Abstract
A 44-year-old man presented to hospital 24 h after an intentional overdose of metformin and gliclazide. He had a critical metabolic acidosis on presentation with a pH of 6.88, and very rapidly deteriorated into distributive shock refractory to large volume fluid resuscitation and massive doses of vasopressors. We introduced a methylene blue infusion as a rescue therapy in an attempt to improve the patient's haemodynamics, which was successful. The patient made a full recovery with no long-term sequelae.
Background
In presenting this case, we hope to highlight the potential use of methylene blue in cases of severe distributive shock refractory to conventional therapies. With the increasing incidence of type 2 diabetes mellitus in first world countries, and metformin as a common first-line treatment, presentations of severe metformin-induced lactic acidosis and refractory distributive shock may increase. We present a case demonstrating the near-fatal consequences that can arise from metformin overdose and discuss methylene blue as a potential adjunct in the management of refractory distributive shock. Methylene blue has been well described in cases of vasoplegic shock in cardiac surgery patients post-cardiopulmonary bypass,1 and in sepsis,2–5 but documentation of its use in a wider population of patients with distributive shock from other causes is limited. We report this case to highlight the use of methylene blue for severe distributive shock when other conventional methods have failed.
Case presentation/investigations/treatment
The patient is a 44-year-old Caucasian man with type 2 diabetes mellitus, managed on metformin (extended-release) 1 g two times per day, gliclazide (modified release) 60 mg once daily and exenatide 10 µg two times per day. His history includes a fundoplication procedure for gastroesophageal reflux disease.
The patient trialled desvenlafaxine, prescribed by his general practitioner 6 months prior to presentation, for depressive symptoms. After 6 weeks, he self-ceased this medication feeling that his symptoms (‘anger issues’) were unchanged. There is no other mental health history. He is a non-smoker and lives with his wife, 16-year-old daughter and 13-year-old son. For the preceding 6 months, he was working as a supermarket forklift driver.
Three days prior to presentation, the patient was seen in his diabetic outpatient clinic and was well. One day prior to presentation, he was arrested for driving with a suspended license. His license suspension was increased from 6 months to 2 years and he was likely to receive a large fine and potentially lose income. Later that night, he ingested all of his gliclazide and metformin medication, thought to be approximately 35 tablets of each and equal to 2.1 and 35 g, respectively.
The patient vomited the following morning; however, he was largely asymptomatic until the following evening, 20 h following the ingestion. At this time he developed severe abdominal pain and disclosed the overdose to his wife. On arrival in the emergency department he was normotensive with a Glasgow Coma Scale of 15, however, he was hypoglycaemic with a blood sugar level of 2.1 mmol/L and his initial venous blood gas revealed a pH of 6.88, a bicarbonate level of 4 mmol/L, lactate of 29 mmol/L, and a high anion gap of 36 mmol/L. He had partial respiratory compensation with a PaCO2 of 23 mm Hg.
The patient was urgently transferred to the intensive care unit (ICU). His rapidly worsening encephalopathy necessitated intubation and ventilation to allow institution of necessary management strategies, the most urgent being renal replacement therapy. Blood tests showed an acute kidney injury with creatinine of 326 µmol/L and urea 9.3 mmol/L. Paracetamol and salicylate levels were undetectable.
The patient was immediately started on the mode of haemodialysis that we use in our unit—sustained low efficiency daily dialysis (SLEDD)—against a high bicarbonate dialysate. Despite optimising minute ventilation, it was not possible to achieve a PaCO2 to offset the metabolic acidosis.
The patient's second major issue was severe haemodynamic instability secondary to distributive shock, compounded by severe acidaemia. Cardiovascular support in the form of norepinephrine and epinephrine was required to maintain a mean arterial pressure (MAP) of +/− 55. Owing to tachyarrhythmias, the presence of significant lactic acidaemia and a clinical scenario consistent with profound vasodilatory shock, the epinephrine was ceased and vasopressin was initiated. In the setting of refractory shock, stress dose steroids (hydrocortisone 50 mg q6 h) and empiric antibiotics were started.
At 08:00 h on day 1 of his ICU admission, approximately 10 h post presentation and 8 h post commencement of renal replacement therapy, the patient had a MAP of 50–55 mm Hg maintained by infusions of norepinephrine at 120 µg/min (1.7 µg/kg/min) and vasopressin at 0.06 units/min. During the first 8 h he received significant volume resuscitation guided by clinical and echocardiographic findings. To assess fluid responsiveness, we utilised passive leg raise. The changes in radial pulse pressure during this test have been found to be significantly correlated with changes in stroke volume, which in turn are significantly correlated with changes in stroke volume secondary to rapid fluid loading.6
A toxicologist was consulted and an N-acetylcysteine infusion started based on a possibility that the undetectable paracetamol level may have been unreliable given the ingestion was over 24 h previously.
A transthoracic echocardiogram showed a reasonable left ventricular ejection fraction of 50% with no valvular abnormality. There was significant pulmonary hypertension, however, with a right ventricular systolic pressure of 70 mm Hg and globally reduced right ventricular (RV) systolic function.
At this stage, the patient had severe distributive shock requiring massive doses of vasopressor support and barely maintaining a MAP of 50. Methylene blue was implemented as a rescue therapy with a bolus of 2 mg/kg followed by an infusion at 0.25 mg/kg/h for approximately 20 h (figure 1). This decision was carefully considered, as despite some favourable anecdotal evidence for methylene blue and its effectiveness in increasing blood pressure (BP) and systemic vascular resistance (SVR), there are several studies that have demonstrated increased mean pulmonary arterial pressure (MPAP) and therefore pulmonary vascular resistance in response to methylene blue,7–10 which was particularly pertinent in this case. All other therapies were exhausted; however, fortunately, not only did the patient's haemodynamics improve, his acid-base status also improved (figure 2).
Figure 1.
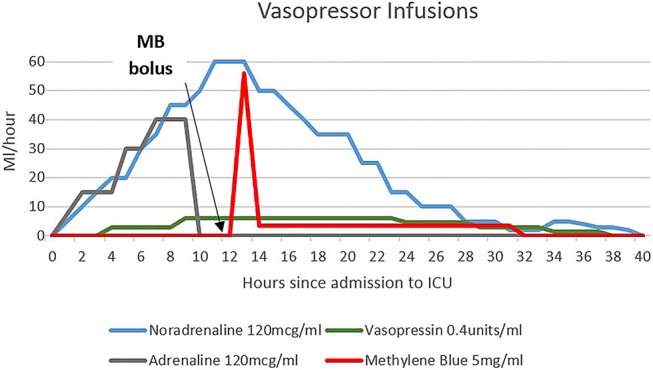
Haemodynamic support during the first 40 h of the ICU admission (ICU, intensive care unit; MB, methylene blue).
Figure 2.
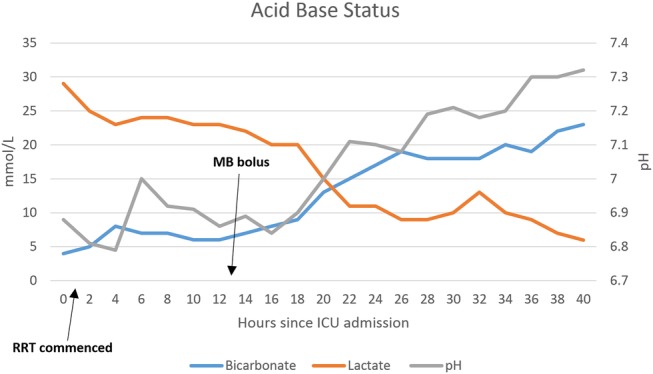
Improvement in the patient’s acid-base status following initiation of MB (ICU, intensive care unit; MB, methylene blue; RRT, renal replacement therapy).
Throughout day 1 of the ICU admission, fluid overload, acute lung injury and RV dysfunction all contributed to a rapidly worsening gas exchange. A chest X-ray revealed bilateral infiltrate and ruled out readily correctable causes of respiratory deterioration such as pneumothorax or endobronchial intubation. Fluid removal via dialysis in an attempt to improve gas exchange was hampered considerably by severe haemodynamic instability, as was the use of positive end-expiratory pressure and other recruitment manoeuvres. Despite optimising ventilation and using nebulised prostacyclin to decrease MPAP, critical respiratory failure was proving to be the most imminently life-threatening issue.
At this juncture, we proned the patient in an attempt to mitigate the need for extracorporeal membrane oxygenation (ECMO), which is not available at our hospital. Prior to proning the patient, his case was discussed with the ECMO retrieval centre. The patient was a suitable ECMO candidate, and if he failed to improve despite proning, he was to be retrieved by ECMO specialists for further advanced management of his combined cardiorespiratory failure.
Mortality has been shown to be decreased in severely hypoxaemic patients who are proned.11 The 2013 Proning Severe ARDS Patients (PROSEVA) trial demonstrated a statistically significant improvement in 28-day and 90-day mortality in patients with acute respiratory distress syndrome (ARDS) who were proned.12 At the time of proning, our patient was on 100% oxygen with a pH of 7.05, a PaO2 of 52 mm Hg and a PaCO2 of 71 mm Hg (figure 3). He was proned for 18 h in keeping with the PROSEVA trial and his dramatic improvement in gas exchange nullified the need for a second period of proning.
Figure 3.
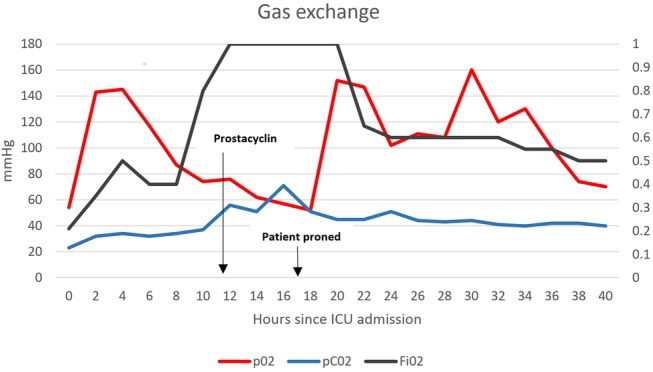
Trends in gas exchange following initiation of nebulised prostacyclin and proning (ICU, intensive care unit).
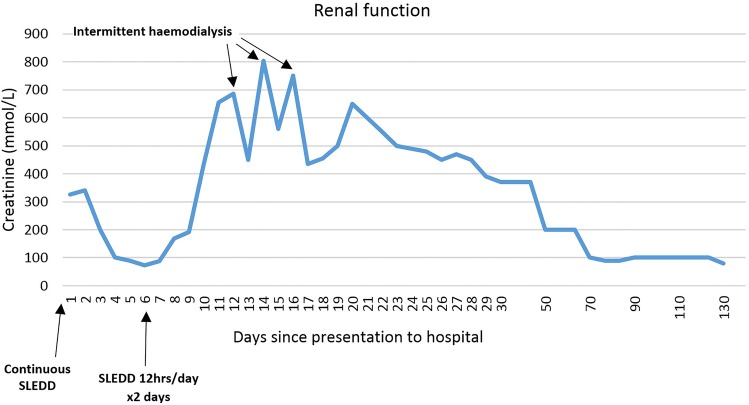
Over the following 24 h, the vasopressors were slowly weaned. The methylene blue infusion continued for 20 h and the only side effect observed was blue discolouration of the small amount of urine he passed. All haemodynamic support was ceased completely by day 2 of his ICU admission and he was successfully extubated on day 6. He was discharged to the renal ward following a 9-day ICU admission, and after three sessions of intermittent haemodialysis, renal replacement therapy was no longer required (figure 4).
Figure 4.
Weaning of (SLEDD) began on day 5 of admission. The final dose of intermittent haemodialysis was on day 16 of the patient's hospital admission (SLEDD, sustained low efficiency daily dialysis).
Outcome and follow-up
The patient is receiving ongoing psychological support in the community and continues the antidepressant initiated by the psychiatrist during his admission. His last review in the general medical outpatient clinic was 6 months following his discharge from hospital.
Discussion
Methylene blue has many well-recognised applications in various areas of medicine, including as a dye in radiographic medicine and as an effective treatment for methaemoglobinaemia.13 There are several case reports of methylene blue helping to restore and maintain a satisfactory BP in cases of severe distributive shock secondary to sepsis, and in vasoplegic syndrome following cardiopulmonary bypass.14 Less well recognised, but nevertheless promising, uses for methylene blue are documented in various toxicology settings such as β-blocker and calcium channel blocker overdose, and Quetiapine overdose.15–17 Our case is another compelling example of its utility in toxicology, and we propose that further research into the use of methylene blue as an adjunctive therapy in cases of refractory distributive shock, secondary to a variety of aetiologies, is warranted. At present, there is insufficient evidence to support the use of methylene blue as a first-line agent above conventional vasopressors.
Distributive shock is a term commonly used to describe the profound peripheral vasodilation associated with many types of shock, to varying degrees. One of the most commonly seen types of shock in clinical practice is secondary to sepsis. The pathogenesis of septic shock is thought to be a complex response of cellular activation that releases a multitude of proinflammatory mediators, which include excessive nitric oxide.18
Nitric oxide seems to play a vital role in the pathophysiological basis of the low-resistance state found in distributive shock. Increased production or release of this prevalent mediator is believed to be responsible for vasodilation and the reduced response to vasopressors.19 Metformin causes an increase in peripheral perfusion and glucose uptake in diabetes, and recent studies have suggested the mechanism for this may be increased activation of endothelial nitric oxide synthase (eNOS).20 The severe haemodynamic instability seen in this case may have been largely contributed to by a combination of the effects of metformin on eNOS and the role that nitric oxide plays in peripheral vasodilation seen in distributive shock. This has been hypothesised in one similar case report of metformin-associated lactic acidosis leading to dramatic haemodynamic instability, in which methylene blue also proved effective in restoring satisfactory haemodynamics.21
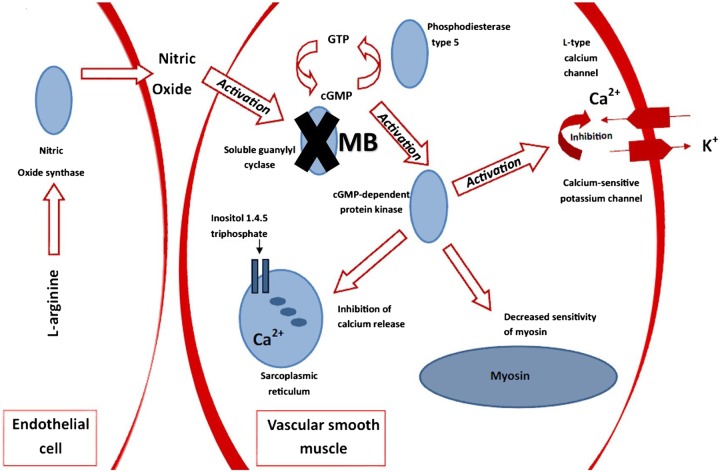
It has been proposed that reduction of nitric oxide synthesis, by inhibiting nitric oxide synthase, may be effective in attenuating hypotension and reverting vascular hyporesponsiveness,22 however, studies in animals and humans have resulted in increased mortality.23 24 This is believed to be related to an imbalance between the protective and pathological actions of nitric oxide.25 Methylene blue works by a different mechanism; it is an inhibitor of guanylyl cyclase, which is the target enzyme in the endothelium-dependent relaxation mediated by nitric oxide.26 Nitric oxide plays a role in guanylyl cyclase enzyme activation and cyclic guanosine monophosphate production (figure 5), and the role of nitric oxide as an important mediator in vascular smooth muscle relaxation has been well described. By inhibiting guanylyl cyclase, a downstream effector of nitric oxide, methylene blue is effective in increasing SVR and improving vascular responsiveness to standard catecholamines, without affecting other protective actions of nitric oxide.
Figure 5.
By inhibiting guanylyl cyclase, methylene blue prevents the activation of cGMP-dependent protein kinases. This leads to inhibition of calcium-sensitive potassium channels, decreased sensitivity of myosin, inhibition of calcium release and, ultimately, less vascular smooth muscle relaxation (cGMP, cyclic guanosine monophosphate; GTP, guanosine 5′-triphosphate).
On the basis of existing data, the dosing regimen for methylene blue in cases of distributive shock is not clear. Various case reports on anaphylactic shock27–32 and septic shock18 have documented the use of single or repeated boluses or an infusion with or without a bolus. Dosing of methylene blue for methaemoglobinaemia is typically 1–2 mg/kg of a 1% solution, intravenously.33 We used this practice, and the documented incidence of adverse effects at higher doses, as the rationale for giving our patient a 2 mg/kg bolus followed by a low-dose infusion, although we acknowledge there is limited evidence to support this dosing regimen, especially when administered concurrently with renal replacement therapy.
One prospective, randomised, double-blind, single centre study compared escalating doses of methylene blue in 15 mechanically ventilated patients with septic shock and found that although increasingly higher doses (1 mg/kg, 3 mg/kg or 6 mg/kg as an infusion over 20 min) further enhance global haemodynamics, there is a risk of compromising splanchnic perfusion and they should therefore be avoided.34 Other studies have reported further adverse effects of methylene blue at higher doses (5–7 mg/kg), such as ECG abnormalities (T-wave inversion and diminished R waves), dyspnoea, chest discomfort, nausea, diarrhoea, diaphoresis and abdominal discomfort.13 35 Large bolus doses may also potentially worsen pulmonary function,21 which may be due to its potential to cause pulmonary vasoconstriction and increase pulmonary vascular resistance.
One of the most commonly reported side effects of methylene blue is blue discolouration of the skin, urine or faeces, which is reversible on cessation of the drug. Ideally, it should be administered via central venous access as tissue necrosis may occur on extravasation.36 The use of methylene blue is particularly treacherous in patients with glucose-6-phosphate dehydrogenase deficiency as, in this population, it confers an increased risk of haemolytic anaemia.13 It is a strong monoamine oxidase inhibitor and therefore particular caution should be taken if administering concomitantly with antidepressant medication due to the potential of serotonergic crises.37
The evidence to support or refute the use of methylene blue in severe distributive shock is largely from case series or small clinical trials, and as such it is almost exclusively employed as a last resort therapy in cases where standard treatments have failed. Some authors have surmised, however, that methylene blue may be of more therapeutic benefit if used earlier in the course of illness.38 39 There is no high-level evidence at present to support the use of methylene blue prior to conventional vasopressors, however, we hope to encourage further research into its use as a relatively safe and feasible management strategy for severe distributive shock secondary to a variety of aetiologies.
Conclusion
In this case of severe distributive shock following a large metformin overdose, methylene blue proved to be an effective rescue therapy in a setting not previously well described. Cases similar to ours may become more prevalent as the incidence of type 2 diabetes increases in the western world and metformin continues to be recommended for first-line management in the majority of newly diagnosed type 2 diabetes.40
We hypothesise that the introduction of methylene blue was a significant contributor to the resolution of shock in this patient, with minimal adverse effects. However, significant adverse effects have been reported at a variety of doses. It is because of this uncertainty that further research is required into the use of methylene blue and its ideal dosing regimen, before its use in clinical practice becomes routine. If this uncertainty can be resolved through sufficiently powered trials, it may well prove to be an effective management strategy for refractory distributive shock secondary to a wide range of aetiologies.
Learning points.
Metformin overdose may present up to 24 h following ingestion and can very rapidly deteriorate into an acutely life-threatening situation.
Severe distributive shock, secondary to a variety of aetiologies and characterised by refractory hypotension, may be effectively treated with the introduction of a methylene blue infusion.
The procedure of proning a patient has significant risks and should not be undertaken without due consideration of all possible therapies in consultation with all relevant specialists.
Footnotes
Contributors: REG drafted the original article and was involved in the care of the patient for several continuous days at the beginning of his admission. She is the guarantor. MC devised the initial plan to report the case and was heavily involved in the care of the patient throughout his admission. She also revised several draft articles and made adjustments. JW was heavily involved in the care of the patient also and made several valuable suggestions and revisions during the writing of the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Levin RL, Degrange MA, Bruno GF et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg 2004;77:496–9. 10.1016/S0003-4975(03)01510-8 [DOI] [PubMed] [Google Scholar]
- 2.Donati A, Conti G, Loggi S et al. Does methylene blue administration to septic shock patients affect vascular permability and blood volume? Crit Care Med 2002;30:2271–7. 10.1097/00003246-200210000-00015 [DOI] [PubMed] [Google Scholar]
- 3.Edmund S, Kwok H, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med 2006;21:359–63. 10.1177/0885066606290671 [DOI] [PubMed] [Google Scholar]
- 4.Kirov MY, Evgenov OV, Evgenov NV et al. Infusion of methylene blue in human septic shock: a pilot, randomised, controlled study. Crit Care Med 2001;29:1860–7. 10.1097/00003246-200110000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Dumbarton T, Minor S, Yeung C et al. Prolonged methylene blue infusion in refractory septic shock: a case report. Can J Anesth 2011;58:401–5. 10.1007/s12630-011-9458-x [DOI] [PubMed] [Google Scholar]
- 6.Boulain T, Achard J, Teboul J et al. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 2002;121:1245–52. 10.1378/chest.121.4.1245 [DOI] [PubMed] [Google Scholar]
- 7.Andresen M, Dougnac A, Diaz O et al. Use of methylene blue in patients with refractory septic shock: impact on haemodynamics and gas exchange. J Crit Care 1998;13:164–8. 10.1016/S0883-9441(98)90001-6 [DOI] [PubMed] [Google Scholar]
- 8.Gachot B, Bedos J, Veber B et al. Short-term effects of methylene blue on haemodynamics and gas exchange in humans with septic shock. Intensive Care Med 1995;21:1027–31. 10.1007/BF01700666 [DOI] [PubMed] [Google Scholar]
- 9.Schneider F, Lutun P, Hasselmann M et al. Methylene blue increases systemic vascular resistance in human septic shock: preliminary observations. Intensive Care Med 1992;18:309–11. 10.1007/BF01706481 [DOI] [PubMed] [Google Scholar]
- 10.Weingartner R, Oliveira E, Oliveira ES et al. Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a short infusion of methylene blue. Braz J Med Biol Res 1999;32:1505–13. 10.1590/S0100-879X1999001200009 [DOI] [PubMed] [Google Scholar]
- 11.Sud S, Friedrich JO, Taccone P et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010;36:585–99. 10.1007/s00134-009-1748-1 [DOI] [PubMed] [Google Scholar]
- 12.Guérin C, Reignier J, Richard J et al. Prone positioning in severe acute respiratory distress syndrome. The New Eng J of Med 2013;368:2159–68. 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 13.Clifton J II, Leikin J. Methylene blue. Amer J Ther 2003;10:289–91. 10.1097/00045391-200307000-00009 [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam G. Vasoplegic syndrome- the role of methylene blue. Eur J Cardiothorac Surg 2005;28:705–10. 10.1016/j.ejcts.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 15.Jang DH, Nelson LS, Hoffman RS. Methylene blue in the treatment of refractory shock from an amlodipine overdose. Ann Emerg Med 2011;58:565–7. 10.1016/j.annemergmed.2011.02.025 [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal N, Kupfer Y, Seneviratne C et al. Methylene blue reverses recalcitrant shock in beta-blocker and calcium channel blocker overdose. BMJ Case Rep 2013;2013:pii: bcr2012007402 10.1136/bcr-2012-007402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher J, Taori G, Braitberg G et al. Methylene blue used in the treatment of refractory shock resulting from drug poisoning. Clin Toxicol 2013;52:63–5. 10.3109/15563650.2013.870343 [DOI] [PubMed] [Google Scholar]
- 18.Jang D, Nelson L, Hoffman R. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013;9:242–9. 10.1007/s13181-013-0298-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasin L, Umbrello M, Greco T et al. Methylene blue as a vasopressor: a meta-analysis of randomised trials. Crit Care Resusc 2013;15:42–8. [PubMed] [Google Scholar]
- 20.Davis BJ, Xie Z, Viollet B et al. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006;55:496–505. 10.2337/diabetes.55.02.06.db05-1064 [DOI] [PubMed] [Google Scholar]
- 21.Plumb B, Parker A, Wong P. Feeling blue with metformin-associated lactic acidosis. BMJ Case Rep 2013;2013:pii: bcr2013008855 10.1136/bcr-2013-008855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petros A, Lamb G, Leone A et al. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res 1994;28:34–9. 10.1093/cvr/28.1.34 [DOI] [PubMed] [Google Scholar]
- 23.Cobb JP, Natanson C, Hoffman WD et al. N omega-amino-L-arginine, an inhibitor of nitric oxide synthase, raises vascular resistance but increases mortality rates in awake canines challenged with endotoxin. J Exp Med 1992;176:1175–82. 10.1084/jem.176.4.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez A, Lorente JA, Steingrub J et al. Multiple-center, randomised, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 2004;32:21–30. 10.1097/01.CCM.0000105581.01815.C6 [DOI] [PubMed] [Google Scholar]
- 25.Wright CE, Rees DD, Moncada S. Protective and pathological roles of nitric oxide in endotoxic shock. Cardiovasc Res 1992;26:48–57. [DOI] [PubMed] [Google Scholar]
- 26.Ginimuge P, Jyothi S. Methylene blue: revisited. J Anaesthesiol Clin Pharmacol 2010;26:517–20. [PMC free article] [PubMed] [Google Scholar]
- 27.Ingeborg WS, Rengelshausen J, Oberwittler H et al. High absolute oral bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol 2009;65:179–89. 10.1007/s00228-008-0563-x [DOI] [PubMed] [Google Scholar]
- 28.Oliveira Neto A, Duarte N, Vicente W et al. Methylene blue: an effective treatment for contrast medium-induced anaphylaxis. Med Sci Monit 2003;9:CS102–6. [PubMed] [Google Scholar]
- 29.Del Duca D, Sheth SS, Clarke AE et al. Use of methylene blue for catecholamine-refractory vasoplegia from protamine and aprotinin. Ann Thorac Surg 2009;87:640–2. 10.1016/j.athoracsur.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues JM, Pazin Filho A, Rodrigues AJ et al. Methylene blue for clinical anaphylaxis treatment: a case report. Sao Paulo Med J 2007;125:60–2. 10.1590/S1516-31802007000100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissgerber AJ. Methylene blue for refractory hypotension: a case report. AANA J 2008;76:271–4. [PubMed] [Google Scholar]
- 32.Grayling M, Deakin CD. Methylene blue during cardiopulmonary bypass to treat refractory hypotension in septic endocarditis. J Thorac Cardiovasc Surg 2003;125:426–7. 10.1067/mtc.2003.140 [DOI] [PubMed] [Google Scholar]
- 33.Lo J, Darracq M, Clark R. A review of methylene blue treatment for cardiovascular collapse. J Emerg Med 2014;46:670–9. 10.1016/j.jemermed.2013.08.102 [DOI] [PubMed] [Google Scholar]
- 34.Juffermans N, Vervloet M, Daemen-Gubbels C et al. A dose-finding study of methylene blue to inhibit nitric oxide actions in the haemodynamics of human septic shock. Nitric Oxide 2010;22:275–80. 10.1016/j.niox.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 35.Plotkin J, Buell J, Njoku M. Methemoglobinemia associated with dapsone treatment in solid organ transplant recipients: a two-case report and review. Liver Transpl Surg 1997;3:149–52. 10.1002/lt.500030207 [DOI] [PubMed] [Google Scholar]
- 36.Dumbarton T, Gorman S, Minor S et al. Local cutaneous necrosis secondary to a prolonged peripheral infusion of methylene blue in vasodilatory shock. Ann Pharmacother 2012;46:e6 10.1345/aph.1Q560 [DOI] [PubMed] [Google Scholar]
- 37.Rowley M, Riutort K, Shapiro D et al. Methylene-blue associated serotonin syndrome: a ‘green’ encephalopathy after parathyroidectomy. Neurocrit Care 2009;11:88–93. 10.1007/s12028-009-9206-z [DOI] [PubMed] [Google Scholar]
- 38.Evora PR, Jose Rodrigues A, Celotto AC. “Methylene blue should be relegated to rescue use and not as first-line therapy” cannot become a paradigm. J Cardiovasc Anesth 2014;28:11–12. 10.1053/j.jvca.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 39.Evora PR. Methylene blue does not have to be considered only as rescue therapy for distributive shock. J Med Toxicol 2013;9:426 10.1007/s13181-013-0333-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Royal Australian College of General Practitioners. General practice management of type 2 diabetes—2014–15. Melbourne: The Royal Australian College of General Practitioners and Diabetes Australia, 2014:47–8. (accessed Sep 2014). [Google Scholar]