Abstract
Resection of brain tumors is followed by chemotherapy and radiation to ablate remaining malignant cell populations. Targeting these populations stands to reduce tumor recurrence and offer the promise of more complete therapy. Thus, improving access to the tumor, while leaving normal brain tissue unscathed, is a critical pursuit. A central challenge in this endeavor lies in the limited delivery of therapeutics to the tumor itself. The blood-brain barrier (BBB) is responsible for much of this difficulty but also provides an essential separation from systemic circulation. Due to the BBB’s physical and chemical constraints, many current therapies, from cytotoxic drugs to antibody-based proteins, cannot gain access to the tumor. This review describes the characteristics of the BBB and associated changes wrought by the presence of a tumor. Current strategies for enhancing the delivery of therapies across the BBB to the tumor will be discussed, with a distinction made between strategies that seek to disrupt the BBB and those that aim to circumvent it.
Keywords: blood-brain barrier, focused ultrasound, convection-enhanced delivery, P-glycoprotein
Management of most primary brain tumors includes maximal safe resection or biopsy followed by radiation and chemotherapy to target the remaining and potentially invasive tumor cells. However, delivering effective adjuvant treatment to these residual cell populations without damaging physiological brain tissue is a major challenge. One critical obstacle to effective treatments is the blood-brain barrier (BBB). This dynamic structure protects the CNS from environmental toxins and mediates physiological responses, effectively isolating the brain from the systemic circulation. Although many of the constituent cells and molecules of the BBB manifest throughout the body, in the brain they are combined into a unique construction that severely restricts entry into the brain.
Improved drug delivery stands to enhance existing treatments, mediate tumor recurrence, and provide an opportunity to therapeutically target tumors not amenable to resection. Thus, the motivation to enhance drug delivery is powerful and has led to the development of diverse methodologies to target and evade the BBB. In this review, we discuss normal BBB physiology and pathological changes wrought by tumors, and detail therapeutic methods to disrupt, modulate, and circumvent the BBB.
Blood-Brain Barrier and Tumor-Associated Changes
The BBB refers to both passive and active mechanisms used by the brain endothelium to regulate access to the brain. This barrier is modulated in the context of brain tumors, evidenced by the penetration of Gd through the BBB on MRI of patients with glioblastoma.98 Gadolinium enhancement increases as a function of WHO grade of astrocytoma, suggesting that BBB dysfunction is related to increasing histological grade in astrocytomas.66 Although BBB dysfunction is observed in many gliomas, the disruption is often heterogeneous and the vasculature remains grossly intact in brain regions where infiltrating cells are found, underscoring the need for tumor-specific methods to bypass the BBB.123 In this section we review the cellular structure of normal BBB and the impact of brain tumors on BBB coherence.
Endothelial Cells, Tight Junctions, and Extracellular Matrix
The BBB exists as a selective barrier formed by tight junctions between cerebral capillary endothelial cells, and is a critical regulator of brain homeostasis96 (Fig. 1). Endothelial cells in the cerebral vasculature share properties with peripheral endothelial cells but also have important differences. Small gaseous molecules such as O2 and CO2 can diffuse through the lipid membranes of the BBB, as can small lipophilic molecules. However, the BBB tightly controls homeostasis by exclusion of harmful xenobiotics. One unique feature of brain endothelial cells is the existence of specific transport systems that regulate the entry of compounds necessary for brain metabolism, and chief among these are ATP-binding cassette (ABC) transporters. 12,52 BBB endothelial cells also have a lower number of endocytic vesicles and increased number of tight junctions, limiting transcellular and paracellular flow. Additionally, a host of intra- and extracellular enzymes provide further resistance by metabolizing substances ranging from peptides to toxic compounds.34
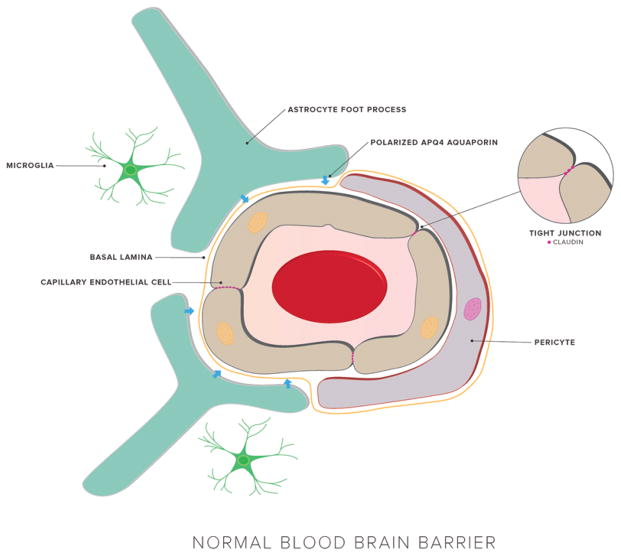
FIG. 1.
The normal, physiological BBB structure maintains strict control over CNS penetration. The major components of the BBB are cerebral endothelial cells bound together by tight junctions. The endothelium is surrounded by the basal lamina, pericytes, astrocytic endfeet, and microglia. These diverse cell types give rise to a dynamic environment that regulates entry into the brain.
Tight junctions—the links between capillary endothelial cells—in the brain are more complex than those found in peripheral tissues and serve to prevent paracellular diffusion. Two critical components of these tight junctions are occludins and claudins. Occludins are 60- to 65-kD proteins involved in tight junction regulation that are capable of binding zona occludens protein 1 (ZO-1).129 Claudin-3, claudin-5, and potentially claudin-12 contribute to the BBB’s restriction of small ions;125 other key components include ZO-1, ZO-2, ZO-3, cingulin, and 7H6 (Fig. 2).
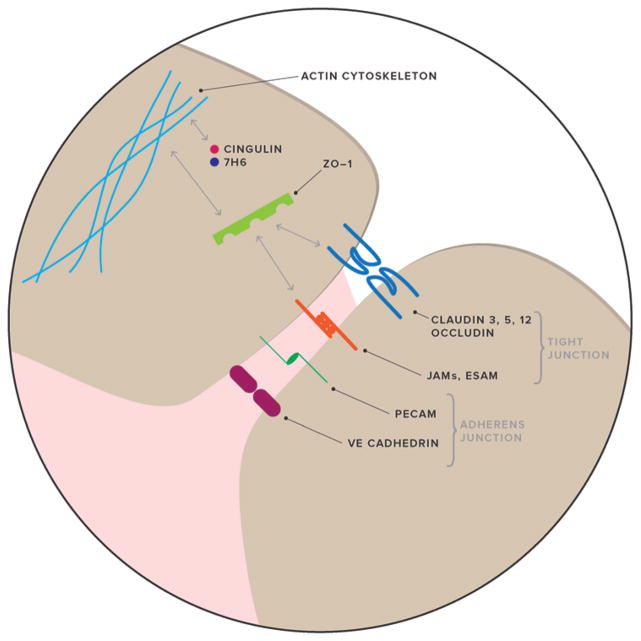
FIG. 2.
Molecular composition of the cerebrovascular endothelial tight-junction structure. Claudins and occludin are critical junctional components. Two other components of the tight junction are junctional adhesion molecules (JAMs) and the endothelial selective adhesion molecule (ESAM). ZO-1 serves as an adaptor molecule in the cytoplasm with the ability to bind membrane proteins. Other important adaptor molecules are cingulin and 7H6. These adaptor proteins, in conjunction with other regulatory proteins, foster communication between membrane junctional molecules and the cytoskeleton. A second junctional complex is the adherens junction, consisting of vascular endothelial cadherin (VE-cadherin) and the platelet–endothelial cell adhesion molecule (PECAM).
Wolburg et al. found that a key component of BBB tight junctions, claudin-3, is lost in glioblastoma.126 This finding implicated claudin-3 as an effector in the leakiness of glioblastoma vessels. There is further evidence implicating claudin-1 loss in tumor microvessels, as well as downregulation of claudin-5 and occludin in hyperplastic vasculature. These perturbations result in a phenotypic change in BBB function due to leaky tight junctions and hyperpermeable endothelial cells.70
The extracellular matrix (ECM) is also modulated by tumors. Rascher et al. demonstrated that agrin, an important component of the BBB basal lamina, is absent if claudin-1, claudin-5, and occludin are downregulated.95 The authors also noted that loss of agrin correlated with upregulation of tenascin, an ECM molecule not normally expressed in brain vessels. Although the specific mechanisms underlying alterations in tight-junction and ECM components remain unclear, these correlative studies suggest some phenotypic relationship.
Astrocytes
The neurovascular unit is formed by endothelial cells surrounded by basal lamina and astrocytic endfeet. BBB maintenance is orchestrated by astrocytes, which serve as a cellular link to neurons. From studies of astroglial-endothelial co-culture, a number of receptors, transporters, and ligands have been identified that are involved in the bidirectional induction involved in BBB maintenance.2
Aquaporin-4 (AQP4) is an aquaporin water channel that is believed to have an important role in glioblastoma-related edema. Astrocytes express AQP4 and Warth et al. found that AQP4 redistribution is correlated with loss of agrin in cerebral capillary basal laminae in human glioblastoma. The authors reported that the distribution of AQP4 shifted from the glial membrane in contact with mesenchymal space to cover the entire surface of glioma cells.118 In normal astrocytes, AQP4 is arranged as orthogonal arrays of particles, but this array arrangement is lost in glioblastoma. The functional consequence of this loss of astrocyte polarization is yet unknown, but the strong evidence for the role of astrocytes in glioblastoma makes this an important topic for further investigation.
A recent study by Watkins et al. used a mouse model to demonstrate that glioma cells displace astrocytic endfeet from their position alongside endothelial cells. This is a significant breach of the BBB that disrupts communication between the astrocytes and vasculature. Glioma cells were able to co-opt regulation of vascular tone. The authors demonstrated that single glioma cells were sufficient to produce local BBB opening.119 A study by Ndoum et al. demonstrated disruption of the astrocyte–endothelial cell association in intratumoral vessels in the enhancing regions of high-grade gliomas. Moreover, the authors found that low-grade gliomas, as well as the nonenhancing regions of high-grade gliomas, displayed intact astrocyte–endothelial cell relationships, as would be observed in unperturbed BBB.82
Pericytes
Cerebral pericytes are an additional component of the BBB that occupy the perivascular space. In triculture experiments with endothelial cells and astrocytes, capillary-like structures are realized. Endothelial cells that form these structures in the presence of pericytes demonstrate resistance to apoptosis, supporting a stabilizing function of pericytes in angiogenesis.95 Further studies recapitulated the pericyte role in vascular tone, stability, repair, and angiogenesis,64 as well as in modulation of astrocyte function.110
Abnormal pericyte distribution has been observed in established tumors.78 Given that brain pericytes can support BBB function through transforming growth factor-β production, a role may exist for pericyte loss in glioma-related BBB dysfunction.31 A more general role for pericytes in tumor vessel formation was highlighted by the discovery that glioblastoma stem cells can differentiate into pericytes during angiogenesis.25
P-Glycoprotein
A major player in maintaining the integrity and polarity of the BBB is through an efflux pump known as P-glycoprotein (P-gp). This 170-kD transmembrane protein belongs to the ABC transporter family and is encoded by the ABCB1 (or MDR1) gene.6 On the BBB, P-gp is localized on the apical membrane that facilitates transport in a unidirectional manner.38,112 The expression pattern of P-gp suggests that its normal physiological role is to protect the body from xenobiotic compounds by effluxing cytotoxic molecules into luminal spaces for elimination. A characteristic feature of P-gp is broad substrate specificity. A partial list of substrates in relation to CNS tumors is summarized in Table 1.
TABLE 1.
Summary of current FDA-approved pharmacological treatments for CNS tumors
| Drug | Indications* | Molecular Weight (Da) | ABC Transporter Substrate |
|---|---|---|---|
| Carmustine | Glioblastoma37 | 214 | No |
| Cisplatin | Medulloblastoma40 | 300 | ABCC2, ABCC6 |
| Cyclophosphamide | Medulloblastoma | 261 | No |
| Etoposide | Glioblastoma | 588 | ABCB1 |
| Irinotecan | Glioblastoma | 586, 623 (HCl), 677 (HCl trihydrate) | ABCB1 |
| Lomustine | Medulloblastoma, Grade III glioma108 | 233 | No |
| Procarbazine | Grade III glioma | 221 | No |
| Temozolomide | Glioblastoma | 194 | ABCB1 |
| Vincristine | Medulloblastoma, Grade III glioma | 824 | ABCB1 |
| Bevacizumab | Glioblastoma | 149,000 | No |
In addition to the normal physiological role of P-gp, overexpression of P-gp is a feature common to many multidrug-resistant tumors.55,85 P-gp expression was demonstrated in glioma, and expression levels were correlated with multidrug resistance and tumor grade.44,68,74 In relation to the BBB, P-gp activity is disrupted at the necrotic core of glioblastoma but preserved at the tumor border.28 This is clinically significant for glioblastoma following resection, because residual border cells with an intact barrier and potential P-gp overexpression limit drug uptake and often relapse into larger and more aggressive tumors.26
Therapeutic Implications
Early efforts to increase drug delivery to the brain have focused on disruption of key cellular components. However, disruptive efforts have become more refined and are joined by efforts to circumvent and modulate the BBB. In this section we detail current efforts in each of these therapeutic strategies.
BBB Disruption
Osmotic Disruption
The concept of hyperosmolar BBB disruption was first reported by Rapoport et al. in 1972.93 Following delivery of the hyperosmotic agent, water leaves endothelial cells, resulting in shrinkage and tight-junction dysfunction, leading to increased permeability of the BBB allowing for a therapeutic window of several hours.94 A variety of substances have been used as osmotic disruptors of the BBB, but mannitol has been most commonly used for this purpose.1,13,17,92 Studies suggest that this method increases the concentrations of various chemotherapeutic agents in the brain up to 90-fold.124 Furthermore, in a 1991 study of 30 patients with primary CNS lymphoma, BBB disruption via mannitol and cyclophosphamide before irradiation improved mean survival from 17.8 months to 44.5 months compared with controls receiving radiotherapy alone.83
There exists some debate regarding the effectiveness of this method due to conflicting reports about its differential effect on BBB permeability. Studies in multiple animal models reported that hypertonic solutions did not selectively disrupt the BBB local to the tumor.42,80,130 The increase in BBB permeability in a nonselective manner is problematic and raised concerns of systemic toxicity throughout the CNS.58 Nonetheless, recent studies support the method’s safety and efficacy in humans.19,32 More work is needed to better understand the potential therapeutic value of this strategy.
MRI-Guided Focused Ultrasound
The feasibility of focused ultrasound (FUS) to disrupt the BBB was first demonstrated more than 10 years ago.48 Subsequent studies have confirmed FUS as a valuable method to introduce focal and transient BBB disruption. 46,47 This technique has several advantages over other approaches because it is readily repeatable, noninvasive, and able to disrupt the BBB in a targeted way. Studies suggest that FUS may increase cerebrovascular permeability by producing shear stress in cells or by activation of signaling pathways involved in the regulation of permeability. 41,51,115,116 The disruption of tight junction proteins by FUS may also contribute to this method’s mechanism of action.104–106
The technique can be used in conjunction with intravenously administered microbubbles to lower the ultrasound energy required to induce BBB disruption.48 Nonhuman primate studies have shown that microbubble-enhanced FUS can successfully induce local BBB opening with minimal side effects.73,75,114 The safety of FUS therapy is promising as it is not associated with significant tissue damage.10,49,76 The use of MRI with FUS allows for the targeting and evaluation of BBB opening,48 and several groups have developed methods that aim to monitor acoustic emissions from microbubbles in real time.7,53,57
Although this approach is in the preclinical phase, it is of high clinical relevance as various FDA-approved chemotherapy drugs such as doxorubicin, carmustine, trastuzumab, and temozolomide have been successfully introduced across the BBB through this approach.8,71,77,122 Focused ultrasound has also been combined with nanoparticle platforms to enhance diagnostic and treatment capabilities. In the study by Diaz et al., gold nanoparticles were safely introduced to the tumor periphery with MRI-guided FUS in a mouse brain tumor model, augmenting surface-enhanced Raman scattering capability. Furthermore, the authors demonstrated that nanoparticles coated with anti–epidermal growth factor receptor antibody or nonspecific human immunoglobulin-G had increased uptake in glioma cells.30
While FUS shows promise in animal models, a limitation is signal attenuation and distortion from the skull. A study in rabbits sought to measure BBB disruption by applying FUS directly to the brain surface through a device implanted in a skull bur hole.11 Further study is necessary to gauge the feasibility of this approach in humans.
Bradykinin Administration
Bradykinin administration has been shown to upregulate caveolin-1 and caveolin-2 at the BBB.72 The upregulation of these compounds serves to increase endothelial cell permeability, increasing the chance of appropriate drug delivery. The potential of bradykinin, and synthetic analogs, to disrupt the BBB has been widely explored.16,35,50,102 A central limitation is that the effect of the upregulation is exceedingly transient.72 One clinical trial showed minimal therapeutic benefit of using carboplatin with lobradimil, a synthetic bradykinin analog, to treat brain tumors in a pediatric population.117 A greater understanding of the cellular mechanisms at the BBB stands to improve the efficacy of administering bradykinin with chemotherapy drugs.
Radiation-Induced Disruption
The use of radiation therapy to induce DNA damage and subsequent cell death has become an important treatment modality for brain tumors. Recent innovations in radiation therapy have improved precision, tumor definition with imaging, and radiation delivery through beam shaping.24 In addition to its current utility, radiation therapy may play a role in selectively disrupting the BBB. Studies in both animals and humans have demonstrated that radiation therapy can induce focal BBB disruption with minimal effects on normal vasculature.23,67,86,89 These results suggest that BBB disruption may be an additional utility of radiation therapy.
BBB Circumvention
Convection-Enhanced Delivery
Convection-enhanced delivery (CED), first described by Bobo et al. in 1994, involves the use of surgically implanted catheters that enable continuous delivery of chemotherapy directly into the tumor through positive pressure microperfusion.18 Various antineoplastic agents, mostly immunotoxins, are under investigation for use through CED.59,62,84,100,107 Another approach involves chemotherapeutic delivery via CED of nanoparticles.14,128 Although these studies have demonstrated effectiveness in vivo, more work must be conducted to investigate the long-term effects of potential accumulations of the nanoparticles in the brain. CED can be used following resection or to treat inoperable tumors.56 The major drawbacks of CED include operative risks and limited drug distribution due to backflow.91,101,111 Despite the promise of this novel approach in enhancing the delivery of therapeutics, its safety and efficacy has yet to be clearly determined, as several Phase III clinical trials have failed to meet clinical end points.63,87,90,99,120,121
Viral-Mediated Circumvention
Viral vectors to deliver therapeutic drugs have also been examined for glioblastoma treatment. The goal of these strategies is to specifically target tumor cells via cell surface receptors and use virus replication derivatives to combat cancer growth. The value of using viruses as vehicles is partly due to their small size, allowing for permeability across the BBB. Such methods also have promise in combating cancers that have acquired chemotherapy and drug resistance. In vivo studies with the measles virus demonstrated a cytopathic effect on glioma stem cells and prolonging survival in a mouse model.4 Viruses can be created with soluble peptide markers to monitor spread in vivo, and viral vectors may have synergistic activity when combined with conventional treatments, such as CED or radiation therapy.5,27,56 Adding an amphotropic retroviral replicating vector can similarly be used to target glioma cells. Toca 511, a retroviral replicating vector, has been shown to safely deliver a cytosine deaminase gene and improve survival for glioblastoma models in vivo.45 When combined with radiation therapy or CED, this approach has promise for future steps in combating glioblastoma growth.
Carrier Molecules
Other treatment strategies aim to use carrier molecules to transport drugs across the BBB. In creating these compounds, the surface coating can be engineered to optimize transport and targeting abilities. Other factors such as core polymer, drug, and stabilizer formulation have also been shown to influence nanoparticle delivery.39 Particle systems such as poly(lactic-coglycolic acid) and dendrimer nanoparticles have been studied in the context of brain cancer.39 Another synthetic peptide, K16ApoE, carries chemotherapeutic compounds into the brain via a ligand-receptor system.103 Although it is difficult to accurately monitor dosage, as well as systemic toxicological effects, these systems offer greater promise for drug delivery. Studies have reviewed optimal nanoparticle sizing, but future research on ligand-receptor interactions at the BBB and the ideal surface characteristics of nanoparticle delivery mechanisms is necessary.81,127 Nanotherapeutic approaches also have used magnetic therapy to localize drug-carrying molecules.29 In this method, a carrier molecule with iron residues is guided to the tumor location with an external magnetic field. Such an approach is encouraging, as a drug can be administered directly to the brain and with sustained release. Advances in biomaterials will also be able to increase the half-life of the encapsulated drug, improving efficacy.29
Liposomal Delivery
Liposomes contain a drug of interest within a lipophilic vesicle, facilitating endocytosis and uptake into brain tissue. These compounds hold great promise for glioblastoma, offering more surface area for passive diffusion. Various liposome preparations have been explored and combined with CED in previous studies.43,60 Liposomal delivery has been extensively studied for doxorubicin, showing disease stabilization and low systemic toxicity.36 A recent study using a rat glioma model found that the surface charge of liposomes is a significant factor for deposition within the brain.54 The beneficial effect was noted independent of techniques disrupting BBB permeability, offering a safer and simpler method of administration. Other studies have added compounds such as wheat germ agglutinin (WGA) to the liposome surface. WGA has been shown to aid in adsorptive endocytosis in the BBB, as this glycoprotein binds to negatively charged residues in the epithelial membrane. 33 Liposomes modified with WGA have been shown to reliably target glioma tumors both in vitro and in vivo, offering a possible area of research for glioblastoma treatment. 69 Limitations of this delivery mechanism include the large size of liposomes and controlled release of the encapsulated drugs from the vesicles
Polymer Wafers
Polymer wafers that are implanted into the resection cavity after surgery allow for the localized administration of drugs that would otherwise be unable to access the tumor site due to the BBB.21,22 This approach has renewed interest in therapeutics originally believed to be of limited use due to their inability to penetrate the BBB or due to their toxicity.15 The Gliadel wafer (Eisai) is a critical example of this strategy and received FDA approval for use in 2003 for newly diagnosed and recurrent malignant gliomas. 9,20,56,79 However, its use is not generally recommended as subsequent studies demonstrated marginally increased survival in patients with glioblastoma and a high incidence of associated complications such as seizures, cerebral edema, and infection. Bregy and colleagues reviewed 795 patients with newly diagnosed high-grade glioma treated with Gliadel wafers in 19 studies and reported an overall complication rate of 42.7%.20 Thus, more work must be completed to reduce complications associated with this approach and additional polymer delivery methods must be developed.
P-gp Targeting and Modulation
Modulation of specific surface proteins on capillary endothelial cells can offer more specific and less disruptive strategies to deliver drugs into the CNS. Pharmacological interventions often fail in the brain setting due in part to P-gp–mediated efflux of small molecules out of brain tissue back into the capillary lumen. Strategies have been developed to circumvent the BBB through either inhibition of P-gp or the modulation of its expression and/or trafficking.
Direct inhibition of P-gp through small molecules and other pharmaceutical methods have been initially met with limited efficacy and safety in a clinical setting; however, recent advances in drug discovery have elucidated promising new molecules with nanomolar specificity and acceptable tolerability.65,113 The most promising drug to result from this process is tariquidar, which binds P-gp noncompetitively at nanomolar concentrations.97 This drug has been shown to sufficiently inhibit P-gp at the BBB in vivo. Kreisl et al. showed greater uptake of 11C-N-desmethylloperamide by PET, a known P-gp substrate.61 Acceptable tolerability is achieved in combination with dose–linear responses, tariquidar shows promise for inhibiting P-gp at the human BBB and allowing effective CNS drug delivery.
Pinzón-Daza et al. has elucidated the role of crosstalk between canonical and noncanonical Wnt pathways and its relationship to P-gp expression in the human BBB.88 The authors found that downregulation of β-catenin led to a decrease in P-gp expression. It was also shown in vitro that the inhibition of β-catenin enhanced delivery of doxorubicin, a P-gp substrate, across a BBB epithelial monolayer against glioblastoma cells.
Modulation of P-gp has attracted much attention in disrupting the BBB in a noninvasive, specific, and rapid manner. However, despite numerous clinical trials involving P-gp inhibitors, none have been performed in any patients with primary or metastatic neoplasms of the CNS.109 Outcomes to explore would be whether co-administration of P-gp inhibitors along with chemotherapy can stop tumor growth and/or reduce tumor size, result in prolonged survival, and result in an outcome that avoids any long-term sequelae.3
Conclusions
A growing body of evidence implicates the BBB as critical in fully understanding brain tumor pathophysiology. Future studies hold potential for both fundamental biological knowledge and for critical therapeutic discoveries. However, the question remains whether BBB disruption coupled with targeted therapy will improve patient survival. Several investigators across multiple disciplines are working collaboratively to improve the ability to penetrate the BBB to allow novel therapeutics to infiltrate further into the tumor and the surrounding brain.
ABBREVIATIONS
- ABC
ATP-binding cassette
- AQP4
aquaporin-4
- BBB
blood-brain barrier
- CED
convection-enhanced delivery
- ECM
extracellular matrix
- FUS
focused ultrasound
- P-gp
P-glycoprotein
- WGA
wheat germ agglutinin
- ZO
zona occludens
Footnotes
Author Contributions
Conception and design: Azad, Pan. Acquisition of data: Azad. Analysis and interpretation of data: Azad. Drafting the article: all authors. Critically revising the article: Remington, Wilson, Grant.
Disclosure: Dr. Grant is supported by a grant from the NIH (no. 7K08NS075144-04).
References
- 1.Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc Brain Metab Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- 2.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17:2793–2802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen C, Opyrchal M, Aderca I, Schroeder MA, Sarkaria JN, Domingo E, et al. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2013;20:444–449. doi: 10.1038/gt.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen C, Paraskevakou G, Liu C, Iankov ID, Msaouel P, Zollman P, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin Biol Ther. 2008;8:213–220. doi: 10.1517/14712598.8.2.213. (Erratum in Expert Opin Biol Ther 8:855, 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambudkar SV, Lelong IH, Zhang J, Cardarelli CO, Gottesman MM, Pastan I. Partial purification and reconstitution of the human multidrug-resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc Natl Acad Sci U S A. 1992;89:8472–8476. doi: 10.1073/pnas.89.18.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS ONE. 2012;7:e45783. doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashby LS, Ryken TC. Management of malignant glioma: steady progress with multimodal approaches. Neurosurg Focus. 2006;20(4):E3. [PubMed] [Google Scholar]
- 10.Baseri B, Choi JJ, Tung YS, Konofagou EE. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol. 2010;36:1445–1459. doi: 10.1016/j.ultrasmedbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beccaria K, Canney M, Goldwirt L, Fernandez C, Adam C, Piquet J, et al. Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits. J Neurosurg. 2013;119:887–898. doi: 10.3171/2013.5.JNS122374. [DOI] [PubMed] [Google Scholar]
- 12.Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 13.Bellavance MA, Blanchette M, Fortin D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10:166–177. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernal GM, LaRiviere MJ, Mansour N, Pytel P, Cahill KE, Voce DJ, et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine (Lond Print) 2014;10:149–157. doi: 10.1016/j.nano.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biddlestone-Thorpe L, Marchi N, Guo K, Ghosh C, Janigro D, Valerie K, et al. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Adv Drug Deliv Rev. 2012;64:605–613. doi: 10.1016/j.addr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black KL, Cloughesy T, Huang SC, Gobin YP, Zhou Y, Grous J, et al. Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J Neurosurg. 1997;86:603–609. doi: 10.3171/jns.1997.86.4.0603. [DOI] [PubMed] [Google Scholar]
- 17.Blanchette M, Pellerin M, Tremblay L, Lepage M, Fortin D. Real-time monitoring of gadolinium diethylenetriamine penta-acetic acid during osmotic blood-brain barrier disruption using magnetic resonance imaging in normal wistar rats. Neurosurgery. 2009;65:344–351. doi: 10.1227/01.NEU.0000349762.17256.9E. [DOI] [PubMed] [Google Scholar]
- 18.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boockvar JA, Tsiouris AJ, Hofstetter CP, Kovanlikaya I, Fralin S, Kesavabhotla K, et al. Safety and maximum tolerated dose of superselective intraarterial cerebral infusion of bevacizumab after osmotic blood-brain barrier disruption for recurrent malignant glioma. Clinical article. J Neurosurg. 2011;114:624–632. doi: 10.3171/2010.9.JNS101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bregy A, Shah AH, Diaz MV, Pierce HE, Ames PL, Diaz D, et al. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev Anticancer Ther. 2013;13:1453–1461. doi: 10.1586/14737140.2013.840090. [DOI] [PubMed] [Google Scholar]
- 21.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 22.Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339. doi: 10.1016/s0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Tsien CI, Shen Z, Tatro DS, Ten Haken R, Kessler ML, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–4136. doi: 10.1200/JCO.2005.07.144. [DOI] [PubMed] [Google Scholar]
- 24.Chacko AM, Li C, Pryma DA, Brem S, Coukos G, Muzykantov V. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide. Expert Opin Drug Deliv. 2013;10:907–926. doi: 10.1517/17425247.2013.808184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheshier SH, Kalani MY, Lim M, Ailles L, Huhn SL, Weissman IL. A neurosurgeon’s guide to stem cells, cancer stem cells, and brain tumor stem cells. Neurosurgery. 2009;65:237–250. doi: 10.1227/01.NEU.0000349921.14519.2A. [DOI] [PubMed] [Google Scholar]
- 27.Debinski W, Tatter SB. Convection-enhanced delivery to achieve widespread distribution of viral vectors: predicting clinical implementation. Curr Opin Mol Ther. 2010;12:647–653. [PubMed] [Google Scholar]
- 28.Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 29.del Burgo LS, Hernández RM, Orive G, Pedraz JL. Nanotherapeutic approaches for brain cancer management. Nanomedicine (Lond Print) 2014;10:905–919. doi: 10.1016/j.nano.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Diaz RJ, McVeigh PZ, O’Reilly MA, Burrell K, Bebenek M, Smith C, et al. Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: potential for targeting experimental brain tumors. Nanomedicine (Lond Print) 2014;10:1075–1087. doi: 10.1016/j.nano.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, et al. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637–647. doi: 10.1002/(sici)1097-0142(20000201)88:3<637::aid-cncr22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Du J, Lu WL, Ying X, Liu Y, Du P, Tian W, et al. Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood-brain barrier and survival of brain tumor-bearing animals. Mol Pharm. 2009;6:905–917. doi: 10.1021/mp800218q. [DOI] [PubMed] [Google Scholar]
- 34.el-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol (Noisy-le-grand) 1999;45:15–23. [PubMed] [Google Scholar]
- 35.Emerich DF, Snodgrass P, Pink M, Bloom F, Bartus RT. Central analgesic actions of loperamide following transient permeation of the blood brain barrier with Cereport (RMP-7) Brain Res. 1998;801:259–266. doi: 10.1016/s0006-8993(98)00571-x. [DOI] [PubMed] [Google Scholar]
- 36.Fabel K, Dietrich J, Hau P, Wismeth C, Winner B, Przywara S, et al. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer. 2001;92:1936–1942. doi: 10.1002/1097-0142(20011001)92:7<1936::aid-cncr1712>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 37.Fadul CE, Wen PY, Kim L, Olson JJ. Cytotoxic chemotherapeutic management of newly diagnosed glioblastoma multiforme. J Neurooncol. 2008;89:339–357. doi: 10.1007/s11060-008-9615-4. [DOI] [PubMed] [Google Scholar]
- 38.Fung KL, Pan J, Ohnuma S, Lund PE, Pixley JN, Kimchi-Sarfaty C, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res. 2014;74:598–608. doi: 10.1158/0008-5472.CAN-13-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74:157–163. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Gerber NU, Mynarek M, von Hoff K, Friedrich C, Resch A, Rutkowski S. Recent developments and current concepts in medulloblastoma. Cancer Treat Rev. 2014;40:356–365. doi: 10.1016/j.ctrv.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 41.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Groothuis DR, Warkne PC, Molnar P, Lapin GD, Mikhael MA. Effect of hyperosmotic blood-brain barrier disruption on transcapillary transport in canine brain tumors. J Neurosurg. 1990;72:441–449. doi: 10.3171/jns.1990.72.3.0441. [DOI] [PubMed] [Google Scholar]
- 43.Gupta B, Levchenko TS, Torchilin VP. TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncol Res. 2007;16:351–359. doi: 10.3727/000000006783980946. [DOI] [PubMed] [Google Scholar]
- 44.Henson JW, Cordon-Cardo C, Posner JB. P-glycoprotein expression in brain tumors. J Neurooncol. 1992;14:37–43. doi: 10.1007/BF00170943. [DOI] [PubMed] [Google Scholar]
- 45.Huang TT, Hlavaty J, Ostertag D, Espinoza FL, Martin B, Petznek H, et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013;20:544–551. doi: 10.1038/cgt.2013.51. [DOI] [PubMed] [Google Scholar]
- 46.Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 47.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive opening of BBB by focused ultrasound. Acta Neurochir Suppl. 2003;86:555–558. doi: 10.1007/978-3-7091-0651-8_113. [DOI] [PubMed] [Google Scholar]
- 48.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 49.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105:445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 50.Inamura T, Black KL. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab. 1994;14:862–870. doi: 10.1038/jcbfm.1994.108. [DOI] [PubMed] [Google Scholar]
- 51.Jalali S, Huang Y, Dumont DJ, Hynynen K. Focused ultrasound-mediated bbb disruption is associated with an increase in activation of AKT: experimental study in rats. BMC Neurol. 2010;10:114. doi: 10.1186/1471-2377-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones RM, O’Reilly MA, Hynynen K. Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: a simulation study. Phys Med Biol. 2013;58:4981–5005. doi: 10.1088/0031-9155/58/14/4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi S, Singh-Moon R, Wang M, Chaudhuri DB, Ellis JA, Bruce JN, et al. Cationic surface charge enhances early regional deposition of liposomes after intracarotid injection. J Neurooncol. 2014;120:489–497. doi: 10.1007/s11060-014-1584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 56.Juratli TA, Schackert G, Krex D. Current status of local therapy in malignant gliomas—a clinical review of three selected approaches. Pharmacol Ther. 2013;139:341–358. doi: 10.1016/j.pharmthera.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Kaye EA, Chen J, Pauly KB. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med. 2011;65:738–743. doi: 10.1002/mrm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemper EM, Boogerd W, Thuis I, Beijnen JH, van Tellingen O. Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30:415–423. doi: 10.1016/j.ctrv.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Kioi M, Husain SR, Croteau D, Kunwar S, Puri RK. Convection-enhanced delivery of interleukin-13 receptor-directed cytotoxin for malignant glioma therapy. Technol Cancer Res Treat. 2006;5:239–250. doi: 10.1177/153303460600500307. [DOI] [PubMed] [Google Scholar]
- 60.Krauze MT, Forsayeth J, Yin D, Bankiewicz KS. Convectionenhanced delivery of liposomes to primate brain. Methods Enzymol. 2009;465:349–362. doi: 10.1016/S0076-6879(09)65018-7. [DOI] [PubMed] [Google Scholar]
- 61.Kreisl WC, Liow JS, Kimura N, Seneca N, Zoghbi SS, Morse CL, et al. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med. 2010;51:559–566. doi: 10.2967/jnumed.109.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–111. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 63.Kunwar S, Pai LH, Pastan I. Cytotoxicity and antitumor effects of growth factor-toxin fusion proteins on human glioblastoma multiforme cells. J Neurosurg. 1993;79:569–576. doi: 10.3171/jns.1993.79.4.0569. [DOI] [PubMed] [Google Scholar]
- 64.Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009;77:235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lampidis TJ, Krishan A, Planas L, Tapiero H. Reversal of intrinsic resistance to adriamycin in normal cells by verapamil. Cancer Drug Deliv. 1986;3:251–259. doi: 10.1089/cdd.1986.3.251. [DOI] [PubMed] [Google Scholar]
- 66.Larsson HB, Stubgaard M, Frederiksen JL, Jensen M, Henriksen O, Paulson OB. Quantitation of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn Reson Med. 1990;16:117–131. doi: 10.1002/mrm.1910160111. [DOI] [PubMed] [Google Scholar]
- 67.Lemasson B, Serduc R, Maisin C, Bouchet A, Coquery N, Robert P, et al. Monitoring blood-brain barrier status in a rat model of glioma receiving therapy: dual injection of low-molecular-weight and macromolecular MR contrast media. Radiology. 2010;257:342–352. doi: 10.1148/radiol.10092343. [DOI] [PubMed] [Google Scholar]
- 68.Leweke F, Damian MS, Schindler C, Schachenmayr W. Multidrug resistance in glioblastoma. Chemosensitivity testing and immunohistochemical demonstration of P-glycoprotein. Pathol Res Pract. 1998;194:149–155. doi: 10.1016/S0344-0338(98)80015-0. [DOI] [PubMed] [Google Scholar]
- 69.Li XT, Ju RJ, Li XY, Zeng F, Shi JF, Liu L, et al. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget. 2014;5:6497–6511. doi: 10.18632/oncotarget.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 71.Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW, et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 72.Liu LB, Xue YX, Liu YH. Bradykinin increases the permeability of the blood-tumor barrier by the caveolae-mediated transcellular pathway. J Neurooncol. 2010;99:187–194. doi: 10.1007/s11060-010-0124-x. [DOI] [PubMed] [Google Scholar]
- 73.Marquet F, Tung YS, Teichert T, Ferrera VP, Konofagou EE. Noninvasive, transient and selective blood-brain barrier opening in non-human primates in vivo. PLoS ONE. 2011;6:e22598. doi: 10.1371/journal.pone.0022598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsumoto T, Tani E, Kaba K, Shindo H, Miyaji K. Expression of P-glycoprotein in human glioma cell lines and surgical glioma specimens. J Neurosurg. 1991;74:460–466. doi: 10.3171/jns.1991.74.3.0460. [DOI] [PubMed] [Google Scholar]
- 75.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28:871–880. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 78.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, Mc-Donald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagpal S. The role of BCNU polymer wafers (Gliadel) in the treatment of malignant glioma. Neurosurg Clin N Am. 2012;23:289–295. ix. doi: 10.1016/j.nec.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Nakagawa H, Groothuis D, Blasberg RG. The effect of graded hypertonic intracarotid infusions on drug delivery to experimental RG-2 gliomas. Neurology. 1984;34:1571–1581. doi: 10.1212/wnl.34.12.1571. [DOI] [PubMed] [Google Scholar]
- 81.Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu Q, Swaminathan G, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119. doi: 10.1126/scitranslmed.3003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nduom EK, Yang C, Merrill MJ, Zhuang Z, Lonser RR. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J Neurosurg. 2013;119:427–433. doi: 10.3171/2013.3.JNS122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neuwelt EA, Goldman DL, Dahlborg SA, Crossen J, Ramsey F, Roman-Goldstein S, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9:1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 84.Parney IF, Kunwar S, McDermott M, Berger M, Prados M, Cha S, et al. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102:267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 85.Pastan I, Gottesman MM. Multidrug resistance. Annu Rev Med. 1991;42:277–286. doi: 10.1146/annurev.me.42.020191.001425. [DOI] [PubMed] [Google Scholar]
- 86.Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13:1675–1683. doi: 10.1158/1078-0432.CCR-06-2489. [DOI] [PubMed] [Google Scholar]
- 87.Patel SJ, Shapiro WR, Laske DW, Jensen RL, Asher AL, Wessels BW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56:1243–1253. doi: 10.1227/01.neu.0000159649.71890.30. [DOI] [PubMed] [Google Scholar]
- 88.Pinzón-Daza ML, Salaroglio IC, Kopecka J, Garzòn R, Couraud PO, Ghigo D, et al. The cross-talk between canonical and non-canonical Wnt-dependent pathways regulates P-glycoprotein expression in human blood-brain barrier cells. J Cereb Blood Flow Metab. 2014;34:1258–1269. doi: 10.1038/jcbfm.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qin DX, Zheng R, Tang J, Li JX, Hu YH. Influence of radiation on the blood-brain barrier and optimum time of chemotherapy. Int J Radiat Oncol Biol Phys. 1990;19:1507–1510. doi: 10.1016/0360-3016(90)90364-p. [DOI] [PubMed] [Google Scholar]
- 90.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6:2157–2165. [PubMed] [Google Scholar]
- 91.Rapoport SI. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin Investig Drugs. 2001;10:1809–1818. doi: 10.1517/13543784.10.10.1809. [DOI] [PubMed] [Google Scholar]
- 92.Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20:217–230. doi: 10.1023/a:1007049806660. [DOI] [PubMed] [Google Scholar]
- 93.Rapoport SI, Hori M, Klatzo I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am J Physiol. 1972;223:323–331. doi: 10.1152/ajplegacy.1972.223.2.323. [DOI] [PubMed] [Google Scholar]
- 94.Rapoport SI, Robinson PJ. Tight-junctional modification as the basis of osmotic opening of the blood-brain barrier. Ann N Y Acad Sci. 1986;481:250–267. doi: 10.1111/j.1749-6632.1986.tb27155.x. [DOI] [PubMed] [Google Scholar]
- 95.Rascher G, Fischmann A, Kröger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104:85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 96.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roe M, Folkes A, Ashworth P, Brumwell J, Chima L, Hunjan S, et al. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg Med Chem Lett. 1999;9:595–600. doi: 10.1016/s0960-894x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- 98.Sage MR, Wilson AJ. The blood-brain barrier: an important concept in neuroimaging. AJNR Am J Neuroradiol. 1994;15:601–622. [PMC free article] [PubMed] [Google Scholar]
- 99.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 100.Sampson JH, Akabani G, Friedman AH, Bigner D, Kunwar S, Berger MS, et al. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus. 2006;20(4):E14. doi: 10.3171/foc.2006.20.4.9. [DOI] [PubMed] [Google Scholar]
- 101.Sampson JH, Archer G, Pedain C, Wembacher-Schröder E, Westphal M, Kunwar S, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113:301–309. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 102.Sanovich E, Bartus RT, Friden PM, Dean RL, Le HQ, Brightman MW. Pathway across blood-brain barrier opened by the bradykinin agonist, RMP-7. Brain Res. 1995;705:125–135. doi: 10.1016/0006-8993(95)01143-9. [DOI] [PubMed] [Google Scholar]
- 103.Sarkar G, Curran GL, Sarkaria JN, Lowe VJ, Jenkins RB. Peptide carrier-mediated non-covalent delivery of unmodified cisplatin, methotrexate and other agents via intravenous route to the brain. PLoS ONE. 2014;9:e97655. doi: 10.1371/journal.pone.0097655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43:364–369. doi: 10.1007/s12031-010-9451-9. [DOI] [PubMed] [Google Scholar]
- 105.Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–1104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 107.Sonabend AM, Carminucci AS, Amendolara B, Bansal M, Leung R, Lei L, et al. Convection-enhanced delivery of etoposide is effective against murine proneural glioblastoma. Neuro Oncol. 2014;16:1210–1219. doi: 10.1093/neuonc/nou026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stupp R, Tonn JC, Brada M, Pentheroudakis G. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 (Suppl 5):v190–v193. doi: 10.1093/annonc/mdq187. [DOI] [PubMed] [Google Scholar]
- 109.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 110.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89:1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 111.Tanner PG, Holtmannspotter M, Tonn JC, Goldbrunner R. Effects of drug efflux on convection-enhanced paclitaxel delivery to malignant gliomas: technical note. Neurosurgery. 2007;61:E880–E882. doi: 10.1227/01.NEU.0000298922.77921.F2. [DOI] [PubMed] [Google Scholar]
- 112.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Contr. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 114.Tung YS, Marquet F, Teichert T, Ferrera V, Konofagou EE. Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Appl Phys Lett. 2011;98:163704. doi: 10.1063/1.3580763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Wamel A, Kooiman K, Emmer M, ten Cate FJ, Versluis M, de Jong N. Ultrasound microbubble induced endothelial cell permeability. J Control Release. 2006;116:e100–e102. doi: 10.1016/j.jconrel.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 116.VanBavel E. Effects of shear stress on endothelial cells: possible relevance for ultrasound applications. Prog Biophys Mol Biol. 2007;93:374–383. doi: 10.1016/j.pbiomolbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 117.Warren K, Jakacki R, Widemann B, Aikin A, Libucha M, Packer R, et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children’s Oncology Group. Cancer Chemother Pharmacol. 2006;58:343–347. doi: 10.1007/s00280-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 118.Warth A, Kröger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol. 2004;107:311–318. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]
- 119.Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64:125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 121.Weber FW, Floeth F, Asher A, Bucholz R, Berger M, Prados M, et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl. 2003;88:93–103. doi: 10.1007/978-3-7091-6090-9_15. [DOI] [PubMed] [Google Scholar]
- 122.Wei KC, Chu PC, Wang HY, Huang CY, Chen PY, Tsai HC, et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS ONE. 2013;8:e58995. doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 124.Williams PC, Henner WD, Roman-Goldstein S, Dahlborg SA, Brummett RE, Tableman M, et al. Toxicity and efficacy of carboplatin and etoposide in conjunction with disruption of the blood-brain tumor barrier in the treatment of intracranial neoplasms. Neurosurgery. 1995;37:17–28. doi: 10.1227/00006123-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 126.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 127.Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol. 2014;4:126. doi: 10.3389/fonc.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xi G, Robinson E, Mania-Farnell B, Vanin EF, Shim KW, Takao T, et al. Convection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomedicine (Lond Print) 2014;10:381–391. doi: 10.1016/j.nano.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 129.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 130.Zünkeler B, Carson RE, Olson J, Blasberg RG, DeVroom H, Lutz RJ, et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J Neurosurg. 1996;85:1056–1065. doi: 10.3171/jns.1996.85.6.1056. [DOI] [PubMed] [Google Scholar]