Abstract
The advent of highly active antiretroviral therapy (HAART) has transformed human immunodeficiency virus (HIV)/AIDS into a manageable chronic disorder. Clinical care, however, needs to address the metabolic, anthropometric, and cardiovascular changes associated with HIV infection and HAART. Studies in developing countries suggest an increasing incidence of HIV-associated cardiometabolic syndrome (CMS), especially in urban settings. Predictions indicate that the greatest increase in the prevalence of diabetes will occur in Africa over the next 2 decades due to lifestyle changes. This, coupled with increased access to HAART, may exponentially increase the prevalence of CMS in developing countries, where HIV infection is prevalent. Appropriate evaluation and intervention programs need to be implemented in the developing world, especially sub-Saharan Africa, to curtail HIV-related CMS. This should include routine cardiovascular risk assessments, management of HIV infection with more “metabolically friendly” HAART, and encouragment of lifestyle modifications, particularly smoking cessation, weight management, regular exercise, and adherence to a healthy diet.
A well-known African aphorism states, “During the course of drumming and dancing the rhythm of the leading drum causes the steps of the dancers to change.” This aphorism embodies the dramatic change in the course of the global human immunodeficiency virus (HIV) epidemic with the introduction of highly active antiretroviral therapy (HAART) leading to dramatic improvements in morbidity and mortality rates in Western countries.1 HAART has improved immunologic and virologic outcomes,2 as well as the quality of life of HIV-positive (HIV+) patients.3 HAART regimens, however, particularly protease inhibitors (PIs), have been implicated in the development of metabolic complications, such as impaired glucose tolerance, dyslipidemia, abdominal obesity, and hypertension4 and HIV infection is associated with exercise intolerance,5 all conditions associated with an elevated risk for cardiovascular disease (CVD) in the general population.6 Whether this risk in HIV+ individuals is similar to or greater than the general population is now a high-priority research area.
The prevalence of HIV is highest in developing countries, particularly sub-Saharan Africa (Table I).7 The negative social, economic, and health implications of rising HIV prevalence rates are fully understood. Although access to HAART is increasing in developing countries, barriers to the development of programs for improving access to HAART in developing countries from underresourced health infrastructures exist.8 Further, such programs need to address the potential cardiometabolic consequences of HIV, HAART, and lifestyle factors. If not, the absence of these programs in accessible standard HIV-related care in developing countries may ultimately result in an epidemic of cardiometabolic complications.9 In developing countries, however, effective initiatives will require data on the prevalence of HIV-associated cardiometabolic syndrome (CMS) and its effects on drug adherence, morbidity, and mortality. Current data are limited. This review discusses HIV treatment, CMS management strategies, and the role of clinicians in the developing world where HIV and CMS are emerging epidemics.
Table I.
Regional Statistics for Global Prevalence of the Human Immunodeficiency Virus
| No. of Persons With HIV | No. of New Infections, 2006 | No. of AIDS Deaths, 2006 | Adult Prevalence, % | |
|---|---|---|---|---|
| Sub-Saharan Africa | 24.7 million | 2.8 million | 2.1 million | 5.9 |
| South and South East Asia | 7.8 million | 860,000 | 590,000 | 0.6 |
| East Asia | 750,000 | 100,000 | 43,000 | 0.1 |
| Latin America | 1.7 million | 140,000 | 65,000 | 0.5 |
| North America | 1.4 million | 43,000 | 18,000 | 0.8 |
| Western and Central Europe | 740,000 | 22,000 | 12,000 | 0.3 |
| Eastern Europe and Central Asia | 1.7 million | 270,000 | 84,000 | 0.9 |
| Middle East and North Africa | 460,000 | 68,000 | 36,000 | 0.2 |
| Caribbean | 250,000 | 27,000 | 19,000 | 1.2 |
| Oceania | 81,000 | 7100 | 4000 | 0.4 |
| Total | 39.5 million | 4.3 million | 2.9 million | – |
Adapted from the Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization.
HIV Treatment in the Developing World
In the past, access to HAART has been limited in low- and middle-income countries of Africa, Asia, and South America, where 90% of persons with HIV infection live.10 Gradually, however, HAART has become more available due to price reductions for propriety drugs, increasing availability of generic formulations, and the launch of various initiatives by international agencies. Thus, the World Health Organization’s (WHO’s) “3 by 5” program; The Global Fund to Fight AIDS, Tuberculosis, and Malaria; and the United States President’s Emergency Plan for AIDS Relief (PEPFAR) have promoted access of HAART to HIV populations in the Third World.11 In particular, generic, fixed-dose HAART regimens, recommended by the WHO for use in resource-limited settings,7 have substantially improved the life expectancy and prognosis of HIV+ patients.12 Furthermore, in low- and middle-income countries, coverage for access to HAART has improved from 7%–23% to 24%–75% in some regions.7
Obviously, increased access to HAART in sub-Saharan Africa is essential to combat the HIV epidemic. HIV and HAART, however, may lead to metabolic complications in a region where the prevalence of non–HIV-related type 2 diabetes is already predicted to increase 161% from 2000 to 2030 (Table II).13 This is likely a result of increased urbanization among developing countries and undesirable lifestyle changes.14 Thus, it is possible that Africa will confront 2 separate epidemics, each leading to an increase in the prevalence of CMS. Recent data imply that CVD may be increasing within African populations: from 1975 to 1980 only 59 persons with acute cardiac events were admitted to the coronary care unit at the Chris Hani-Baragwanath Hospital in Soweto, South Africa, while in 2004, 154 persons were admitted.15 Similar upward trends in the prevalence of CVD have also been noted in other sub-Saharan African countries.16
Table II.
Estimated Changes in Number of Persons With Type 2 Diabetes From Year 2000 to 2030
| Region | 2000 | 2030 | % Increase |
|---|---|---|---|
| Middle East | 20 | 53 | 163 |
| Sub-Saharan Africa | 7 | 19 | 161 |
| India | 32 | 79 | 151 |
| Asia and Islands | 22 | 58 | 148 |
| Latin America | 13 | 33 | 148 |
| China | 21 | 42 | 104 |
| Established Economies | 44 | 68 | 54 |
| East Europe | 12 | 14 | 20 |
| Total | 171 | 366 | 114 |
Values are expressed in millions. Adapted from Wild et al.13
HIV-Associated Cardiometabolic Abnormalities
Infection with HIV and HAART treatment are associated with CMS.17,18 One characteristic of HIV-associated CMS is lipodystrophy or fat redistribution (peripheral fat loss and/or central adiposity), which occurs in about 18% to 83% of Western HAART-treated HIV+ patients.19 Variability in the reported prevalence is thought to be due to the lack of definitive diagnostic criteria and differences among the HAART regimens used. Unlike the genetic lipodystrophy syndromes,20 body fat redistribution observed in HIV-associated lipodystrophy may not precede the metabolic abnormalities. Thus, in a study conducted in Rwanda, fasting glucose levels were higher in HIV-infected individuals receiving HAART than in HIV-negative individuals, irrespective of whether they had HIV-related lipodystrophy.21 Moreover, an Italian study reported that the prevalence of impaired fasting glucose was the same in HIV+ patients receiving or not receiving HAART but was higher than that in HIV-negative patients.22 The prevalence of dyslipidemia in developed countries is lower in HIV-seronegative patients than in HAART-treated HIV+ patients and is highest in HAART-treated HIV+ patients with lipodystrophy.23 In Western countries, the prevalence of hypertension in HIV+ patients was estimated to be 20% to 25% before the introduction of HAART,24 and the prevalence may be as high as 74% in patients with HAART-associated CMS, especially in the presence of hyper-triglyceridemia and insulin resistance.25 Thus, metabolic risk factors for heart disease are more prevalent in Western patients receiving HAART, and the risk of CVD increases with each year of HAART exposure.26 Furthermore, when compared with HIV-negative patients, twice as many HAART-treated HIV+ patients have an estimated 10-year cardiovascular heart disease risk >20%,27 and CVD ranks among the leading causes of death in HIV+ patients in developed countries.28
Data on the prevalence of HIV metabolic and anthropometric complications from sub-Saharan African populations are limited. A cross-sectional study of 571 HIV-infected Rwandans receiving WHO-recommended first-line HAART regimens showed that the prevalence of lipodystrophy was 34%, and in patients taking HAART for longer than 18 months, it was 69.6%.21 The prevalence of lipodystrophy was higher in the urban (48.5%) than the rural (17.3%) population, indicating the potential role of nutrition and physical exercise in HIV-related CMS. Lipodystrophy in this population was characterized by both peripheral lipoatrophy and abdominal adiposity and was associated with elevated total cholesterol levels and a higher prevalence of metabolic syndrome when compared with HIV+ Rwandans without lipodystrophy and with healthy HIV-negative Rwandans. The HIV+ patients, regardless of the presence or absence of lipodystrophy, had higher fasting glucose levels and a higher prevalence of impaired fasting glucose (17.3% vs 2%) compared with the healthy controls.21 Hypercholesterolemia (fasting cholesterol level >5.0 mmol/L) and hyper-triglyceridemia (fasting triglyceride level >1.7 mmol/L) were not observed in either HIV+ nonlipodystrophic or HIV-negative patients but were present in 14% and 9%, respectively, of HIV+ patients with lipodystrophy.21 These low fasting lipid levels are not unexpected; previous reports have found lower lipid levels in African populations than in European populations.28,29 Thus, although CMS is more prevalent in African patients with HIV-associated lipodystrophy than those without lipodystrophy, it may be less atherogenic than observed in Europeans due to the lower lipid levels of the general African population. HIV-negative African patients, however, are more insulin resistant than body mass index–matched healthy white patients,30 and the increased prevalence of impaired fasting glucose in African HIV+ patients receiving HAART21 suggests that the diabetogenic effect of HAART must be closely investigated in future longitudinal studies.
Untreated HIV infection, which is associated with pericardial disease and cardiomyopathy,9 worsens as the infection progresses and affects cardiometabolic health in studies conducted in both developed31 and developing areas of the world.32 A recent Italian study demonstrated that the prevalence of the metabolic syndrome in HAART-naive HIV+ patients is 20.8% and is higher than in HIV-negative patients (15.8%) mainly due to lower high-density lipoprotein cholesterol levels and impaired fasting glucose.22 A study in Rwanda reports that 17.7% of HAART-naive HIV+ patients have HIV-associated dilated cardiomyopathy.33 Finally, untreated HIV infection is characterized by hypertriglyceridemia and low high-density lipoprotein and low-density lipoprotein cholesterol concentrations.34 Thus, the combined effects of HIV infection plus HAART on lipid and glucose metabolism may increase the prevalence of CVD, diabetes, and CMS observed in this population.
Management of CMS in HIV+ Patients
Management strategies for CMS from Westernized countries are usually targeted at the particular anthropometric and metabolic abnormalities. The management of lipodystrophy in patients receiving HAART usually involves switching from a thymidine analog–based HAART regimen (eg, stavudine) to a different nucleoside or non-nucleoside–based regimen.35 Strategies for managing glucose intolerance and insulin resistance have involved insulin sensitizers and secretagogues (metformin and thiazolidinediones).36 Initial treatment for fasting hyperinsulinemia, impaired glucose tolerance, and type 2 diabetes should include increased physical activity and dietary modification. Selection of PIs (especially indinavir) that are known to contribute to insulin resistance should be avoided if possible.36 Rosiglitazone improves insulin sensitivity and does not increase arm or leg fat content, but it does increase triglyceride and total cholesterol levels in HIV+ patients receiving HAART.37 Rosiglitazone should not be administered to patients with compromised heart function or heart failure (eg, New York Heart Association classification III–IV). Metformin reduces insulin levels, central adiposity, and diastolic blood pressure in HIV-infected patients taking HAART.19 These treatment strategies need to be further examined for safety and efficacy in persons living with HIV.
Plastic surgery offers good cosmetic results for facial lipoatrophy. Surgical interventions, however, including soft tissue augmentation such as poly-L-lactic acid use or injectable liquid silicone, are not sustainable in the developing world because they are expensive procedures that require considerable expertise. Serious complications such as edema, nodule formation, and cellulitis may also occur.38
Management of dyslipidemia in patients from developing countries who receive HAART, as in the general population, should follow the guidelines of the National Cholesterol Education Program until further research demonstrates otherwise.39 Although more data on potential interactions between fibrates and statins with PIs are needed,36 these drugs can reduce but rarely normalize triglyceride and cholesterol levels in HIV-infected persons.
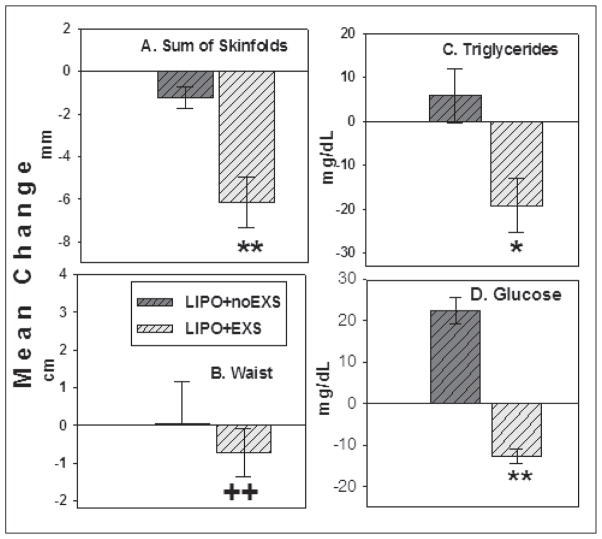
In the developing world, appropriate pharmacologic treatments for HIV-related metabolic and anthropomorphic complications have not been adequately investigated. In a study from Rwanda, HAART-treated HIV+ patients with lipodystrophy were randomized to a 6-month exercise training program or to no intervention.40 Exercise training reduced the waist-to-hip ratio by approximately 9%, fasting glucose by approximately 3%, and triglyceride levels, while in the control patients, waist-to-hip ratio increased 0.5%, fasting glucose levels increased 10%, and triglycerides were unchanged (Figure). In this study, exercise training reduced central adiposity and improved metabolic profiles in HIV-infected African patients treated with HAART. Because HIV lipodystrophy adversely affects quality of life in HIV+ African patients receiving HAART,41 effective treatments should improve physical and psychological well-being. Simpler behavioral and lifestyle interventions such as physical exercise and nutrition counseling may be more practical and appropriate in Africa where health care resources and pharmaceutical-based interventions are limited.
Figure.
Six-month changes in anthropometric and metabolic variables. *P<.05. **P<.0001. ++P=.001, vs lipodystrophy and no exercise (LIPO+noEXS) patients. Adapted from Mutimura et al.40
Conclusions
The substantial benefits of HAART in terms of decreased morbidity and mortality outweigh the increased risk of CVD and diabetes observed with the use of such treatments. It is important, however, for HIV treatment initiatives, particularly in sub-Saharan Africa, to include effective monitoring and management strategies for HIV- and HAART-associated cardiometabolic abnormalities. This is difficult in resource-poor countries, where access to HIV treatment is steadily improving, but minimal resources are available to manage evolving cardiometabolic complications. Therefore, government agencies and international partners need to support and improve the efforts of scientists and clinicians working in the field of HIV/AIDS to set up mechanisms to study and address HIV- and HAART-associated CMS. Furthermore, clinicians need to be made more aware of the metabolic disorders related to antiretroviral therapy and to treat them as modifiable CVD risk factors by promoting smoking cessation and increased physical activity in patients. Patients with elevated lipid and glucose levels should be advised to initiate treatment with glucose- or lipid-lowering agents if feasible, and the effect of their HAART regimen on these parameters should be evaluated. Finally, a patient-centered care approach that involves patients in therapeutic decisions and the monitoring of patients’ perceptions of the effects of HAART on body shape may optimize adherence to antiretroviral therapy and improve patients’ quality of life.
Footnotes
Disclosures: Dr Mutimura acknowledges the support of Kigali Health Institute and the Commission Nationale de Lutte Contre le SIDA and Multi-Sectorial AIDS Program, Rwanda. Professor Crowther acknowledges financial support from the National Health Laboratory Service, the South African Medical Research Council, the National Research Foundation, the South African Sugar Association, and the University of the Witwatersrand. Professor Stewart acknowledges support from the South African Medical Research Council and the University of the Witwatersrand. Professor Cade acknowledges support from National Institutes of Health grant No. KDK074343A.
References
- 1.Palella FJ, Delaney KM, Moorenman AC. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Hogg R, Heath K, Yip B. Improved survival among HIV-infected individuals following initiation therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Ostrow D, Detels R, et al. Impacts of HIV infection and HAART use on quality of life. Qual Life Res. 2006;15:941–949. doi: 10.1007/s11136-005-5913-x. [DOI] [PubMed] [Google Scholar]
- 4.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV–infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 5.Cade WT, Fantry LE, Nabar S, et al. Decreased peak arterio-venous oxygen difference during treadmill exercise testing in individuals infected with the human immunodeficiency virus. Arch Phys Med Rehabil. 2003;84:1595–1603. doi: 10.1053/s0003-9993(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 6.Assmann G, Guerra R, Fox G, et al. Harmonizing the definition of the Metabolic Syndrome: Comparison of the Criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States of American and European populations. Am J Cardiol. 2007;99:541–548. doi: 10.1016/j.amjcard.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/ AIDS (UNAIDS) [Accessed May 18, 2007];Report on the global AIDS epidemic. http://www.unaids.org/en/hiv_data/2006globalreport/defaut.asp.
- 8.Van Damme W, Kober K, Laga M. The real challenges for scaling up ART in sub-Saharan Africa. AIDS. 2006;20:653–656. doi: 10.1097/01.aids.0000216364.44409.b1. [DOI] [PubMed] [Google Scholar]
- 9.Venter WDF, Sanne IM. The cardiovascular consequences of HIV and antiretroviral therapy. Cardiovasc J S Afr. 2003;14:225–229. [PubMed] [Google Scholar]
- 10.Egger M, Dabis F, Schechter M, et al. Mortality of HIV-1-Infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 11.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 12.Porter K, Babiker A, Bhaskaran K, et al. Determinants of Survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 13.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 14.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Wilkinson D, Becker A, et al. Mapping the emergence of heart disease in a black, urban population in Africa: the Heart of Soweto Study. Int J Cardiol. 2006;108:101–108. doi: 10.1016/j.ijcard.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Akinboboye O, Idris O, Akinkugbe O. Trends in coronary artery disease and associated risk factors in sub-Saharan Africans. J Hum Hypertens. 2003;17:381–387. doi: 10.1038/sj.jhh.1001562. [DOI] [PubMed] [Google Scholar]
- 17.Jericó C, Knobel H, Montero M, et al. Metabolic syndrome among HIV-infected patients. Diabetes Care. 2005;28:144–149. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- 18.Bergersen BM, Schumacher A, Sandvik L, et al. Important differences in components of metabolic syndrome between HIV patients with and without highly antiretroviral therapy and health controls. Scand J Infect Dis. 2006;38:682–689. doi: 10.1080/00365540500361302. [DOI] [PubMed] [Google Scholar]
- 19.Grinspoon S, Carr A. Cardiovascular risk and body fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 20.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 21.Mutimura E, Stewart A, Rheeder P, et al. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active anti-retroviral therapy (HAART) J Acquir Immune Defic Syndr. 2007;46:451–455. doi: 10.1097/qai.0b013e318158c0a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonfanti P, Giannattasio C, Ricci E, et al. HIV and metabolic syndrome: a comparison with the general population. J Acquir Immune Defic Syndr. 2007;45:426–431. doi: 10.1097/QAI.0b013e318074ef83. [DOI] [PubMed] [Google Scholar]
- 23.Friis-Møller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy: results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 24.Aoun S, Ramos E. Hypertension in the HIV-infected patient. Curr Hypertens Rep. 2000;2:478–481. doi: 10.1007/s11906-000-0031-1. [DOI] [PubMed] [Google Scholar]
- 25.Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationship to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377–1382. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 26.Friis-Møller N, Sabin CA, Weber R, et al. Combination of antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 27.Bergersen BM, Sandvik L, Bruun JN, et al. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23:625–630. doi: 10.1007/s10096-004-1177-6. [DOI] [PubMed] [Google Scholar]
- 28.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 29.Adedeji OO. The plasma lipid concentrations of healthy Nigerians. Trop Geogr Med. 1994;46:23–26. [PubMed] [Google Scholar]
- 30.Ferris WF, Naran NH, Crowther NJ, et al. The relationship between insulin sensitivity and serum adiponectin levels in 3 population groups. Horm Metab Res. 2005;37:695–701. doi: 10.1055/s-2005-870580. [DOI] [PubMed] [Google Scholar]
- 31.Velasquez EM, Glancy DL. Cardiovascular disease in patients infected with human immunodeficiency virus. J La State Med Soc. 2003;155:314–324. [PubMed] [Google Scholar]
- 32.Longo-Mbenza B, Seghers LV, Vita EK, et al. Assessment of ventricular diastolic function in AIDS patients from Congo: a Doppler echocardiographic study. Heart. 1998;80:184–189. doi: 10.1136/hrt.80.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twagirumukiza M, Nkeramihigo E, Seminega B, et al. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res. 2007;5:129–137. doi: 10.2174/157016207779316288. [DOI] [PubMed] [Google Scholar]
- 34.Grunfeld C, Pang M, Doerrler W, et al. Lipid, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 35.Tebas P, Zhang J, Yarasheski K, et al. Switching to a protease inhibitor-containing, nucleoside-sparing regimen (lopinavir/ritonavir plus efavirenz) increases limb fat but raises serum lipid levels: results of a prospective randomized trial (AIDS clinical trial group 5125s) J Acquir Immune Defic Syndr. 2007;45:193–200. doi: 10.1097/QAI.0b013e318042e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capeau J. From lipodystrophy and insulin resistance to metabolic syndrome: HIV infection, treatment and aging. Curr Opin HIV AIDS. 2007;2:247–252. doi: 10.1097/COH.0b013e3281e66919. [DOI] [PubMed] [Google Scholar]
- 37.Carr A, Workman C, Carey D, et al. No effect of rosiglitazone for treatment of HIV-1 lipodystrophy: randomised, double-blind, placebo-controlled trial. Lancet. 2004;363:429–438. doi: 10.1016/S0140-6736(04)15489-5. [DOI] [PubMed] [Google Scholar]
- 38.Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 suppl):77S–84S. doi: 10.1097/01.prs.0000234919.25096.67. [DOI] [PubMed] [Google Scholar]
- 39.Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidaemia in human immunodeficiency virus (HIV) infected adults receiving antiretroviral therapy: recommendations of the HIV Medicine Association of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 40.Mutimura E, Stewart A, Cade WT, et al. Exercise training reduces central adiposity and improves metabolic indices in HAART-treated HIV-positive subjects in Rwanda: a randomized controlled trial. AIDS Res Hum Retroviruses. doi: 10.1089/aid.2007.0023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutimura E, Stewart A, Crowther NJ. Assessment of quality of life in HAART-treated HIV-positive subjects with body fat redistribution in Rwanda. AIDS Res Ther. 2007;4:19. doi: 10.1186/1742-6405-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]