Abstract
A man in his late 40s with sickle cell anaemia (HbSS) presented to the emergency department with 2 weeks of diffuse oedema, increased abdominal girth and dyspnoea. His anasarca was thought to be indicative of an acute decompensation of his known liver cirrhosis with transfusion-induced haemosiderosis. While his anasarca improved with diuresis, his direct hyperbilirubinaemia suddenly worsened without any signs of haemolysis, biliary disease or obstruction. He also developed an acute worsening in serum creatinine (1.17–7.0 mg/dL in 7 days) despite subsequent treatment for presumed hepatorenal syndrome (HRS). Given his clinical decline, the patient's goals of care were transitioned to comfort measures only. His clinical presentation and rapid liver and renal deterioration were most typical of sickle cell intrahepatic cholestasis (SCIC). SCIC can lead to rapid deterioration in renal function and can be mistaken for HRS. When SCIC is suspected, consideration of exchange transfusions should be made early.
Background
When managing a patient with sickle cell disease and acute liver failure, it is important to consider sickle cell-induced aetiologies of acute decompensation, such as sickle cell intrahepatic cholestasis (SCIC) complicated by renal failure. The pathophysiological and clinical similarities between SCIC and hepatorenal syndrome (HRS) make diagnosing of these acute conditions challenging. In this case report, we review the literature on potential aetiologies of acute decompensation of liver disease in patients with sickle cell anaemia (HbSS) and cirrhosis. We also explain the pathophysiological similarities and differences between HRS and SCIC complicated by renal failure.
Case presentation
A man in his late 40s with HbSS presented to the emergency department with 2 weeks of diffuse oedema, increased abdominal girth and dyspnoea. The patient had a history of requiring 4–6 hospitalisations per year for pain crises. He had received more than 100 packed red blood cell (pRBC) transfusions over his lifetime. Of note, he was not adherent to his recommended chelation therapy or hydroxyurea. One year prior to presentation, he had been diagnosed with cirrhosis of the liver with diffuse haemosiderosis seen on liver histology, but did not follow-up for outpatient treatment. His last sickle cell crisis was approximately 6 months prior to the current presentation.
Investigations
On arrival, the patient was afebrile with a blood pressure of 138/63 mm Hg, a pulse of 100 bpm and a respiratory rate of 18 breaths/min. His oxygen saturation on room air was 93%. He was in no acute distress and oriented to person, place and time. His physical examination was notable for scleral icterus and a distended abdomen that was tender to palpation in the upper quadrants bilaterally. His liver edge was palpated 2 cm below the costal margin. No rebound, guarding, or Murphy's sign was appreciated on abdominal examination. His extremities were warm and well perfused, but he did have 3+ pitting oedema up to the thighs, bilaterally. The remainder of the physical examination was unremarkable.
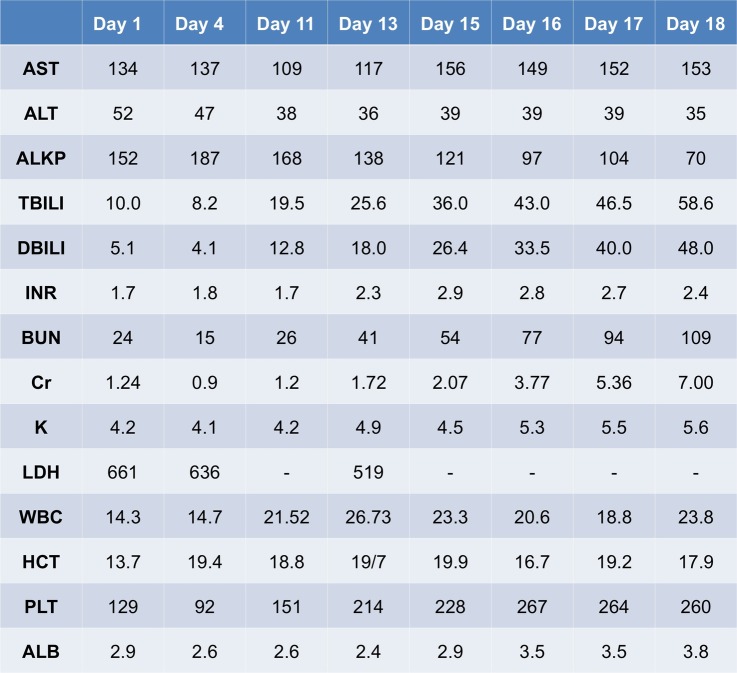
As outlined in figure 1, the patient's admission laboratory findings were notable for a white cell count (WCC) of 14×103 cells/μL, haematocrit 13.7% (mean corpuscular volume 90 fL), platelet count 129×103 cells/μL and reticulocyte count 18.16% (reticulocyte index of 2.4). He received 1 unit of pRBCs, and his haematocrit subsequently stabilised at 20%. He was also found to have a total bilirubin level of 10 mg/dL (direct bilirubin 5.1 mg/dL) with mildly elevated liver function enzymes (aspartate transaminase (AST) 134 U/L, alanine transaminase (ALT) 52 U/L, alkaline phosphatase (ALKP) 152 U/L) and an elevated prothrombin time with an international normalised ratio (INR) of 1.7. His albumin was 2.8 g/dL. His serum ferritin was >5000 µg/L. Abdominal ultrasound revealed cirrhosis of the liver with a trace amount of ascites. CT scan of the abdomen showed a constellation of findings consistent with iron overload, including a cirrhotic appearing, hyperdense liver, hyperdense lymphadenopathy and cardiomegaly. An echocardiogram and urine protein/creatinine ratio were normal. An infectious workup at this time was unrevealing as hepatitis viruses A, B and C, Epstein-Barr virus and Cytomegalovirus tests were all negative. Autoimmune panels were also negative.
Figure 1.
Pertinent laboratory values since admission (ALB, albumin; ALKP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; DBILI, serum direct bilirubin; INR, international normalised ratio; HCT, haematocrit; LDH, lactate dehydrogenase; PLT, platelet count; TBILI, serum total bilirubin; WCC, white cell count).
Treatment
Initially, this patient's anasarca was thought to be indicative of an acute decompensation of his known cirrhosis. He was given intravenous furosemide for 7 days and was also started on aldactone for diuresis. His serum creatinine remained stable while receiving diuretics. However, after 7 days, the patient continued to report of abdominal pain and distension, and his diuretics were stopped. At this time, his blood work demonstrated acutely worsening, predominately direct, hyperbilirubinaemia (total bilirubin/direct bilirubin up to 19.5/12.8 mg/dL on day 11, rising to 58.6/48 mg/dL within a week). His INR rose from 1.7 to 2.9 in the same time period. His haematocrit was stable, and his reticulocyte count and peripheral smear argued against active haemolysis. Repeat abdominal ultrasound was stable from initial presentation, without cholecystitis or common bile duct dilation. MR cholangiopancreatography (MRCP) showed no biliary disease or obstruction. In the setting of progressive leucocytosis (WCC to 27×103 cells/μL), the patient was treated empirically for spontaneous bacterial peritonitis (SBP) with ceftriaxone. The patient began to develop an acute worsening in serum creatinine (1.2–7.0 mg/dL in 7 days).
Outcome and follow-up
The patient's renal dysfunction was initially thought to be secondary to HRS. He was given albumin infusion without improvement in his renal function. Despite subsequent treatment with octreotide, midodrine and ongoing albumin infusion, the patient's renal function continued to deteriorate acutely with rapidly rising serum creatinine. He also developed encephalopathy in the setting of his worsening liver and renal function.
Our suspicion for SCIC was high given the constellation of his symptoms, including right upper quadrant pain and nausea, progressive development of jaundice, renal impairment and encephalopathy. His laboratory findings, including pronounced hyperbilirubinaemia, elevated lactate dehydrogenase (LDH), prolongation of the prothrombin time and partial thromboplastin time, and rapid elevation of serum creatinine, also supported our hypothesis (figure 1). In addition, our patient presented with multiple predisposing factors for SCIC, including male gender and hepatic fibrosis secondary to iron overload. In haemosiderosis, non-transferrin-bound iron can induce reactive oxygen species, which can result in cellular and endothelial dysfunction; this, in turn, promotes intrahepatic sickling.1 This intrahepatic destruction in the setting of baseline haemosiderosis likely exacerbated the acuity of our patient's clinical failure.
On day 16 of hospitalisation, the patient's goals of care were transitioned to focusing on comfort. Although our suspicion for SCIC was high, we were unable to make further diagnostic or management attempts, including exchange transfusions and dialysis. The patient expired on day 18 of hospitalisation with his family declining autopsy.
Discussion
This case illustrates a patient with HbSS complicated by cirrhosis secondary to iron overload, who presents with acute hepatic insult manifesting as hyperbilirubinaemia and ascites. The patient's hospital course was complicated by acute renal failure, leading to rapid deterioration and, ultimately, death.
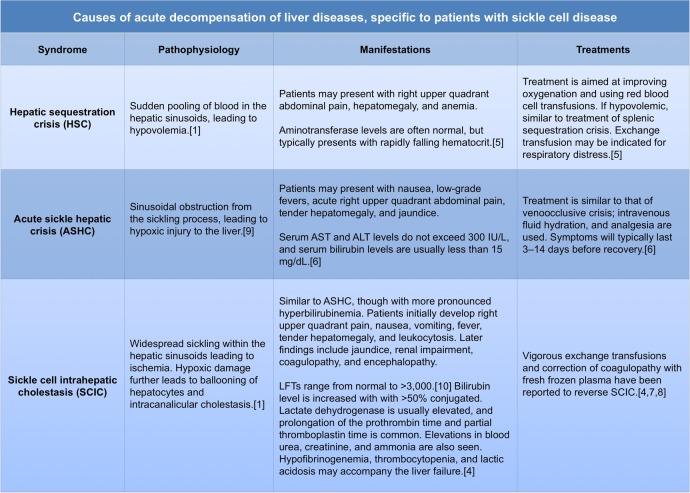
Chronic liver disease in HbSS is common. Approximately 16–29% of patients with HbSS present with cirrhosis at autopsy.2 3 In most cases, cirrhosis develops as a consequence of chronic hepatitis B or C infection, or iron overload due to multiple blood transfusions.4 Patients with HbSS and cirrhosis may present with acute decompensation of their liver disease due to one of several different aetiologies, as outlined in figure 2.
Figure 2.
Causes of acute decompensation of cirrhosis in patients with SCD (ALT, alanine transaminase; AST, aspartate transaminase; LFTs, liver function test; SCD, sickle cell disease).
One common cause of cirrhosis in HbSS is hepatorenal sequestration crisis (HSC), which typically presents with right upper quadrant abdominal pain from a sudden pooling of blood in the hepatic sinusoids, leading to hypovolaemia (figure 2). Blood chemistry laboratory values are notable for rapidly falling haematocrit and normal aminotransferase levels.5 Our patient's haematocrit remained within normal range while his aminotransferase levels were elevated, making this the unlikely aetiology for him.
In contrast, patients with acute sickle hepatic crisis (ASHC) present with elevated aminotransferase and serum bilirubin levels. The pathophysiology of ASHC is thought to be from sinusoidal obstruction from the sickling process, which leads to hypoxic injury to the liver. Liver biopsy shows sickle thrombi in sinusoids with RBC engorgement, occasional findings of Kupffer cell hypertrophy, mild centrilobular necrosis and cholestasis.6
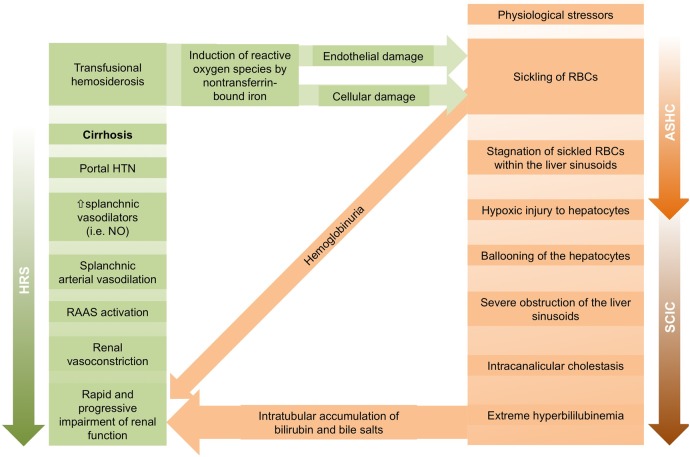
As shown in figure 3, ASHC can further develop into SCIC, which is a rare but potentially fatal complication of HbSS. SCIC is a distinct clinical syndrome characterised by severe hyperbilirubinaemia and cholestatic jaundice with coagulopathy in the absence of another obvious aetiology for acute liver disease. It is often accompanied by fever and coagulopathy. Renal impairment is common in SCIC and is postulated to be due to acute tubular necrosis secondary to the combination of hyperbilirubinaemia, intravascular volume depletion, and haemoglobinuria. Liver biopsy typically shows ballooning of hepatocytes, Kupffer cell hyperplasia, sickled RBCs in hepatic sinusoids, erythrophagocytosis, intracanalicular cholestasis with bile plugs, anoxic necrosis and varying degrees of fibrosis.2 5 Immediate, vigorous exchange transfusions and correction of coagulopathy with fresh frozen plasma have been reported to reverse SCIC.4 7 8
Figure 3.
Pathophysiology of renal impairment in patients with acute decompensation of cirrhosis from HbSS. Mechanisms include (1) SCIC leading to acute tubular necrosis and direct injury from haemoglobinuria, and (2) HRS (ASHC, acute sickle hepatic crisis; HbSS, sickle cell anaemia; HRS, hepatorenal syndrome; HTN, hypertension; NO, nitric oxide; RAAS, renin-angiotensin-aldosterone system; RBCs, red blood cells; SCIC, sickle cell intrahepatic cholestasis).
SCIC has several clinical and pathophysiological similarities to HRS, posing a real diagnostic challenge. Figure 3 illustrates the pathophysiological differences between HRS and SCIC complicated by renal failure. In HRS, dilation of splanchnic circulation activates the renin-angiotensin-aldosterone system and triggers renal vasoconstriction, with rapidly progressive renal impairment. In contrast, ASHC occurs when stagnation of sickled RBCs within the liver sinusoids leads to hypoxic injury to the hepatocytes, which then in turn induces further sickling.8 ASHC then progresses to SCIC as liver sinusoids become severely obstructed, resulting in intracanalicular cholestasis and extreme hyperbilirubinaemia. Bilirubin and bile salts accumulate in the renal tubules, causing acute tubular necrosis. Sickling of RBCs additionally produces haemoglobinuria, which increases the tubular debris and amplifies the impairment of renal function.2 8
We present the case of a young man with HbSS and a history of receiving more than 100 pRBC transfusions, leading to haemosiderosis. The acuity of the patient's demise from liver failure compounded by renal failure was striking and suggested an acute, superimposed insult on chronic liver disease. When managing a patient with sickle cell disease and acute liver failure, it is important to consider sickle cell-induced aetiologies of acute decompensation, which have high morbidity and mortality if not treated promptly. The consideration of exchange transfusions should be made early while managing this patient population. Although it is feasible to have liver biopsy during the disease course to confirm the pathophysiology of sickle cell-induced acute liver failure, this process must not delay initiation of exchange transfusions. Moreover, potential candidates for liver transplantation should be managed in a transplant centre with contributions from haematology and hepatology. While some studies have reported its use, particularly in the paediatric literature, further research needs to be carried out in the role of transplantation in the HbSS population to avoid liver failure and its mortality.9–11 While it has not been shown to affect the prognosis of HbSS disease, it may help with management of this challenging complication of the disease.
Learning points.
When managing a patient with sickle cell disease and acute liver failure, it is important to consider sickle cell-induced aetiologies of acute decompensation, such as sickle cell intrahepatic cholestasis (SCIC).
SCIC typically presents with hyperbilirubinaemia, renal impairment, coagulopathy and encephalopathy.
SCIC can lead to profound and rapid deterioration in renal function and can be mistaken for hepatorenal syndrome.
When SCIC is suspected, consideration of exchange transfusions should be made early.
Acknowledgments
Dr Karin Andersson provided editorial assistance with the preparation of this manuscript.
Footnotes
Contributors: DDI analysed the data, conducted the literature review, and drafted and revised the manuscript. UE collected data and revised the draft paper. JWD contacted the patient's relatives and revised the draft paper. VC initiated the collaborative project, monitored the data collection process and revised the draft paper.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gardner K, Suddle A, Kane P et al. How we treat sickle hepatopathy and liver transplantation in adults. Blood 2014;123:2302–7. 10.1182/blood-2013-12-542076 [DOI] [PubMed] [Google Scholar]
- 2.Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7. 10.1016/S0002-9343(80)80008-8 [DOI] [PubMed] [Google Scholar]
- 3.Song YS. Hepatic lesions in sickle cell anemia. Am J Pathol 1957;33:331–51. [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee S, Owen C, Chopra S. Sickle cell hepatopathy. Hepatology 2001;33:1021–8. 10.1053/jhep.2001.24114 [DOI] [PubMed] [Google Scholar]
- 5.Hatton CS, Bunch C, Weatherall DJ. Hepatic sequestration in sickle cell anaemia. Br Med J 1985;290:744–5. 10.1136/bmj.290.6470.744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheehy TW. Sickle cell hepatopathy. South Med J 1977;70:533–8. 10.1097/00007611-197705000-00008 [DOI] [PubMed] [Google Scholar]
- 7.Ahn H, Li CS, Wang W. Sickle cell hepatopathy: clinical presentation, treatment, and outcome in pediatric and adult patients. Pediatr Blood Cancer 2005;45:184–90. 10.1002/pbc.20317 [DOI] [PubMed] [Google Scholar]
- 8.Shao SH, Orringer EP. Sickle cell intrahepatic cholestasis: approach to a difficult problem. Am J Gastroenterol 1995;90:2048–50. [PubMed] [Google Scholar]
- 9.Blinder MA, Geng B, Lisker-Melman M et al. Successful orthotopic liver transplantation in an adult patient with sickle cell disease and review of the literature. Hematol Rep 2013;5:1–4. 10.4081/hr.2013.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurtova M, Bachir D, Lee K. Transplantation for liver failure in patients with sickle cell disease: challenging but feasible. Liver Transpl 2011;17:381–92. 10.1002/lt.22257 [DOI] [PubMed] [Google Scholar]
- 11.Mekeel KL, Langham MR Jr, Gonzalez-Peralta R et al. Liver transplantation in children with sickle-cell disease. Liver Transpl 2007;13:505–8. 10.1002/lt.20999 [DOI] [PubMed] [Google Scholar]