Highlights
-
•
In a motor imagery based BCI system to control FES, practicing imagery both before and during FES additionally increases intensity of event related desynchronisation throughout the whole period of electrical stimulation.
-
•
Discontinuing to practice motor imagery following the onset of FES, reduces subsequent event-related desynchronisation.
-
•
Motor imagery and FES produce event-related desynchronisation in similar frequency ranges.
Keywords: Motor imagination, Functional electrical stimulation, Rehabilitation, Brain computer interface, Event-related synchronisation/desynchronisation
Abstract
Objective
Motor imagination (MI) and functional electrical stimulation (FES) can activate the sensory-motor cortex through efferent and afferent pathways respectively. Motor imagination can be used as a control strategy to activate FES through a brain–computer interface as the part of a rehabilitation therapy. It is believed that precise timing between the onset of MI and FES is important for strengthening the cortico-spinal pathways but it is not known whether prolonged MI during FES influences cortical response.
Methods
Electroencephalogram was measured in ten able-bodied participants using MI strategy to control FES through a BCI system. Event related synchronisation/desynchronisation (ERS/ERD) over the sensory-motor cortex was analysed and compared in three paradigms: MI before FES, MI before and during FES and FES alone activated automatically.
Results
MI practiced both before and during FES produced strongest ERD. When MI only preceded FES it resulted in a weaker beta ERD during FES than when FES was activated automatically. Following termination of FES, beta ERD returns to the baseline level within 0.5 s while alpha ERD took longer than 1 s.
Conclusions
When MI and FES are combined for rehabilitation purposes it is recommended that MI is practiced throughout FES activation period.
Significance
The study is relevant for neurorehabilitation of movement.
1. Introduction
The adult brain is capable of adapting to environmental challenges, such as learning new skills, and to functional disabilities produced by a lesion to the nervous system (Celnik and Cohen, 2004). In able-bodied individuals activity dependant neuroplasticity is driven by a voluntary activation of the cortex resulting in activation of muscles in conjunction with feedback from sensory receptors activated by that movement. The existence of these adaptation processes stimulated the development of neurorehabilitation interventions geared to enhance neuroplasticity when it plays a beneficial role and to inhibit it when it is detrimental (Celnik and Cohen, 2004). Neuroplasticity generally manifests as an increase in the excitability of corticospinal circuits which over time strengthens the connectivity of the cortico-spinal pathways. This strengthening is associated with improved motor learning (McDonnell and Ridding, 2006), improved motor function following stroke (Powell et al., 1999; Conforto et al., 2002), spinal cord injury (Hoffmann and Field-Fotte, 2007) and other central nervous system damages (Everaert et al., 2010; Stein et al., 2013).
In recent years Brain Computer Interface (BCI) has been proposed as a tool for promoting neurorehabilitation of motor functions in patients with stroke and spinal cord injury (Dobkin, 2007; Grosse-Wentrup et al., 2011; Keiser et al., 2014). Typically a BCI control strategy is motor imagination (MI) which is a mental simulation of an action (Jeannerod, 2001; Mulder, 2007). BCI can be used to control a functional electrical stimulator (FES) applied to the patient’s upper or lower limb muscles (Tam et al., 2011; Dally et al., 2009; Vuckovic et al., 2014) while the patient performs MI of the limb’s movement. Such a setup is called BCI controlled FES, or simply, BCI–FES. The purpose of MI is dual: to provide a BCI command signal to control FES and to activate the efferent motor pathways. The purpose of FES is to activate the afferent pathways. It is believed that MI timely preceding the FES can induce activity dependant plasticity in patients incapable of performing an overt (executed) movement (Dobkin, 2007; Keiser et al., 2014). A study on able-bodied participants by Mratchacz et al. (2012) demonstrated that a timely combination of MI and FES is crucial for the strengthening of Cortico-Spinal (CS) pathways. They measured cortical evoked potential during MI and delivered FES at different phases of MI. A subsequent motor evoked potential was maximal when FES was delivered during maximum negative phase of movement-related cortical potentials.
Although it is believed that the precise timing between the onset of MI and FES is important for strengthening the CS pathways (Mratchacz et al., 2012), it is not known whether a prolonged MI during FES affects the cortical activity induced by FES. In stroke patients and patients with incomplete SCI, MI produces stronger activation of the sensory-motor cortex than FES (Szameitat et al., 2012). Therefore a sustained motor imagery during FES might result in a stronger and more sustained activation of the sensory-motor cortex. Yet BCI–FES studies typically do not specify to participants whether or not to continue with MI once they activate the FES. Motor imagery is a cognitively demanding condition (Jannerod, 2001; Mulder, 2007), therefore most BCI users would probably stop performing MI once they activate FES, unless told otherwise.
Multiple EEG studies compared brain activation during different modalities of covert and overt movements: imagination of movement, observation of movement, passive movement and movement caused by electrical stimulation. Alegre et al. (2002) analysed event related synchronisation/desynchronisation (ERS/ERD) (Pfurtscheller and Lopes da silva, 1999) during passive movements and found beta ERD during the passive movements and post-movement beta ERS. Cho et al. (2011) compared EEG patterns during active movements, passive robotic movements, MI, FES producing only sensation and FES producing sensation and muscle contraction. They found similar ERS/ERD patterns in the lower beta range for all modalities except for MI of movement. Müller et al. (2003) showed that a major time–frequency difference between active and FES induced movements was the presence of ERD prior to movement onset in the active case. Although multiple EEG studies compared ERS/ERD patterns of MI and FES, these two modalities have typically been analysed separately. Therefore there is no study looking into the influence of MI on FES, which is important to consider should these two modalities be used together.
A study by Saito et al. (2013) demonstrated that electrical stimulation with intensity above the motor threshold, delivered during prolonged repetitive MI, increases the excitability of the cortico-spinal tract. While MI in that study was not used to control the electrical stimulator, the study supports the idea of practicing MI during FES. On the other hand, studies on repetitive or sustained movements (Cassim et al., 2000; Erbil and Ungan, 2007) showed that prolonged movements do not necessarily produce prolonged ERD throughout the whole movement. Therefore, it is possible that the contribution of ERD induced by prolonged MI during FES is very small.
In this study we used MI based BCI to compare ERS/ERD responses based on three covert movements paradigms: MI before FES, MI before and during FES and FES alone activated automatically. We show that continuing MI during FES produces the strongest ERD over the sensory-motor cortex. When MI preceded FES, but was not practiced during the FES, it resulted in weaker beta ERD during FES than when FES was applied on its own. The results are relevant for designing neurorehabilitation therapies which combine synchronous activation of sensory and motor pathways.
2. Methods
2.1. Participants and procedures
Ten able-bodied volunteers (ages 27 ± 5, 5F, 5M) participated in the study. Ethical permission was obtained from the College of Science and Engineering Ethical Committee. All participants signed an informed consent form.
2.2. EEG recording
The EEG was recorded using the gtec EEG amplifier (Guger Technologies, Austria). A ground electrode was attached to participants’ left ear. EEG was recorded bipolarly from the electrodes located at CF3-CP3, CFz-CPz and CF4-CP4. Because analysis was performed only for the right hand in this study, CF3-CP3 and CF4-CP4 will be referred to as the contralateral and ipsilateral side respectively. The location CFz-CPz will be referred to as the central area. The sampling frequency was 256 samples/s and the EEG signal was filtered between 5 and 60 Hz using 5th order Butterworth filter within the g-Usbamp device, set through a graphical user interface. Impedance was kept under 5 kΩ.
2.3. Experimental protocol
The experiment comprised two parts, an off-line and an on-line study. In the first, off-line study, participants were asked to perform cue-based MI of their left and right hand and these data were used to build a BCI classifier. In the on-line study part, the classifier built during the off-line part was used to implement a BCI controlled with MI of only the right hand waving movement.
2.3.1. Off-line cue-based BCI classifier
An experimental protocol that instructed participants to imagine left and right hand movements was devised using visual cues. Participants were seated at a desk, approximately 1.5 m in front of a computer monitor. Participants were instructed to look at the centre of the monitor and were instructed to respond to a sequence of visual cues. At t = 0 s a readiness cue (a cross +), which remained on for 4 s, appeared on the computer monitor. At t = 1 s an initiation cue, presented as an arrow, was displayed for 1.25 s, pointing to the left (←) or to the right (→). The left and right arrows corresponded to the left and right hand kinaesthetic MI of waving respectively. Participants were asked to continue with MI until the cross disappeared from the screen (3 s after the initiation cue appeared). Although later analysis will be performed only for MI of the right hand, an experimental paradigm with MI of both hands was chosen to assure that motor preparation did not start following the readiness cue (a cross). In total there were 40 trials comprising 20 trials for the left and 20 trials for the right hand MI with the left and right cues presented to the participants in a random order. To avoid tiredness the participants rested between the trials for a period of 3–5 s between the trials. The recorded data were inspected and trials containing artifacts (e.g. EOG, EMG) were removed. In general, due to the bipolar recording technique, artifacts were minimised and no more than 1–2 trials had to be removed. The off-line paradigm and subsequent signal processing was performed using rtsBCI (Scherer, 2005) and the BioSig Open source toolboxes (Vidaurre et al., 2011).
2.3.2. Feature extraction and initial classifier computation based on off-line BCI
Participant-specific frequency bands were selected by inspecting the ERS/ERD maps of the EEG from the channels. Here ERD and ERS refer to a decrease and increase respectively of EEG power relative to a baseline period within a narrow frequency band. The baseline/ reference period was a 1 s long, from 0.5 s to 1.5 s prior to the warning sign. Movement related cortical processes like those during MI and physical execution can be quantified with ERD across the sensorimotor cortex. ERS/ERD, sometimes referred to as event related spectral perturbation (Makeig, 1993), will be used in this text as a general term to refer to both ERD and ERS when necessary. For off-line BCI, ERS/ERD maps were created using a bandpower method, which is a standard method in Biosig programme used to perform the off-line analysis. The specific frequency bands were chosen to improve classification accuracy because although the alpha and beta bands (sensory-motor rhythms) are most reactive to MI, previous studies showed that there might be slight variations among the individuals (Neuper et al., 2009; Vuckovic and Osuagwu, 2013). Statistical significance of the ERS/ERD values was determined by applying a t-percentile bootstrap algorithm (Graimann et al., 2002) with a significance level p = 0.05. The selected bands were those visually showing significant ERD. To obtain the bandpower features the data in each channel was bandpass filtered (again 5th order IIR Butterworth) in the selected bands and was then squared and smoothed/averaged over a one second sliding window. This provided 6 features in total used to build a BCI classifier. The trials in the feature domain were split into baseline and active periods and each period (baseline and active) was then split into smaller segments of 0.4 s.
A linear discriminant analysis (LDA) (Duda et al., 2001) classifier in the feature space was computed between the active period for the right hand MI and its corresponding baseline period for each of the 0.4 s segments. To validate the classifiers, the leave-one-trial-out cross validation approach was adopted due to the relatively small number of trials. A ratio of correctly classified trials compared to the total number of trials was adopted as a measure of the classification accuracy. The classifier obtained from the 0.4 s segment with maximum classification accuracy was used in the online BCI.
2.3.3. On-line experimental paradigm
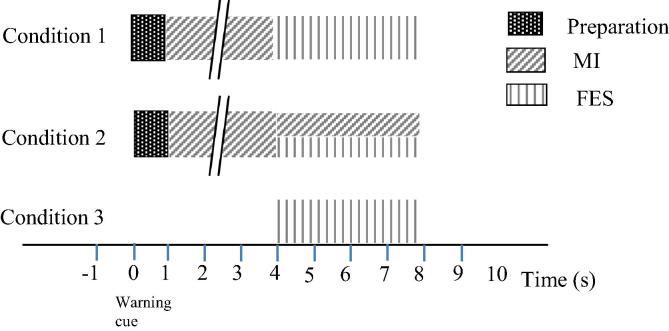
There were three conditions in which the FES was activated (Fig. 1). In the first condition, participants had to perform MI of the right hand to activate the FES and stop MI as soon as the FES was activated. In the second condition, participants were instructed to perform MI of the right hand to activate the FES and to carry on with the MI while the FES was active. In the third condition, they were not required to perform MI but FES was automatically activated.
Fig. 1.
Experimental paradigms for conditions 1–3 for the on-line BCI control of FES. The period of MI is of variable duration, maximum duration 10 s. The preparation period in conditions 1 and 2 lasted 3 s and FES was always applied for 4 s.
In the first two conditions, the participants were presented with a cross at t = 0 s on a computer screen. The cross warned the participants to get ready. At t = 3 s an execution cue which was an arrow pointing to the right, was presented on the screen. In all cases FES was automatically deactivated after 4 s following the onset (Fig. 1).
The participants were instructed to imagine waving with their right hand with a frequency of about 0.5 Hz as soon as they saw the execution cue. A scale, providing a continuous on-line information about MI, was shown in the left upper corner of the screen (Fig. 2). It was explained to participants that the FES would be activated when the indicator of the scale reached zero. They were not specifically instructed to look at the indicator, and could choose to concentrate on either the scale or on their own hand while imagining moving their right hand. Therefore for conditions 1 and 2 the participants voluntarily activated the FES using kinaesthetic MI.
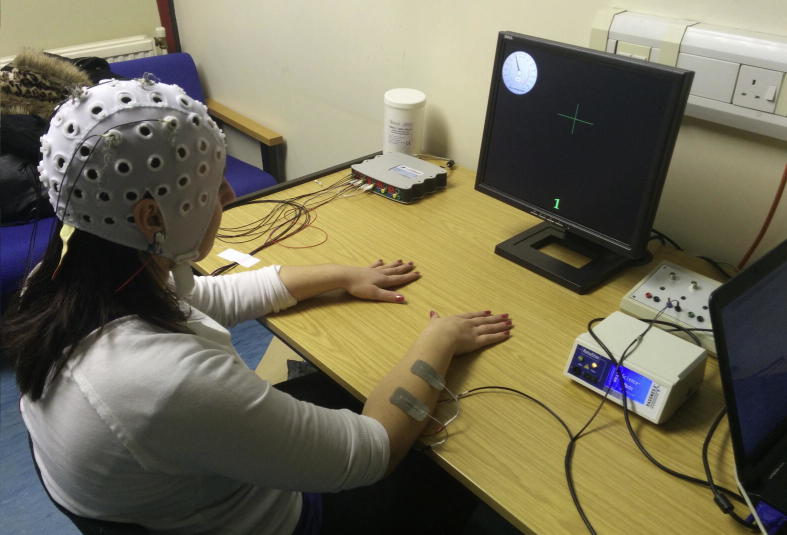
Fig. 2.
Experimental setup for the on-line experiment. Participants sit in front of a screen with forearms in front of him/her. Bipolar electrodes for FES are attached to participants′ right forearm. A warning sign (a cross) in the middle of the screen instructs the participant to start MI of the right hand. A scale in the left upper corner is proportional to the control parameter of the BCI and moves to the left, towards 0 during MI. This setup was used for conditions 1 and 2.
For condition 3, FES was activated automatically after a semi-random period of 10–20 s. The semi-random period was chosen to avoid anticipation of FES. In this condition there was no computer screen in front of participants, and participants were instructed to relax and look in front or them.
All participants were naive to the conditions and therefore had one probation run for condition 1 to familiarise themselves with the conditions. For each condition, 45 trials (separated into three runs of 15 trials) were obtained. Different conditions were separated in different runs, and runs containing different conditions were organised in semi-random order to avoid the effect of tiredness on any particular condition. There was a 10 s break between two runs to avoid muscle fatigue. Participants had up to 15 s to accomplish the condition; otherwise the trial was considered unsuccessful and was repeated.
FES stimulation parameters (the frequency of stimulus, stimulus amplitude and duration) were kept constant throughout the experiment, for all three conditions. Electrical stimulation (Rehastim, Hasomed, Switzerland) was applied using bipolar electrodes placed on the right hand extensor muscles. The following stimulation parameters were constant for all participants: Duration of FES 4 s, frequency of stimulus 30 Hz and pulse duration 200 μs. The stimulation amplitude varied among participants between 10 mA and 17 mA and was set to produce a visible wrist extension.
2.3.4. Time–frequency analysis
To create ERS/ERD maps it was necessary to choose a baseline where there was no condition-related EEG activity. For conditions 1 and 2, the baseline period was chosen 1 s prior to a warning cue (a cross). Because it took variable time to activate FES, all trials were aligned with respect to the moment when FES was activated. For conditions 1 and 2, one 9 s long epoch consisted of 1 s of the baseline period, 3 s before FES activation, and 5 s after FES activation (this includes 4 s during FES and 1 s after FES). For condition 3 the same epoch length was chosen, 4 s before FES and 5 s after FES activation, with a baseline taken from the first second. A structure of epochs for different conditions is shown in Fig. 1.
To perform group analysis, EEGLAB (Delorme and Makeig, 2004) was used to visualize and compare the ERS/ERD arising from the three conditions. ERS/ERD was computed using EEGLAB routines. The Morlet Wavelet transform was used to perform time frequency analysis of the EEG data in the frequency band 5–41 Hz with a Hanning-tapered window applied and the number of cycles set to 3. The ERS/ERD was computed as power changes in decibels relative to a baseline period (1 s before the warning cue). The average ERS/ERD was calculated over 10 participants using the structure STUDY in EEGLAB. The full description of ERS/ERD method is given in the EEGLAB’s methods by Delorme and Makeig (2004).
Statistical analysis was performed to compare between conditions 1 and 2, conditions 2 and 3 and conditions 1 and 3. To compare between means of two variables a non-parametric permutation test, based on resampling, was implemented in EEGLAB with a significance level set to p = 0.05. To compare between the conditions, a common baseline period was calculated. Correction for multiple comparisons was performed using the Holm-Bonferroni method (Holm, 1979).
3. Results
Table 1 shows frequency bands chosen to build off-line classifiers and the corresponding classification accuracy between the right hand MI and the baseline. A minimum of 75% classification accuracy was required for a classifier to be used in the on-line phase. Although EEG was filtered with a high pass filter at 5 Hz during recording (Butterworth, 5th order), some frequency content on lower frequencies still remained due to a relatively low order of the on-line filter. That resulted in a visible ERD in wider bands including lower frequencies. Therefore in two volunteers lower frequency bands starting from 2 Hz were included to create a classifier.
Table 1.
Information about off-line BCI classifier for each participants: frequency bands used to calculate classification features and corresponding classification accuracy.
| 1 | 8–12, 12–16 Hz | 75% |
| 2 | 6–12, 16–20 Hz | 85% |
| 3 | 4–12, 16–24 Hz | 75% |
| 4 | 2–8, 8–12 Hz | 76% |
| 5 | 2–8, 8–16 Hz | 78% |
| 6 | 4–10, 10–16 Hz | 85% |
| 7 | 8–12, 18–24 Hz | 76% |
| 8 | 10–16, 20–26 Hz | 77% |
| 9 | 8–14, 20–26 Hz | 75% |
| 10 | 4–8, 8–12 Hz | 83% |
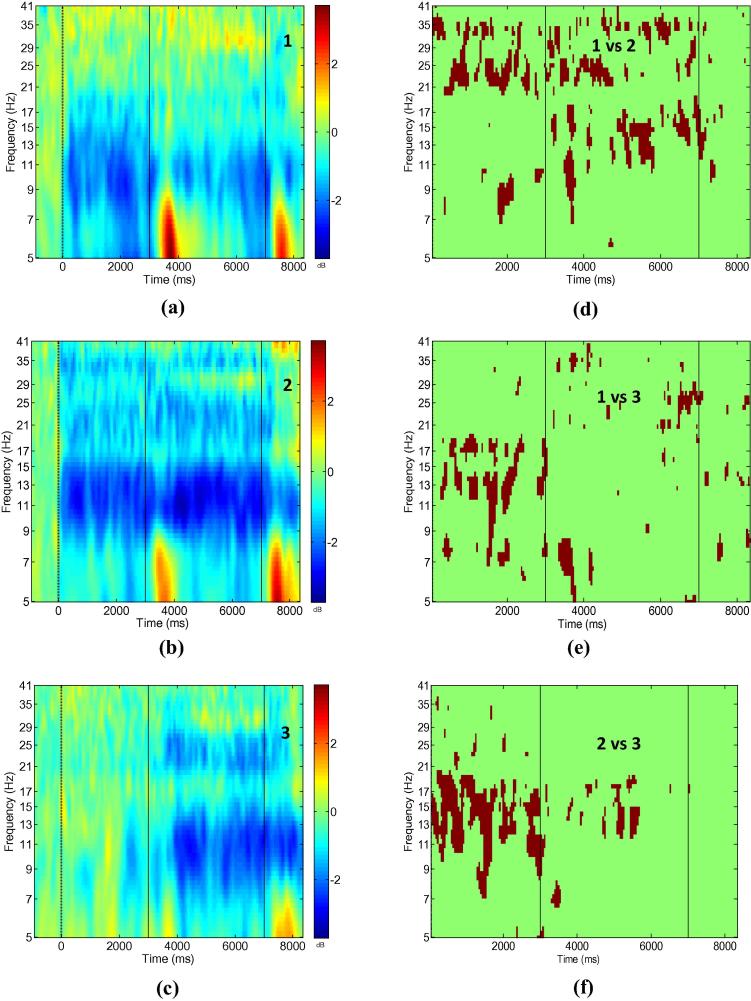
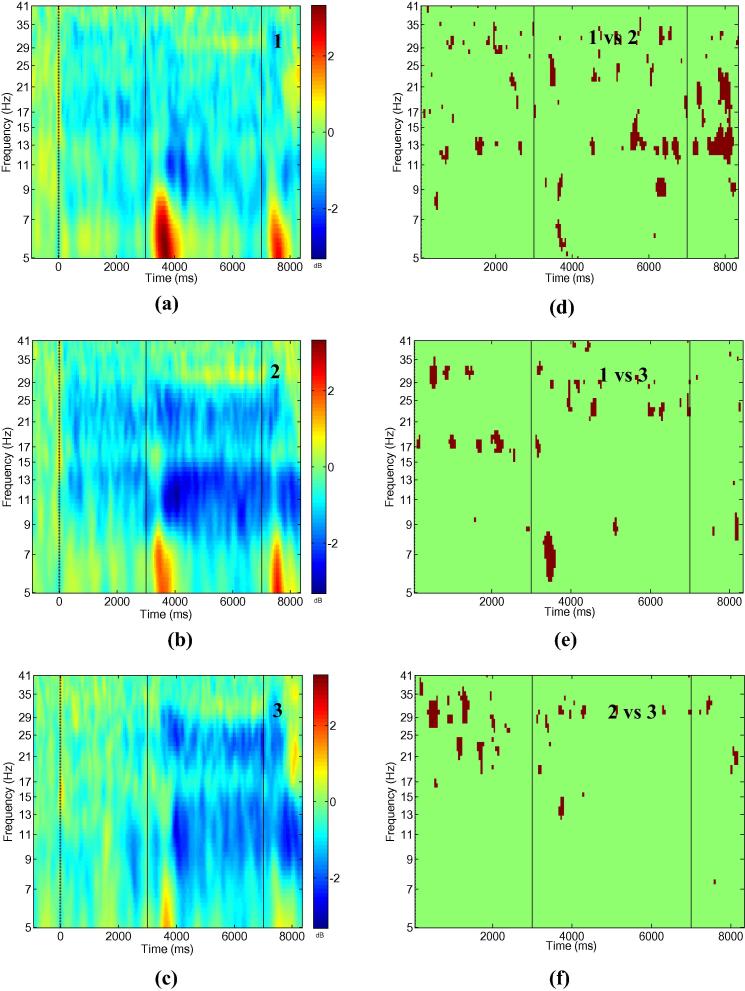
Fig. 3 shows ERS/ERD maps for all three experimental conditions (left column) and areas of statistically significant differences in the time–frequency domain between the conditions (right column) at electrode location CF3-CP3.
Fig. 3.
Bipolar electrode recording CF3-CP3; Event related synchronisation/desynchronisation for conditions 1–3 (a–c) and regions of statistically significant difference between conditions (d–f). Numbers in the left column figures correspond to the conditions, numbers in the right column figures correspond to pairs of conditions being compared. Significance level p = 0.05, with Holm–Bonferroni corrections for multiple comparison.
In condition 1 (Fig. 3a) strong ERD could be noticed in the alpha and lower beta band (8–16 Hz). This ERD is also noticeable before the electrical stimulation (t = 3000 ms) because participants were imagining to move their hands, and FES was activated when the classifier detected MI activity. In condition 2 (Fig. 3b), stronger ERD can be noticed both before and upon FES. ERD was present in three frequency bands: 8–16 Hz as in condition 1, and in the higher beta band and the lower gamma band (30–35 Hz). ERD was significantly stronger in condition 2 in all three frequency bands (Fig. 3d). It is interesting that ERD was also stronger in the period before FES (with respect to the common baseline). This might indicate that MI was more effective, producing stronger ERD, when participants carried on with MI following FES activation. Although ERD in the gamma band was present in condition 2 only, it could also be a harmonic of the alpha band ERD which was strongest for this condition.
In condition 3 (Fig. 3c), ERD can be noticed during FES in the alpha/lower beta band (8–16 Hz) and in the higher beta band. Statistical analysis revealed a significant difference between conditions 1 and 3 in a period before FES and during FES (Fig. 3e). Significant differences in a period before FES arise from the absence of ERD in condition 3. During FES significant differences can be noticed in the theta and the beta bands. The difference in the theta band is caused by the absence of ERS in condition 3, probably because participants did not know when to expect the FES. The difference in the higher beta band reflects weaker ERD in condition 1. This is a surprising result, indicating that stopping to imagine hand movements once the electrical stimulation is perceived, results in weaker ERD evoked by a subsequent electrical stimulation.
Statistical significant differences between conditions 2 and 3 (Fig. 3f) were found in a period before FES and during FES. A significant difference in a period before FES arises from the absence of ERD in condition 3. A significant difference in a period following FES can be noticed in the theta and the alpha/lower beta (8–16 Hz) band, because of the absence of theta ERS in condition 3 and due to stronger alpha/low beta ERD in condition 2. Electrical stimulation caused an extension of the hand extensor muscles. A beta ERS (around 30 Hz) that was visible during FES in three out of 10 participants, is probably caused by termination of extension of the wrist. In Fig. 3, weak beta ERS is visible in all three conditions and it occurs in parallel with stronger alpha ERD. The ERD occurring after the termination of FES (t = 7000–8000 ms) could be attributed to post-movement ERD that can be noticed following prolonged repetitive movements (Erbil and Ungan, 2007). In all three conditions beta ERD returns to its resting value during the first 0.5 s while alpha ERD remained during the whole analysed post-stimulus period.
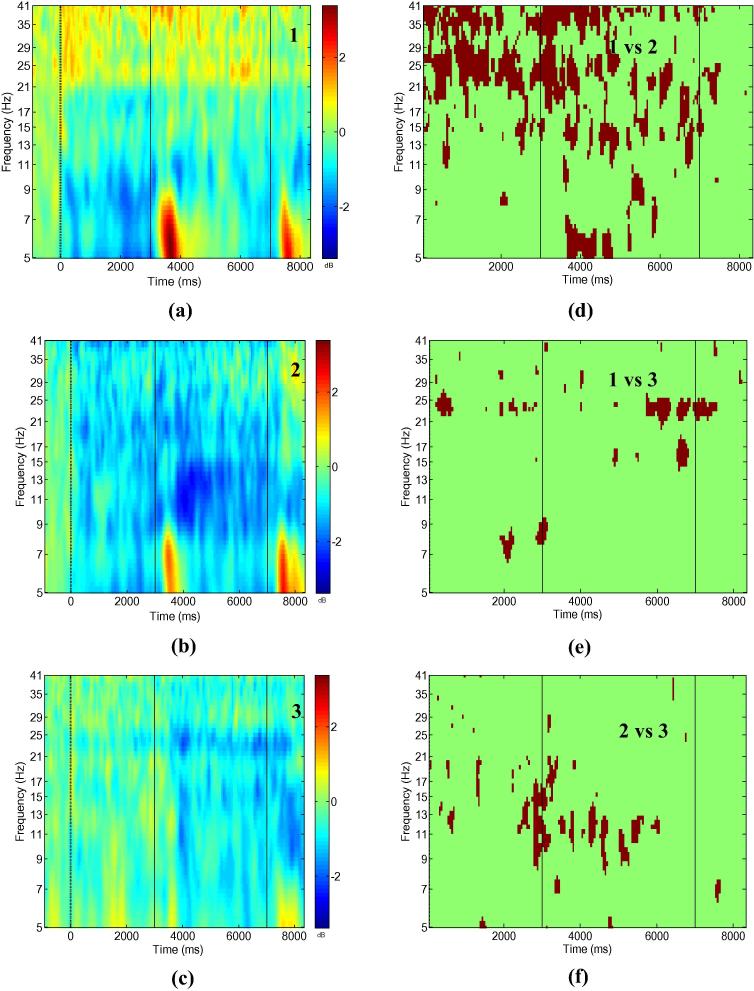
Fig. 4 shows ERS/ERD maps for all three experimental conditions (left column) and areas of statistical significant differences in the time–frequency domain between the conditions (right column) at electrode location CF4-CP4. Weak ERD can be noticed during FES in both conditions 1 and 3, indicating that FES does not affect the ipsilateral cortex very much. Prominent ERD during FES can be noticed for condition 2. Therefore statistical significant differences were larger between conditions 1 and 2, and 2 and 3 (Fig. 4d and f) than between conditions 1 and 3 (Fig. 4e).
Fig. 4.
Bipolar electrode recording CF4-CP4; Event related synchronisation/desynchronisation for conditions 1–3 (a–c) and regions of statistically significant difference between conditions (d–f). Numbers in the left column figures correspond to the conditions, numbers in the right column figures correspond to pairs of conditions being compared. Significance level p = 0.05, with Holm–Bonferroni corrections for multiple comparison.
Fig. 5 shows ERS/ERD maps for all three experimental conditions (left column) and areas of statistical significant differences in the time–frequency domain between all three conditions (right column) at electrode location CFz-CPz. The weakest ERD upon FES can be noticed for condition 1, significantly lower than in condition 2 (Fig. 5d) over the 8–16 Hz band and lower than condition 3 in the higher beta band (Fig. 5e). Although visually, condition 2 seems to have stronger ERD than condition 3 (Fig. 5f), statistical analysis revealed significant differences mostly in the period before FES. Post FES beta ERD is followed by beta ERS and is most prominent for condition 3.
Fig. 5.
Bipolar electrode recording CFz-CPz; Event related synchronisation/desynchronisation for conditions 1–3 (a–c) and regions of statistically significant difference between conditions (d–f). Numbers in the left column figures correspond to the conditions, numbers in the right column figures correspond to pairs of conditions being compared. Significance level p = 0.05, with Holm–Bonferroni corrections for multiple comparison.
A short-lasting increase in power in the theta band, can be noticed in all three electrode location in conditions 1 and 2 upon FES and in all three conditions after cessation of FES, probably related to expectation and processing of sensory sensation. Its intensity looks stronger in condition1 than in condition 2, though the difference was not statistically significant. The analysis of individual ERS/ERD maps showed that this phenomenon was present in seven out of 10 participants.
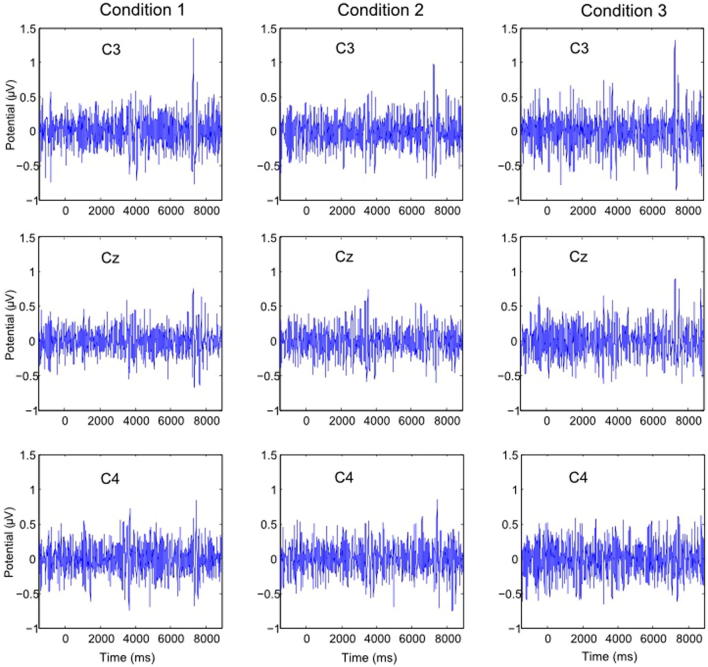
To check whether this short lasting increase in power corresponds to ERS or to an Event Related Potential (ERP) component evoked by switching FES on and off, a post hoc time domain analysis was performed by averaging EEG signal across trials (Fig. 6). Although this averaging cannot provide a proper quantitative ERP analysis because the signal was high-pass filtered at 5 Hz during EEG recording and is quite noisy due to the insufficient number of trials, it can provide a qualitative comparison between ERP and ERS/ERD. It can be noticed that a positive peak at about t = 7500 ms corresponds to a power increase in ERS/ERD maps upon switching FES off (Figs. 3–5). The peak is most prominent at C3. Another much less prominent positive peak, most consistently noticeable in condition 1 over all three electrodes, can be noticed at about t = 3500 ms corresponding to ERS visible upon switching FES on (Figs. 3–5). This indicates that a short lasting increase in power that appears in the theta band in ERS/ERD maps is actually a phase-locked ERP component, which is not frequency specific.
Fig. 6.
Averaged EEG in time domain. Columns correspond to different conditions while rows correspond to different electrode location. Time scale corresponds to the time scale in Figs. 3–5.
4. Discussion
This study shows the influence of motor imagination on ERS/ERD patterns during combined motor imagery and functional electrical stimulation. MI and FES are often combined modalities in BCI studies aiming at neurorehabilitation. Although MI is used to activate FES it is typically not specified to a participant whether to continue or discontinue with MI once the FES is activated.
The study showed that performing MI before and during FES produces stronger ERD than when MI was terminated upon activation of FES. Interestingly, on the contralateral side, we found larger differences between conditions 1 and 2 (MI before FES and MI before and during FES) than between conditions 2 and 3 (MI before and during FES, and FES alone). We also found weaker ERD in the higher beta band in condition 1 (MI until FES) than in condition 3 (FES alone). Conditions 2 and 3 had ERD in very similar frequency bands. The onset of FES in condition 2, did not change the frequency bands in which ERD was noticed during MI preceding the FES. This result is in accordance with (Saito et al., 2013) showing that efferent and afferent stimulation of the sensory-motor cortex results in ERD in the same frequency bands.
It was a surprising finding a weaker ERD in condition 1 because it is believed that stronger beta oscillations (thus weaker ERD) is associated with keeping ‘status quo’ (Engel and Fries, 2010) which in this study would correspond to condition 2. In experiments with go/no-go task ERS was noticed during no-go task in which monkeys were asked to sustain pressing a lever, while ERD was noticed in a go task when they were asked to release a lever (Zhang et al., 2008). On the contrary, in the current study, keeping ‘status quo’ (continue with MI upon FES) maintained ERD in condition 2 and changing motor status in condition 1 (stop with MI upon FES) effectively resulted in ERS. The difference is that Zhang’s et al. study comprised motor task only while in the current study, motor task was followed by a sensory stimulus which produced ERD at the same frequency band as MI. As a result of motor task being combined with a sensory stimulus, beta ERD present either during MI combined with FES (condition 2) or during FES alone (condition 3) was diminished in condition 1.
On the ipsilateral side, intensity of ERD was significantly stronger in condition 2 compared to both conditions 1 and 3. In condition 3, ERD was visible in the higher beta band only. This demonstrates that while MI results in alpha ERD both contra and ipsilaterally, FES causes alpha ERD mostly on the contralateral side. Beta ERS noticed around 30 Hz on the contralateral side during FES was not noticed on the ipsilateral side. Short increase in power in the theta band, upon the onset of FES was significantly higher in condition 1 than in condition 2 and absent in condition 3.
In the central area, located over the sensory-motor cortex of legs, strong ERD was noticed in the alpha and beta bands in both condition 2 and 3 and was in the same frequency range for MI and for FES. The increase in power in the theta range was noticeable in all three conditions but was again strongest in condition 1. Although studies of MI of legs demonstrated a phenomena called ‘focal ERD/surround ERS’ (Pfurtscheller and Lopes da silva, 1999), current study and some previous BCI studies based on MI of hands (Neuper et al., 2009) reported ERD over the central area, which for MI of hands would correspond to the ‘neighbouring/surrounding region’ of the area activated by MI.
Cassim et al. (2000) reported sustained alpha and beta ERD upon termination of a prolonged repetitive movement. In the current study, alpha ERD could still be noticed 1 s upon termination of FES (longer periods were not analysed) while beta returned to its baseline value much faster in all conditions and over all recorded electrode locations. Over the central location, post-movement beta ERD was also followed by beta ERS.
The short lasting increase in power in the theta band upon initiation and termination of FES, over both contra and ipsilateral sites, had a delay of 300–400 ms with respect to FES. We performed both time and time–frequency analyses to determine whether this was an induced, time locked activity (ERS/ERD), or an evoked, time and phase locked activity (ERP) related to sensory processing of FES. Although ERS/ERD maps showed the frequency specific increase in power, that could be interpreted as theta ERS, time domain analysis showed peaks located approximately at the same time instances as the increase in theta power, closely following the events of activation and deactivation of FES. That indicates that this phenomenon likely presents ERP rather than ERS. Previous studies also showed that neural processing of infrequent but expected FES produces somatosensory P300 (Bruyant et al., 1993).
In general, stronger ERP were noticed upon cessation than upon initiation of FES in all three conditions. Strongest ERP was noticed in condition 1. In time–frequency analysis, the strongest short lasting increase in power was noticed in condition 1, when participants were instructed to terminate MI upon sensing electrical stimulation (i.e. upon activating FES). This is a supporting evidence that patients′ preparation for a sensory stimulus as a cue for an action alters their subsequent brain response.
A drawback of this study is that participants were asked to continue MI of a repetitive movement during FES while FES actually produced a sustained extension of the wrist. The study only models a simple MI-FES scenario. In case of FES used for rehabilitation, patients would practice a functional movement assisted by FES, so they could practice MI of movements produced by FES. Results of the current study are not consistent with results of previous studies on repetitive movements, that showed decline of ERD within 5 s following the initiation of movements (Cassim et al., 2000). In the current study in condition 2, a sustained alpha ERD can be seen throughout MI, before MI, during MI with FES and also following termination of FES, for a total period of 8 s. An explanation for this could be that the initial MI was facilitated and sustained by the visual feedback and that later ERD was sustained by both MI and FES. In this study the excitability of the cortico-spinal tract was not tested. However highest intensity of ERD in condition 2 supports the idea of increased cortical excitability by combining MI and electrical stimulation (Saito et al., 2013).
Mrachacz-Kersting et al. (2012) demonstrated that MI preceding FES strengthens cortico-spinal pathways, thus implying that practicing MI during FES is not relevant. The difference between the current study and study by Mratchacz is that in their study, participants were asked to perform one ballistic movement, rather than to perform MI of a repetitive movement. Their study was based on the analysis of movement-related cortical potential, with a well-defined morphology, rather than of ERS/ERD in time–frequency domain. It is however debatable which type of MI, short ballistic or slower and repetitive, would be more intuitive for neurorehabilitation using BCI and FES. It addition, it is not known if precise initial timing of MI with FES as in Mrachacz-Kersting et al. (2012), followed by prolonged MI during FES would potentially have even stronger rehabilitation effect.
In summary, this study demonstrated the influence of the MI-FES experimental paradigm on the activation of the sensory-motor cortex, as measured by ERS/ERD. Increased cortical activation is of interest in BCI–FES for effective rehabilitation, therefore users should be encouraged to continue with MI throughout the whole period of afferent stimulation of muscles.
Acknowledgements
This work was partially supported by the Engineering and Physical Sciences Research Council (EPSRC), United Kingdom grant EP/P505534/1. The authors wish to thank to all volunteers in the study for their participation.
Conflict of interest: The authors disclose any financial or non-financial interest in the study.
Contributor Information
Clare Reynolds, Email: clare_t_reynolds@hotmail.com.
Bethel A. Osuagwu, Email: b.osuagwu.1@research.gla.ac.uk.
Aleksandra Vuckovic, Email: aleksandra.vuckovic@glasgow.ac.uk.
References
- Alegre M., Labarga A., Gurtubay I.G., Iriarte J., Malanda A., Artieda J. Beta electroencephalograph changes during passive movements: sensory afferences contribute to beta event-related desynchronization in humans. Neurosci Lett. 2002;331:29–32. doi: 10.1016/s0304-3940(02)00825-x. [DOI] [PubMed] [Google Scholar]
- Bruyant P., García-Larrea L., Mauguière F. Target side and scalp topography of the somatosensory P300. Electroencephalogr Clin Neurophysiol. 1993;88:468–477. doi: 10.1016/0168-5597(93)90036-o. [DOI] [PubMed] [Google Scholar]
- Cassim F., Szurhaj W., Sediri H., Devos D., Bourriez J., Poirot I. Brief and sustained movements: differences in event-related (de)synchronization (ERS/ERD) patterns. Clin Neurophysiol. 2000;111:2032–2039. doi: 10.1016/s1388-2457(00)00455-7. [DOI] [PubMed] [Google Scholar]
- Celnik P.A., Cohen L.G. Modulation of motor function and cortical plasticity in health and n disease. Restor Neurol Neurosci. 2004;22:261–268. [PubMed] [Google Scholar]
- Cho W, Vidaurre C, Hoffmann U, Birbaumer N, Ramos-Murguialday A. Afferent and efferent activity control in the design of brain computer interfaces for motor rehabilitation. In: Conf Proc IEEE Eng Med Biol Soc, 30 Aug–3 Sept 2011. Edited by EBMS; 2011. p. 7310–5. [DOI] [PubMed]
- Conforto A.B., Kaelin-Lang A., Cohen L.G. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- Daly J.J., Cheng R., Rogers J., Litinas K., Horvat K., Dohring M. Feasibility of a new application of noninvasive Brain Computer Interface (BCI): a case study of training for recovery of volitional motor control after stroke. J Neurol Phys Ther. 2009;33:203–211. doi: 10.1097/NPT.0b013e3181c1fc0b. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dobkin B.H. Brain–computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J Physiol. 2007;579:637–642. doi: 10.1113/jphysiol.2006.123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda R.O., Hart P.E., Stork D.G. Pattern classification. 2nd ed. Wiley Interscience; 2001. Linear discriminant functions; pp. 215–281. [Google Scholar]
- Engel A.K., Fries P. Beta band oscillations—signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Erbil N., Ungan P. Changes in the alpha and beta amplitudes of the central EEG during the onset, continuation, and offset of long-duration repetitive hand movements. Brain Res. 2007;1169:44–56. doi: 10.1016/j.brainres.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Everaert D.G., Thompson A.K., Chong S.L., Stein R.B. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010;24:168–177. doi: 10.1177/1545968309349939. [DOI] [PubMed] [Google Scholar]
- Graimann B., Huggins J.E., Levine S.P., Pfurtscheller G. Visualization of significant ERS/ERD patterns in multichannel EEG and ECoG data. Clin Neurophysiol. 2002;113:43–47. doi: 10.1016/s1388-2457(01)00697-6. [DOI] [PubMed] [Google Scholar]
- Grosse-Wentrup M., Mattia D., Oweiss K. Using brain–computer interfaces to induce neural plasticity and restore function. J Neural Eng. 2011;8:025004. doi: 10.1088/1741-2560/8/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L.R., Field-Fote E.C. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther. 2007;87:208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Kaiser V., Bauernfeind G., Kreilinger A., Kaufmann T., Kübler A., Neuper C. Cortical effects of user training in a motor imagery based brain–computer interface measured by fNIRS and EEG. Neuroimage. 2014;85:432–444. doi: 10.1016/j.neuroimage.2013.04.097. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the eeg spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Ridding M.C. Afferent stimulation facilitates performance on a novel motor condition. Exp Brain Res. 2006;170:109–115. doi: 10.1007/s00221-005-0192-x. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N., Kristensen S.R., Niazi I.K., Farina D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J Physiol. 2012;590:1669–1682. doi: 10.1113/jphysiol.2011.222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder T. Motor imagination and action observation: cognitive tools for rehabilitation. J Neural Transm. 2007;114:1265–1278. doi: 10.1007/s00702-007-0763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G.R., Neuper C., Rupp R., Keinrath C., Gerner H.J., Pfurtscheller G. Event-related beta EEG changes during wrist movements induced by functional electrical stimulation of forearm muscles in man. Neurosci Lett. 2003;340:143–147. doi: 10.1016/s0304-3940(03)00019-3. [DOI] [PubMed] [Google Scholar]
- Neuper C., Scherer R., Wriessnegger S., Pfurtscheller G. Motor imagery and action observation: modulation of sensorimotor brain rhythms during mental control of a brain–computer interface. Clin Neurophysiol. 2009;120:239–247. doi: 10.1016/j.clinph.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Powell J., Pandyan A.D., Granat M., Cameron M., Stott D.J. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30:1384–1389. doi: 10.1161/01.str.30.7.1384. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Saito K., Yamaguchi T., Yoshida N., Tanabe S., Kondo K., Sugawara K. Combined effect of motor imagery and peripheral nerve electrical stimulation on the motor cortex. Exp Brain Res. 2013;227:333–342. doi: 10.1007/s00221-013-3513-5. [DOI] [PubMed] [Google Scholar]
- Scherer R. rtsBCI – a collection of methods and functions for real-time data acquisition, storage, signal processing and visualization based on Matlab/Simulink. Available online: <http://biosig.sf.net> [accessed September 2014].
- Stein R.B., Everaert D.G., Roy F.D., Chong S., Soleimani M. Facilitation of corticospinal connections in able-bodied people and people with central nervous system disorders using eight interventions. J Clin Neurophysiol. 2013;30:66–78. doi: 10.1097/WNP.0b013e31827ed6bd. [DOI] [PubMed] [Google Scholar]
- Szameitat A.J., Shen S., Conforto A., Sterr A. Cortical activation during executed, imagined, observed, and passive wrist movements in healthy volunteers and stroke patients. Neuroimage. 2012;62:266–280. doi: 10.1016/j.neuroimage.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Tam W., Tong K., Fei M., Gao S. A minimal set of electrodes for motor imagination BCI to control an assistive device in chronic stroke participants: a multi-session study. IEEE Trans Neural Syst Rehabil Eng. 2011;19:617–627. doi: 10.1109/TNSRE.2011.2168542. [DOI] [PubMed] [Google Scholar]
- Vidaurre C., Sander T.H., Schlögl A. BioSig: the free and open source software library for biomedical signal processing. Comput Intell Neurosci. 2011;2011:935364. doi: 10.1155/2011/935364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic A., Osuagwu B.A. Using a motor imagery questionnaire to estimate the performance of a brain–computer interface based on object oriented motor imagery. Clin Neurophysiol. 2013;124:1586–1595. doi: 10.1016/j.clinph.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Vuckovic A., Hasan M.A., Fraser M., Conway B.A., Nasseroleslami B., Allan D.B. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J Pain. 2014;15:645–655. doi: 10.1016/j.jpain.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen Y., Bressler S.L., Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience. 2008;156:238–246. doi: 10.1016/j.neuroscience.2008.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]