Abstract
Animals maximize fitness by modulating sleep and foraging strategies in response to changes in nutrient availability. Wild populations of the fruit fly, Drosophila melanogaster, display highly variable levels of starvation and desiccation resistance that differ in accordance with geographic location, nutrient availability, and evolutionary history. Further, flies potently modulate sleep in response to changes in food availability, and selection for starvation resistance enhances sleep, revealing strong genetic relationships between sleep and nutrient availability. To determine the genetic and evolutionary relationship between sleep and nutrient deprivation, we assessed sleep in flies selected for desiccation or starvation resistance. While starvation resistant flies have higher levels of triglycerides, desiccation resistant flies have enhanced glycogen stores, indicative of distinct physiological adaptations to food or water scarcity. Strikingly, selection for starvation resistance, but not desiccation resistance, leads to increased sleep, indicating that enhanced sleep is not a generalized consequence of higher energy stores. Thermotolerance is not altered in starvation or desiccation resistant flies, providing further evidence for context-specific adaptation to environmental stressors. F2 hybrid flies were generated by crossing starvation selected flies with desiccation selected flies, and the relationship between nutrient deprivation and sleep was examined. Hybrids exhibit a positive correlation between starvation resistance and sleep, while no interaction was detected between desiccation resistance and sleep, revealing that prolonged sleep provides an adaptive response to starvation stress. Therefore, these findings demonstrate context-specific evolution of enhanced sleep in response to chronic food deprivation, and provide a model for understanding the evolutionary relationship between sleep and nutrient availability.
Introduction
Sleep is a near universal animal behavior, with highly conserved functional and molecular properties [1,2]. Sleep duration and timing vary greatly between species in accordance with ecological, environmental, and evolutionary history [3,4]. Animals modulate sleep in response to a number of factors, including environmental stressors, developmental stage, and aging [5–7]. While sleep is clearly influenced by many environmental factors, sleep timing and duration are closely related to nutrient availability and foraging strategy [8,9]. Both flies and mammals suppress sleep in response to starvation, presumably in order to forage for food. This indicates a functional trade-off between sleep duration and feeding [7,10,11]. Conversely, one proposed function of sleep is energy conservation, suggesting prolonged sleep may improve survival in the absence of nutrients [12]. Although there are likely evolutionary interactions between sleep and nutrient availability, these interactions are not well understood.
The fruit fly Drosophila melanogaster presents a powerful model for investigating genetic interactions between sleep and metabolic processes [13,14]. Resistance to nutrient deprivation is associated with enhanced metabolic stores, as well as physiological or behavioral adaptations that conserve energy [15–17]. Water and food represent two primary nutrient sources, and Drosophila appear to have developed distinct mechanisms to cope with the deprivation of each nutrient source. Starvation and desiccation resistance in wild populations of Drosophila have been studied extensively across many geographic ranges and are often found to be strongly correlated with the lipid or glycogen content of the flies [15,18,19]. Many traits associated with stress resistance vary greatly due to naturally occurring genetic variation, providing the opportunity to identify genetic regulators of these traits. Genomic analyses of fully sequenced inbred lines and quantitative genetic approaches have provided insight into the genetic basis for resistance to environmental and physiological stress [20,21]. While these studies have provided insight into the molecular underpinnings of many traits related to stress resistance, the functional and evolutionary interactions between sleep and nutrient deprivation remains unclear. Here, we examine the evolutionary relationship between sleep duration resistance to food and water deprivation.
Experimental evolution in wild-caught Drosophila melanogaster provides a powerful approach to study the evolutionary basis for, and interaction between, traits [22, 23]. Previous work has demonstrated changes in sleep and activity in flies selected for starvation resistance [24], but it is not clear whether these represent generalized adaptations to stress or selective changes to prolong survival in response to starvation. We have utilized experimental evolution to generate flies with enhanced resistance to starvation and desiccation, providing the opportunity to examine the evolutionary and functional relationship between these traits. Three populations of wild-caught flies were independently selected over 60 generations under conditions of starvation resistance (SR) or desiccation resistance (DR), allowing for the examination of repeated evolutionary changes in response to distinct forms of nutrient stress [23,24]. Flies selected for starvation resistance survive up to 18 days in the absence of food, while non-selected controls survive an average of four days [23,24]. Selection for desiccation resistance results in flies that survive up to 4 days in the absence of water, nearly twice the survival time of non-selected controls [23,24]. Here, we examine the sleep and activity phenotypes of flies selected for SR and DR to determine whether conserved or distinct changes in activity contribute to the generation of resistance to starvation and desiccation.
Both energy stores and resistance to nutrient deprivation differ between flies selected for DR and SR, suggesting that independent genetic mechanisms regulate evolutionary changes that result from chronic nutrient deprivation. Selection for SR, but not DR, results in flies with prolonged sleep, suggesting that change in sleep is not a generalized response to environmental stress. F2 hybrids generated from SR-DR selected flies display an interaction between sleep and starvation resistance, but not sleep and desiccation resistance, supporting the notion that prolonged sleep duration is an evolutionarily adaptive response to surviving starvation stress, specifically. These findings provide evidence for the context-dependent evolution of metabolic and behavioral adaptations in response to nutrient deprivation and introduce a framework for understanding the evolutionary basis for interactions between sleep and food availability.
Materials and Methods
Generation of starvation and desiccation resistant flies
The wild-derived stocks used in this study were collected with owner’s permission from Terhune Orchards in Princeton, N.J. in 1999 and have been maintained as outbred stocks at 25°C on standard corn meal medium since this time. The generation of DR and fed control FDR flies have previously been described as Td and Tf flies, respectively ([23] and Fig 1B). These have been renamed in this manuscript for clarity, with the three replicated DR populations being designated as DRa, DRb, and DRc, and the three fed control populations as FDRa, FDRb, and FDRc. Briefly, these three populations of DR flies were selected from the founding stock that had been maintained on standard food conditions. Selection for desiccation resistance in DR flies occurred by transferring populations of ~7,500 adult flies to a population cage containing silica gel desiccant alone. The silica gel was replaced with fly food when ~15% of the flies survived. Eggs were then collected from the progeny, and this cycle was repeated for 30 generations to develop the previously described DR lines. The DR lines used in this paper have been maintained under reduced desiccation selection (24 hours under desiccation for each generation) for ~110 generations. The FDR flies used in this paper were three replicate fed control populations maintained on food throughout the selection process (Fig 1B). Because desiccation selection involves removal of both food and water, an additional population of lines was generated where food deprivation was yoked to the desiccation selected Drosophila. These flies were provided agar instead of the silica gel desiccant, and flies were transferred at the same times as DR group flies [23]. Of the three groups originally generated, only two remain, and these flies have been renamed DRCTRLa and DRCTRLb for clarity.
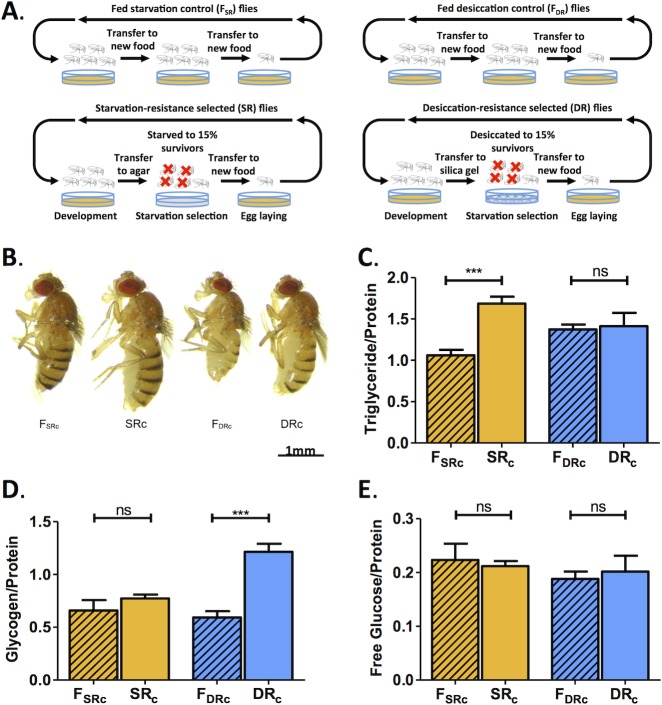
Fig 1. SR and DR selection increases body size and alters metabolic profile.
A) Schematic of selection processes for SR and DR flies. Adult outbred flies were placed under desiccation or starvation conditions until ~15% of the flies survived. The flies were then moved to food. This process was repeated over >80 generations. The FDR controls were placed on agar during desiccation selection to account for food deprivation in DR selected flies. There were three replicated SR populations (designated SRa, SRb and SRc) and three fed control populations (FSRa, FSRb and FSRc). For DR experiments there were three replicated groups (designated DRa, DRb and DRc) and three fed control populations (FDRa, FDRb and FDRc). B) Flies from the C Group. SRc and DRc flies are visibly larger than FSRc and FDRc controls. C) Triglyceride levels are elevated in the SRc flies compared to FSRc controls. No differences are observed between DRc flies and FDRc controls (P<0.001; See S1 Table). D) Glycogen levels were greater in DRc flies than in FDRc controls. No differences were present between SRc flies and FSRc controls (P<0.001; See S1 Table). E) Free glucose levels did not differ between SRc or DRc flies and their respective controls (P>0.05; See S1 Table).
The SR and fed control FSR populations were derived from two control treatments for desiccation-selected populations described in [23]. These SR lines and controls were previously described in [24]. For the selection process, approximately 8,000 experimental flies for each of the three starvation selected groups were maintained in constant light at room temperature (~23°C) on 1% agar until only 15–20% of the original population survived. Surviving flies were then placed on food to lay eggs. The next generation of adults was selected for starvation resistance in the same manner. Flies assayed for behavior experiments described in this manuscript ranged between generations 55 and 70 of selection. FSR populations were maintained on food while the SR populations were starved. There were three replicated SR populations (designated SRa, SRb, and SRc) and three fed control populations (FSRa, FSRb, and FSRc). It should be noted that fed control populations are also demographic controls, as they are maintained in the same generation time as their associated stress-selected populations. All selection occurred in the laboratory of Allen Gibbs (UNLV).
Drosophila maintenance
Flies taken off of the selection process for behavioral experiments were maintained and tested in humidified incubators at 25°C and 65% humidity (Powers Scientific). Flies were reared on a 12:12 light-dark cycle for 2–6 generations following selection prior to behavioral analysis. All flies were maintained on Jazz-Mix Drosophila Food (Fisher Scientific).
Protein, glycogen, and triglyceride measurements
For quantifying triglyceride, glycogen and protein content of flies two female flies aged 3–5 days were homogenized in HCl, pH 7.4, 0.1% Triton-X, 1X protease inhibitor cocktail (Sigma Aldrich, P8340). Triglyceride concentration was measured using the Stanbio Liquicolor Kit (Boerne, TX, S2200), and protein concentrations were measuring using a BCA Protein Assay Kit (Pierce Scientific, 23225). Total glucose levels were determined using the Glucose Oxidase Reagent (Pointe Scientific, G7519) in samples previously treated with 8 mg/mL 48 amyloglucosidase in 0.2M Sodium Citrate buffer, pH 5.0 (Boston BioProducts, BB-88). Free glucose was measured in samples not treated with amyloglucosidase, and then glycogen concentrations were determined by subtracting the free glucose from total glucose concentrations. Both glycogen and triglyceride concentrations were standardized to the total protein content of each sample containing two flies.
Sleep and activity analysis
Activity monitoring using Drosophila Activity Monitoring (DAM) System
Fly activity was monitored using DAM2 Drosophila activity monitors (Trikinetics, Waltham, MA) as previously described [25,26]. Female flies were briefly anesthetized using CO2 within 1 hour of lights on at Zeitgeber Time 0 (ZT0) and placed into plastic tubes containing standard food. The DAM system measures activity by detecting infrared beam crossings for each animal. These data were used to calculate sleep information by extracting immobility bouts of 5 minutes or more using the Drosophila Sleep Counting Macro [27]. Multiple variables of sleep were analyzed, including total sleep duration, sleep bout number, and average sleep bout length as previously described [27,28]. For experiments examining the effects of starvation on sleep, activity was recorded for one day on food prior to transferring flies into tubes containing 1% agar (Fisher Scientific). Flies were then transferred every 7 days onto fresh agar tubes for the remainder of the experiment. For experiments examining the effects of desiccation on sleep, activity was recorded for one day on food prior to transferring flies into tubes containing dry Kimwipes (Fisher Scientific).
Stress survival
Flies subjected to stress survival tests were first acclimated in DAM2 monitor tubes containing standard fly food for 24 hours. For experiments examining the effects of stress on longevity, flies were then transferred into individual DAM2 tubes and were assayed under starvation, desiccation, or heat shock conditions. A 1% agar (Fisher Scientific) solution was made to replicate starvation selection conditions; kimwipes were used to represent desiccation conditions; and a temperature increase to 35°C was used to generate heat stress conditions. Activity was recorded in DAM2 monitors and measured using the Sleep Counting Macro [27]. Death was manually determined at the last activity time point from the final recorded activity bout for each individual fly. For analysis, we applied Kaplan-Meier analysis by grouping each stress resistant population to its respective control.
Statistics
Statistical analyses were performed using InStat software (GraphPad Software 5.0 Inc.) or IBM SPSS 22.0 software (IBM, Somers, NY, USA). We employed two-way ANOVA for most of the comparative analyses, followed by posthoc analysis if necessary. In the slope analysis, we used ANOVA to compare the slopes of grouped FSR (FSRa, FSRb, and FSRc) and SR (SRa, SRb, and SRc) populations. In the figures, graph bars are mean values and error bars represent the standard error of the mean. All statistics are fully reported in S1 Table.
Results
Altered energy stores in starvation and desiccation resistant flies
Three independent groups of flies were derived for starvation (SR) or desiccation (DR) resistance from a previously described outbred population [23, 24]. SR flies were generated by maintaining flies on agar until ~15% of flies survived, while fed control flies (FSR) were maintained on food (Fig 1A). DR selected flies were maintained on silica gel desiccant until ~15% survived, while fed control flies (FDR) were maintained on food [23] (Fig 1A). Consistent with previous reports, both SR and DR selection resulted in increased body size compared to FSR and FDR group controls for all three replicates tested ([23,24] Fig 1B and data not shown). Triglyceride and glycogen represent the primary energy stores in Drosophila. Triglycerides provide a more efficient method of energy storage, while glycogen provides a source of metabolic water. This raises the possibility that the two selection processes result in a differential accumulation of these energy stores [16]. We found that triglyceride levels are significantly higher in all three groups of SR flies compared to FSR control flies, while no difference was observed between DR selected flies and FDR controls, indicating that only selection for starvation resistance results in increased triglyceride accumulation (Fig 1C and S1 Fig). Glycogen levels are elevated in DR flies in all groups compared to FDR control flies, while no differences are observed between SR and FSR controls in Groups B and C. However, SR flies in Group A do have increased glycogen levels, along with their increase in triglyceride levels (Fig 1D and S1 Fig). No significant differences in free glucose were observed between any of the lines tested. However, for Group A, free glucose is elevated in SR and reduced in DR selected groups compared to controls, indicating that selection primarily effects triglyceride energy stores in this group (Fig 1E and S1 Fig). These findings reveal distinct differences in physiological phenotypes between independently selected DR and SR lines. Therefore, selection for starvation resistance results in enhanced triglyceride levels, while selection for desiccation resistance results in increased glycogen stores. These findings suggest that distinct metabolic phenotypes are associated with the evolution of resistance to starvation and desiccation stress.
Selection for starvation and desiccation resistance has differential effects in response to nutrient deprivation
To determine whether each selection protocols generally enhanced stress resistance or increased survival to nutrient deprivation in a context-dependent fashion we measured longevity of SR and DR selected flies under starvation and desiccation conditions. Following 24hrs of acclimation on food, flies were transferred to tubes containing 1% agar or dry Kimwipes. Survival time was measured using the Drosophila Activity Monitor (DAM) system [29]. Under starvation conditions, all three SR groups survived longer than FSR and FDR controls and DR flies (Fig 2A and S2 Fig). However, two groups of desiccation selected DR flies survived longer than associated controls, suggesting that selection for desiccation resistance may confer moderate starvation resistance (Fig 2A and S2 Fig). Under desiccation conditions, all three groups of SR flies survived longer than FSR group controls, and all three groups of desiccation selected DR flies survived longer than FDR controls (Fig 2B and S2 Fig). Therefore, experimental selection for starvation or desiccation resistance has differential effects on the evolution of resistance to nutrient deprivation.
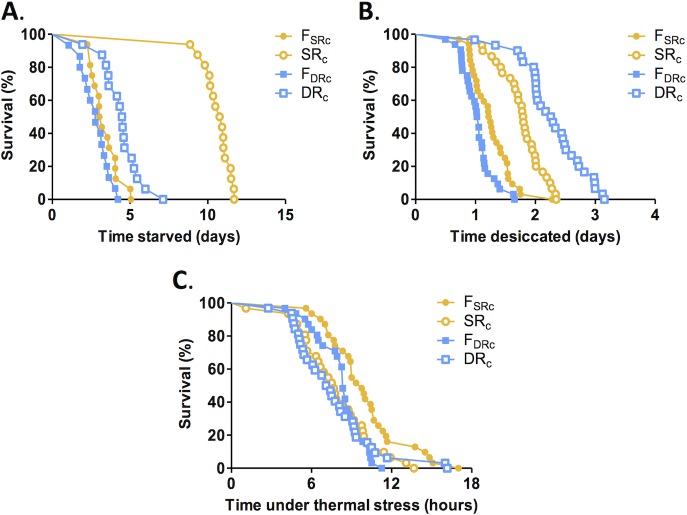
Fig 2. Distinct resistance to nutrient deprivation in SR and DR flies.
Survival of flies placed in activity monitors under starvation conditions. A) Flies from the SRc lines survived longer than FSRc controls, whereas DR lines do not differ from FDRc controls (SR lines: P < 0.001 in all groups; DR lines: P>0.05). B) DRc flies survive longer than FDRc controls under desiccation conditions. SRc flies were also resistant to desiccation compared to FSRc controls (DRc line: P<0.001; SRc line: P<0.001, See S1 Table.) C) SRc flies did not live as long as FSRc controls, and no difference in longevity was observed in DRc flies and controls, under thermal stress conditions (SR lines: P = 0.01; DR lines P>0.05; See S1 Table).
It is possible that SR and DR selection results in flies that are selectively resistant to nutrient deprivation, or that are generally resistant to stress. Previous reports indicate that DR selected flies showed a generalized resistance to stressors, including chemical, heat, and radiation stressors [16,17]. To differentiate between these possibilities, the longevity of SR and DR flies under conditions of high-temperature stress was assessed. Flies were maintained at 35°C, and longevity was measured. No increased longevity was observed between SR or DR flies and their controls, suggesting that their enhanced survival in response to nutrient deprivation is not generalizable to other stressors (Fig 2C and S2 Fig). Therefore, the enhanced resistance to nutritional deprivation following the selection protocol used to generate the flies in this study does not result from generalized stress resistance.
Sleep is not altered in flies selected for desiccation resistance
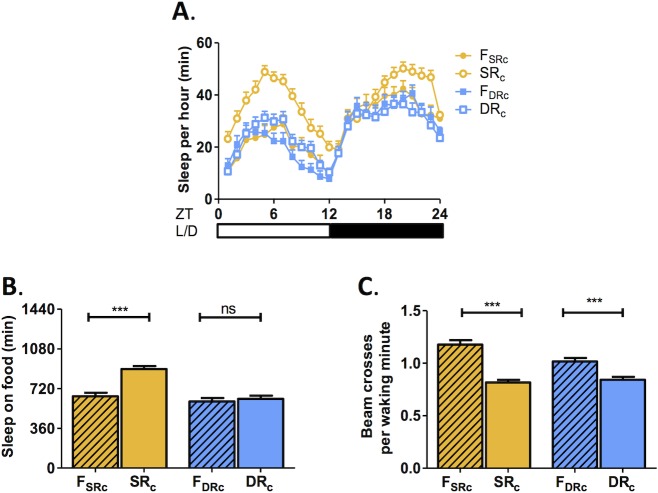
SR flies sleep longer than their controls, raising the possibility that prolonged sleep is adaptive for survival under conditions of chronic nutrient deprivation [24]. It is possible that the evolution of prolonged sleep either occurs specifically under conditions of starvation, or is a general response to nutrient deprivation. To differentiate between these two possibilities we measured sleep in flies selected for desiccation resistance. There was no difference in the sleep duration between DR and FDR flies (Fig 3 and S3 Fig). In agreement with previous findings, all three SR lines slept longer than FSR controls, but no DR line slept longer than its respective FDR control. This confirms that evolutionary selection for SR, but not DR, results in prolonged sleep (Fig 3 and S3 Fig). Sleep can be differentiated from lethargy or hyperactivity by measuring the amount of activity exhibited when an animal is awake [26]. We measured beam crossings per waking minute to infer waking activity in DR flies to determine if they conserve energy by reducing activity, rather than by extending sleep. Waking activity was reduced in all three DR lines compared to FDR controls, while waking activity was not changed (Group A and B) or reduced (Group C) in SR files (Fig 3C and S3 Fig). Therefore, selection for DR does not result in prolonged sleep, but does reduce activity, providing evidence for distinct energy conservation strategies in response to starvation or desiccation conditions.
Fig 3. Selection for DR does not alter sleep.
A) Sleep profiles depicting hourly sleep reveal that sleep in SRc flies is increased during both day and night compared to the DRc flies and respective controls (N = 64 for all groups). B) The total sleep duration over 24hrs on food is significantly longer in SRc flies than in FSRc flies. No differences are observed between DRc flies and FDRc flies (SRc group: P<0.001; DRc lines: P>0.05; See S1 Table). C) Beam crossings per waking minute are reduced in DRc and SRc flies compared to respective controls (SRc group: P<0.001; DRc lines: P<0.001; See S1 Table).
DR phenotypes are not due to starvation during selection
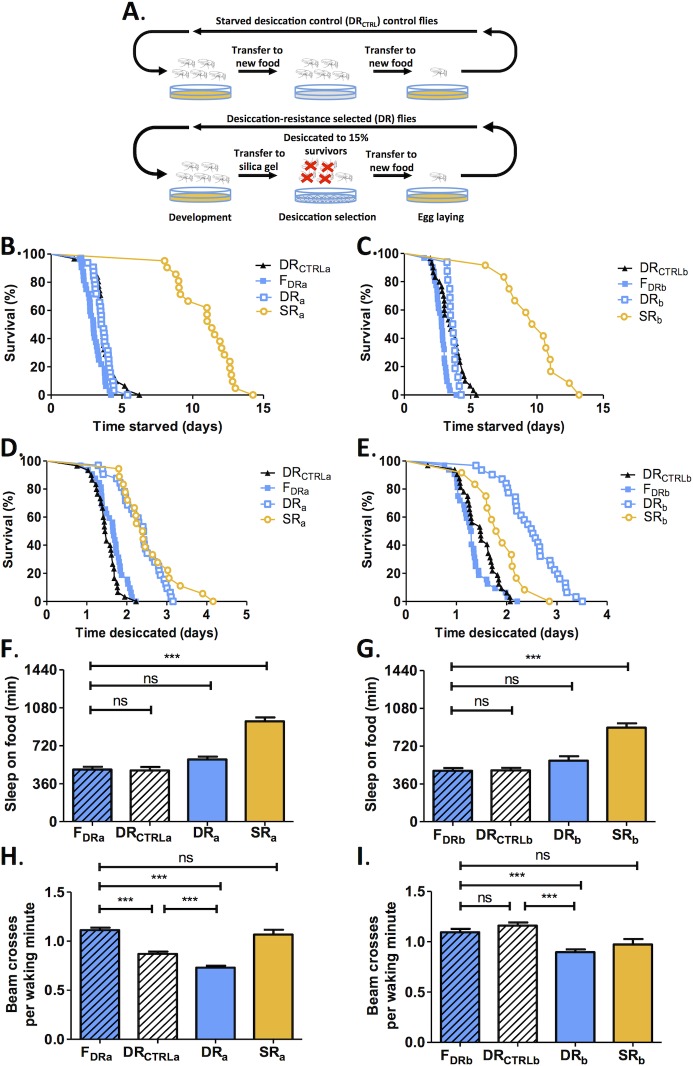
The selection protocol used to generate DR flies creates a state of both food and water deprivation, raising the possibility that resistance to nutrient deprivation and the altered activity levels of DR flies are due to starvation. To account for this possibility we assayed yoked-control flies (DRCTRL) that were starved for the period that DR flies were desiccated throughout DR selection (Fig 4A). Only two of the three originally selected DRCTRL groups remain. Survival under starvation conditions (Fig 4B and 4C) and desiccation conditions (Fig 4D and 4E) did not differ between DRCTRL flies or FDR control flies, suggesting that the resistance to nutrient deprivation observed in DR flies results from desiccation selection specifically. Flies from the SRa and SRb groups survived significantly longer than their FDR and DRCTRL controls. This indicates that the relatively short starvation selection time used for DR selection (~3–4 days) is insufficient to confer changes in starvation resistance (Fig 4B and 4C). Further, DRa and DRb group flies survived longer under desiccation conditions than their DRCTRL flies, confirming that survival under desiccation conditions in DR flies is not due to starvation during the selection procedure.
Fig 4. Resistance to nutrient deprivation in DR is not confounded by starvation during selection.
A) Schematic of selection process for yoked-control flies (DRCTRLa and DRCTRLb) that were starved during the selection period for DR flies. Of the three groups originally generated, only two remain. B, C) Survival of DRCTRLa and DRCTRLb flies does not differ from respective FDR controls under starvation conditions. D, E) Survival of DRCTRLa and DRCTRLb flies does not differ from respective FDR controls under desiccation conditions. F, G) Sleep is not increased in the DRCTRLa and DRCTRLb flies compared to their respective FDR controls or DR selected experimental groups. Sleep is significantly less than respective SR selected flies. H) Beam crossings per waking minute, an meausre of activity while awake, were reduced in DRCTRLa flies compared to FDRa controls, and was significantly greater than SRa selected flies. I) Beam crossings per waking minute did not differ between DRCTRLb and FDRb flies and was significantly greater than both DRb and SRb selected lines. *** denotes P<0.001.
To determine whether starvation during desiccation impacted behavior, we tested DRCTRL flies for sleep and waking activity. Sleep did not differ between FDR and DRCTRL flies, indicating that the selection period was not long enough do induce the increased sleep phenotype observed in all three SR groups (Fig 4F and 4G). Beam crossings per waking minute were reduced in the DRCTRLa control flies compared to FDRa control flies, though not to the levels of the DRa flies, suggesting that starvation partially contributes to the reduced waking activity for Group A (Fig 4H). Conversely, no differences in waking activity, measured by beam crossings per waking minute, was observed between FDRb and DRCTRLb control flies, suggesting that the reduced activity of the DRb flies is not due to starvation during the desiccation selection process (Fig 4I). Taken together, these results fortify the notion that selection under conditions of nutrient deprivation results in differences in survival and behavior that are directly dependent on that water or food loss during the selection process.
Sleep provides an adaptive response to prolonged starvation
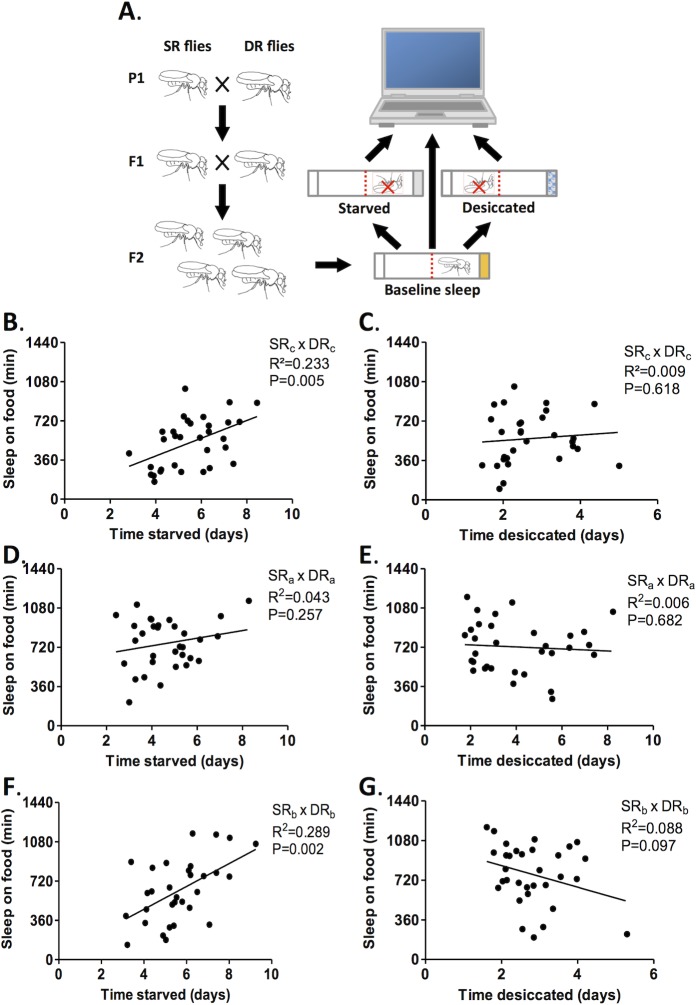
The enhanced sleep duration of SR flies raises the possibility that extended sleep is advantageous under conditions of chronic starvation, but not desiccation. To directly test this hypothesis we generated F2 hybrids between SR and DR flies. Individual F2 flies were tested for sleep duration on food, then transferred to starvation or desiccation conditions, and the relationship between sleep on food and resistance to nutrient deprivation was determined (Fig 5A). A significant positive correlation between sleep and starvation resistance was observed between SRb-DRb and SRc-DRc populations, suggesting that prolonged sleep is adaptive in food-deprived conditions (Fig 5B and 5F). However, there was no correlation in SRa-DRa hybrids, raising the possibility that the advantageous effects of sleep in response to starvation are more dependent on genetic background and evolutionary history (Fig 5D). No relationship was observed between sleep and desiccation resistance for any of the pairings tested (Fig 5C, 5E and 5G). Therefore, prolonged sleep appears to explain up to 30% of the resistance to starvation. Taken together with the prolonged sleep of SR flies, these findings support the notion that adaptations in response to starvation and desiccation conditions result in distinct behavioral and physiological alterations.
Fig 5. Sleep enhances starvation resistance in SR-DR hybrid flies.
A) Schematic of behavioral analysis. F2 progeny were generated by crossing SR and DR parental lines. Individuals were then tested for sleep on food over 24hrs, followed by longevity under starvation or desiccation conditions. B) Correlation analysis for SRc-DRc F2 hybrids reveals a correlation between sleep duration on food and starvation resistance (N = 32 for starved; P<0.01; R2 = 0.482). C) No correlation is observed between desiccation resistance and sleep duration on food in SRc-DRc F2 hybrid flies (N = 30 for desiccated; P>0.05; R2 = 0.01). D, E) No correlation between sleep duration and longevity was observed in flies from SRa-DRa crosses for either starvation or desiccation (N = 32; P>0.05; R2 = 0.043; N = 32; P>0.05; R2 = 0.006). F, G) Sleep duration was correlated with starvation resistance in SRb-DRb F2 hybrid flies (N = 31; P<0.05; R2 = 0.289), while no correlation was observed between sleep duration and desiccation resistance (N = 32; P>0.05; R2 = 0.068).
Discussion
Adapting to changes in water and food availability is a central challenge for survival. Animals have developed diverse physiological and behavioral traits to cope with both chronic and acute nutrient shortage [30]. Three primary methods for survival in the absence of nutrients are conservation of energy, elevated accumulation of energy stores, and increased tolerance to energy depletion [31]. Previous studies have indicated developmental, behavioral, and metabolic changes that are associated with starvation resistance [17,24,32]. Desiccation resistance has been linked to increased glycogen stores and changes in cuticular hydrocarbons that prevent water loss [33]. Total carbohydrate content and an increase in hemolymph volume has also been associated with desiccation resistance, suggesting that diverse physiological changes underlie the evolution of this process [16,23,32,34]. Further, a previous study indicated that exposure to chronic stressors, including mechanical and light stimulation, result in a reduction of triglyceride and glycogen stores, raising the possibility that energy stores are generally protective against environmental stressors [35]. Therefore, adaptation to desiccation or starvation conditions likely involves complex interactions between developmental, physiological, and behavioral traits.
In this study, we examine the role of energy conservation through changes in sleep, physiology, and activity in starvation and desiccation resistance. We have utilized experimental evolution to directly investigate the effects of selection for starvation and desiccation resistance on metabolic function and behavior. We identify differences in metabolic function, sleep, and activity response to nutrient deprivation between flies selected for starvation and desiccation resistance, suggesting specialized adaptations to SR or DR conditions. Previous studies examining DR selected flies have identified a generalized resistance to stressors, including chemical stress, heat stress, and radiation stress [16,17]. Our findings suggest that the B and C groups of DR flies are less susceptible to starvation, while starvation resistance does not differ in DRa flies when compared to their controls. None of the DR groups gained resistance to heat stress. These findings highlight how multiple mechanisms likely underlie the evolution of DR, with at least some of these mechanisms being conducive to starvation resistance.
A comparison of energy stores between starvation and desiccation resistant fly lines reveals significant differences between two of the three groups, suggesting distinct survival-promoting mechanisms in response to starvation and desiccation conditions. Selection for starvation resistance increases triglyceride levels in all three groups, while selection for desiccation resistance results in increased glycogen stores in all three groups. Triglycerides are richer in energy compared to glycogen, and mutants with enhanced triglyceride stores have increased SR, suggesting that these promote long-term survival in the absence of food [36]. Glycogen appears to be involved in water binding, allowing animals to increase water weight, making it the more suitable energy store under desiccation conditions [37]. Interestingly, SRa flies had increases in both glycogen and triglyceride levels and were resistant to both starvation and desiccation, fortifying the notion that triglyceride stores promote starvation resistance, while glycogen stores promote desiccation resistance. A number of previous studies have linked starvation and desiccation to enhanced lipid and glycogen levels. However, another study found no relationship between lipid levels and starvation resistance, raising the notion that multiple mechanisms are available for inducing starvation resistance [16,38].
Behavioral changes, including reduced movement and increased sleep duration, may conserve energy and prolong survival during nutrient deprivation. We had previously shown that the SR flies sleep longer than controls, but it was not clear whether this phenotype was a specific response to starvation or a more general response to stress [24]. Two lines of evidence suggest that prolonged sleep is not a generalized adaptation to stress. First, no increase in sleep is observed in desiccation resistant flies, suggesting functional differences between starvation and desiccation resistance. Second, neither selection for desiccation nor starvation resistance affects survival in response to high-heat stress. The findings that SR flies have higher triglyceride stores raise the possibility that triglycerides (fat storage) modulate sleep. However, we previously rescued the body size and triglyceride levels of SR flies by removing larvae from food prior to pupation and found no effect on sleep [24]. Further, we report increased body size of DR flies that sleep normally compared to controls, suggesting that the enhanced sleep in SR flies is independent of energy stores or body size. Therefore, the sleep increase in SR flies is likely due to changes in genetic factors that regulate behavior.
Nutrient availability during development potently affects adult behavior and physiology [39]. It is therefore likely that selection for resistance to nutrient deprivation during the larval or adult state have effects throughout the animal’s life cycle. In this study, both starvation resistant and desiccation resistant larvae were raised on standard fly food. However, selection for resistance to poor food quality during larval development results in reduced larval foraging activity that is influenced by polymorphisms in the foraging locus [40]. Further, larvae raised in nutrient poor conditions display many phenotypes associated with reduced food quality, including reduced adult size and prolonged development [40]. Therefore, selection for resistance to nutrient deprivation at the adult or larval stage appears to reduce foraging, although it is unclear whether shared genetic mechanism are involved in these processes. The increased body size of SR flies is, at least partially, due to prolonged larval development, raising the possibility that the starvation and desiccation phenotypes observed may be affected by nutrient availability in the larval stage (Reynolds and Gibbs, Personal Communication). The robust difference of both SR and DR populations to nutrient deprivation may provide a model for investigating the contributions of larval development to these processes.
Multiple studies link total activity to water loss due to increased respiration [33,41], raising the possibility that reduced activity promotes desiccation resistance through decreased respiration. It has been previously reported that flies selected for starvation and desiccation resistance have reduced activity that is uncoupled from respiration, suggesting these two are separable [17,42]. Our findings show reduced activity in SR and DR groups. Reduced waking activity was observed in all three groups of SR selected flies compared to fed controls. All three DR groups displayed significantly reduced activity, or at least trended towards this phenotype, compared to non-selected FDR control flies. Therefore, independent mechanisms appear to have evolved to reduce total activity, whereby selection for SR results in reduced sleep and waking activity, while selection for DR results in reduced waking activity without affecting sleep. While the reasons for this are unclear, we speculate that there is a greater pressure for reduced activity in SR flies, resulting in multiple adaptive strategies, including increased sleep.
It has previously been proposed that sleep or prolonged immobility allows for energy conservation in the absence of food. For example, many animals enhance their sleep or hibernate during winter periods when food is scarce [12]. We generated F2 hybrids from SR and DR flies to directly test the assertion that increased sleep is linked to starvation resistance. We found that sleep on food is correlated with starvation resistance for two of the three hybrid groups tested, while there was no correlation between sleep duration and desiccation resistance. Therefore, these findings provide evidence that sleep represents an adaptive behavior that enhances survival in the absence of food, but not in the absence of water.
In conclusion, we have used experimental evolution to examine the effects of desiccation and starvation selection on metabolism and behavior. Flies selected for desiccation or starvation resistance show differences in energy stores, behavioral response to nutrient deprivation, and sleep duration. Sleep duration is enhanced in flies selected for starvation resistance, but no differences are observed in desiccation resistant flies. Longevity under starvation conditions is linked to sleep, supporting the notion that prolonged sleep represents an adaptive evolutionary response to long-term starvation. Therefore, these findings reveal an evolutionary capacity for outbred flies to adapt to distinct forms of nutrient stress, and establish starvation resistant flies as a model for understanding the evolutionary relationship between sleep and survival under nutrient poor conditions.
Supporting Information
A, B) Triglyceride levels in A and B group flies. Triglyceride levels were elevated in SRa and SRb flies compared to FSR controls. No differences in triglyceride levels were observed between DRa and DRb flies and FDR controls (N = 10 and P<0.0001 for all A groups; N = 20 and P<0.01 for FSRb and SRb; N = 10 and P>0.05 for FDRb and DRb). C, D) Glycogen levels were increased in both SRa and DRa flies compared to respective controls. No differences in glycogen levels were apparent in SRb flies compared to FSRb controls, while glycogen levels were increased in DRb flies compared to FDRb controls (N = 20 for FSRa, SFa, and DRa groups; N = 18 for FDRa; P<0.001 for FSRa and SRa; P = 0.002 for FDRa and DRa; N = 10 for FSRb; N = 7 for SRb; N = 9 for FDRb and DRb; P>0.05 for FSRb and SRb; P<0.001 for FDRb and DRb). E, F) Slight to no differences in free glucose were observed between the lines tested (N = 20 for FSRa, SFa, and DRa groups; N = 18 for FDRa; P<0.05 for all A groups; N = 10 for FSRb; N = 7 for SRb; N = 9 for FDRb and DRb; P>0.05 for all B groups).
(PDF)
Survival of flies placed in activity monitors under starvation, desiccation, and heat stress conditions. A, B) Flies from the SRa and SRb groups survived longer than DR counterparts and both controls under starvation conditions. No differences were observed between DRa flies and controls, while DRb flies survived longer than controls under starvation conditions (N = 16 for all A groups and SRb, FDRb, and DRb; N = 14 for FSRb; P<0.001 for FSRa and SRa, FSRb and SRb, FDRb and DRb; P>0.05 for FDRa and DRa). C, D) SRa and DRa flies survive longer than controls under desiccation conditions. DRb flies survived longer than SRb flies and both controls under desiccation conditions (N = 32 for FSRa, SRa, FDRa, and DRb; N = 31 for DRa and SRb; N = 30 for FSRb; N = 29 for FDRb; P<0.001 for both FDR vs. DR groups; P<0.01 for FSRa vs. SRa; P<0.05 for FSRb vs. SRb). E, F) No differences were observed between SR and DR flies under thermal stress conditions (N = 32 for all groups; P>0.05 for all groups).
(PDF)
A, B) The total sleep duration over 24hrs on food is significantly longer in SRa and SRb flies compared to FSRa controls. No differences were observed between DRa and DRb flies and control lines (N = 64 for all groups; P<0.0001 for all groups). C, D) Sleep profiles depicting hourly sleep reveal sleep in SRa and SRb flies is increased during both day and night periods compared to the DR groups and both controls (N = 64 for all groups; P<0.001 for both FSR vs. SR groups; P>0.05 for both FDR vs. DR groups). E, F) Waking activity is reduced in DR flies, but not in SR flies, when compared to controls (N = 64 for all groups; P>0.05 for both FSR vs. SR groups; P = 0<0.001 for both FDR vs. DR groups).
(PDF)
The number of replicates (N) and statistical values are presented for each figure within the main text. ‘NS’ denotes non-significant differences between experimental group and control. * denotes P<0.05, ** denotes P<0.01, *** denotes P<0.001.
(DOCX)
Data Availability
All relevant analyzed data are within the paper and its Supporting Information files. Original data files can be obtained by contacting ACK directly.
Funding Statement
This work was supported by NIH grants R15 NS080155 and R01 NS0825152 to ACK; R15-GM100395 to AG; and P20 RR-016464 supporting PM.
References
- 1. Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8: 269–300. 10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- 2.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011. pp. 194–207. 10.1016/j.cell.2011.07.004 [DOI] [PMC free article] [PubMed]
- 3. Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution (N Y). 2008;62: 1764–1776. 10.1111/j.1558-5646.2008.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18: R670–R679. 10.1016/j.cub.2008.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koh K, Evans JM, Hendricks JC, Sehgal A. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006;103: 13843–13847. 10.1073/pnas.0605903103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kayser M, Yue Z, Shegal A. A critical period of sleep for development of courtship circuitry and behavior in Drosophila.No Title. Science (80-). 2014;344: 269–74. 10.1126/science.1250553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167: 311–323. 10.1534/genetics.167.1.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDonald DM, Keene AC. The sleep-feeding conflict: Understanding behavioral integration through genetic analysis in Drosophila. Aging (Albany, NY Online). 2010;2: 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Capellini I, Nunn CL, McNamara P, Preston BT, Barton RA. Energetic constraints, not predation, influence the evolution of sleep patterning in mammals. Funct Ecol. 2008;22: 847–853. 10.1111/j.1365-2435.2008.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borbély AA. Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res. 1977;124: 457–471. 10.1016/0006-8993(77)90947-7 [DOI] [PubMed] [Google Scholar]
- 11. Keene AC, Duboué ER, McDonald DM, Dus M, Suh GSB, Waddell S, et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20: 1209–1215. 10.1016/j.cub.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berger RJ, Phillips NH. Energy-Conservation and Sleep. Behav Brain Res. 1995;69: 65–73. [DOI] [PubMed] [Google Scholar]
- 13. Erion R, DiAngelo JR, Crocker A, Sehgal A. Interaction between sleep and metabolism in Drosophila with altered octopamine signaling. J Biol Chem. 2012;287: 32406–32414. 10.1074/jbc.M112.360875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yurgel M, Masek P, DiAngelo JR, Keene A. Genetic dissection of sleep-metabolism interactions in the fruit fly. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014;epub ahead. [DOI] [PMC free article] [PubMed]
- 15. Marron MT, Markow TA, Kain KJ, Gibbs AG. Effects of starvation and desiccation on energy metabolism in desert and mesic Drosophila. J Insect Physiol. 2003;49: 261–270. 10.1016/S0022-1910(02)00287-1 [DOI] [PubMed] [Google Scholar]
- 16. Chippindale AK, Gibbs AG, Sheik M, Yee KJ, Djawdan M, Bradley TJ, et al. Resource acquisition and the evolution of stress resistance in Drosophila melanogaster. Evolution (N Y). 1998;52: 1342–1352. 10.2307/2411304 [DOI] [PubMed] [Google Scholar]
- 17. Harshman LG, Hoffmann AA, Clark AG. Selection for starvation resistance in Drosophila melanogaster: Physiological correlates, enzyme activities and multiple stress responses. J Evol Biol. 1999;12: 370–379. 10.1046/j.1420-9101.1999.00024.x [DOI] [Google Scholar]
- 18. Parkash R, Aggarwal DD. Trade-off of energy metabolites as well as body color phenotypes for starvation and desiccation resistance in montane populations of Drosophila melanogaster. Comp Biochem Physiol—A Mol Integr Physiol. 2012;161: 102–113. 10.1016/j.cbpa.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 19. Ballard JWO, Melvin RG, Simpson SJ. Starvation resistance is positively correlated with body lipid proportion in five wild caught Drosophila simulans populations. J Insect Physiol. 2008;54: 1371–1376. 10.1016/j.jinsphys.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 20. Vieira C, Pasyukova EG, Zeng ZB, Hackett JB, Lyman RF, Mackay TF. Genotype-environment interaction for quantitative trait loci affecting life span in Drosophila melanogaster. Genetics. 2000;154: 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harbison ST, Yamamoto AH, Fanara JJ, Norga KK, Mackay TFC. Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics. 2004;166: 1807–1823. 10.1534/genetics.166.4.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burke MK, Rose MR. Experimental evolution with Drosophila. Am J Physiol Regul Integr Comp Physiol. 2009;296: R1847–54. 10.1152/ajpregu.90551.2008 [DOI] [PubMed] [Google Scholar]
- 23. Gefen E, Marlon AJ, Gibbs AG. Selection for desiccation resistance in adult Drosophila melanogaster affects larval development and metabolite accumulation. J Exp Biol. 2006;209: 3293–3300. 10.1242/jeb.02397 [DOI] [PubMed] [Google Scholar]
- 24.Masek P, Reynolds L a, Bollinger WL, Moody C, Mehta A, Murakami K, et al. Altered regulation of sleep and feeding contribute to starvation resistance in Drosophila. J Exp Biol. 2014; 10.1242/jeb.103309 [DOI] [PMC free article] [PubMed]
- 25. Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25: 129–138. 10.1016/S0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- 26. Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287: 1834–1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- 27. Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Processing sleep data created with the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc. 2010;2010: pdb.prot5520. 10.1101/pdb.prot5520 [DOI] [PubMed] [Google Scholar]
- 28. Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441: 753–756. 10.1038/nature04739 [DOI] [PubMed] [Google Scholar]
- 29. Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Locomotor activity level monitoring using the Drosophila activity monitoring (DAM) system. Cold Spring Harb Protoc. 2010;5 10.1101/pdb.prot5518 [DOI] [PubMed] [Google Scholar]
- 30. Sibley RM. The life-history approach to physiological ecology. Funct Ecol. 1991;5: 184–191. 10.2307/2389256 [DOI] [Google Scholar]
- 31. Rion S, Kawecki TJ. Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol. 2007;20: 1655–1664. 10.1111/j.1420-9101.2007.01405.x [DOI] [PubMed] [Google Scholar]
- 32. Djawdan M, Sugiyama T. Metabolic aspects of the trade-off between fecundity and longevity in Drosophila melanogaster. Physiol …. 1996;69: 1176–1195. Available: http://roselab.bio.uci.edu/Publications/48 Djawdan Sugiyama Schlaeger Bradley Rose 1996.pdf [Google Scholar]
- 33. Gibbs AG, Fukuzato F, Matzkin LM. Evolution of water conservation mechanisms in Drosophila. J Exp Biol. 2003;206: 1183–1192. 10.1242/jeb.00233 [DOI] [PubMed] [Google Scholar]
- 34. Folk DG, Han C, Bradley TJ. Water acquisition and partitioning in Drosophila melanogaster: effects of selection for desiccation-resistance. J Exp Biol. 2001;204: 3323–3331. [DOI] [PubMed] [Google Scholar]
- 35. Harbison ST, Sehgal A. Energy stores are not altered by long-term partial sleep deprivation in Drosophila melanogaster. PLoS One. 2009;4 10.1371/journal.pone.0006211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1: 323–330. 10.1016/j.cmet.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 37. Archer MA, Bradley TJ, Mueller LD, Rose MR. Using Experimental Evolution to Study the Physiological Mechanisms of Desiccation Resistance in Drosophila melanogaster. Physiol Biochem Zool. 2007;80: 386–98. 10.1086/518354 [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann AA, Hallas R, Anderson AR, Telonis-Scott M. Evidence for a robust sex-specific trade-off between cold resistance and starvation resistance in Drosophila melanogaster. Journal of Evolutionary Biology. 2005. pp. 804–810. 10.1111/j.1420-9101.2004.00871.x [DOI] [PubMed] [Google Scholar]
- 39. Tu M-P, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2: 327–333. 10.1046/j.1474-9728.2003.00064.x [DOI] [PubMed] [Google Scholar]
- 40.Vijendravarma RK, Narasimha S, Kawecki TJ. Evolution of foraging behaviour in response to chronic malnutrition in Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences. 2012. pp. 3540–3546. 10.1098/rspb.2012.0966 [DOI] [PMC free article] [PubMed]
- 41. Lehmann FO, Dickinson MH, Staunton J. The scaling of carbon dioxide release and respiratory water loss in flying fruit flies (Drosophila spp.). J Exp Biol. 2000;203: 1613–1624. [DOI] [PubMed] [Google Scholar]
- 42. Williams A, Rose M, Bradley T. The respiratory pattern in Drosophila melanogaster selected for desiccation resistance is not associated with the observed evolution of decreased locomotory activity. Physiol Biochem Zool. 2004;77: 10–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, B) Triglyceride levels in A and B group flies. Triglyceride levels were elevated in SRa and SRb flies compared to FSR controls. No differences in triglyceride levels were observed between DRa and DRb flies and FDR controls (N = 10 and P<0.0001 for all A groups; N = 20 and P<0.01 for FSRb and SRb; N = 10 and P>0.05 for FDRb and DRb). C, D) Glycogen levels were increased in both SRa and DRa flies compared to respective controls. No differences in glycogen levels were apparent in SRb flies compared to FSRb controls, while glycogen levels were increased in DRb flies compared to FDRb controls (N = 20 for FSRa, SFa, and DRa groups; N = 18 for FDRa; P<0.001 for FSRa and SRa; P = 0.002 for FDRa and DRa; N = 10 for FSRb; N = 7 for SRb; N = 9 for FDRb and DRb; P>0.05 for FSRb and SRb; P<0.001 for FDRb and DRb). E, F) Slight to no differences in free glucose were observed between the lines tested (N = 20 for FSRa, SFa, and DRa groups; N = 18 for FDRa; P<0.05 for all A groups; N = 10 for FSRb; N = 7 for SRb; N = 9 for FDRb and DRb; P>0.05 for all B groups).
(PDF)
Survival of flies placed in activity monitors under starvation, desiccation, and heat stress conditions. A, B) Flies from the SRa and SRb groups survived longer than DR counterparts and both controls under starvation conditions. No differences were observed between DRa flies and controls, while DRb flies survived longer than controls under starvation conditions (N = 16 for all A groups and SRb, FDRb, and DRb; N = 14 for FSRb; P<0.001 for FSRa and SRa, FSRb and SRb, FDRb and DRb; P>0.05 for FDRa and DRa). C, D) SRa and DRa flies survive longer than controls under desiccation conditions. DRb flies survived longer than SRb flies and both controls under desiccation conditions (N = 32 for FSRa, SRa, FDRa, and DRb; N = 31 for DRa and SRb; N = 30 for FSRb; N = 29 for FDRb; P<0.001 for both FDR vs. DR groups; P<0.01 for FSRa vs. SRa; P<0.05 for FSRb vs. SRb). E, F) No differences were observed between SR and DR flies under thermal stress conditions (N = 32 for all groups; P>0.05 for all groups).
(PDF)
A, B) The total sleep duration over 24hrs on food is significantly longer in SRa and SRb flies compared to FSRa controls. No differences were observed between DRa and DRb flies and control lines (N = 64 for all groups; P<0.0001 for all groups). C, D) Sleep profiles depicting hourly sleep reveal sleep in SRa and SRb flies is increased during both day and night periods compared to the DR groups and both controls (N = 64 for all groups; P<0.001 for both FSR vs. SR groups; P>0.05 for both FDR vs. DR groups). E, F) Waking activity is reduced in DR flies, but not in SR flies, when compared to controls (N = 64 for all groups; P>0.05 for both FSR vs. SR groups; P = 0<0.001 for both FDR vs. DR groups).
(PDF)
The number of replicates (N) and statistical values are presented for each figure within the main text. ‘NS’ denotes non-significant differences between experimental group and control. * denotes P<0.05, ** denotes P<0.01, *** denotes P<0.001.
(DOCX)
Data Availability Statement
All relevant analyzed data are within the paper and its Supporting Information files. Original data files can be obtained by contacting ACK directly.