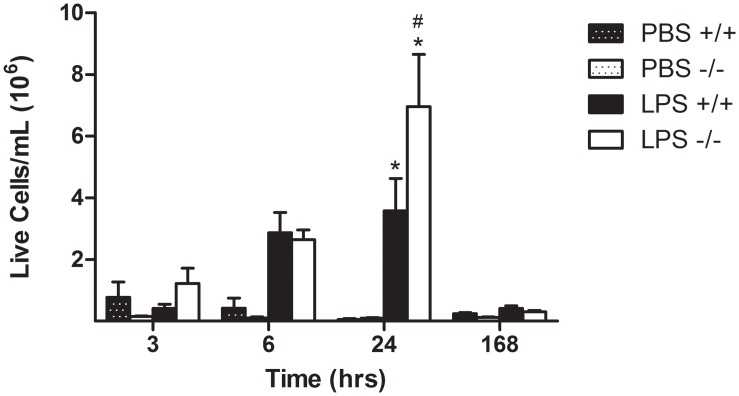
To evaluate the LPS-induced influx of inflammatory cells in the lung over time, total live cell counts were analyzed and compared between Duoxa -/- and Duoxa +/+ mice at 3h, 6h, 24h, and 7 days (Fig 2). Both Duoxa -/- and Duoxa +/+ mice demonstrated increasing live cell counts at each timepoint up to 24h which subsided at 7 days. While Duoxa -/- and Duoxa +/+ mice had similar trends in live cell counts for all timepoints, Duoxa -/- mice had significantly increased live cell counts at 24h when compared to Duoxa +/+ mice (p ≤0.05).
Fig 2. Time course of LPS-induced airway inflammation.
Leukocytes were collected from the airway compartment by BAL at various timepoints up to 7 days (168h) after intratracheal instillation of 1μg LPS. The number of live cells was determined by trypan blue exclusion. Live cell counts are displayed for PBS control and LPS-exposed Duoxa -/- (-/-) and Duoxa +/+ (+/+) mice as indicated. Data are shown as mean ± SEM for six mice in each group; * = p< 0.05 between LPS-treated and PBS-treated controls, # = p<0.05 between LPS-treated Duoxa +/+ and Duoxa -/- mice.
LPS-induced Neutrophil Influx
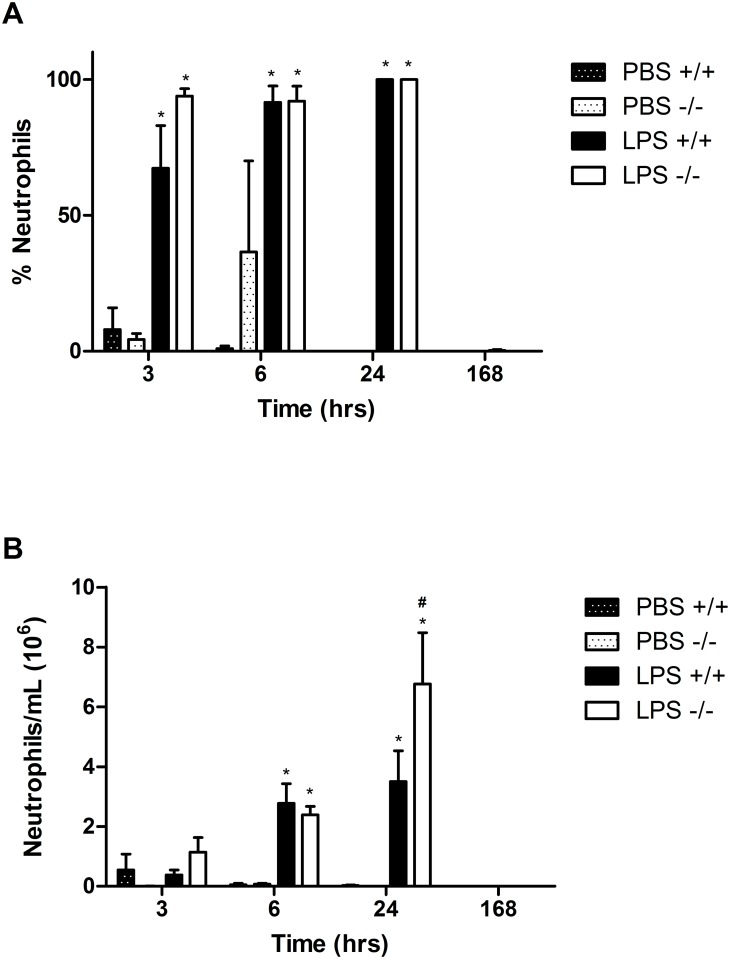
We analyzed the BALF for macrophages, neutrophils, eosinophils and lymphocytes at 3h, 6h, and 24h after LPS exposure to determine the cell populations recruited by LPS in Duoxa -/- and Duoxa +/+mice. As expected, we observed predominant neutrophilic inflammation in LPS-exposed Duoxa +/+mice (Fig 3A). Surprisingly, Duoxa -/- mice had similar levels of neutrophilic inflammation after LPS exposure (Fig 3A), which conflicts previous reports. Similar to the live cell counts, both Duoxa -/- and Duoxa +/+ mice demonstrated steadily increasing absolute neutrophils counts that peaked at 24h and subsided at 7 days. However, counter to what we would predict a priori, Duoxa -/- mice had a statistically significant increase in neutrophils at the 24h timepoint (Fig 3B).
Fig 3. LPS induces predominantly neutrophilic inflammation in both Duoxa -/- and Duoxa +/+ mice.
Leukocytes were collected from the airway compartment by BAL at various timepoints up to 7 days (168h) after intratracheal instillation of 1μg LPS. Cell differentials were determined visually based on cell morphology and the percent of neutrophils (A) was compared between Duoxa -/- (-/-) and Duoxa +/+ (+/+) mice. Absolute neutrophil counts (B) were calculated by multiplying neutrophil percentage with total cell number. Data are shown as mean±SEM from six mice in each group; * = p< 0.05 between LPS-treated and PBS-treated controls, # = p<0.05 between LPS-treated Duoxa +/+ and Duoxa -/- mice.
Analysis of BALF seven days after LPS exposure demonstrated a return to a macrophage-predominant cell profile with a slightly elevated lymphocyte population in both Duoxa -/- and Duoxa +/+mice with no significant differences between the two groups of animals (data not shown).
LPS-induced Cytokine Production
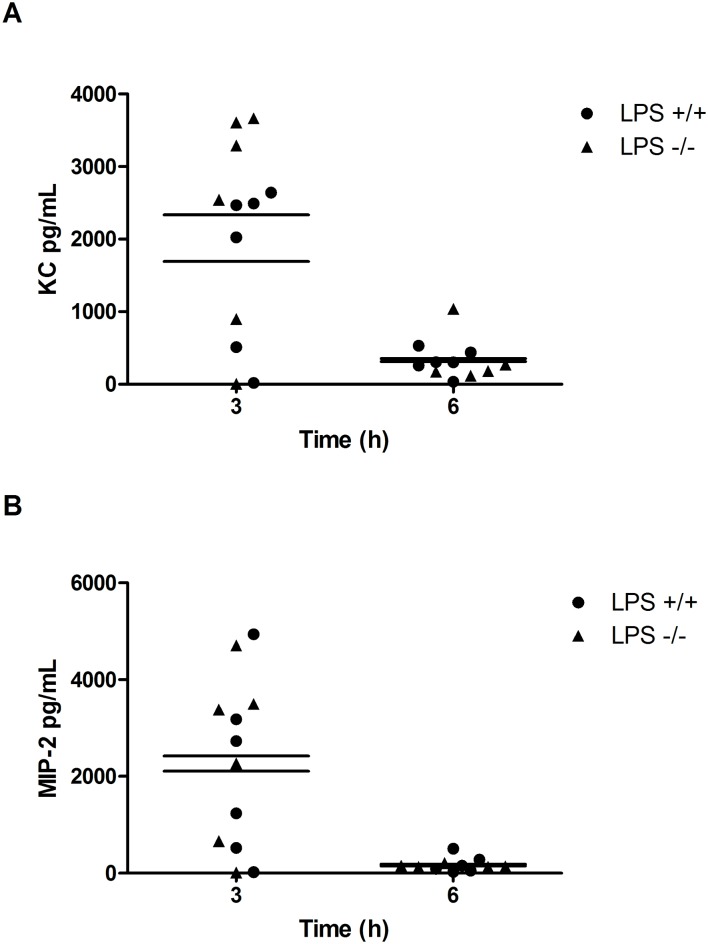
Typically, the binding of LPS to the TLR-4 receptor activates a signaling cascade that leads to increased IL-8 production and subsequent neutrophil recruitment[17], and DUOX-derived hydrogen peroxide has been shown to play a role in LPS-induced IL-8 production[10,11,13]. Therefore, we measured changes in the IL-8 mouse homologs KC and MIP-2[18] in BALF from Duoxa -/- and Duoxa +/+ mice after LPS instillation to evaluate the impact of DUOX-derived hydrogen peroxide in neutrophil chemotaxis. Both KC and MIP-2 levels peaked at 3h consistent with the canonical LPS-TLR4-IL-8 signaling pathway. Surprisingly, LPS induced similar levels of IL-8 homologues in the Duoxa -/- mice compared with the Duoxa +/+ mice (Fig 4). Alternatively, previous studies suggested LPS initiates DUOX-dependent upregulation of TGF-α signaling in airway epithelium, which may be primarily responsible for neutrophil influx into the airway[10,11,13,14]. To evaluate this possibility, we compared TGF-α levels in BALF from Duoxa -/- and Duoxa +/+ mice and found no induction of TGF-α in Duoxa -/- or Duoxa +/+ mice (data not shown). This supported our observation that LPS-induced neutrophil recruitment occurred through modulated expression of KC and MIP-2 independent of TGF-α signaling.
Fig 4. Cytokine levels are similar between LPS-exposed Duoxa +/+ and Duoxa-/- mice.
BALF was collected at various timepoints from Duoxa +/+ (+/+) and Duoxa-/- (-/-) mice followed by measurement of KC (A) or MIP-2 (B) cytokine levels in the supernatant by ELISA. Cytokine concentration was determined by comparison to standard controls for each cytokine. Data from six animals in each group are shown. Mean cytokine values from Duoxa +/+ (+/+) and Duoxa-/- (-/-) mice are shown as a vertical line. Cytokine levels for LPS treatment differed significantly than PBS controls at three hours in both groups of mice (data not shown).
Discussion
Intratracheal LPS administration is known to recruit neutrophils into the lungs of mice[17,19,20]. This occurs through LPS serving as a ligand for TLR-4, subsequently activating transcription of the major neutrophil chemokine IL-8[21] in humans, or KC and MIP-2, mouse homologs of IL-8, in mice[18,22]. We previously identified DUOX as a major source of epithelial-derived H2O2 in isolated murine epithelium, and an important enzyme for neutrophil recruitment in a mouse model of allergic asthma. [9]. Based on these results and the existing literature, we hypothesized that DUOX-derived hydrogen peroxide would act as an important signaling molecule for neutrophil migration into the lungs following LPS exposure. Surprisingly, our data suggest that LPS-mediated neutrophil recruitment in the mouse lung does not require DUOX-dependent H2O2 signaling.
H2O2, the primary product of DUOX enzymatic activity, is known to be a critical factor for multiple intracellular signaling pathways[23,24]. In this report, we investigated the role of DUOX-generated H2O2 in LPS-mediated neutrophil recruitment to the lung. Previously, other groups have demonstrated associative correlations between DUOX and LPS-mediated neutrophil signaling through either IL-8 or TGF-α[10,11,14], and we investigated both possibilities in our model.
Nakanaga et al. characterized DUOX-dependent LPS signaling in NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line. They demonstrated that DUOX-derived hydrogen peroxide activated TGF-α converting enzyme (TACE), followed by TGF-α release, binding to epidermal growth factor receptor (EGFR), and subsequent increases in IL-8 production in vitro[10]. Similarly, Boots et al., utilizing HBE1 cells, an immortalized human bronchial epithelial cell line, found that DUOX-generated H2O2 was necessary for TGF-α-mediated constitutive EGFR activity and increased IL-8 production[11]. In contrast, we did not observe any differences in TGF-α, KC, or MIP-2 between Duoxa -/- or Duoxa +/+ mice after LPS exposure (Fig 4 and data not shown). Our data suggest that LPS is able to signal neutrophils through increased KC or MIP-2 independent of either DUOX isoform. Additionally, TGF-α had no apparent role in LPS-induced neutrophil recruitment.
Because these previous studies were done in cell culture models, it is possible that our conflicting findings are due to compensatory mechanisms available in the in vivo system that do not occur during short-term DUOX inhibition in cell culture systems, the use of alternative NOX isoforms in vivo, or both [25,26]. We did not observe compensatory increases in NOX4, the most likely alternative NOX to be expressed in the absence of DUOX expression[26], in the airway epithelium of these mice (data not shown), but we cannot exclude an unusual upregulation of other NOX isoforms.
Alternatively, the specific stimulus may be the primary difference. Although there is clear evidence that DUOX is required for neutrophil recruitment in multiple model systems[7,9,14,27], in vivo models that evaluate mechanisms of DUOX-mediated neutrophil recruitment in the airway are limited. Li et al. reported that LPS-induced neutrophil chemotaxis into the lung of mice was reduced with the general NADPH oxidase inhibitor DPI[28,29]. Because DPI is relatively non-specific for all flavin proteins, these results may not account for potential alternative NOX(s) that are functioning in lieu of DUOX[30,31]. Similarly, Ryu et al. demonstrated that TLR-4 signaling induced features of allergic asthma that were dependent upon murine DUOX2-generated reactive oxygen species[32], but their studies did not exclude TLR-4-independent pathways that may be primarily responsible for DUOX-dependent neutrophil recruitment. Here, we specifically evaluated how LPS signals through DUOX in vivo, but directly demonstrated that neither DUOX isoform is required for LPS-mediated neutrophil recruitment. Together, our data suggest that DUOX is not required for LPS-TLR-4-dependent neutrophil recruitment, but is required for allergy-induced neutrophil recruitment.
We speculate that our observations are due to differences in the primary source of neutrophil recruitment. A primary mechanism for LPS-induced neutrophil recruitment in the lung involves interactions between neutrophils, vascular endothelial cells, and alveolar macrophages [33,34], which predominantly express Nox1 or Nox2. Mechanisms for ovalbumin-induced neutrophilic inflammation are not as well characterized, but potentially rely on airway specific proteins such as TNF-related apoptosis inducing ligand (TRAIL) [35–37]. Nonspecific inhibition of Nox proteins subsequently will inhibit both LPS- and OVA-induced neutrophil recruitment, whereas selective inhibition of DUOX proteins will only effect OVA-induced, or airway epithelium-dependent, mechanisms of neutrophilic inflammation. Further investigation of these differences potentially will reveal important novel pathways of neutrophil recruitment.
Surprisingly, we observed significantly more neutrophils recruited into the airway in Duoxa -/- mice compared to Duoxa +/+ mice. These results suggest that LPS-induced neutrophil influx is enhanced in the absence of functional DUOX, in contrast to ova-induced neutrophil influx, where DUOX is required for neutrophil recruitment[9]. Because ROS are known regulators of cell differentiation[38], and DUOX expression has been found to increase with age in the developing lung[39,40], it is possible that the Duoxa -/- mice have impaired epithelial structural integrity or repair mechanisms, skewed epithelial cell populations, or altered cell-cell junctions to explain this observation. For example, neutrophils are known to cause lung epithelial damage during extravasation into the airway[41,42] and DUOX-derived hydrogen peroxide is crucial in lung epithelial repair and wound closure[13,43–46]. Without an intact DUOX-mediated repair mechanism, this “leaky” epithelium may allow an increased number of neutrophils to migrate from the blood vessels to the airways after LPS-triggered signaling.
Conclusion
In contrast to previous studies, we have demonstrated that DUOX does not contribute specifically to LPS-mediated neutrophil recruitment. These differences may be due to compensatory mechanisms that occur in the long-term absence of both DUOX isoforms. Importantly, there is strong evidence that DUOX is important in allergy-induced neutrophilic inflammation[9,32] and future studies exploring these contrasting findings will likely reveal novel mechanisms of allergy-induced neutrophil influx.
Supporting Information
(PDF)
Acknowledgments
This work was supported by grants NHLBI R01 HL085311 and NHLBI T32 HL007013 from the National Institutes of Health (Bethesda, MD).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants NHLBI R01 HL085311 and NHLBI T32 HL007013 from the National Institutes of Health (Bethesda, MD) (http://www.nhlbi.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. De Deken X, Corvilain B, Dumont JE, Miot F (2014) Roles of DUOX-Mediated Hydrogen Peroxide in Metabolism, Host Defense, and Signaling. Antioxid Redox Signal 20: 2776–2793. 10.1089/ars.2013.5602 [DOI] [PubMed] [Google Scholar]
- 2. Rada B, Leto TL (2010) Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett 584: 917–922. 10.1016/j.febslet.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB Jr., Nauseef WM, et al. (2007) A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 175: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, et al. (2010) Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A 107: 3546–3551. 10.1073/pnas.0914351107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klyubin IV, Kirpichnikova KM, Gamaley IA (1996) Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur J Cell Biol 70: 347–351. [PubMed] [Google Scholar]
- 6. Enyedi B, Niethammer P (2013) H2O2: a chemoattractant? Methods Enzymol 528: 237–255. 10.1016/B978-0-12-405881-1.00014-8 [DOI] [PubMed] [Google Scholar]
- 7. Niethammer P, Grabher C, Look AT, Mitchison TJ (2009) A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999. 10.1038/nature08119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Oliveira S, Boudinot P, Calado A, Mulero V (2015) Duox1-Derived H2O2 Modulates Cxcl8 Expression and Neutrophil Recruitment via JNK/c-JUN/AP-1 Signaling and Chromatin Modifications. J Immunol 194: 1523–1533. 10.4049/jimmunol.1402386 [DOI] [PubMed] [Google Scholar]
- 9. Chang S, Linderholm A, Franzi L, Kenyon N, Grasberger H, Harper R. (2013) Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic Biol Med 65C: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX (2007) Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol. [DOI] [PubMed] [Google Scholar]
- 11. Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. (2009) ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem 284: 17858–17867. 10.1074/jbc.M809761200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joo JH, Ryu JH, Kim CH, Kim HJ, Suh MS, Kim JO, et al. (2011) Dual Oxidase 2 is Essential for the Toll-Like Receptor 5-Mediated Inflammatory Response in Airway Mucosa. Antioxid Redox Signal. [DOI] [PubMed] [Google Scholar]
- 13. Koff JL, Shao MX, Kim S, Ueki IF, Nadel JA (2006) Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. J Immunol 177: 8693–8700. [DOI] [PubMed] [Google Scholar]
- 14. Li W, Yan F, Zhou H, Lin X, Wu Y, Chen C, et al. (2013) P. aeruginosa lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly through Duox1 in vitro and in vivo. PLoS One 8: e63945 10.1371/journal.pone.0063945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, et al. (2002) Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol 168: 810–815. [DOI] [PubMed] [Google Scholar]
- 16. Grasberger H, De Deken X, Mayo OB, Raad H, Weiss M, Liao XH, et al. (2012) Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol Endocrinol 26: 481–492. 10.1210/me.2011-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reutershan J, Basit A, Galkina EV, Ley K (2005) Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 289: L807–815. [DOI] [PubMed] [Google Scholar]
- 18. Call DR, Nemzek JA, Ebong SJ, Bolgos GR, Newcomb DE, Wollenberg GK, et al. (2001) Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock 15: 278–284. [DOI] [PubMed] [Google Scholar]
- 19. Yi ES, Remick DG, Lim Y, Tang W, Nadzienko CE, Bedoya A, et al. (1996) The intratracheal administration of endotoxin: X. Dexamethasone downregulates neutrophil emigration and cytokine expression in vivo. Inflammation 20: 165–175. [DOI] [PubMed] [Google Scholar]
- 20. Ulich TR, Watson LR, Yin SM, Guo KZ, Wang P, Thang H, et al. (1991) The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol 138: 1485–1496. [PMC free article] [PubMed] [Google Scholar]
- 21. Scarcella DL, Chow CW, Gonzales MF, Economou C, Brasseur F, Ashley DM. (1999) Expression of MAGE and GAGE in high-grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. Clin Cancer Res 5: 335–341. [PubMed] [Google Scholar]
- 22. Rovai LE, Herschman HR, Smith JB (1998) The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J Leukoc Biol 64: 494–502. [DOI] [PubMed] [Google Scholar]
- 23. Veal E, Day A (2011) Hydrogen peroxide as a signaling molecule. Antioxid Redox Signal 15: 147–151. 10.1089/ars.2011.3968 [DOI] [PubMed] [Google Scholar]
- 24. Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15: 247–254. [DOI] [PubMed] [Google Scholar]
- 25. Kim HJ, Park YD, Moon UY, Kim JH, Jeon JH, Lee JG, et al. (2008) The role of Nox4 in oxidative stress-induced MUC5AC overexpression in human airway epithelial cells. Am J Respir Cell Mol Biol 39: 598–609. 10.1165/rcmb.2007-0262OC [DOI] [PubMed] [Google Scholar]
- 26. Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, et al. (2009) Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med 47: 1450–1458. 10.1016/j.freeradbiomed.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Oliveira S, Lopez-Munoz A, Candel S, Pelegrin P, Calado A, Mulero V. (2014) ATP modulates acute inflammation in vivo through dual oxidase 1-derived H2O2 production and NF-kappaB activation. J Immunol 192: 5710–5719. 10.4049/jimmunol.1302902 [DOI] [PubMed] [Google Scholar]
- 28. Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, et al. (2003) Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 284: L26–38. [DOI] [PubMed] [Google Scholar]
- 29. Forteza R, Salathe M, Miot F, Conner GE (2005) Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 462–469. [DOI] [PubMed] [Google Scholar]
- 30. Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, et al. (2008) Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9: 686–696. [DOI] [PubMed] [Google Scholar]
- 31. Tazzeo T, Worek F, Janssen L (2009) The NADPH oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal Ca(2+) pump. Br J Pharmacol 158: 790–796. 10.1111/j.1476-5381.2009.00394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryu JH, Yoo JY, Kim MJ, Hwang SG, Ahn KC, Ryu JC, et al. (2012) Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. [DOI] [PubMed] [Google Scholar]
- 33. Fan J (2010) TLR Cross-Talk Mechanism of Hemorrhagic Shock-Primed Pulmonary Neutrophil Infiltration. Open Crit Care Med J 2: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan J, Frey RS, Malik AB (2003) TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest 112: 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tisato V, Garrovo C, Biffi S, Petrera F, Voltan R, Casciano F, et al. (2014) Intranasal administration of recombinant TRAIL down-regulates CXCL-1/KC in an ovalbumin-induced airway inflammation murine model. PLoS One 9: e115387 10.1371/journal.pone.0115387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robertson NM, Rosemiller M, Lindemeyer RG, Steplewski A, Zangrilli JG, Litwack G. (2004) TRAIL in the airways. Vitam Horm 67: 149–167. [DOI] [PubMed] [Google Scholar]
- 37. Robertson NM, Zangrilli JG, Steplewski A, Hastie A, Lindemeyer RG, Planeta MA, et al. (2002) Differential expression of TRAIL and TRAIL receptors in allergic asthmatics following segmental antigen challenge: evidence for a role of TRAIL in eosinophil survival. J Immunol 169: 5986–5996. [DOI] [PubMed] [Google Scholar]
- 38. Ushio-Fukai M, Urao N (2009) Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal 11: 2517–2533. 10.1089/ARS.2009.2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, et al. (2007) Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1506–1514. [DOI] [PubMed] [Google Scholar]
- 40. Weng T, Chen Z, Jin N, Gao L, Liu L (2006) Gene expression profiling identifies regulatory pathways involved in the late stage of rat fetal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1027–1037. [DOI] [PubMed] [Google Scholar]
- 41. Grommes J, Soehnlein O (2011) Contribution of neutrophils to acute lung injury. Mol Med 17: 293–307. 10.2119/molmed.2010.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Looney MR, Matthay MA (2006) Animal models of transfusion-related acute lung injury. Crit Care Med 34: S132–136. [DOI] [PubMed] [Google Scholar]
- 43. Gorissen SH, Hristova M, Habibovic A, Sipsey LM, Spiess PC, Janssen-Heininger YM, et al. (2012) DUOX1 is Required for Airway Epithelial Cell Migration and Bronchiolar Re-Epithelialization Following Injury. Am J Respir Cell Mol Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Razzell W, Evans IR, Martin P, Wood W (2013) Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 23: 424–429. 10.1016/j.cub.2013.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A (2007) Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282: 3213–3220. [DOI] [PubMed] [Google Scholar]
- 46. van der Vliet A, Janssen-Heininger YM (2013) Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? J Cell Biochem [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.