Abstract
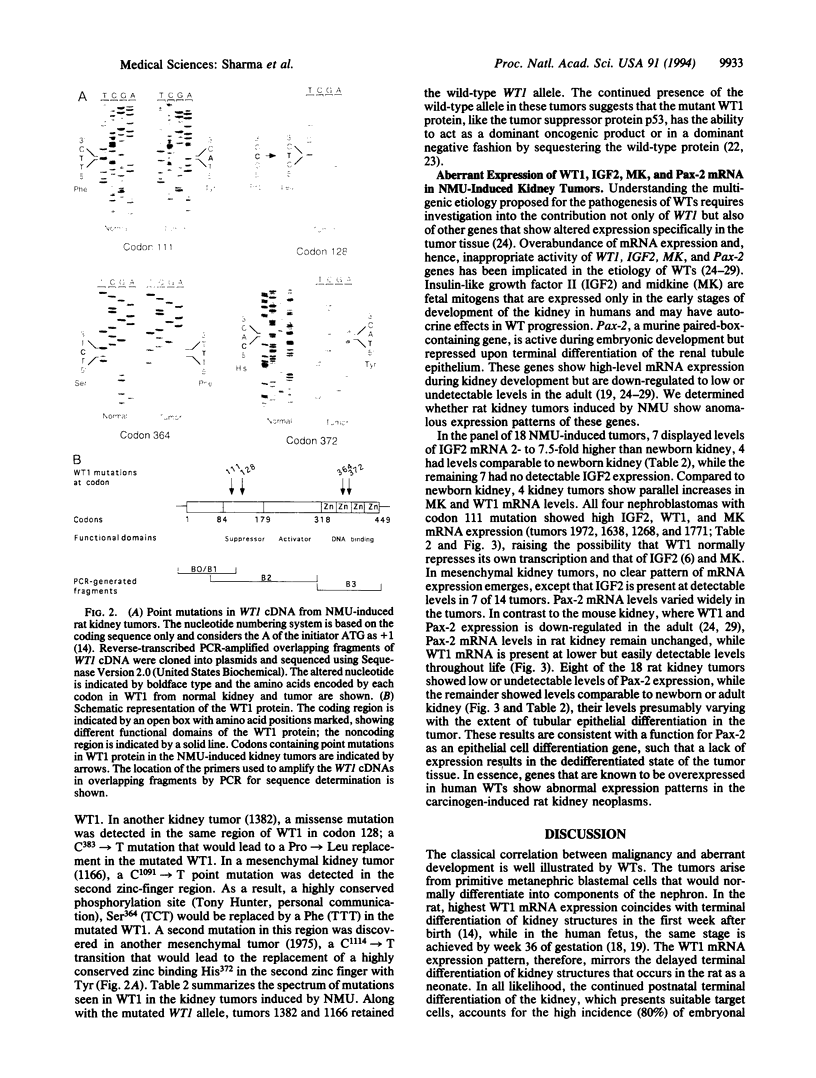
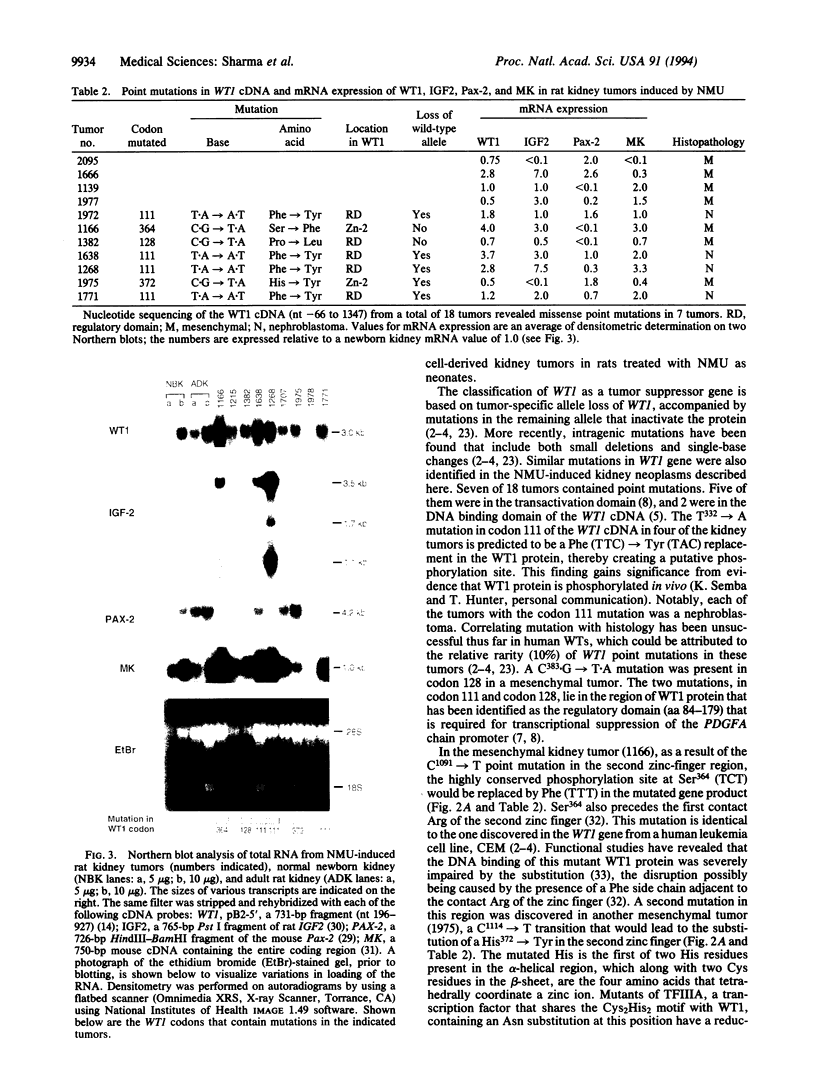
Embryonal kidney cell tumors develop in rats given the alkylating agent N-nitroso-N'-methylurea as neonates. These tumors resemble the childhood Wilms tumors in their histopathology. Deletions and mutations in the Wilms tumor suppressor gene, WT1, are present in up to 6% of childhood nephroblastomas. To investigate the role of WT1 in rat kidney tumorigenesis, we studied the genetic alterations in WT1 and its target genes. Point mutations were found in WT1 cDNA in 7 of 18 kidney tumors. Mesenchymal tumors contained G-->A transition mutations in codons 128, 364, and 372, typical of the methylating action of N-nitroso-N'-methylurea on DNA. Each of the four nephroblastomas contained the same T-->A mutation at codon 111 of WT1, reflective of transversion mutagenesis by N-nitroso-N'-methylurea in vivo. Like Wilms tumors, mRNA levels of WT1, IGF2, Pax-2, and MK genes were higher than newborn kidney in the majority of the tumors. The histopathology of the rat kidney tumors and the genetic alterations are reminiscent of those observed in Wilms tumors, establishing this as a relevant model system for the human disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Beckwith J. B., Kiviat N. B., Bonadio J. F. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol. 1990;10(1-2):1–36. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- Bhanot O. S., Grevatt P. C., Donahue J. M., Gabrielides C. N., Solomon J. J. In vitro DNA replication implicates O2-ethyldeoxythymidine in transversion mutagenesis by ethylating agents. Nucleic Acids Res. 1992 Feb 11;20(3):587–594. doi: 10.1093/nar/20.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore W. A., Oghene K., Little M. H., Seawright A., van Heyningen V., Hastie N. D. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992 Jul 10;257(5067):235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Breslow N., Beckwith J. B., Ciol M., Sharples K. Age distribution of Wilms' tumor: report from the National Wilms' Tumor Study. Cancer Res. 1988 Mar 15;48(6):1653–1657. [PubMed] [Google Scholar]
- Clapp W. L., Abrahamson D. R. Regulation of kidney organogenesis: homeobox genes, growth factors, and Wilms tumor. Curr Opin Nephrol Hypertens. 1993 May;2(3):419–429. [PubMed] [Google Scholar]
- Coombs L. M., Pigott D., Proctor A., Eydmann M., Denner J., Knowles M. A. Simultaneous isolation of DNA, RNA, and antigenic protein exhibiting kinase activity from small tumor samples using guanidine isothiocyanate. Anal Biochem. 1990 Aug 1;188(2):338–343. doi: 10.1016/0003-2697(90)90617-i. [DOI] [PubMed] [Google Scholar]
- Del Rio S., Setzer D. R. The role of zinc fingers in transcriptional activation by transcription factor IIIA. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):168–172. doi: 10.1073/pnas.90.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G. R., Douglass E. C. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Gashler A. L., Bonthron D. T., Madden S. L., Rauscher F. J., 3rd, Collins T., Sukhatme V. P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T., Lane J., Housman D. A mouse model of the aniridia-Wilms tumor deletion syndrome. Science. 1990 Nov 9;250(4982):823–827. doi: 10.1126/science.2173141. [DOI] [PubMed] [Google Scholar]
- Hard G. C. Differential renal tumor response to N-ethylnitrosourea and dimethylnitrosamine in the Nb rat: basis for a new rodent model of nephroblastoma. Carcinogenesis. 1985 Nov;6(11):1551–1558. doi: 10.1093/carcin/6.11.1551. [DOI] [PubMed] [Google Scholar]
- Hastie N. D. Wilms' tumour gene and function. Curr Opin Genet Dev. 1993 Jun;3(3):408–413. doi: 10.1016/0959-437x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Huff V., Saunders G. F. Wilms tumor genes. Biochim Biophys Acta. 1993 Dec 23;1155(3):295–306. doi: 10.1016/0304-419x(93)90011-z. [DOI] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kumar R., Sukumar S., Barbacid M. Activation of ras oncogenes preceding the onset of neoplasia. Science. 1990 Jun 1;248(4959):1101–1104. doi: 10.1126/science.2188364. [DOI] [PubMed] [Google Scholar]
- Little M. H., Williamson K. A., Mannens M., Kelsey A., Gosden C., Hastie N. D., van Heyningen V. Evidence that WT1 mutations in Denys-Drash syndrome patients may act in a dominant-negative fashion. Hum Mol Genet. 1993 Mar;2(3):259–264. doi: 10.1093/hmg/2.3.259. [DOI] [PubMed] [Google Scholar]
- Maheswaran S., Park S., Bernard A., Morris J. F., Rauscher F. J., 3rd, Hill D. E., Haber D. A. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Tomlinson G. E., Timmons C. F., Huff V., Cohn S. L., Strong L. C., Saunders G. F. RNA expression of the WT1 gene in Wilms' tumors in relation to histology. J Natl Cancer Inst. 1992 Feb 5;84(3):181–187. doi: 10.1093/jnci/84.3.181. [DOI] [PubMed] [Google Scholar]
- Park S., Bernard A., Bove K. E., Sens D. A., Hazen-Martin D. J., Garvin A. J., Haber D. A. Inactivation of WT1 in nephrogenic rests, genetic precursors to Wilms' tumour. Nat Genet. 1993 Dec;5(4):363–367. doi: 10.1038/ng1293-363. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K., Fleming S., Davidson D., Bickmore W., Porteous D., Gosden C., Bard J., Buckler A., Pelletier J., Housman D. The candidate Wilms' tumour gene is involved in genitourinary development. Nature. 1990 Jul 12;346(6280):194–197. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd The WT1 Wilms tumor gene product: a developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J. 1993 Jul;7(10):896–903. [PubMed] [Google Scholar]
- Reeve A. E., Eccles M. R., Wilkins R. J., Bell G. I., Millow L. J. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985 Sep 19;317(6034):258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- Saxén L., Lehtonen E. Embryonic kidney in organ culture. Differentiation. 1987;36(1):2–11. doi: 10.1111/j.1432-0436.1987.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Sharma P. M., Bowman M., Madden S. L., Rauscher F. J., 3rd, Sukumar S. RNA editing in the Wilms' tumor susceptibility gene, WT1. Genes Dev. 1994 Mar 15;8(6):720–731. doi: 10.1101/gad.8.6.720. [DOI] [PubMed] [Google Scholar]
- Sharma P. M., Yang X., Bowman M., Roberts V., Sukumar S. Molecular cloning of rat Wilms' tumor complementary DNA and a study of messenger RNA expression in the urogenital system and the brain. Cancer Res. 1992 Nov 15;52(22):6407–6412. [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Sukumar S. An experimental analysis of cancer: role of ras oncogenes in multistep carcinogenesis. Cancer Cells. 1990 Jul;2(7):199–204. [PubMed] [Google Scholar]
- Sukumar S., Barbacid M. Specific patterns of oncogene activation in transplacentally induced tumors. Proc Natl Acad Sci U S A. 1990 Jan;87(2):718–722. doi: 10.1073/pnas.87.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tomomura M., Kadomatsu K., Matsubara S., Muramatsu T. A retinoic acid-responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J Biol Chem. 1990 Jun 25;265(18):10765–10770. [PubMed] [Google Scholar]
- Tsutsui J., Kadomatsu K., Matsubara S., Nakagawara A., Hamanoue M., Takao S., Shimazu H., Ohi Y., Muramatsu T. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms' tumor and other human carcinomas. Cancer Res. 1993 Mar 15;53(6):1281–1285. [PubMed] [Google Scholar]
- Turusov V. S., Alexandrov V. A., Timoshenko I. V. Nephroblastoma and renal mesenchymal tumor induced in rats by N-nitrosoethyl- and N-nitrosomethylurea. Neoplasma. 1980;27(3):229–235. [PubMed] [Google Scholar]
- Van Heyningen V., Hastie N. D. Wilms' tumour: reconciling genetics and biology. Trends Genet. 1992 Jan;8(1):16–21. doi: 10.1016/0168-9525(92)90019-z. [DOI] [PubMed] [Google Scholar]
- Varanasi R., Bardeesy N., Ghahremani M., Petruzzi M. J., Nowak N., Adam M. A., Grundy P., Shows T. B., Pelletier J. Fine structure analysis of the WT1 gene in sporadic Wilms tumors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3554–3558. doi: 10.1073/pnas.91.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Deuel T. F. The Wilms' tumor gene product WT1 activates or suppresses transcription through separate functional domains. J Biol Chem. 1993 May 5;268(13):9172–9175. [PubMed] [Google Scholar]
- Weller A., Sorokin L., Illgen E. M., Ekblom P. Development and growth of mouse embryonic kidney in organ culture and modulation of development by soluble growth factor. Dev Biol. 1991 Apr;144(2):248–261. doi: 10.1016/0012-1606(91)90419-4. [DOI] [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield H. J., Bruni C. B., Frunzio R., Terrell J. E., Nissley S. P., Rechler M. M. Isolation of a cDNA clone encoding rat insulin-like growth factor-II precursor. Nature. 1984 Nov 15;312(5991):277–280. doi: 10.1038/312277a0. [DOI] [PubMed] [Google Scholar]






