Abstract
The cytomegalovirus (CMV) disease spectrum is very wide, with symptomatic infections being rare in immunocompetent hosts. We present the case of a 31-year-old immunocompetent man diagnosed with CMV gastritis in the context of primary infection. The most important laboratory abnormalities leading to diagnosis were: elevation of liver enzymes (3–4× the upper limit of normal), thrombocytopenia (133 G/L), lymphocytosis (55%–4.2 G/L) with activated lymphocytes, CMV IgM positive (negative IgG), CMV viral load of 5700 copies/mL (real-time PCR); autoimmunity study showed antiparietal cell antibodies; abdominal ultrasonography detected homogenous splenomegaly (14.6×13.4 cm) and endoscopy unveiled superficial erosions of the gastric antrum that were biopsied. Anatomopathology and immunohistochemistry of the samples identified cytomegalic inclusions in endothelial cells. Cellular and humoral immunity deficits were excluded. As the patient developed severe asthaenia, adynamia and epigastric pain, he was administered gancyclovir 5 mg/kg intravenously twice daily for 7 days, with resolution of symptoms and gastric lesions confirmed by re-evaluation through endoscopy.
Background
Cytomegalovirus (CMV) is the largest virus infecting humans.1 It is an enveloped virus, with icosahedral symmetry containing a large genome of double stranded DNA.2 As in other members of the herpes virus family, it can establish latent infection after acute infection resolution.1–3 Risk of reactivation is higher in immunocompromised patients (those under chemotherapy, transplant recipients, those on long term corticosteroid therapy, HIV-infected patients, the elderly), for whom CMV is recognised as an important pathogen.3 However, severe trauma, sepsis, shock, burns, cirrhosis and other critic conditions can reactivate CMV in non-immunocompromised patients.2 Although CMV infection4 in immunocompetent hosts is generally asymptomatic or presenting as a viral or mononucleosis-like syndrome, gastrointestinal, neurological, haematological, skin, cardiac or ocular CMV disease can also occur, mostly as reactivation.3 5 In these cases, it can cause severe organ damage, resulting in significant morbidity and mortality.3
We present a rare case of an immunocompetent patient with CMV gastritis (biopsy proven), as manifestation of an acute CMV infection.
Case presentation
In July 2011, a 31-year-old man presented to the emergency department with severe occipital headache, fever (39°C), dysesthaesia of the scalp and odynophagia, which had lasted for the past 3 days. He was observed by a neurologist and underwent a lumbar puncture (LP), which showed no abnormalities. He was then observed by an otorhinolaryngologist who diagnosed an acute tonsillitis, discharging him with a prescription of amoxicillin/clavulanate and an analgaesic.
He had a history of lymphocytic meningitis and had been diagnosed post-LP syndrome in February of the same year, but was taking no medication. He mentioned contact with sheep, goats, birds and probably rats, at a farm.
Three days later, he consulted an infectious diseases specialist. He had neither fever nor odynophagia, but was nauseous, vomiting, with severe headache, blurred vision, and phonophobia and photophobia, which were interpreted as a new post-LP syndrome. He was admitted to the infectious disease unit.
He remained symptomatic for 2–3 days and did not respond to the medication. An angio-CT of the cranium was performed, excluding vascular brain malformations.
On the 6th day of hospitalisation, he developed fever (38°C) without evident origin. Amoxicillin/clavulanate administration was interrupted and replaced by ceftriaxone 1 g q12 h and doxycycline 100 mg q12 h, which were administered for 8 days.
During the following 10 days, the patient remained febrile, with migraine-like headache, night sweating and shivers. No cough, dyspnoea, thoracic pain or gastrointestinal or urinary symptoms were observed.
The following aspects should be highlighted from the complementary study developed: mild thrombocytopenia (133 G/L), lymphocytosis (55%–4.2 G/L) with activated lymphocytes, 3–4× elevation of aspartate aminotransferase and alanine aminotransferase. Rickettsia conorii serology was positive with a titre of 1/640, CMV IgM positive 2.27 (negative <0.70—immunofluorescence assay), with negative IgG, HSV 1 e 2 IgM with only HSV 1 IgG positive. HIV, hepatitis C virus, hepatitis E virus, Coxiella, Leptospira, Borrelia, Toxoplasma, Brucella, syphilis, Mycoplasma, Chlamydia and Legionella serologies all came back negative. Immunity to hepatitis B virus and Epstein-Barr was confirmed. Mantoux test was anergic. Blood and urine cultures were negative. CMV culture in blood and urine (by shell-vial) was negative. HIV viral load was negative. CD4/CD8 inversion was present, with normal CD4 count. CMV viral load was detectable, 5700 copies/mL (real-time PCR) (result only known after discharge).
While some results were pending, the hypothesis of tumoural or autoimmune pathology was considered. Thus, anti-mitochondrial, antismooth muscle, antinuclear, antiparietal cells, antigliadin and antineutrophil cytoplasmic antibodies were searched for. Only antiparietal cell antibodies came back positive. This triggered a study of the intestinal tract. Abdominal ultrasonography showed homogenous splenomegaly (14.6×13.4 cm). Endoscopy showed shallow erosions of the gastric antrum, which were biopsied. The anatomopathological and immunohistochemistry study of the biopsied samples identified cytomegalic inclusions in endothelial cells of the lamina propria, which confirmed gastric CMV disease (figures 1 and 2). Cellular and humoral immunity deficits were searched for and excluded.
Figure 1.
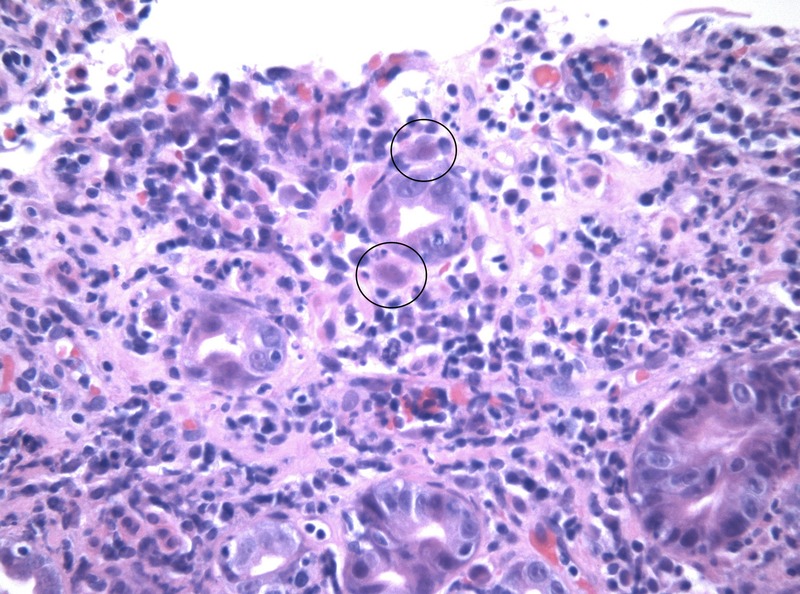
H&E stain showing cytomegalic inclusions (×400).
Figure 2.
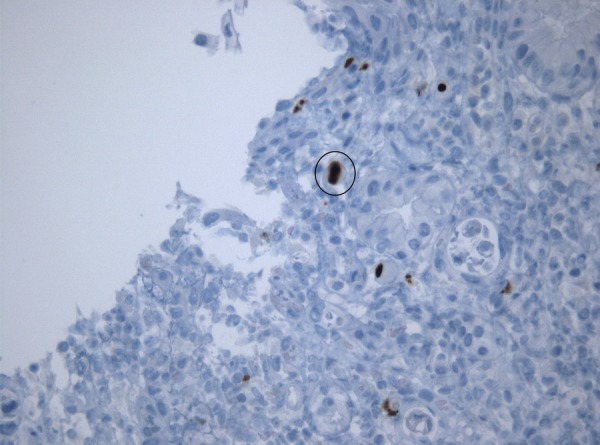
Immunohistochemical identification of cytomegalic inclusions (×400).
A positron emission tomography (PET)-scan was also performed, revealing hypermetabolic involvement of laterocervical and splenic ganglion.
The patient was discharged 3 days after the endoscopy, therefore the biopsy result was only known at the follow-up appointment, 2 weeks after discharge. At the same time, repeated CMV serologies showed seroconversion, with IgG of 51.4 UA/mL (negative <6 UA/mL), and IgM still positive with low avidity CMV IgG, which supported recent infection. In this consultation, the patient presented severe asthaenia, adynamia and epigastric pain, and was readmitted for treatment of cytomegalic gastritis in the context of primary CMV infection.
The patient completed 7 days of treatment with gancyclovir 5 mg/kg intravenously twice daily with good clinical evolution and resolution of gastric lesions confirmed by endoscopic evaluation.
Differential diagnosis
In the case presented, the initial symptoms of the patient were very acute. This, together with the recent episode of meningitis, led to the exclusion of a central nervous system infection, triggering a post-LP syndrome.
The first laboratory test results after initial admission presented non-specific alterations in blood cell count and liver enzymes, which did not help to clarify the diagnosis.
At first, the fact that this case was diagnosed during the summer (in July) and that the patient had rural activity, made us think of zoonosis as the cause of the complaints. Even though the high initial R. conorii serology titre could favour this diagnosis, the lack of response to treatment and the later found IgG kinetics excluded this hypothesis. Therefore, less frequent pathologies had to be sought out. As the patient remained feverish without evident cause, and was not responding to therapy, autoimmune and tumoural hypotheses were considered. After the results of the autoimmunity study, further evaluation was carried out aimed at the digestive tract, both by ultrasonography and endoscopy, which revealed the aforementioned erosions. The common association of CMV infection with any kind of immunocompromise led to the exclusion of acute HIV infection as well as of humoral or cellular immunity deficits. The biopsy result and CMV viral load were only known after patient discharge, which caused further delay in the treatment and led to a PET-scan, with the hope of finding a primary location of a tumour.
Outcome and follow-up
One month later, at an outpatient visit, the patient was asymptomatic, with normalised liver function. Rickettsia serology and HSV 1 and 2 IgM were both negative (probable cross-reaction in the first test). He had restarted his professional activity and had no limitations.
During the following 6 months, he attended consultations and was continuously asymptomatic. CMV serologies showed a five-fold IgG increase and steady IgM decrease.
Discussion
CMV infection in immunocompetent patients is usually asymptomatic or, if symptomatic, it most frequently causes a mononucleosis-like syndrome.2 3 Invasive disease is rare in these patients, rarely documented, and usually caused by reactivation of CMV infection. Invasive disease as primary CMV infection is rare, with few cases described.6
Recent data show that life-threatening complications of CMV infection in immunocompetent hosts may not be as rare as previously thought. Severe trauma, sepsis, shock, burns, cirrhosis and other critic conditions can play a role in reactivating CMV in non-immunocompromised patients, which in turn extends length of hospital stay and the probability of nosocomial infections.2 The most common site of infection is the gastrointestinal tract, followed by the central nervous system and haematological manifestations. Less frequent sites are the eye, liver and lung.5
Gastrointestinal lesions caused by CMV are not specific and range from patchy erythaema, exudates and microerosions to diffusely oedematous mucosa, multiple mucosal erosions, deep ulcers and pseudotumours.4 7 CMV gastric ulcers are difficult to differentiate from Helicobacter pylori and non-steroidal anti-inflammatory drug-related ulcers.8 Diagnosis of a CMV disease needs clinical findings suggestive of organ involvement, together with demonstration of CMV by viral isolation (culture, detection of CMV proteins or CMV DNA amplification), histopathological testing, immunohistochemical analysis or in situ hybridisation of a clinical sample from the site of involvement.2 CMV serology may help distinguish primary infection (seronegative patients) from CMV reactivation (seropositive patients).4
The pathway followed by our diagnosis has already been mentioned in the case presentation and differential diagnosis.
As it is mostly a self-limited disease, antiviral treatment is usually not recommended in immunocompetent CMV infection,2 3 except in cases of meningoencephalitis, ocular or lung involvement.5 In our case, as the patient was very symptomatic in the follow-up consultation, we opted for treatment. The response was positive, with full recovery.
The common positive outcome of treated patients may also be due to the self-limiting nature of the disease.2 5 Further studies are needed to clarify the beneficial role of treatment in immunocompetent patients.
Learning points.
Cytomegalovirus (CMV) disease in immunocompetent patients is generally asymptomatic or may present as a viral or mononucleosis-like syndrome.
Severe life threatening complications of CMV infection in immunocompetent patients may not be as rare as previously thought.
In these patients, CMV can affect any organ, with the gastrointestinal tract being the most common site of infection, followed by the central nervous system and haematological manifestations. Less frequent sites are the eye, liver and lung.
As CMV gastrointestinal lesions are not specific, the diagnosis of a CMV disease needs clinical findings suggestive of organ involvement, together with demonstration of CMV by viral isolation, histopathological testing, immunohistochemical analysis or in situ hybridisation.
Treatment of CMV disease in immunocompetent hosts is not consensual, but it is recommended in meningoencephalitis, ocular or lung involvement.
Acknowledgments
The authors thank To Dra Conceição Ventura, Professor Doutor Vitor Duque who also cared for the patient and Dr Joaquim Oliveira, who helped drafting and revising the manuscript.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Cumpacker CS II, Zhang JL. Cytomegalovirus. In: Mandell GL, Bennett JE, Dolin R eds. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 7th edn Philadelphia: Churchill Livingstone Elsevier, 2010;Chapter 138:1971–87. [Google Scholar]
- 2.Jain M, Duggal S, Chugh TD. Cytomegalic infection in non-immunosuppressed critically ill patients. J Infect Dev Ctries 2011;5:571–9. [DOI] [PubMed] [Google Scholar]
- 3.Friel TJ. Epidemiology, clinical manifestations, and treatment of cytomegalovirus in immunocompetent hosts [Internet]. 2014. [updated 13 May 2014; cited 17 Jul 2014]. http://www.uptodate.com
- 4.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002;34:1094–7. 10.1086/339329 [DOI] [PubMed] [Google Scholar]
- 5.Rafailidis PI, Mourtzoukou EG, Varbobitis IC et al. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J 2008;5:47 10.1186/1743-422X-5-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bencharif L, Cathébras P, Bouchou K et al. Exudative gastroenteropathy revealing primary CMV infection in an immunocompetent adult. Rev Med Interne 1998;19:288–90. 10.1016/S0248-8663(97)89335-6 [DOI] [PubMed] [Google Scholar]
- 7.Maiorana A, Baccarini P, Foroni M et al. Human cytomegalovirus infection of the gastrointestinal tract in apparently immunocompetent patients. Hum Pathol 2003;34:1331–6. 10.1016/j.humpath.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Ebisutani C, Kawamura A, Shibata N et al. Gastric ulcer associated with cytomegalovirus in an immunocompetent patient: method for diagnosis. Case Rep Gastroenterol 2012;6:365–8. 10.1159/000339714 [DOI] [PMC free article] [PubMed] [Google Scholar]


