Abstract
Transforming growth factor (TGF-β1) is among the strongest factors of liver fibrogenesis, but its association with Schistosoma-caused liver fibrosis is controversial. Tissue transglutaminase (tTG) is the principal enzyme controlling TGF-β1 maturation and contributes to Sj-infected liver fibrosis. Here we aim to explore the consistency between tTG and TGF-β1 and TGF-β1 source and its correlation with liver fibrosis after Sj-infection. TGF-β1 was upregulated at weeks 6 and 8 upon liver fibrosis induction. During tTG inhibition, TGF-β1 level decreased in sera and liver of infected mice. TGF-β1 showed positive staining in liver containing Sj adult worms and eggs. TGF-β1 was also detected in Sj adult worm sections, soluble egg antigen and Sj adult worm antigen, and adult worms' culture medium. The TGF-β1 mature peptide cDNA sequence and its extended sequence were amplified through RT-PCR and RACE-PCR using adult worms as template, and sequence is analyzed and loaded to NCBI GenBank (number GQ338152.1). TGF-β1 transcript in Sj eggs was higher than in adult worms. In Sj-infected liver, transcriptional level of TGF-β1 from Sj, but not mouse liver, correlated with liver fibrosis extent. This study provides evidence that tTG regulates TGF-β1 and illustrates the importance of targeting tTG in treating Sj infection-induced fibrosis.
1. Introduction
Schistosomiasis is one of the nine neglected tropical diseases that received much attention over the last several years. After Schistosoma cercariae penetrate the hosts' skin and develop into adult worms, they reside in tributaries of the portal vasculature where they continuously release eggs. The portal blood flow then carries the eggs into the liver where they induce production of inflammatory granuloma and, subsequently, tissue repair and fibrosis. Schistosoma japonicum (Sj) mainly damages mammalian hosts by producing liver granuloma and fibrosis [1].
Transforming growth factor (TGF-β1) is one of the strongest factors that lead to liver fibrosis. TGF-β1 promotes hepatic stellate cell (HSC) proliferation and collagen synthesis in the activated HSC [2–4] or modulates deposition of extracellular matrix (ECM) components and immune functions [5]. However, the relationship between TGF-β1, liver fibrosis, and Schistosoma infection is controversial. Alves Oliveira et al. [6] and Kaviratne et al. [7] have demonstrated that IL-13, but not TGF-β1, is strongly associated with fibrosis during S. mansoni (Sm) infection. However, Techau et al. [8] discovered that pigs prenatally exposed to Sj showed higher levels of TGF-β1 mRNA expression in the liver than postnatally infected and noninfected pigs. TGF-β1 has sometimes been accepted as the key factor inducing liver granuloma and fibrosis during Sj infection because some researchers have recognized TGF-β1 inhibition as one of the factors that can be used to evaluate the antifibrotic effects of drugs on hosts infected with Sj [9–11]. However, systemic studies that reveal whether liver fibrosis caused by Sj infection is dependent or not dependent on TGF-β1 are lacking.
In vertebrates, the TGF-β superfamily is a structurally conserved but functionally diverse group of proteins with at least 35 members, including the prototypic TGF-β subfamily (comprising TGF-β1, TGF-β2, and TGF-β3), an extensive bone morphogenetic protein (BMP) subfamily (with 20 members), the growth and differentiation factor subfamily (at least 9 members), and the activin/inhibin subfamily (InACT). A common feature shared by the members of this family is that the mature bioactive forms are homo- or heterodimers corresponding to the cleaved carboxyterminal regions of larger preproproteins [12]. The activated TGF-β1 binding with specific receptors in the cell membrane through the Smad signal transduction pathways plays the biological role [13]. Some members of TGF-β superfamily, including InACT, BMP, receptors of TGF-β [14–18] and Smad1, Smad2, and Smad4, and their signaling pathway-associated molecules have been identified in Schistosoma [19–22]. InAct plays important roles in Sm development and embryogenesis [23]. In addition, Hirata et al. [24] revealed the expression of TGF-β-like molecules in Sj cercariae, schistosomula, eggs, and adult worms by using antibodies against anti-mouse TGF-β1, TGF-β2, and TGF-β3, respectively. However no study has revealed that members of TGF-β subfamily exist in Sj, as well as their roles in the parasite development or pathogenesis, even though genomes of Sj and Sm have already been analyzed [25, 26].
Molecular mechanisms of host-parasite interaction are complex and involve much molecular cross talk, including ligands and receptors, substrates, and enzymes, which are either from the host or from the parasite. Up to now, many studies have indicated that tissue transglutaminase (tTG) and TGF-β1 are closely related. TGF-β1 dimer is synthesized intracellularly and combines with latency-associated peptide (LAP) to form the small latent TGF-β complex (SLC). The mature inactive SLC then forms the large latent TGF-β complex (LLC) by covalent bonding with the large latent TGF-β binding protein (LTBP-1) and is stored in the ECM [27, 28]. Latent TGF-β activation in the ECM involves tTG as the principal enzyme that covalently cross-links LBTP to major ECM proteins, such as fibronectin, thereby controlling the rate of TGF-β maturation [29–31]. Upregulation of extracellular tTG increases the levels of active TGF-β both in cell-culture models and in vivo in various pathological states [32, 33].
Our previous research showed that tTG is involved in the development of Sj-infection-induced liver fibrosis in mice, and the underlying mechanism may be associated with tTG-regulated IL-13 expression [34]. In this study, we investigated the association between tTG and TGF-β1 that originated from the host or from Sj using Sj-infected mice as liver fibrosis model. We showed that tTG-regulated TGF-β1 in the parasite is related to mouse liver fibrosis after Sj-infection.
2. Materials and Methods
2.1. Ethics Statement
This study was performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of State Scientific and Technological Commission. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Guangzhou Medical University (permit number: SCXK(Guangdong)2011-0029). All surgeries were performed under sodium pentobarbital anesthesia, and every effort was made to minimize suffering.
2.2. Parasites, Animals, and Culture Medium of Sj Adult Worms
Female BABL/c mice (6 weeks old to 8 weeks old; from the Experimental Animal Center of Sun Yat-Sen University, Guangzhou, China) were maintained according to the guidelines approved by the Guangzhou Medical University Animal Experiment and Care Committee. Cercariae of Sj Chinese mainland strain were obtained from the infected Oncomelania hupensis (Jiangsu Institute for Schistosomiasis Control, Wuxi, China). Adult schistosomes were recovered by hepatic-portal perfusion from BABL/c mice that had been percutaneously exposed to 20 ± 3 cercariae. Adult parasites and eggs were collected and were maintained in phosphate-buffered saline (PBS) for soluble worm antigen (SWA) and soluble egg antigen (SEA) preparation. Twenty pairs of freshly washed adult worms were transferred to 2 mL RPMI 1640 medium supplemented with 1 mM glutamine, 1000 units/mL penicillin, and 1000 μg/mL streptomycin for 2 h. Worms were finally cultured in 2 mL sterile RPMI 1640 medium supplemented with 20% sterile fetal bovine serum (FBS), 1 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin for 16 h. The adult Sj culture medium and negative control medium (sterile RPMI 1640 medium supplemented with 20% FBS, 1 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin) were collected.
2.3. Reagents
TGF-β1 ELISA kit was obtained from R&D systems. The following antibodies were used for Western blot analysis or immunohistochemistry (IHC) assay: anti-TGF-β1(V) (sc-146, Santa Cruz Biotechnology), anti-GAPDH (Cell Signaling Technology), anti-alpha SMA (BOSTER), anti-Smad2 (sc-101153, Santa Cruz Biotechnology), and anti-phosphospecific Smad2 (sre465/476, MILLIPORE).
2.4. Parasite Infection, Cystamine (CTM) Administration, and Sample Collection
Forty BABL/c mice were infected cutaneously with 20 ± 3 Sj cercariae for 5, 6, 8, or 12 weeks (10 mice in each time course), and 10 uninfected mice served as the control. CTM (Sigma-Aldrich, St. Louis, USA, tTG inhibitor) treatment in mice was shown in our previous study [34]. CTM (10−2 mM) was administered in each mouse once per day for 7 d, whereas PBS was used as control. Blood sera for ELISA were collected from each group by cutting the caudal vein of the mice. Perfusions of the hepatic portal system of Sj-infected mice were performed to collect adult worms, as described previously. Meanwhile, liver lobes were prepared for Western blot analysis, IHC or RT-PCR, and qPCR. Mouse infection, CTM administration, and sample collection were repeated at least twice.
2.5. TGF-β1 Detection by ELISA
Blood samples were collected by cutting the tail veins of mice in each group and placed into EP tube with 1000 IU/mL heparin (10 μL), after which blood plasma was collected (3000 rpm, 10 min centrifugation). Blood plasma was diluted 1 : 4 ratio and was immediately used in experiments. TGF-β1 level were detected using the mouse DuoSet ELISA Development kit (R&D Systems: DY1679) according to the manufacturer's instructions.
2.6. Western Blot
The liver of Sj-infected mouse was collected and was ground into powder in liquid nitrogen, and moderate amount of protein lysis solution (RIPA from Shanghai Bocai Biological Technology Co., Ltd.) was added for liver tissue protein preparation. Protein concentration was determined by Bradford assay (Bio-Rad, Redmond, WA). Tissue lysates (30 μg) were separated by 10% SDS-PAGE and then transferred onto polyvinylidene fluoride membranes (Amersham, Bucks, UK). The membranes were blocked with 5% nonfat dried milk before incubation with target-specific antibodies. Protein bands were detected with ECL reagents.
2.7. RT-PCR, Rapid Amplification of cDNA Ends-PCR (RACE-PCR), and Real-Time Quantitative Polymerase Chain Reaction (Q-PCR)
Liver tissues of mice or Sj adult worms from Sj-infected mice were homogenized in Trizol (Invitrogen, Carlsbad, CA), and total RNA was extracted according to the manufacturer's protocol. RNA purity was assessed by spectrophotometry. Reverse transcription reactions for cDNA synthesis were performed using PrimeScript RT Master Mix (TAKARA). Relative expression level of mRNA was determined by Q-PCR with SYBR Green I PCR Master (TAKARA) using ABI7500. Data were normalized with mouse GAPDH and Sj tubulin-α. PCR products were analyzed by electrophoresis on 1% agarose gels containing ethidium bromide, and Q-PCR results were expressed as fold amplification using the 2−ΔΔCt method. Each experiment was repeated three times.
2.8. Statistics
All experiments were repeated at least twice with similar results. Data were compared by Student's t-test. Results were expressed as mean ± SD. P < 0.05 was considered significant.
3. Results
3.1. TGF-β1 Is Upregulated in Sj-Infected Mice
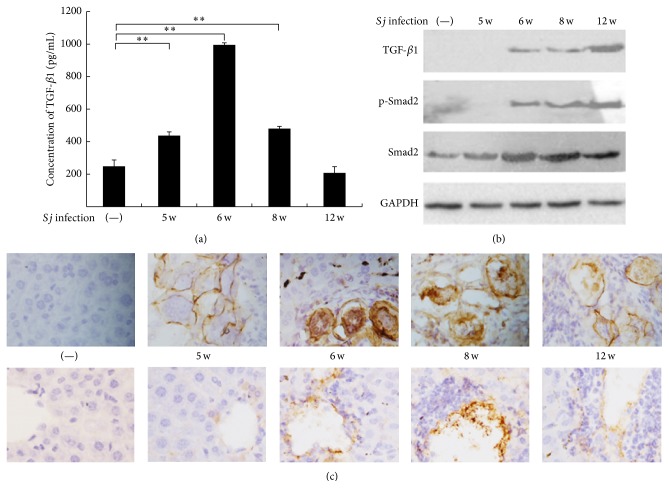
We previously reported a high extent of post-Sj-infection hepatic fibrosis in mice. Liver granuloma began at week 5, and fibrosis progressed most seriously at week 8, whereas chronic liver fibrosis appeared at week 12 [34]. TGF-β1 was usually the key factor in inducing liver fibrosis compared with other causes [2, 3]. TGF-β1 concentration in mouse serum increased 5, 6, and 8 weeks after Sj infection, and the highest level was observed at week 6 (Figure 1(a)). Western blot analysis and IHC assay revealed that TGF-β1 protein level in Sj-infected mouse liver also increased (Figures 1(b) and 2(c)). In addition, Smad2, the downstream signaling protein of TGF-β1 pathway, was also activated in mice liver after Sj infection (Figure 1(b)). TGF-β1 could be involved in liver fibrosis in a Smad2-dependent manner during Sj infection. TGF-β1 was localized either in the cells of blood vessels where Sj adult worms reside or in the eggs of Sj and in the cells of liver tissue where eggs are deposited (Figure 1(c)). The results suggested that TGF-β1 likely promoted hepatic fibrosis after Sj infection.
Figure 1.
TGF-β1 level increased gradually in mice liver during the courses of Sj infection. (a) TGF-β1 level in serum was determined by ELISA in BALB/c mice with 20 ± 3 infective Sj cercariae for 5, 6, 8, and 12 weeks, and uninfected mice were used as control. Data are shown as means ± SD of 10 mice/group. Experiment was performed four times (∗ P < 0.05; and ∗∗ P < 0.01 compared with uninfected group). (b) Equal amounts of proteins of mouse liver tissue lysates at indicated time points were used in the Western blot assay to detect protein expression levels of TGF-β1, p-Smad2, and Smad2. GAPDH was used as loading control. (c) Mouse livers at indicated time points were fixed in paraformaldehyde, embedded in paraffin, and then sliced and immunohistochemically stained for TGF-β1. Representative staining for TGF-β1 is shown at ×400 magnification. Top: TGF-β1 expression in egg, Sj egg granuloma, and the surrounding tissue. Bottom: TGF-β1 expression in hepatic cell and liver tissue around the liver sinusoid.
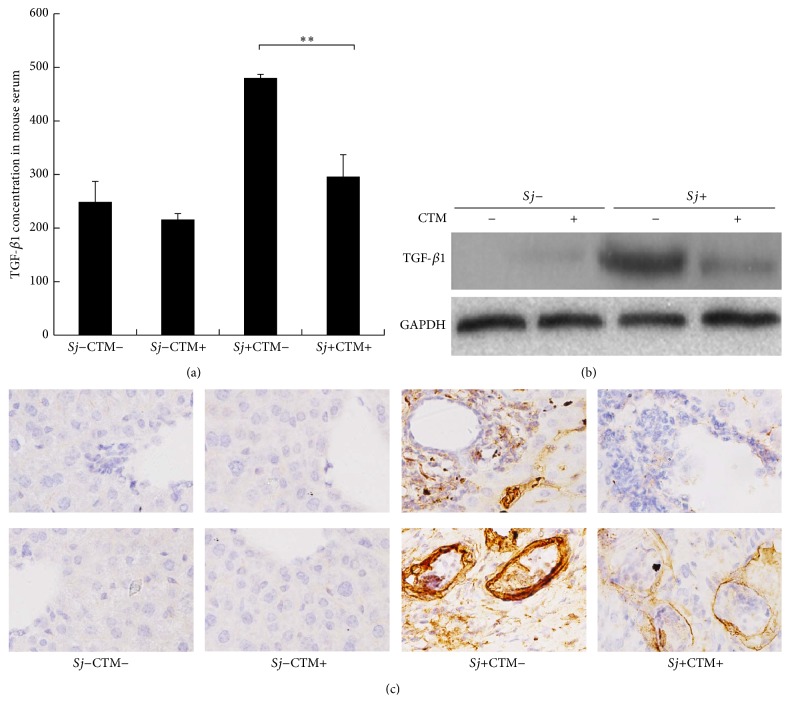
Figure 2.
CTM reduced TGF-β1 expression profile in the liver of Sj-infected mice. tTG activity of BABL/c mouse liver was inhibited by CTM intraperitoneal injection from day 3 to day 10 after Sj infection. Mice were sacrificed at week 8 after infection. (a) Activated TGF-β1 concentration in mouse serum of normal or Sj-infected mice with or without CTM treatment was detected using ELISA. Data were presented as mean ± SD from 10 mice per each group. ∗ P < 0.05; and ∗∗ P < 0.01. (b) Activated TGF-β1 protein levels in mouse liver of normal or Sj-infected mice with or without CTM treatment were detected by Western blot analysis, and GAPDH was used as the internal control. (c) Mouse liver samples collected at indicated time points were fixed in paraformaldehyde, embedded in paraffin, sliced, and immunohistochemically stained for TGF-β1. Representative stainings for TGF-β1 were shown at 40x magnification. Bottom: TGF-β1 expression in egg, Sj egg granuloma, and the surrounding tissue. Top: TGF-β1 expression in hepatic cell and liver tissue around the liver sinusoid; “−” = without, “+” = with.
3.2. Mature TGF-β1 Level Is Decreased along with Alleviation of tTG Activity
To clarify whether tTG induces TGF-β1 maturation during Sj infection, tTG activity inhibitor CTM was used to block tTG activity. The extent of liver fibrosis was suppressed after Sj-infected mice were treated with CTM, and no effect was observed in untreated mice [34]. ELISA results showed that the concentrations of TGF-β1 mature peptide were 296.21 and 480.35 pg/mL in mouse sera with and without CTM treatment, respectively (Figure 2(a)) (P < 0.05). Western blot analysis results revealed that TGF-β1 protein expression level in CTM-treated Sj-infected mice liver in situ was lower compared with that in Sj-infected mice that were not subjected to CTM treatment (Figure 2(b)). IHC assay results showed that, in mice liver in situ, the intensity of positive stain, which indicated active TGF-β1, was remarkably reduced in CTM-treated Sj-infected mice liver compared with Sj-infected mice without CTM treatment (Figure 2(c)). Moreover, this reduction was mainly observed around hepatic sinusoids where Sj adult worms reside, as well as around and in egg granulomas where Sj eggs are deposited. TGF-β1 in Sj eggs was also reduced in Sj-infected mice subjected to CTM treatment (Figure 2(c)). These results indicated that tTG-regulated TGF-β1 promoted hepatic fibrosis in mice during Sj infection, and TGF-β1 proteins located in Sj are partially regulated by tTG of host origin.
3.3. TGF-β1 Protein Was Detected in Sj
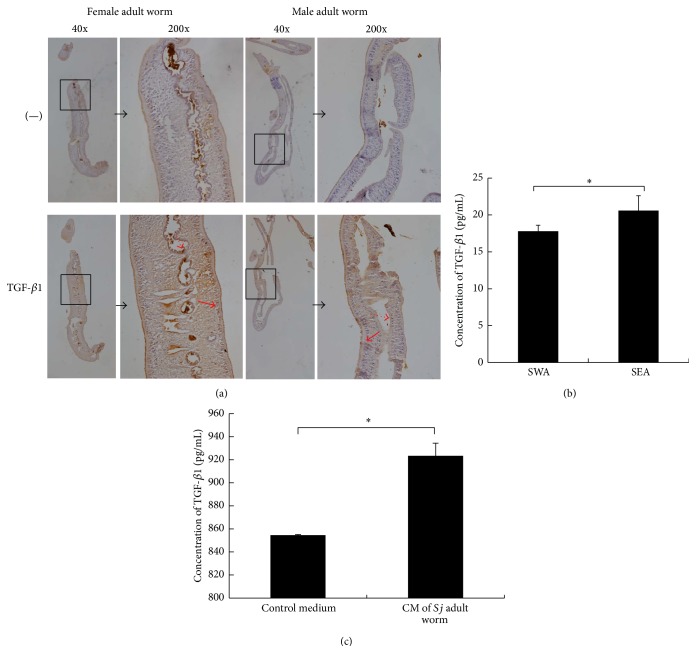
Similar to the findings of Hirata et al. [24], our results showed that TGF-β subfamily immunoreactive molecules are probably expressed in adult worms and eggs of Sj (Figure 2(c)). We validated these results through an IHC assay using sections of male and female adult worms (Figure 3(a)). Moreover, we detected TGF-β1 protein in the SEA, SWA of Sj, and in the culture medium of Sj adult worm using ELISA (Figures 3(b) and 3(c)). Figure 3(a) shows that TGF-β1 immunoreactivity was apparent in subtegumental cells and the lining gut epithelial cells of male and female worms, especially in female worms. TGF-β1 concentrations in SEA and SWA were 17.9 and 20.7 pg/mL, respectively (Figure 3(b)). Furthermore, higher concentration of TGF-β1 was secreted in culture medium of adult worms than in the control medium (Figure 3(c)).
Figure 3.
TGF-β1 protein was expressed in Sj and was secreted. (a) Sj adult female and male worms were fixed in paraformaldehyde, embedded in paraffin, and then sliced and stained for TGF-β1. Representative staining (brown) is shown at 40x magnification (large panel) and ×200 (inset). Dotted red arrow: gut epithelial cells; solid red arrow: subtegumental cells. (b) Equal amounts of Sj soluble adult worm antigen and soluble egg antigen were tested for TGF-β1 by ELISA. Data were shown as means ± SD. Experiment was performed four times (∗ P < 0.05; and ∗∗ P < 0.01 compared with negative PBS control). (c) Twenty pairs of adult worms were freshly collected, washed thrice using PBS, transferred into 2 mL sterile RPMI 1640 medium supplemented with 1 mM glutamine, 1000 units/mL penicillin, and 1000 μg/mL streptomycin for 2 h, and finally cultured in 2 mL sterile RPMI 1640 medium supplemented with 20% FBS, 1 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin for 16 h. Sj worm culture medium was collected for TGF-β1 detection by ELISA, and condition medium (sterile RPMI 1640 medium supplemented with 20% FBS, 1 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin) was used as control. Experiment was performed four times (∗ P < 0.05; and ∗∗ P < 0.01 compared with control medium without Sj adult worms).
3.4. Amplification of TGF-β1 cDNA Sequence Selectively Using Sj as Template
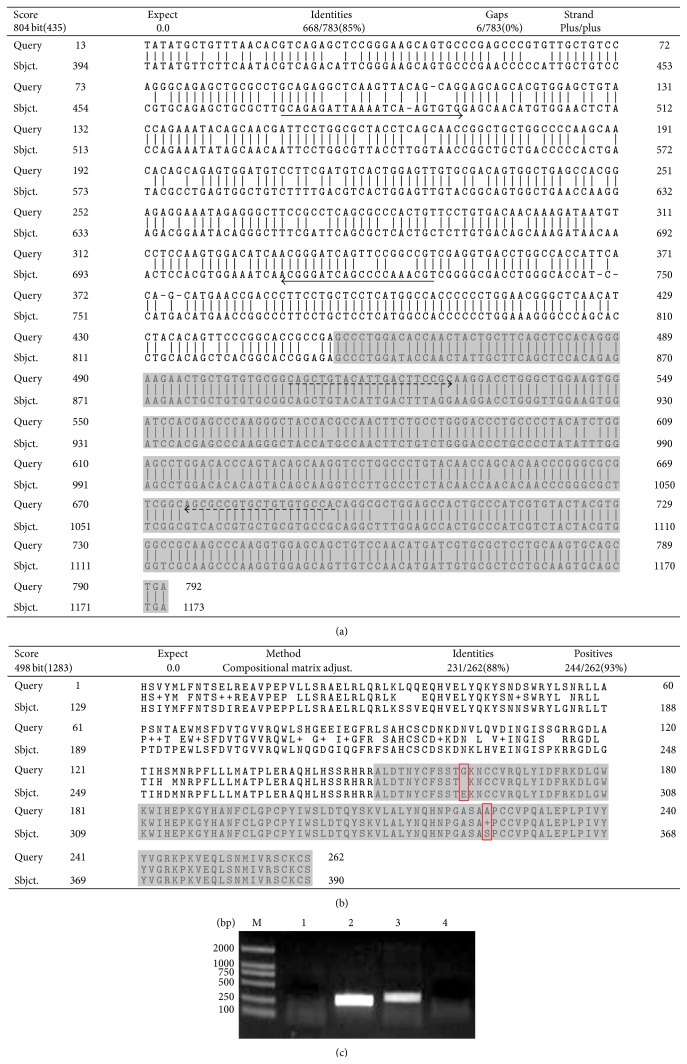
To clarify whether or not TGF-β1 gene exists in Sj, we designed a pair of primers according to the cDNA sequence of mouse TGF-β1 mature peptide because the amino acid sequence of TGF-β1 mature peptide is highly conserved. This primer pair and Sj adult worm cDNA isolated from mice as template were used for PCR amplification of Sj TGF-β1 mature peptide cDNA, and a 339 bp product was obtained, sequenced, and analyzed using bioinformatics. The 5′ and 3′ ends of the fragment were extended via RACE-PCR using primers designed within the known sequence. The extended TGF-β1 cDNA sequence (792 bp long) was loaded into National Center for Biotechnology Information (NCBI) GenBank with number GQ338152.1. The deduced amino acid sequence comprised 263 amino acid residues and contained partial sequence of TGF-β1 propeptide and complete TGF-β1-like domain. The nucleotide sequence of extended TGF-β1 gene from Sj was 85% identical to that from mouse (Figure 4(a)), whereas the amino acid sequence of TGF-β1 was 88% identical (Figure 4(b)), as revealed by BLAST search results. The nucleotide sequence of Sj TGF-β1 mature peptide was different from that of mouse by multiple nucleotides, but the amino acid sequence of Sj TGF-β1 mature peptide had merely two amino acid differences compared with that of mouse (Figure 4(b)). To identify species-specific TGF-β1, we designed and identified primers of Sj and mouse-specific primers (the primer sequences have been underlined in solid and dashed lines, resp., as shown in Figure 4(a)). Figure 4(c) shows that positive bands appeared in the agarose gel only when we used specific primer pairs and the corresponding templates for PCR amplification. Moreover, the transcription level of TGF-β1 was higher in eggs than in adult Sj worms. These results suggested the existence of TGF-β1 gene in Sj adult worms and eggs.
Figure 4.
TGF-β1 gene in Sj was cloned and identified. (a) TGF-β1 mature peptide cDNA in Sj was amplified using Sj adult worm cDNA collected from infected mice as template and primers designed according to mouse TGF-β1 mature peptide sequence, and the 5′ and 3′ ends of TGF-β1 in Sj were extended via RACE. The alignment of TGF-β1 cDNA sequences of Sj and mouse is shown (Query is Sj TGF-β1 and Sbjct. is mouse TGF-β1 cDNA sequence). Solid lines: specific mouse TGF-β1 primer pairs for PCR amplification; dotted lines: specific Sj TGF-β1 primer pairs for PCR amplification. Gray: TGF-β1 mature peptide cDNA sequence. (b) The alignment of TGF-β1 amino acid sequences of Sj and mouse is shown (Query is Sj TGF-β1 and Sbjct. is mouse TGF-β1 amino acid sequence). Gray: TGF-β1 mature peptide amino acid sequence. (c) TGF-β1 primers of mouse- or Sj-specific source were identified through PCR using Sj adult worms or normal mice liver cDNA as template, respectively. M: DL2000 DNA Marker; 1: mouse cDNA as template and specific Sj TGF-β1 primer; 2: mouse cDNA as template and specific mouse TGF-β1 primer; 3: Sj cDNA as template and specific Sj TGF-β1 primer; 4: Sj cDNA as template and specific mouse TGF-β1 primer.
3.5. High TGF-β1 Transcription Level in Sj Was Consistent with the Extent of Liver Fibrosis in Mice
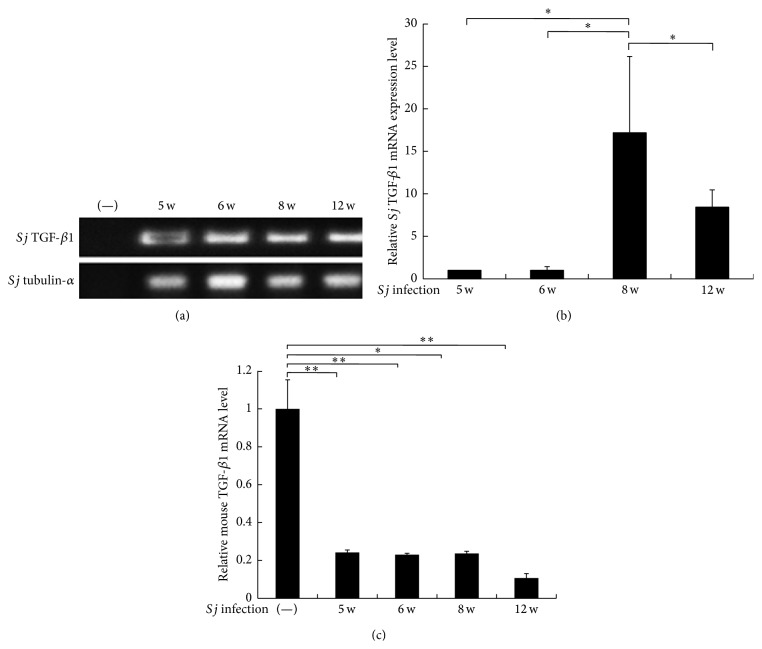
To gain a better understanding of the source of TGF-β1 in hepatic fibrogenesis, the transcriptional levels of TGF-β1 in mice and Sj were evaluated using RT-PCR and SYBR Green quantitative PCR. Sj-infected mouse liver cDNA was prepared as a PCR template, and the specific primer pairs shown in Figure 4(a) were also used. TGF-β1 mRNA expression level in Sj increased, especially at week 8 (Figures 5(a) and 5(b)), and this level was comparable with the TGF-β1 protein level in blood plasma and livers of infected mice, as shown in Figures 1(a) and 1(b). However, the mRNA expression level of mouse TGF-β1 decreased significantly in all time courses after Sj infection (Figure 5(c)). These results suggested that high protein level of TGF-β1 was transcribed mainly from Sj, but not from mice. This finding was consistent with the low transcriptional level of TGF-β1 in the liver during Sj infection, as reported by Bartley et al. [35], although Bartley et al. did not test TGF-β1 from the parasite.
Figure 5.
TGF-β1 is transcribed in Sj and Sj-infected mouse liver. Equal amounts of total RNA from left lobes of BALB/c mice liver with 20 ± 3 infective cercariae of Sj for 5, 6, 8, and 12 weeks were detected for TGF-β1 mRNA expression in Sj by using PCR (a) and SYBR Green-based quantitative PCR (b). Sj tubulin-α was detected as an input control. ∗ P < 0.05, ∗∗ P < 0.01. (c) Equal amounts of total RNA from infected mice livers at indicated time points were detected for mouse TGF-β1 mRNA expression by SYBR Green-based quantitative PCR. GAPDH was detected as an input control. ∗ P < 0.05, ∗∗ P < 0.01.
4. Discussion
Infection with the parasitic helminth Schistosoma accounts for a significant portion of liver fibrosis cases in humans. The causative factors of hepatic fibrogenesis and the host-parasite interaction mechanisms need to be elucidated. TGF-β1 is one of the strongest factors promoting liver fibrosis by activating HSC [2–4]. Sj-infected mouse model did not exhibit high TGF-β1 transcription level, but chronic schistosomiasis patients showed high TGF-β1 transcription level compared with healthy individuals [35, 36]. Hirata et al. [24] revealed the expression of TGF-β1-, TGF-β2-, and TGF-β3-like molecules in Sj. Some members of TGF-β superfamily, including InACT, BMP, receptors of TGF-β, and Smad1, Smad2, and Smad4 and other signaling pathway-associated molecules have been identified in Schistosoma [14–22]. In addition, tTG is the principal enzyme controlling TGF-β maturation rate [29–31]. Upregulation of tTG increases the concentrations of active TGF-β in various pathological states [31–33]. Thus, we systemically detected the level and source of TGF-β1, its relationship with tTG, and the extent of liver fibrosis in mice.
Our results showed that a high level of TGF-β1 mature peptide existed in liver tissue and blood stream, and the overexpression of TGF-β1 in Sj-infected liver section was mainly observed in cells near the Sj adult worms parasites or the deposited Sj eggs. TGF-β1 was downregulated by tTG inhibitor CTM treatment. The protein level of TGF-β1 mature peptide was highly consistent with the level of tTG protein and activity. In mouse models and human patients with alcoholic steatohepatitis, tTG provokes hepatocyte death and is associated with alcohol-induced liver fibrosis [37–39]. Our previous study demonstrated that HSC of Sj-infected-mice liver were activated and the extent of liver fibrosis gradually worsened from week 5 to week 12, and these changes were associated with tTG and IL-13 levels [34]. This study indicated that tTG-regulated TGF-β1 is also correlated with liver fibrosis.
IHC, qPCR, and ELISA results revealed the presence of TGF-β1 in Sj adult worm sections, Sj eggs, and the cultured medium of Sj adult worms. Furthermore, we extended and cloned the sequence of TGF-β1 in Sj. Although Sj whole-genome shotgun (WGS) sequence has been loaded into the NCBI GenBank [40], we failed to find any identical or even similar sequence using NCBI BLASTN to analyze the homology of the extended TGF-β1 cDNA sequence with the Sj WGS sequence. We also could not amplify its DNA sequence using Sj DNA as template and many alternative primer pairs. Parasites were nutritionally dependent on their host organisms and generally have an intimate, long-term physical association with their hosts [41]. Horizontal gene transfer (HGT) is rampant in prokaryotes [42]. Numerous independent studies have implicated schistosomes as agents (donors or recipients) of HGT [43]. Schistosomes cover their body surface with host antigens to avoid being detected by the host's immune system [44, 45] and this form of molecular mimicry might be due to HGTs [46–50]. Retroviruses such as transposable elements (TEs) may have the capacity to transfer genes [51]. Long terminal repeat (LTR) retrotransposons encode envelope-like proteins that provide infective capacity similar to viruses [52, 53]. Schistosome genomes are relatively large and known to be rich in TEs. Approximately half of their genetic material consists of TEs and repeat sequences, including LTR retrotransposons [26, 40, 54]. Therefore, TGF-β1 in Sj might be transferred through HGTs or retroviruses (TEs). Multiple lines of evidence are needed for conclusive documentation while avoiding false positives.
High transcription level of Sj-specific TGF-β1, but not mice-specific TGF-β1, was consistent with the extent of liver fibrosis. tTG, being the principal enzyme, controls the rate of TGF-β1 maturation [29–31]. Our previous report also confirmed that tTG is involved in the development of Sj infection-induced liver fibrosis in mice, and the mechanism may be associated with tTG-regulated IL-13 expression [34]. Thus, we demonstrated the relationship between tTG and TGF-β1. In our study, TGF-β1 is upregulated in Sj-infected mice liver (Figure 1), and TGF-β1 level is suppressed by CTM along with Sj-induced liver fibrosis remission (Figure 2), thereby suggesting that tTG-regulated Sj TGF-β1 is involved in liver granuloma and fibrosis in Sj-infected mice.
In summary, we confirmed that tTG-regulated TGF-β1 and IL-13 were associated with liver fibrosis in mice after Sj-infection; thus, tTG might serve as a potent treatment target. Whether TGF-β1 was of Sj and not of host origin requires further study.
5. Conclusion
In Sj-infected mice, TGF-β1 level in Sj is regulated by tTG and is consistent with the extent of liver granuloma and fibrosis. The origin or transfer route of TGF-β1 gene in Sj needs to be confirmed in the future. Host tTG helps with TGF-β1 maturation in the parasite, thereby showing that tTG is probably a potential drug target.
Acknowledgments
This work was funded by the NSFC (no. 30600516), Science & Technology Planed Projects of Guangzhou (no. 2012J4100009), and Immunology as one of the Key Disciplines of Guangzhou City Universities (no. B127007).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Juanjuan Tang, Xunmin Zhu, and Jingjing Zhao contributed equally to this work. Juanjuan Tang, Xunmin Zhu, and Jingjing Zhao performed the research; Zi Li, Juanjuan Tang and Jingjing Zhao designed the research; Xunmin Zhu, Xiaofang Ji, Yinyan Li, Zhiyan Gao, Xiaomin Li, and Suikai Yan contributed essential reagents or tools; Juanjuan Tang analyzed the data; Juanjuan Tang wrote the paper; and Zi Li, Mingchiu Fung, and Fang Su revised the paper.
References
- 1.Wilson M. S., Mentink-Kane M. M., Pesce J. T., Ramalingam T. R., Thompson R., Wynn T. A. Immunopathology of schistosomiasis. Immunology and Cell Biology. 2007;85(2):148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen T., Jenkins R. H., Fraser D. J. MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. Journal of Pathology. 2013;229(2):274–285. doi: 10.1002/path.4119. [DOI] [PubMed] [Google Scholar]
- 3.Paiva L. A., Maya-Monteiro C. M., Bandeira-Melo C., et al. Interplay of cysteinyl leukotrienes and TGF-β in the activation of hepatic stellate cells from Schistosoma mansoni granulomas. Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids. 2010;1801(12):1341–1348. doi: 10.1016/j.bbalip.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K., Matsuzaki K. Differential regulation of TGF-β/Smad signaling in hepatic stellate cells between acute and chronic liver injuries. Frontiers in Physiology. 2012;3, article 53 doi: 10.3389/fphys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verrecchia F., Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. Journal of Investigative Dermatology. 2002;118(2):211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 6.Alves Oliveira L. F., Moreno E. C., Gazzinelli G., et al. Cytokine production associated with periportal fibrosis during chronic schistosomiasis mansoni in humans. Infection and Immunity. 2006;74(2):1215–1221. doi: 10.1128/iai.74.2.1215-1221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaviratne M., Hesse M., Leusink M., et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. Journal of Immunology. 2004;173(6):4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 8.Techau M. E., Johansen M. V., Aasted B., Lind P., Ornbjerg N., Oswald I. P. Cytokine mRNA profiles in pigs exposed prenatally and postnatally to Schistosoma japonicum . Veterinary Research. 2007;38(1):25–36. doi: 10.1051/vetres:2006042. [DOI] [PubMed] [Google Scholar]
- 9.Chen B.-L., Zhang G.-Y., Wang S.-P., et al. The combined treatment of praziquantel with osteopontin immunoneutralization reduces liver damage in Schistosoma japonicum-infected mice. Parasitology. 2012;139(4):522–529. doi: 10.1017/s0031182011002241. [DOI] [PubMed] [Google Scholar]
- 10.Chu D., Luo Q., Li C., et al. Paeoniflorin inhibits TGF-beta1-mediated collagen production by Schistosoma japonicum soluble egg antigen in vitro. Parasitology. 2007;134(11):1611–1621. doi: 10.1017/s0031182007002946. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y.-J., Luo J., Yuan Q., et al. New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum . PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0020247.e20247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight P. G., Glister C. TGF-β superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 13.Allendorph G. P., Vale W. W., Choe S. Structure of the ternary signaling complex of a TGF-β superfamily member. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7643–7648. doi: 10.1073/pnas.0602558103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leutner S., Oliveira K. C., Rotter B., et al. Combinatory microarray and SuperSAGE analyses identify pairing-dependently transcribed genes in Schistosoma mansoni males, including follistatin. PLoS Neglected Tropical Diseases. 2013;7(11) doi: 10.1371/journal.pntd.0002532.e2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freitas T. C., Jung E., Pearce E. J. A bone morphogenetic protein homologue in the parasitic flatworm, Schistosoma mansoni . International Journal for Parasitology. 2009;39(3):281–287. doi: 10.1016/j.ijpara.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrester S. G., Warfel P. W., Pearce E. J. Tegumental expression of a novel type II receptor serine/threonine kinase (SmRK2) in Schistosoma mansoni . Molecular and Biochemical Parasitology. 2004;136(2):149–156. doi: 10.1016/j.molbiopara.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Osman A., Niles E. G., Verjovski-Almeida S., LoVerde P. T. Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathogens. 2006;2(6, article e54) doi: 10.1371/journal.ppat.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knobloch J., Rossi A., Osman A., Loverde P. T., Klinkert M. Q., Grevelding C. G. Cytological and biochemical evidence for a gonad-preferential interplay of SmFKBP12 and SmTbetaR-I in Schistosoma mansoni. Molecular and Biochemical Parasitology. 2004;138(2):227–236. doi: 10.1016/j.molbiopara.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Carlo J. M., Osman A., Niles E. G., et al. Identification and characterization of an R-Smad ortholog (SmSmad1B) from Schistosoma mansoni . FEBS Journal. 2007;274(16):4075–4093. doi: 10.1111/j.1742-4658.2007.05930.x. [DOI] [PubMed] [Google Scholar]
- 20.Osman A., Niles E. G., LoVerde P. T. Identification and characterization of a Smad2 homologue from Schistosoma mansoni, a transforming growth factor-beta signal transducer. Journal of Biological Chemistry. 2001;276(13):10072–10082. doi: 10.1074/jbc.m005933200. [DOI] [PubMed] [Google Scholar]
- 21.Osman A., Niles E. G., LoVerde P. T. Expression of functional Schistosoma mansoni Smad4: role in Erk-mediated transforming growth factor β (TGF-β) down-regulation. The Journal of Biological Chemistry. 2004;279(8):6474–6486. doi: 10.1074/jbc.m310949200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B.-B., Jiao Y.-W., Cai W.-M., et al. Studies on Smads at transcription level in liver fibrosis of mice with Schistosomiasis japonica . Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2004;22(3):154–156. [PubMed] [Google Scholar]
- 23.Freitas T. C., Jung E., Pearce E. J. TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni . PLoS Pathogens. 2007;3(4, article e52) doi: 10.1371/journal.ppat.0030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata M., Hirata K., Hara T., Kawabuchi M., Fukuma T. Expression of TGF-β-like molecules in the life cycle of Schistosoma japonicum . Parasitology Research. 2005;95(6):367–373. doi: 10.1007/s00436-004-1296-0. [DOI] [PubMed] [Google Scholar]
- 25.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berriman M., Haas B. J., LoVerde P. T., et al. The genome of the blood fluke Schistosoma mansoni . Nature. 2009;460(7253):352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rifkin D. B. Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. Journal of Biological Chemistry. 2005;280(9):7409–7412. doi: 10.1074/jbc.r400029200. [DOI] [PubMed] [Google Scholar]
- 28.Horiguchi M., Ota M., Rifkin D. B. Matrix control of transforming growth factorbeta function. Journal of Biochemistry. 2012;152(4):321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojima S., Nara K., Rifkin D. B. Requirement for transglutaminase in the activation of latent transforming growth factor-β in bovine endothelial cells. Journal of Cell Biology. 1993;121(2):439–448. doi: 10.1083/jcb.121.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorand L., Graham R. M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nature Reviews Molecular Cell Biology. 2003;4(2):140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 31.Verderio E., Gaudry C., Gross S., Smith C., Downes S., Griffin M. Regulation of cell surface tissue transglutaminase: effects on matrix storage of latent transforming growth factor-β binding protein-1. Journal of Histochemistry and Cytochemistry. 1999;47(11):1417–1432. doi: 10.1177/002215549904701108. [DOI] [PubMed] [Google Scholar]
- 32.Oh K., Park H.-B., Byoun O.-J., et al. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. Journal of Experimental Medicine. 2011;208(18):1707–1719. doi: 10.1084/jem.20101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids. 2012;42(2-3):939–949. doi: 10.1007/s00726-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 34.Tang J., Huang H., Ji X., et al. Involvement of IL-13 and tissue transglutaminase in liver granuloma and fibrosis post Schistosoma japonicum infection. Mediators of Inflammation. 2014;2014:11. doi: 10.1155/2014/753483.753483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartley P. B., Ramm G. A., Jones M. K., Ruddell R. G., Li Y., McManus D. P. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. International Journal for Parasitology. 2006;36(9):993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Li L., Wu T., Huang J., et al. Expression of heat shock protein 47, transforming growth factor-beta 1, and connective tissue growth factor in liver tissue of patients with Schistosoma japonicum-induced hepatic fibrosis. Parasitology. 2015;142(02):341–351. doi: 10.1017/s0031182014001115. [DOI] [PubMed] [Google Scholar]
- 37.Tatsukawa H., Kojima S. Recent advances in understanding the roles of transglutaminase 2 in alcoholic steatohepatitis. Cell Biology International. 2010;34(3):325–334. doi: 10.1042/cbi20090130. [DOI] [PubMed] [Google Scholar]
- 38.Kojima S., Kuo T. F., Tatsukawa H., Hirose S. Induction of cross-linking and silencing of Sp1 by transglutaminase during liver injury in ASH and NASH via different ER stress pathways. Digestive Diseases. 2010;28(6):715–721. doi: 10.1159/000324278. [DOI] [PubMed] [Google Scholar]
- 39.Tatsukawa H., Fukaya Y., Frampton G., et al. Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology. 2009;136(5):1783–1795. doi: 10.1053/j.gastro.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogarten J. P. Gene transfer: gene swapping craze reaches eukaryotes. Current Biology. 2003;13(2):R53–R54. doi: 10.1016/s0960-9822(02)01426-4. [DOI] [PubMed] [Google Scholar]
- 42.Gogarten J. P., Doolittle W. F., Lawrence J. G. Prokaryotic evolution in light of gene transfer. Molecular Biology and Evolution. 2002;19(12):2226–2238. doi: 10.1093/oxfordjournals.molbev.a004046. [DOI] [PubMed] [Google Scholar]
- 43.Wijayawardena B. K., Minchella D. J., DeWoody J. A. Hosts, parasites, and horizontal gene transfer. Trends in Parasitology. 2013;29(7):329–338. doi: 10.1016/j.pt.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Damian R. T. Molecular mimicry: antigen sharing by parasite and host and its consequences. The American Naturalist. 1964;98(900):129–149. doi: 10.1086/282313. [DOI] [Google Scholar]
- 45.Salzet M., Capron A., Stefano G. B. Molecular crosstalk in host-parasite relationships: schistosome- and leech-host interactions. Parasitology Today. 2000;16(12):536–540. doi: 10.1016/s0169-4758(00)01787-7. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M., Iwamura Y., Amanuma H., et al. Integration and expression of murine retrovirus-related sequences in schistosomes. Parasitology. 1989;99(1):31–38. doi: 10.1017/s0031182000060996. [DOI] [PubMed] [Google Scholar]
- 47.Iwamura Y., Irie Y., Kominami R., Nara T., Yasuraoka K. Existence of host-related DNA sequences in the schistosome genome. Parasitology. 1991;102(3):397–403. doi: 10.1017/S0031182000064362. [DOI] [PubMed] [Google Scholar]
- 48.Irie Y., Iwamura Y. Host-related DNA sequences are localized in the body of schistosome adults. Parasitology. 1993;107(5):519–528. doi: 10.1017/S0031182000068098. [DOI] [PubMed] [Google Scholar]
- 49.Iwamura Y., Yonekawa H., Irie Y. Detection of host DNA sequences including the H-2 locus of the major histocompatibility complex in schistosomes. Parasitology. 1995;110(2):163–170. doi: 10.1017/s0031182000063927. [DOI] [PubMed] [Google Scholar]
- 50.Imase A., Kobayashi K., Ohmae H., Matsuda H., Iwamura Y. Horizontal and vertical transmission of mouse class I MHC sequence in Schistosoma mansoni . Parasitology. 2001;123(2):163–168. doi: 10.1017/s0031182001008198. [DOI] [PubMed] [Google Scholar]
- 51.Malik H. S., Henikoff S., Eickbush T. H. Poised for contagion: evolutionary origins of the infectious abilities of invertebrate retroviruses. Genome Research. 2000;10(9):1307–1318. doi: 10.1101/gr.145000. [DOI] [PubMed] [Google Scholar]
- 52.Pritham E. J. Transposable elements and factors influencing their success in eukaryotes. Journal of Heredity. 2009;100(5):648–655. doi: 10.1093/jhered/esp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaack S., Gilbert C., Feschotte C. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends in Ecology and Evolution. 2010;25(9):537–546. doi: 10.1016/j.tree.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young N. D., Jex A. R., Li B., et al. Whole-genome sequence of Schistosoma haematobium . Nature Genetics. 2012;44(2):221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]