Abstract
Visceral abdominal aneurysms can originate from multiple disease states, including inflammatory, non-inflammatory and infectious aetiologies. It is important to follow a stepwise approach to make the correct diagnosis, because disease prognosis and management can be substantially different. We describe a 60-year-old Caucasian woman who presented from an outside facility to our University Hospital in a critical state with abdominal bleeding. She had no findings to support a vasculitic process, nor a concern for infectious aetiologies. She required a thoughtful approach and detailed imaging to diagnose a rare non-inflammatory disease as the cause for her mesenteric bleeding—segmental arterial mediolysis (SAM). Through our case and discussion, we describe the importance of recognising this rare entity and of understanding how early recognition can save patients from significant morbidity and unnecessary potential harmful therapeutic options.
Background
Segmental arterial mediolysis (SAM) is a rare non-inflammatory vasculopathy that commonly presents with visceral arterial aneurysmal dilations and is associated with significant mortality.1 2 Given its complicated and broad differential diagnosis with other disorders, many of which have significantly different therapeutic options, early diagnosis and decision-making is vital. We present a case of SAM and the stepwise approach that led us to the correct diagnosis and management.
Case presentation
A 60-year-old Caucasian woman with a medical history of chronic obstructive pulmonary disease was admitted to an outside facility with acute on chronic respiratory failure and pneumonia. She was placed on intravenous antibiotics and a short course of prednisone. Her status improved shortly after treatment was initiated. During her recovery period, she experienced a sudden worsening of hypoxia with hypotension followed by unresponsiveness. She was tachycardic and tachypnoeic, and soon went into cardiopulmonary arrest with pulseless electrical activity. She was stabilised after 15 min of cardiopulmonary resuscitation and a dose of epinephrine. She was intubated, placed on pressor support and transferred to our Academic Health Center for further evaluation and management. On arrival at our facility, she was on a vasopressin drip with stable vital signs. Her physical examination revealed an unconscious sedated woman with an endotracheal tube in place, a mildly distended abdomen and palpable pulses in all extremities. She had a low haemoglobin level of 9.6 g/dL with elevated liver enzymes (aspartate transaminase, alanine transaminase and alkaline phosphatase 3886, 2568 and 61 U/L, respectively), attributed to her shock state, but serum lactic acid was normal at 2.2 mmol/L (N: 0.5–2.2 mmol/L). Electrolytes and renal function were grossly within limits. She was admitted to the surgical intensive care unit for management and close monitoring.
Investigations
On a CT scan with angiogram of her abdomen and pelvis, a large haematoma in the retroperitoneal and intraperitoneal spaces was found, surrounding the liver and spleen, and displacing the pancreas, stomach and proximal duodenum. No active extravasation was seen and over the next few hours, repeat CT scans did not show any progression of the haematoma. In addition, a 2.1 cm proximal superior mesenteric artery (SMA) aneurysm was noted (figure 1).
Figure 1.
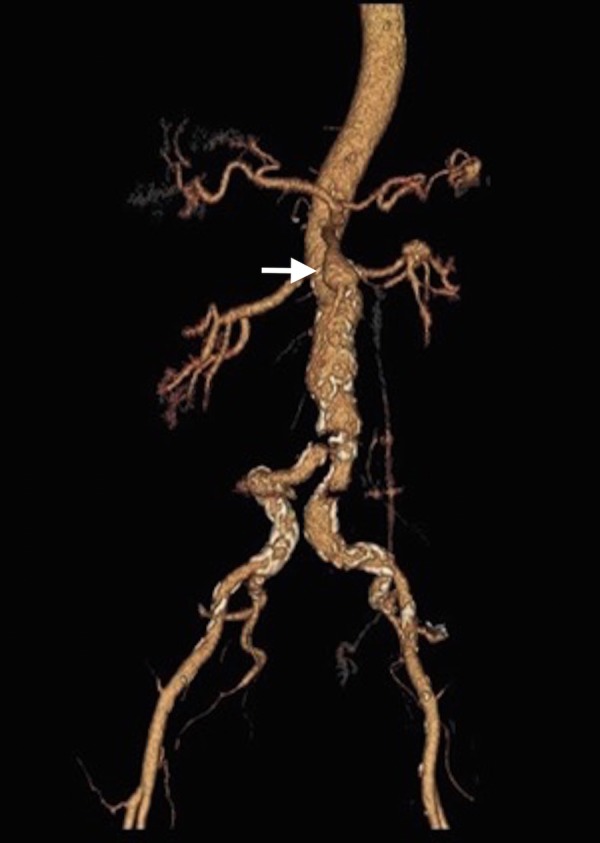
CT angiogram of the abdominal aorta and its branches: a three-dimensional reconstruction image demonstrating the 2.1 cm aneurysm at the proximal end of the superior mesenteric artery (white arrow).
To evaluate her mesenteric vessels better, the patient underwent a conventional mesenteric angiogram (figure 2). A lateral aortogram with selective catheterisation of the coeliac vessel distribution and SMA was performed using right common femoral access. The SMA aneurysm was confirmed. In addition, multiple small microaneurysms distributed in a ‘bead-like fashion’ were identified on selective magnified view of the SMA (figure 2). There were also multiple microaneurysms noted in the coeliac vascular distribution. A concern for polyarteritis nodosa (PAN) or related vasculopathic aetiologies was raised and rheumatology service was consulted.
Figure 2.
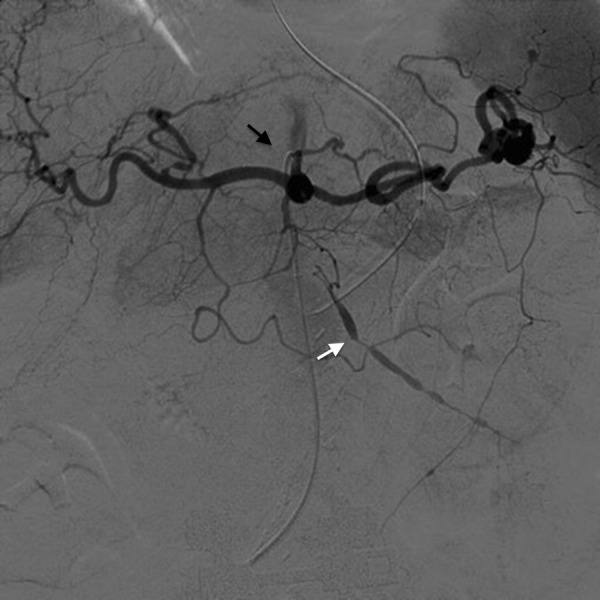
Conventional angiogram of the coeliac plexus (black arrow): note the interesting “string of beads” finding (white arrow) with multiple small aneurysms seen in the same vasculature (the superior mesenteric artery).
Differential diagnosis
Our differential for the patient included inflammatory, infectious and non-inflammatory aetiologies of the SMA aneurysm (box 1). The patient did not fulfil the American College of Rheumatology (ACR) criteria for diagnosis of PAN,3 and did not have any history of systemic features, examination findings or findings on imaging to suggest a similar vasculitic process such as antineutrophil cytoplasm antibody (ANCA)-associated vasculitis, giant cell arteritis, Takayasu's arteritis or Behçet's disease.4 There was no history of rashes or ulcers, headaches, visual symptoms, jaw claudication or large vessel involvement. Laboratory data including an antinuclear antibody (ANA), ANCA panel including immunofluorescence assay screening, and proteinase 3 (PR3) and myeloperoxidase (MPO) antibody screening, ANA comprehensive panel (double-stranded DNA, anti-Smith, RNP, Sjögren's syndrome-related antigen A/B (SSA/SSB) antibodies), Rheumatoid factor and myositis antibody panel were all reported to be negative. An antiphospholipid panel including the anticardiolipin antibody, lupus anticoagulant and β2 glycoprotein were also negative.
Box 1. Differential diagnosis of segmental arterial mediolysis.
- Inflammatory and infectious
- Large/medium vessel vasculitis
- Polyarteritis nodosa
- Large vessel vasculitis (giant cell arteritis, Takayasu's disease)
- Kawasaki disease
- Behçet's disease
- Infectious endocarditis
- Non-inflammatory
- Fibro muscular dysplasia
- Atherosclerosis
- Pseudoxanthoma elasticum
- Neurofibromatosus
- Ehlers-Danlos syndrome type IV
- Marfan's syndrome
We considered the possibility of infections such as mycotic aneurysms, but there was no history of fever or chills, drug use or other concerning physical examination findings. Blood cultures were obtained daily and continued to be negative for aerobic, anaerobic and fungal elements. Stool analysis for ova and parasites was unremarkable. A transthoracic echocardiogram was reported to be within normal limits without any evidence of infective endocarditis (IE).
We next evaluated the patient for non-infectious and non-inflammatory causes. Some disease processes were unlikely (Ehlers-Danlos syndrome (EDS) type IV, neurofibromatosis (NF) and pseudoxanthoma elasticum (PXE)), as they are typically seen in the paediatric age group. Other diagnoses to consider were Marfan's syndrome, fibromuscular dysplasia (FMD), atherosclerosis and SAM. Our patient was a woman without classic marfanoid habitus, and no abnormalities on CT of the chest and echocardiogram suggestive of Marfan's or related disorders. FMD was placed lower on the differential because the typical age group affected is much younger (20 s to 30 s) than our patient, and there is a predilection for renal vascular involvement. Lack of involvement of branch points in vessels for the aneurysms, no involvement of large vasculature and absence of widespread distribution, made atherosclerosis less likely, even though the patient had some demonstrable atherosclerotic plaques on imaging. SAM was clearly the best fitting diagnosis for the patient. A biopsy for confirmation could not be carried out because of the patient's medical status and the nature of the vessels involved.
Treatment
Our patient was treated with careful monitoring, supportive transfusions, and serial imaging and vascular surgery follow-up for future ambulatory endovascular intervention. Since her CT scans did not show an area of blood extravasation from any site, and the haematoma was non-progressive and self-contained, she did not meet any indication for urgent vascular surgical intervention. She went through a ventilator weaning trial, and was subsequently extubated in a few days. However, due to her significant comorbid issues she required prolonged hospitalisation for over a month. She developed ambulatory dysfunction and required physical therapy with a long stay at a rehabilitation unit after discharge. She was asked to continue to follow-up with vascular surgery for elective intervention for her aneurysms and monitoring of her status. On a 1-year follow-up with the patient, she continues to do well and has not experienced any further vascular events or episodes.
Discussion
SAM is an uncommon non-inflammatory disorder of the vascular system, resulting from medial layer degeneration of muscular arteries, sometimes with involvement of accompanying veins.2 5 It was first described by Slavin and Gonzalez-Vitale in 1976, initially as ‘segmental mediolytic arteritis’, and proposed to be changed to its present nomenclature due to the absence of inflammation.2 6 It can predispose to the development of vessel dissection, stenosis, occlusion and aneurysm formation, and is associated with a mortality as high as 50%, in some reports.7
Shenouda et al1 reported a systematic review of 85 patients with SAM in the medical literature between 1976 and 2012. In this report, the median age of presentation was 57 years with a male:female ratio of 1.5:1. Abdominal pain (66%) and haemodynamic shock (29%) were reported to be the most common presenting symptoms. The coeliac axis distribution, and SMA and its branches, were the most common vessels involved, with 47 and 32 patients, respectively. Involvement of the cranial and cerebral vasculature, inferior mesenteric artery and renal vasculature has also been reported.1 8
The aetiology of SAM is not well understood. Pathologically, the affected vessel initially demonstrates vacuolar degeneration of the outer smooth muscle layer.9 As the disease progresses, there is smooth muscle disruption and subsequent ‘mediolysis’ developing into arterial ‘gaps’. These gaps form dissociations in the involved region of the surviving wall, with resultant aneurysm formation and dissection. Subsequent structural changes and reparative fibrosis often lead to vessel stenosis.1 10
Radiological evaluation of SAM is critical and usually a CT angiogram or a conventional angiogram is employed.11 Imaging data typically shows a ‘string of beads’, indicating multiple aneurysms, and absence of inflammatory features. Pathological evidence is difficult to obtain and certainly not a pre-requisite for diagnosis, especially if these characteristic radiographic features are present.1 In the review by Shenouda et al,1 about 31% patients were diagnosed with radiographic evidence alone, and the rest had pathological evidence through resection of the affected area or on autopsy. In most circumstances, the aneurysms may be randomly distributed around the affected vascular bed without predilection for point of origin of the vessel or its bifurcation. This can also be helpful in differentiating it from other disorders such as inflammatory vasculitis and atherosclerotic arterial disease.
The treatment of SAM is significantly different from other diseases that present with similar vascular involvement. The initial approach is supportive and conservative. Although intervention in otherwise asymptomatic individuals remains controversial,1 the treatment of symptomatic aneurysms has been performed with an open surgical approach and, more recently, with endovascular techniques. The latter is minimally invasive and can even be considered for patients in the acute setting as a ‘bailout’ measure.1 In the review by Shenouda et al, coil embolisation was the most common endovascular intervention and reported as successful in 88% patients, with no mortality. The open surgical approach, however, was associated with a 9% mortality rate.1
The complexity in making a diagnosis of SAM lies in its broad differential diagnosis, as many diseases can present with similar features. The therapeutic options and prognosis for these diseases can be very different (box 1). Baker-LePain et al2 have illustrated a comprehensive review of diseases that can mimic as SAM. The differential diagnosis can be broadly categorised into inflammatory or infectious causes, versus non-inflammatory causes. In diagnosis of our case, we considered this broad classification scheme for ease in ruling out the more common disease mimickers.
Inflammatory
The differential for inflammatory causes of SAM includes medium and/or large vessel vasculitides (PAN, ANCA-associated vasculitis, Takayasu's arteritis and giant cell arteritis, Behçet's disease, Kawasaki disease). These can be largely excluded based on presentation and physical examination, imaging and laboratory findings, although biopsy may be essential for a definitive diagnosis.
PAN is a multisystem medium vessel vasculitis and the most common of the vasculitides considered in the differential of SAM. It commonly presents with widespread and systemic involvement. Untreated, the prognosis is very poor, with a 13% 5-year survival.12 13 Therapeutic options involve immediate strong immunosuppression and antiviral therapy if associate with viral aetiology. The ACR classification criteria for PAN are 82.2% sensitive and 86.6% specific if a patient meets at least three of the ten listed criteria.3
Most vasculitides can be classified by evaluating systemic signs, organ systems involved and calibre of vessel involved. Diagnostic testing with autoantibodies including an ANA, ANCA panel with PR3 and MPO antibodies is essential.
Mycotic/infectious
Infection-related vasculopathies are next on the list of potential aetiologies to consider. Mycotic aneurysms, in particular, can present in a similar fashion. Ruling these out with detailed history taking and a physical examination including identification of characteristic signs such as Janeway lesions and splinter haemorrhages, blood cultures and the addition of cardiovascular imaging, namely an echocardiogram, to look for evidence of IE, are critical to the investigation.
Non-inflamamtory
Some of these disorders are seen almost exclusively in the paediatric population; especially EDS type 4, PXE and NF. In addition, their areas of involvement and other characteristic disease features that accompany vascular findings can also help distinguish them from SAM.2
Marfan's syndrome can present at any given age, and elongated fingers, ratio of upper and lower extremities, and associated ocular and cardiovascular features can help distinguish this entity from SAM. Some of these non-inflammatory conditions can now be evaluated using genetic studies.
The final diagnosis of SAM can be made a bit more complicated by other non-inflammatory mimics of this disorder. FMD can present very similarly to SAM, and some authors even consider SAM to be closely associated with FMD.6 FMD, however, presents at an earlier age than SAM, typically in the third and fourth decade of life. It usually has a strong predilection for renal vessels (up to 75%) and cerebrovascular circulation (30%), as well as the propensity to involve multiple sites at the same time.14
Another disorder that mimics SAM closely is atherosclerosis. This can present with involvement of any vessel size, and the age of involvement would usually be in the 60 s and above.2 There are a few differences that can help make the correct diagnosis of SAM in this situation. Even though there may be a coexistence of the diseases, given the similar age predilection, there is a male predominance seen in atherosclerosis, while SAM is reported to affect both the sexes equally, therefore more likely the diagnosis in women. Atherosclerosis also coexists in other organ systems, notably the ocular, cerebrovascular, cardiac or renal vasculature. In contrast to the radiographic finding of ‘string of beads’ in SAM (also seen in FMD to a certain extent), atherosclerosis presents with a shaggy and irregular appearance, and displays widespread involvement of different vascular beds and predisposition to vascular branch off areas.2 11 Apart from this, the pathology findings in SAM (predominantly outer layer of the media) can help distinguish it from the atherosclerotic appearance of fibrous calcified plaques and foamy macrophages.1 2
Summary
Our patient presented in her 60 s, and did not have a presentation, laboratory evaluation or imaging findings to suggest an inflammatory aetiology for her illness. The involvement of the coeliac and SMA vessel distributions in a characteristic beaded pattern, and the absence of diffuse vascular involvement in the rest of the body on imaging studies (especially CTs of the head and chest) were suggestive of SAM. Conservative management led to a positive outcome, with survival, despite significant morbidity due to the patient's prolonged hospitalisation. This case illustrates the importance of early diagnosis and the correct therapeutic management in order to avoid complications/toxicities from a delay in diagnosis or therapy for other possibilities. Age of the patient and the presence of a non-inflammatory process are important delineating factors to consider, as we have depicted in our discussion. Finally, this case illustrates the importance of considering SAM in the differential diagnosis of a patient presenting with multiple abdominal aneurysms, and of the need for appropriate diagnostic testing and intervention as indicated.
Learning points.
Visceral aneurysms can have a wide differential including infectious, inflammatory and non-inflammatory causes.
Segmental arterial mediolysis (SAM) is an unusual but very important cause of non-inflammatory aneurysms.
Age of onset, location of vessel involvement and absence of inflammatory clinical features, laboratory and imaging findings, play an essential role in the diagnosis of this disorder.
Early detection and appropriate management of the patient are essential to prevent potential complications of the disease and to avoid the use of inappropriate therapeutics, which can cause significant morbidity and a high mortality.
Imaging and/or histopathology are required for confirmation of SAM, and patients require close monitoring and possible vascular intervention to prevent the significant morbidity and mortality associated with this disease.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shenouda M, Riga C, Naji Y et al. Segmental arterial mediolysis: a systematic review of 85 cases. Ann Vasc Surg 2014;28:269–77. 10.1016/j.avsg.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Baker-LePain JC, Stone DH, Mattis AN et al. Clinical diagnosis of segmental arterial mediolysis: differentiation from vasculitis and other mimics. Arthritis Care Res 2010;62:1655–60. 10.1002/acr.20294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lightfoot RW, Michel BA, Bloch DA et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 1990;33:1088–93. 10.1002/art.1780330805 [DOI] [PubMed] [Google Scholar]
- 4.Bloch DA, Michel BA, Hunder GG et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Patients and methods. Arthritis Rheum 1990;33:1068–73. 10.1002/art.1780330803 [DOI] [PubMed] [Google Scholar]
- 5.Slavin RE, Inada K. Segmental arterial mediolysis with accompanying venous angiopathy: a clinical pathologic review, report of 3 new cases, and comments on the role of endothelin-1 in its pathogenesis. Int J Surg Pathol 2007;15:121–34. 10.1177/1066896906297684 [DOI] [PubMed] [Google Scholar]
- 6.Slavin RE, Saeki K, Bhagavan B et al. Segmental arterial mediolysis: a precursor to fibromuscular dysplasia? Mod Pathol 1995;8:287–94. [PubMed] [Google Scholar]
- 7.Sakano T, Morita K, Imaki M et al. Segmental arterial mediolysis studied by repeated angiography. Br J Radiol 1997;70:656–8. 10.1259/bjr.70.834.9227264 [DOI] [PubMed] [Google Scholar]
- 8.Tameo MN, Dougherty MJ, Calligaro KD. Spontaneous dissection with rupture of the superior mesenteric artery from segmental arterial mediolysis. J Vasc Surg 2011;53:1107–12. 10.1016/j.jvs.2010.11.034 [DOI] [PubMed] [Google Scholar]
- 9.Slavin RE, Cafferty L, Cartwright J. Segmental mediolytic arteritis. A clinicopathologic and ultrastructural study of two cases. Am J Surg Pathol 1989;13:558–68. 10.1097/00000478-198907000-00003 [DOI] [PubMed] [Google Scholar]
- 10.Kalva SP, Somarouthu B, Jaff MR et al. Segmental arterial mediolysis: clinical and imaging features at presentation and during follow-up. J Vasc Interv Radiol 2011;22:1380–7. 10.1016/j.jvir.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Michael M, Widmer U, Wildermuth S et al. Segmental arterial mediolysis: CTA findings at presentation and follow-up. AJR Am J Roentgenol 2006;187:1463–9. 10.2214/AJR.05.0281 [DOI] [PubMed] [Google Scholar]
- 12.Balow JE. Renal vasculitis. Kidney Int 1985;27:954–64. 10.1038/ki.1985.104 [DOI] [PubMed] [Google Scholar]
- 13.Frohnert PP, Sheps SG. Long-term follow-up study of periarteritis nodosa. Am J Med 1967;43:8–14. 10.1016/0002-9343(67)90144-1 [DOI] [PubMed] [Google Scholar]
- 14.Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med 2004;350:1862–71. 10.1056/NEJMra032393 [DOI] [PubMed] [Google Scholar]


