Abstract
The serum 1,3-beta-D-glucan (BG) assay aids in the early diagnosis of invasive fungal diseases (IFDs) and has been approved for their diagnosis. However, reports on the screening performance of BG are scarce. We performed a meta-analysis of data extracted from only prospective cohort studies to evaluate the screening performance of the BG assay in the diagnosis of IFDs. We specifically searched 4 databases (the PubMed, Web of Science, Elsevier, and Cochrane Collaboration databases) according to EORTC-MSG criteria. A total of 1068 patients in 11 studies were analyzed. Deeks’ funnel plot asymmetry test suggested a low likelihood of publication bias for the included studies (p = 0.055). The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and area under the summary receiver operating characteristic curve, with 95% confidence intervals, were 0.75(0.63,0.84), 0.87(0.81,0.92), 5.85(3.96,8.63), 0.30(0.20,0.45), 19.53(11.16,34.18), and 0.89(0.86,0.91), respectively. The findings of this meta-analysis suggest that the BG assay is a useful screening tool with high sensitivity and specificity for discriminating between patients with and without IFDs. In clinical practice, BG assay results should be evaluated together with clinical and microbiological findings.
Introduction
Invasive fungal diseases (IFDs) are serious complications in patients with disease-related or iatrogenic immunosuppression [1,2] and in patients who are dependent on various types of supportive care [3–5]. In recent years, the incidence of IFDs has been increasing [6,7]. In addition, IFDs are associated with considerable morbidity, including mortality rates of 30–70% due to aspergillosis and of 40–50% due to candidiasis [8,9]. Early administration of antifungal therapy is important [10]. However, the diagnosis of IFDs is challenging because the clinical signs and conventional microbiological and histological techniques are generally not sensitive [11–13].
The serum 1,3-beta-D-glucan (BG) assay, derived from the major cell wall component of various medically important fungi, has been developed and frequently used [14–16]. The results of this assay have been included in the revised diagnostic criteria for IFDs of the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) [17]. However, the test performance varies. Systematic reviews have been conducted to investigate the diagnostic accuracy of the BG assay in the diagnosis of IFDs in patients with Pneumocystis jiroveci pneumonia, invasive candidiasis, and/or invasive aspergillosis [18–20]. In the present study, we performed a meta-analysis of data extracted from only prospective cohort studies to focus on the performance of the BG assay in screening IFDs.
Materials and Methods
Study selection and identification
We searched four databases (the PubMed, Web of Science, Elsevier, and Cochrane Collaboration databases) for records of studies that evaluated the diagnostic performance of serum BG for IFDs from January 2004 through April 2014. The search key words used were as follows: glucan, fungal disease, fungal infection, beta glucan, mycoses, candidiasis, candidemia, aspergillosis, and Aspergillus. The syntax was as follows: “glucan” OR “beta(β) glucan” AND “fungal infection”, “fungal disease”, “mycoses”, “Aspergillus”, “aspergillosis”, “candidiasis”, OR “candidemia”. Using the above search strategy, abstracts were identified and screened by 3 authors (TY.H., SH.W., and SX.L.) without language restrictions. Potentially relevant studies with full text were included based on the following inclusion criteria: (1) the EORTC/MSG criteria were treated as the reference standard for the classification of IFDs as proven, probable, or possible, independent of the BG test results[17,21]; (2) the data extracted as true-positive, false-positive, true-negative, or false-negative results of the BG test were reported independently or could be calculated using the data provided in the manuscript; (3) BG measurements were performed in a homogenous cohort of patients who were at risk for IFDs (data for healthy individuals used as controls were excluded from the analysis to avoid overestimating the specificity of BG testing); and (4) cutoffs of 80 pg/ml for the Fungitell assay, 11 pg/ml for the Wako BG assay, 20 pg/ml for the Fungitec G test, and 10 pg/ml for the GKT-25M were used because these cutoffs are considered to be equivalent [18,19]. Studies including fewer than 10 patients were excluded from the analysis to avoid selection bias.
Two reviewers (WX.J. and DD.L.) judged the study eligibility while screening the citations. The above criteria had to be agreed upon by 3 authors (TY.H., WX.J., and DH.H.) for inclusion in the analysis.
The quality of the included studies was assessed by 3 authors (TY.H., SH.W. and DH.H.) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool, which is based on 14 items that were developed to assess the quality of studies investigating diagnostic tests[22,23]. Each item was scored as “Yes”, “No” or “Unclear”, and the agreement of the 3 authors was required.
Data Analysis
To evaluate the performance of the BG test in screening for IFDs, patients with demonstrated or probable IFDs who were diagnosed according to the EORTC/MSG classification were compared with control patients, and patients with possible IFDs were also included in the analysis. Because we focused on screening performance, a positive test result was defined as 1 positive BG value based on the BG cutoff level used in each study. The pooled sensitivity and specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUC), with 95% confidence intervals (95% CIs), were calculated using a random-effects model [24]. A test for inconsistency (I2) was used to assess heterogeneity [25]. The possibility of publication bias was explored using Deeks’ funnel plot asymmetry test plots for DORs [26]. The presence of a threshold effect on the performance of the BG assay was evaluated using Spearman’s correlation coefficient between the logits of sensitivity and specificity [27]. The Midas module available in Stata software (version 11, Stata Corporation., http://www.stata.com) was used for the analysis. All of the statistical tests were two sided, and P values of 0.05 were considered statistically significant.
Results
Characteristics of the included studies
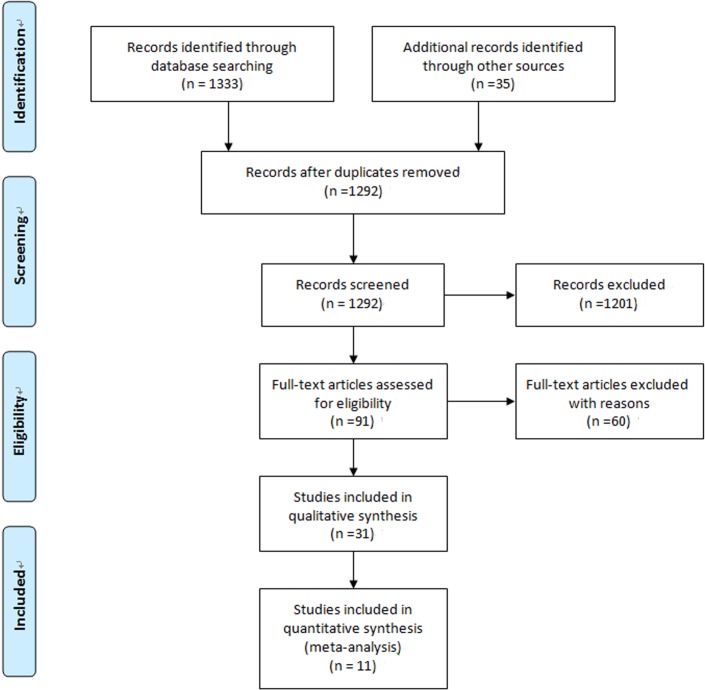
There were 1292 potentially relevant articles, of which 91 full-length articles were selected for a detailed analysis based on their title or abstract. The selection process for study inclusion is shown in Fig 1, and 11 prospective cohort studies met the inclusion criteria [6,7,9,10,12,28–34]. The characteristics of the 11 studies are summarized in Table 1. Among the 11 studies, 5 included patients with hematological malignancy (HM) or other serious tumors [6,12,28,29], 1 included patients who were liver transplant recipients[30], and the remaining 5 studies assessed hospitalized patients who might be at high risk for IFDs. The characteristics of the control groups varied. A screening strategy was used to measure the BG levels in blood samples in 9 studies. The 11 studies included 5 assays: 6 used Fungitell (cutoff value: 80 or 120 pg/ml), 2 used the Fungitec G test (cutoff value: 20 pg/ml), 2 used the Wako BG test (cutoff value: 7 or 11 pg/ml), and 1 used the GKT-25M set (cutoff value: 10 pg/ml). All of the studies, which comprised 1068 patients, employed the EORTC/MSG criteria as the reference standard for IFDs, as shown in Table 2.
Fig 1. Flow chart showing the study selection process.
Table 1. Characteristics of prospective cohort studies including BD Testing for the Diagnosis of IFDs.
| Author/year | Population | Frequency of BG screening | BG assay | Cutoff (pg/ml) | Total No. of patients | proven or probable cases | possible cases | IA | IC |
|---|---|---|---|---|---|---|---|---|---|
| Kawazu/2004 | patients with HM | Once per week | Wako | 11 | 96(149 episodes) | 11 | 13 | 11 | 0 |
| Horiguchi/2004 | patients with HM | Several samples were available for some patients | Fungitec G | 20 | 58(69 episodes) | 8 | 8 | 0 | |
| Pazos/2005 | patients with HM | Twice per week | Fungitell | 80 | 37 | 8 | 3 | 8 | 0 |
| Akamatsu/2007 | living donor liver transplant recipients | once per week for 3 months and once per month for 1 year | Fungitec G | 20 | 180 | 24 | unclear | 5 | 14 |
| Senn/2008 | patients with HM | Twice per week | Wako | 11 | 95(173 episodes) | 32 | 30 | 13 | 15 |
| Hachem/2009 | patients with HM and other tumors | Twice in week 1 and once per week for 12 weeks | Fungitell | 80 | 78 | 62 | unclear | 22 | 23 |
| Zhao/2009 | patients with hematologic or other malignant disorders | Twice per week | GKT-25M | 10 | 130 | 22 | 7 | 4 | 2 |
| Acosta/2011 | patients with various diseases | Twice per week | Fungitell | 80 | 51 | 13 | unclear | 10 | 0 |
| Posteraro/2011 | patients with various diseases | once per week | Fungitell | 80 | 95 | 14 | unclear | 14 | 1 |
| Mohr/2011 | patients with various diseases | Twice per week | Fungitell | 80 | 57 | 9 | 6 | 0 | 15 |
| Bono/2011 | patients with various diseases | Single sample per patient | Fungitell | 80 | 152 | 53 | 47 | 53 | 0 |
IA,invasive aspergillosis;IC,invasive candidiasis
Table 2. Screening performance of invasive fungal infection based on data from different prospective studies.
| Author/year | Reference standard | True positive | False negative | False positive | True negative |
|---|---|---|---|---|---|
| Kawazu/2004 | EORTC/MSG | 6 | 7 | 2 | 134 |
| Horiguchi/2004 | EORTC/MSG | 7 | 1 | 9 | 52 |
| Pazos/2005 | EORTC/MSG | 7 | 1 | 3 | 26 |
| Akamatsu/2007 | EORTC/MSG | 14 | 10 | 26 | 130 |
| Senn/2008 | EORTC/MSG | 30 | 30 | 12 | 101 |
| Hachem/2009 | EORTC/MSG | 37 | 21 | 2 | 18 |
| Zhao/2009 | EORTC/MSG | 18 | 4 | 19 | 89 |
| Acosta/2011 | EORTC/MSG | 11 | 2 | 7 | 31 |
| Posteraro/2011 | EORTC/MSG | 15 | 1 | 5 | 74 |
| Mohr/2011 | EORTC/MSG | 14 | 1 | 14 | 28 |
| Bono/2011 | EORTC/MSG | 79 | 21 | 9 | 43 |
Quality assessment of the included studies
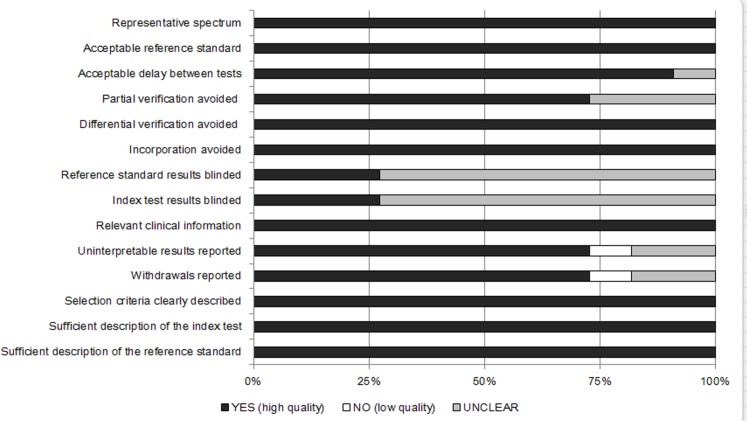
Fig 2 summarizes the findings of the cumulative methodological quality assessment of all 11 studies using the QUADAS tool. All of the studies employed a representative spectrum and an acceptable reference standard and avoided differential verification and incorporation bias. The majority of the studies did not report whether the reference standard results or the index test results were blinded.
Fig 2. Summary of the methodological quality assessment of the reviewed studies according to the 14 QUADAS criteria.
Performance in screening for IFDs
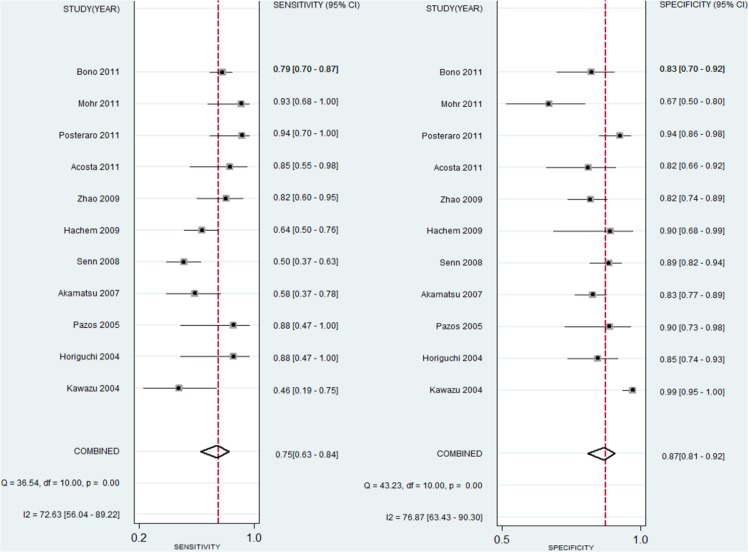
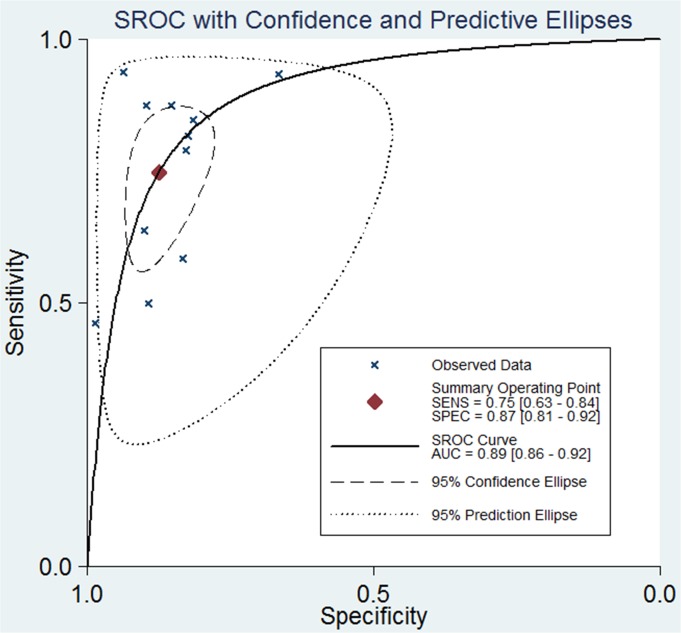
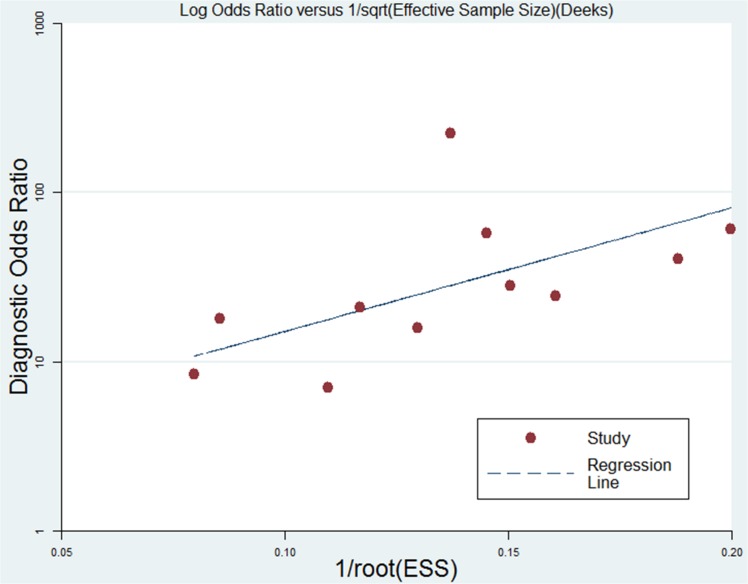
The pooled sensitivity, specificity, PLR, DOR, and AUC, with 95% CIs, are summarized in Table 3 and Fig 3. A similar subgroup analysis was performed (see Table 3). Relatively high inter-study heterogeneity was noted in 11 studies, and the I2 index was74.35% (95% CI: 59.87,98.33%). The SROC curves are displayed in Fig 4. The AUC value was 0.89 (95% CI: 0.86–0.91). Publication bias was evaluated using Deeks’ funnel plot asymmetry test, as shown in Fig 5.
Table 3. Pooled Test Performance of the Studies Included in the Meta-Analysis.
| Test | No.of reports | Pooled SE(95%CI) | Pooled SPE(95%CI) | Pooled PLR(95%CI) | Pooled NLR(95%CI) | Diagnostic Odds Ratio | AUC(95%CI) | |
|---|---|---|---|---|---|---|---|---|
| Kind of mycosis | Candidiasis | 4 | 0.80(0.67,0.90) | 0.77(0.67,0.89) | 3.58(1.22,6.87) | 0.26(0.10,0.57) | 25.43(13.101,49.86) | 0.88(0.83,0.98) |
| Aspergillosis | 6 | 0.73(0.62,0.86) | 0.81(0.64,0.85) | 5.57(3.89,6.23) | 0.34(0.12,0.48) | 23.15(10.4,58.90) | 0.85(0.70,0.95) | |
| Assay type | the Fungitell assay only | 6 | 0.82(0.68,0.90) | 0.86(0.77,0.92) | 5.69(3.46,9.35) | 0.22(0.12,0.39) | 26.52(11.72,60.07) | 0.90(0.88,0.93) |
| Pooled studiesa | 11 | 0.75(0.63,0.84) | 0.87(0.81,0.92) | 5.85(3.96,8.63) | 0.30(0.20,0.45) | 19.53(11.16,34.18) | 0.89(0.86,0.91) | |
NLR, negative likelihood ratio; PLR, positive likelihood ratio; DOR, Diagnostic Odds Ratio; AUC, the area under the summary receiver operating characteristic curve;a:I2 = 74.35%,95% CI:59.87–98.33%
Fig 3. Forest plot of the pooled sensitivity and specificity of the BG assays for the diagnosis of IFDs.
Fig 4. Summary receiver operating characteristic curve plots for the sensitivity and specificity for the diagnosis of IFDs.
Fig 5. Linear regression of Deeks’ funnel plot asymmetry test for DORs (P = 0.055).
Discussion
In this meta-analysis, the I2 of the 11 studies was 74.35% (95% CI: 59.87,98.33%), representing moderate heterogeneity [25,26,35]. In addition, a weak negative correlation was observed between the logits of sensitivity and specificity calculated for each of the 11 studies (Spearman’s correlation coefficient = -0.11), indicating a minor effect of the diagnostic threshold (cutoff level) on the performance of the BG measurement[19]. Regarding Deeks’ funnel plot asymmetry test, as shown in Fig 5, the non-significant slope coefficient (p = 0.055) suggested relative symmetry of the data and a low likelihood of publication bias.
Five types of BG assays, with different cutoff values, were included in the analysis. The different cutoffs for BG levels used may have altered the true-positive results and potentially created a bias toward the assessment of certain patients with false-positive results. In the study inclusion process, if a study reported BG data with different cutoff values, we included data referring to cutoffs of 80 pg/ml for the Fungitell assay, 11 pg/ml for the Wako BG assay, 20 pg/ml for the Fungitec G test, and 10 pg/ml for the GKT-25M. All of these cutoffs were considered to be equivalent, as indicated in several studies [18,19].
This meta-analysis included data for 1068 patients extracted from 11 prospective cohort studies with strict criteria for patient enrollment. In particular, data for healthy individuals used as controls were excluded from the analysis to avoid overestimating the specificity of the BG testing[36]. Therefore, all of the selected patients and controls at high risk for IFDs can be considered as highly representative of the actual clinical setting [9].
When evaluating the various patient categories, we included patients with possible IFDs because they represent a large number of patients in clinical practice. In addition, a certain proportion of possible IFD patients might have a true IFD, and therefore, the exclusion of data on possible IFD cases may lead to overestimating the assay sensitivity[37,38].
In comparison with certain meta-analyses evaluating the diagnostic accuracy of BG assays, including cohort studies and case-control studies, the sensitivity and specificity of the BG measurements in our meta-analysis appeared to be lower (0.75 vs. 0.78) and higher (0.87 vs. 0.80), respectively[19]. The use of case-control studies may bias results[39]. Lamoth et al. conducted a meta-analysis of only 6 cohort studies investigating the performance of BG assays in the diagnosis of IFD in hemato-oncological patients, reporting a lower sensitivity (0.70) and a higher specificity (0.91)[36]. However, the findings of the earlier meta-analysis should be interpreted with the high heterogeneity, ranging from 79% to 96%, in mind.
In general, an AUC value between 0.80 and 0.90 is considered to indicate good screening performance [18,40–41]. Although the AUC values in the 11 prospective cohort studies varied, the present meta-analysis demonstrated that serum BG measurement had good performance in screening for IFDs, with an AUC value of 0.89.
This meta-analysis has certain limitations. First, the quality assessment using the QUADAS tool showed that in the majority of the studies, information on blinded reference standard results and index test results was not reported. Second, there were only 4 studies on the analysis of invasive candidiasis, so the pooled screening performance-related values for invasive candidiasis (such as pooled sensitivity) should be considered carefully. Third, the performance of the BG assay in screening for the Pneumocystis jiroveci pneumonia was not assessed in this study due to a lack of data.
In conclusion, the findings of this meta-analysis suggest that the BG assay is a useful screening tool with high sensitivity and specificity for discriminating between patients with and without IFDs. In clinical practice, BG assay results should be evaluated together with clinical and microbiological findings. Certain issues regarding the optimal utilization of BG testing require further evaluation, especially the optimal sampling strategy for patients who are at high risk [20].
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Enoch DA, Ludlam HA, Brown NM (2006) Invasive fungal infections: a review of epidemiology and management options. Journal of Medical Microbiology 55: 809–818. [DOI] [PubMed] [Google Scholar]
- 2. Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, et al. (2006) Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica-the Hematology Journal 91: 986–989. [PubMed] [Google Scholar]
- 3. Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME (2008) Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: Differences in risk factors and outcome. Anesthesia and Analgesia 106: 523–529. 10.1213/ane.0b013e3181607262 [DOI] [PubMed] [Google Scholar]
- 4. Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, et al. (2010) Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clinical Infectious Diseases 50: 1101–1111. 10.1086/651262 [DOI] [PubMed] [Google Scholar]
- 5. Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. (2010) Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clinical Infectious Diseases 50: 1091–1100. 10.1086/651263 [DOI] [PubMed] [Google Scholar]
- 6. Pazos C, Ponton J, Del Palacio A (2005) Contribution of (1 -> 3)-beta-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. Journal of Clinical Microbiology 43: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Z, Ying W, Yun-fang Z, Bi-ru LI, Jing C, Hui-liang X, et al. (2010) Prospective fungal antigen testing in high-risk pediatric patients. Journal of Chinical Pediatrics 28: 1–6. [Google Scholar]
- 8. Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. (2003) Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 102: 827–833. [DOI] [PubMed] [Google Scholar]
- 9. Acosta J, Catalan M, del Palacio-Perez-Medel A, Lora D, Montejo JC, Cuetara MS, et al. (2011) A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1 -> 3)-beta-D-glucan chromogenic assay in serum samples. Clinical Microbiology and Infection 17: 1053–1060. 10.1111/j.1469-0691.2010.03357.x [DOI] [PubMed] [Google Scholar]
- 10. Posteraro B, De Pascale G, Tumbarello M, Torelli R, Pennisi MA, Bello G, et al. (2011) Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1–>3)-beta-D-glucan assay, Candida score, and colonization index. Crit Care 15: R249 10.1186/cc10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kedzierska A, Kochan P, Pietrzyk A, Kedzierska J (2007) Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1–>3)-beta-D-glucan antigens. Eur J Clin Microbiol Infect Dis 26: 755–766. [DOI] [PubMed] [Google Scholar]
- 12. Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I (2009) Utility of galactomannan enzyme immunoassay and (1,3) beta-D-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol 47: 129–133. 10.1128/JCM.00506-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB (2010) The (1,3){beta}-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol 48: 4083–4088. 10.1128/JCM.01183-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pickering JW, Sant HW, Bowles CAP, Roberts WL, Woods GL (2005) Evaluation of a (1 –> 3)-beta-D-glucan assay for diagnosis of invasive fungal infections. Journal of Clinical Microbiology 43: 5957–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, et al. (1995) PLASMA (1–3)-BETA-D-GLUCAN MEASUREMENT IN DIAGNOSIS OF INVASIVE DEEP MYCOSIS AND FUNGAL FEBRILE EPISODES. Lancet 345: 17–20. [DOI] [PubMed] [Google Scholar]
- 16. Hiyoshi M, Tagawa S, Hashimoto S, Sakamoto C, Tatsumi N (1999) Evaluation of a new laboratory test measuring plasma (1–>3)-beta-D-glucan in the diagnosis of Candida deep mycosis: comparison with a serologic test. Kansenshogaku zasshi The Journal of the Japanese Association for Infectious Diseases 73: 1–6. [DOI] [PubMed] [Google Scholar]
- 17. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 46: 1813–1821. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, et al. (2004) Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clinical Infectious Diseases 39:199–205. [DOI] [PubMed] [Google Scholar]
- 19. Obayashi T, Negishi K, Suzuki T, Funata N (2008) Reappraisal of the serum (1–>3)-beta-D-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis 46: 1864–1870. 10.1086/588295 [DOI] [PubMed] [Google Scholar]
- 20. He S, Hang JP, Zhang L, Wang F, Zhang DC, Gong F, et al. (2014) A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-d-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect.2014 July 28 pii: S1684-1182(14)00120-0. 10.1016/j.jmii.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 21. Ascioglu S, Rex JH, De Pauw B, Bennett JE, Bille J, Crokaert F, et al. (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clinical Infectious Diseases 34: 7–14. [DOI] [PubMed] [Google Scholar]
- 22. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. (2003) Towards complete and,accurate reporting of studies of diagnostic accuracy: the STARD initiative. British Medical Journal 326: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC medical research methodology 3: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chappell FM, Raab GM, Wardlaw JM (2009) When are summary ROC curves appropriate for diagnostic meta-analyses? Statistics in Medicine 28: 2653–2668. 10.1002/sim.3631 [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 26. Lijmer JG, Bossuyt PMM, Heisterkamp SH (2002) Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Statistics in Medicine 21: 1525–1537. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. British Medical Journal 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, et al. (2004) Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked Immunosorbent assay for galactomannan, and a (1 –> 3)-beta-D-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. Journal of Clinical Microbiology 42: 2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horiguchi Y (2004) [The performance of (1, 3)-beta-D-glucan and Aspergillus galactomannan measurement for early diagnosis of invasive aspergillosis in patients with hematological diseases]. Kansenshogaku Zasshi 78: 566–573. [DOI] [PubMed] [Google Scholar]
- 30. Akamatsu N, Sugawara Y, Kaneko J, Tamura S, Makuuchi M (2007) Preemptive treatment of fungal infection based on plasma (1 –> 3)beta-D-glucan levels after liver transplantation. Infection 35: 346–351. [DOI] [PubMed] [Google Scholar]
- 31. Senn L, Robinson JO, Schmidt S, Knaup M, Asahi N, Satomura S, et al. (2008) 1,3-Beta-D-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis 46: 878–885. 10.1086/527382 [DOI] [PubMed] [Google Scholar]
- 32. Mohr JF, Sims C, Paetznick V, Rodriguez J, Finkelman MA, Rex JH, et al. (2011) Prospective survey of (1–>3)-beta-D-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol 49: 58–61. 10.1128/JCM.01240-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, et al. (2008) Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. Journal of Medical Microbiology 57: 287–295. 10.1099/jmm.0.47479-0 [DOI] [PubMed] [Google Scholar]
- 34. Del Bono V, Delfino E, Furfaro E, Mikulska M, Nicco E, Bruzzi P, et al. (2011) Clinical performance of the (1,3)-beta-D-glucan assay in early diagnosis of nosocomial Candida bloodstream infections. Clin Vaccine Immunol 18: 2113–2117. 10.1128/CVI.05408-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leon C, Ruiz-Santana S, Saavedra P, Galvan B, Blanco A, Castro C, et al. (2009) Usefulness of the "Candida score" for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Critical Care Medicine 37: 1624–1633. 10.1097/CCM.0b013e31819daa14 [DOI] [PubMed] [Google Scholar]
- 36. Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, et al. (2012) beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis 54: 633–643. 10.1093/cid/cir897 [DOI] [PubMed] [Google Scholar]
- 37. Koo S, Bryar JM, Page JH, Baden LR, Marty FM (2009) Diagnostic Performance of the (1 – > 3)-beta-D-Glucan Assay for Invasive Fungal Disease. Clinical Infectious Diseases 49: 1650–1659. 10.1086/647942 [DOI] [PubMed] [Google Scholar]
- 38. Onishi A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, et al. (2012) Diagnostic accuracy of serum 1,3-beta-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 50: 7–15. 10.1128/JCM.05267-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshida K, Shoji H, Takuma T, Niki Y (2011) Clinical viability of Fungitell, a new (1 –> 3)-beta-d-glucan measurement kit, for diagnosis of invasive fungal infection, and comparison with other kits available in Japan. Journal of Infection and Chemotherapy 17: 473–477. 10.1007/s10156-010-0198-6 [DOI] [PubMed] [Google Scholar]
- 40.Rose SR, Vallabhajosyula S, Velez MG, Fedorko DP, VanRaden MJ, Gea-Banacloche JC, et al. (2014) The utility of bronchoalveolar lavage beta-D-glucan testing for the diagnosis of invasive fungal infections. J Infect. [DOI] [PMC free article] [PubMed]
- 41. Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME (2011) beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 52: 750–770. 10.1093/cid/ciq206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.