The transcription factor c-Myb plays a role in establishing long-lived plasma cell populations in the bone marrow by affecting migration responses to chemokine gradients. The absence of c-Myb results in an absence of IgG+ antigen-specific plasma cells in the bone marrow following immunization or virus infection.
Abstract
Plasma cell migration is crucial to immunity, but little is known about the molecular regulators of their migratory programs. Here, we detail the critical role of the transcription factor c-Myb in determining plasma cell location. In the absence of c-Myb, no IgG+ antigen-specific plasma cells were detected in the bone marrow after immunization or virus infection. This was correlated with a dramatic reduction of plasma cells in peripheral blood, mislocalization in spleen, and an inability of c-Myb–deficient plasma cells to migrate along a CXCL12 gradient. Therefore, c-Myb plays an essential, novel role in establishing the long-lived plasma cell population in the BM via responsiveness to chemokine migration cues.
Humoral immunity derives from both memory lymphocytes and antibody-secreting plasma cells (ASCs). Specifically, humoral immunity depends on the persistence of these populations for long periods of time, continuous secretion of antibody by BM-resident ASCs, and the ability of memory B cells to rapidly generate ASCs upon reinfection (Tarlinton and Good-Jacobson, 2013). The majority of these cells are generated within germinal centers (GCs) during T-dependent (TD) immune responses (Tarlinton and Good-Jacobson, 2013). GCs are transient structures that expand the population of antigen (Ag)-specific B cell clones. GC B cells undergo affinity maturation, due to selective expansion of those cells with improved Ag binding, resulting from random changes introduced into the variable (V) region genes of the B cell receptor via somatic hypermutation (Victora and Nussenzweig, 2012). Eventually, high-affinity variants are selected to differentiate into recirculating memory B cells, or long-lived ASCs that preferentially migrate to the BM.
The migration of ASCs is an essential component of responses to infection. Migration of ASCs occurs from sites of production, such as the spleen, LNs, and Peyer’s patches, to the BM and/or sites of pathogen residence, with selection to these sites based on differential chemokine receptor expression by the ASC (Cyster, 2003). However, mislocalization of ASCs may contribute to antibody-mediated diseases, highlighting the importance of appropriate regulation of chemokine receptor expression on ASCs, and thus their migration during immune responses. Modulation of chemokine receptor expression on B cells is important at multiple stages of a humoral response. For example, CXCR5 (receptor for CXCL13) is required for migration within a B cell follicle, and CXCR4 (receptor for CXCL12) modulation allows GC B cells to cycle between the light and dark zones of the GC (Allen et al., 2004). Expression of chemokine receptors correlates with the presence of ASCs in either the BM or sites of immunopathology in the body. The chemokine receptors CXCR4 and S1P1 are essential for migration of ASCs to the BM (Hargreaves et al., 2001; Nie et al., 2004; Kabashima et al., 2006). The molecular mechanisms that underlie chemokine responsiveness of ASC, however, remain to be determined.
c-Myb is a transcription factor and a protooncogene that is expressed during B cell development and is essential for continued development and survival (Thomas et al., 2005; Fahl et al., 2009; Greig et al., 2010). c-Myb has been proposed to be important for humoral responses (Lefebvre et al., 2010), although such a role has not yet been explored in vivo. We have investigated the consequences of c-Myb deficiency on the B cell response to Ag using unique genetic tools. Our results reveal that c-Myb expression in B cells is absolutely required for migration of class switched long-lived ASCs to the BM through modulation of chemokine responsiveness, thus revealing a crucial molecular switch underpinning onset of a plasma cell migratory program.
RESULTS AND DISCUSSION
c-Myb is required for establishing Ag-specific ASCs in the BM during a TD response
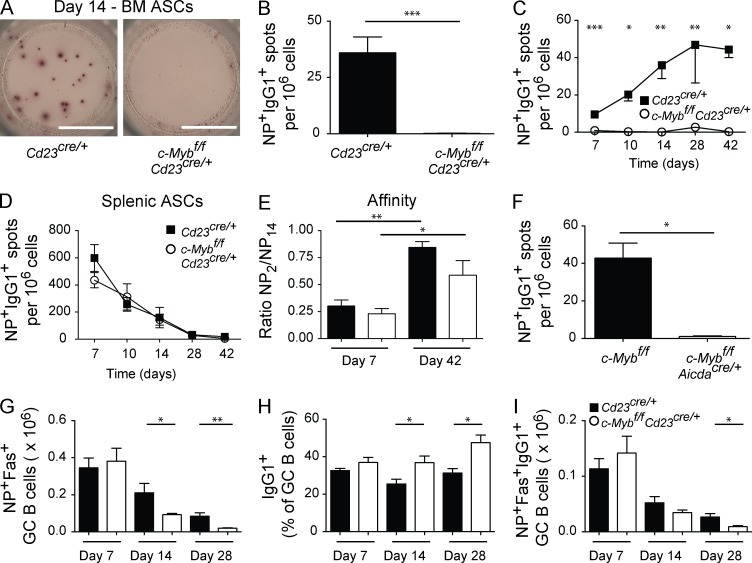
To assess the contribution of c-Myb to a humoral response, we generated c-Mybfl/fl mice carrying a Cd23Cre/+ transgene that deletes c-Myb at the T2 stage of B cell development (Emambokus et al., 2003; Kwon et al., 2008). Strikingly, immunized c-Mybfl/flCd23Cre/+ had no IgG1+ Ag-specific ASCs in the BM (Fig. 1, A–C). Although the frequency of Ag-specific IgG1+ ASCs in the BM of Cd23Cre/+ control mice increased throughout the immune response (Fig. 1 C), there were few or no BM IgG1+ ASCs detected in the absence of c-Myb. This was not due to a lack of production, as assessment of NP+IgG1+ ASCs in the spleen demonstrated comparable frequencies over time (Fig. 1 D). Similarly, affinity maturation of splenic NP+IgG1+ ASCs appeared normal (Fig. 1 E). c-Mybfl/fl mice carrying an AicdaCre/+ allele, in which c-Myb was deleted after Ag activation of mature B cells (Kwon et al., 2008), also revealed a lack of NP+IgG1+ ASCs in the BM during an immune response (Fig. 1 F). This indicates a role for c-Myb during the processes of ASC differentiation and migration rather than in establishing a preexisting condition in naive B cells. NP+ GC B cells formed normally in the absence of c-Myb at day 7 after immunization, but by day 14 there was a twofold decrease, suggesting persistence of these cells was not optimal in the absence of c-Myb (Fig. 1 G). Within the c-Myb–deficient NP+ GC compartment, however, the frequency of IgG1+ cells was increased at day 14 and 28 after immunization compared with controls (Fig. 1 H). Thus, the number of IgG1+NP+ GC B cells, which are arguably the precursors to IgG1+ ASCs in the BM, was similar between knockout and controls at days 7 and 14, and significantly decreased at day 28 (Fig. 1 I). Thus, the lack of ASC in the BM of c-Myb–deficient mice was not caused by an absence of IgG1+NP+ GC B cells.
Figure 1.
Absence of Ag-specific ASCs in the BM during an immune response. Cd23Cre/+ (black bars or closed squares) and c-Mybfl/flCd23Cre/+ (white bars or open circles) mice were immunized with NP-KLH precipitated in alum. (A–E) ELISPOT analysis of NP+IgG1+ ASCs in the BM at day 14 (A and B), over time (C), kinetics in the spleen (D), and affinity of splenic ASCs (E). n ≥ 4 mice per experiment, results are combined from 1 (day 10, day 42), 2 (day 14), or 3 (day 7, day 28) independent experiments per time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (Mann-Whitney nonparametric, two-tailed test; mean ± SEM). Bar, 3 mm. (F) c-Mybfl/flAicdaCre/+ and littermate controls were immunized with NP-KLH in alum and ELISPOT analysis of BM ASCs was performed 14 d after immunization (n ≥ 3 mice per genotype). (G–I) Number of NP+Fas+ GC B cells (G), frequency of NP+Fas+ GC B cells that are IgG1+ (H), and number (I) of IgG1+NP+Fas+ GC B cells assessed by flow cytometry 7 d (n ≥ 11 mice per genotype; combined from three independent experiments), 14 d (n ≥ 6 mice per genotype; combined from two independent experiments), and 28 d (n = 4 mice per genotype) after immunization.
B cell–intrinsic migratory defect
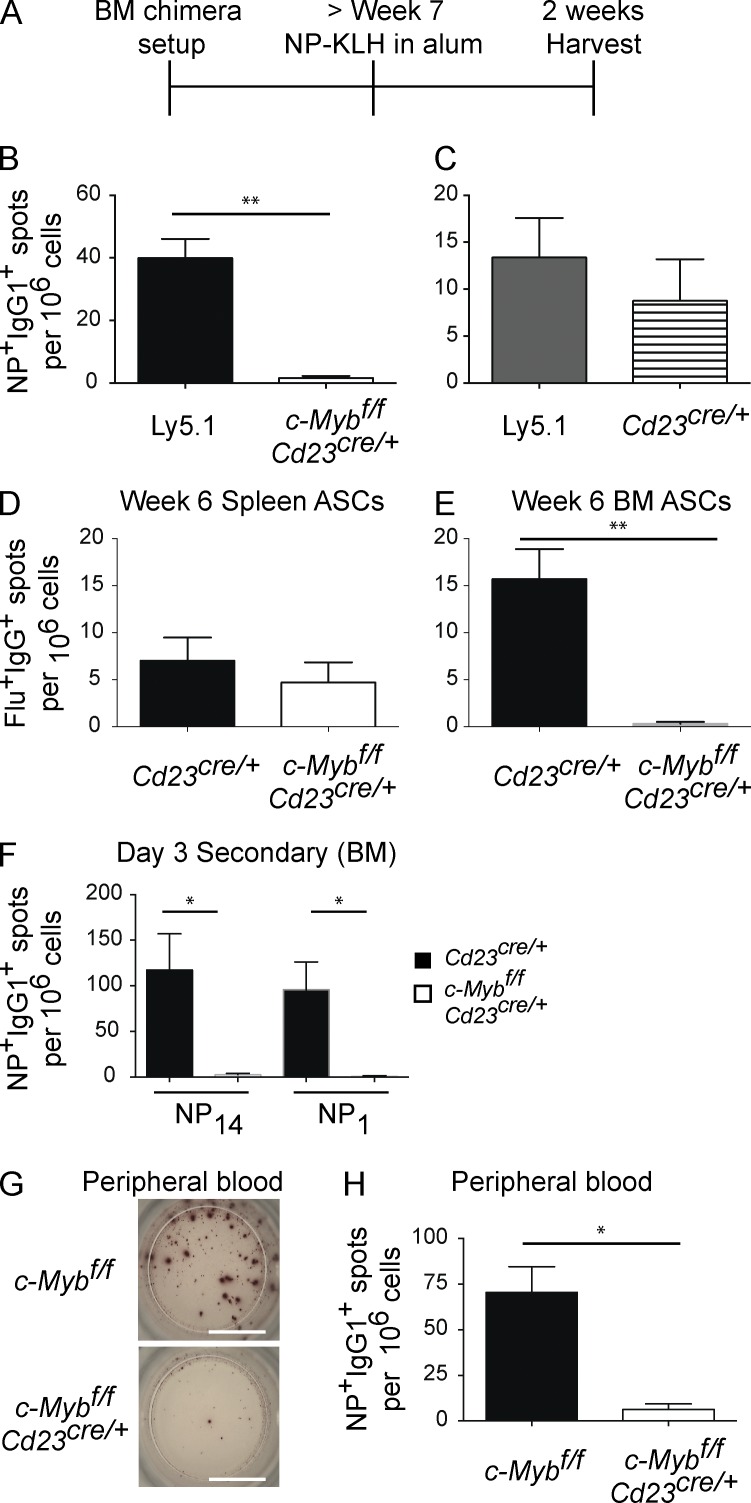
To rule out potential secondary changes in the microenvironment affecting migration of ASCs in c-Mybfl/flCd23Cre/+ mice, mixed BM chimeras were created. Ly5.1 wild-type BM was mixed 1:1 with Ly5.2 c-Mybfl/flCd23Cre/+ (or Cd23Cre/+ only for control chimeras) and used to reconstitute irradiated recipients (Fig. 2 A). Immunization of these chimeras revealed Ly5.1+NP+IgG1+ ASCs were present in the BM (Fig. 2, B and C), as were Cd23Cre/+ Ly5.2+NP+IgG1+ ASCs (Fig. 2 C). In contrast, NP+IgG1+ c-Myb–deficient, BM-resident ASCs were absent (Fig. 2 B). Therefore, although wild-type ASCs migrated to the BM, c-Myb–deficient ASCs did not within the same animal, demonstrating the B cell intrinsic basis of the defect.
Figure 2.
B cell–intrinsic migratory defect. (A) Schematic representation of mixed BM chimera setup. BM from either Cd23Cre/+ (horizontal stripes) or c-Mybfl/flCd23Cre/+ (white bars) were mixed 1:1 with BM from Ly5.1 (black or gray) and injected into irradiated Ly5.1 recipient mice. Mice were rested for at least 7 wk, after which they were immunized with NP-KLH precipitated in alum. (B and C) NP+IgG1+ BM ASCs were assessed at 2 wk after immunization by sort-purification and ELISPOT analysis from 50:50 BM chimeras; n = 5 per genotype; results are representative of two independent experiments. (D and E) Cd23Cre/+ (black bars) and c-Mybfl/flCd23Cre/+ (white bars) mice were infected with HKx31 influenza. Flu-specific IgG+ ASCs were assessed in the spleen (D) and BM (E) 6 wk after infection (n = 5 per genotype). Results are representative of two independent experiments per time point. (F and H) Cd23Cre/+ (black bars) and c-Mybfl/flCd23Cre/+ (white bars) mice were immunized with NP-KLH precipitated in alum, rested for at least 4 wk, and then boosted with NP-KLH in PBS. At day 3–4 after boost, NP+IgG1+ ASCs were assessed in the BM (F) and peripheral blood (G-H). n ≥ 4 per genotype, representative of three independent experiments. Bar, 3 mm. *, P < 0.05; **, P < 0.01 (Mann-Whitney nonparametric, two-tailed test; mean ± SEM).
Infection also fails to induce long-lived BM ASC
To investigate whether the inability of c-Myb–deficient ASCs to seed the BM depended on the immunizing agent, c-Mybfl/flCd23Cre/+ and Cd23Cre/+ control mice were infected with influenza virus. Whereas IgG1 dominates after an NP-KLH in alum immunization, the response to influenza infection is dominated by IgG2c production due to IFN-γ production (Severinson et al., 1990; Collins and Dunnick, 1993; Peng et al., 2002). Mice were analyzed 6 wk after infection to assess the establishment of the antiinfluenza BM ASC population. As with NP-KLH immunization, the frequency of influenza-specific IgG+ ASCs in spleens of c-Myb–deficient mice was comparable to controls (Fig. 2 D). However, whereas Cd23Cre/+ mice generated a population of IgG+ influenza-specific ASCs in BM, no such IgG+ influenza-specific ASCs were detected in the absence of c-Myb (Fig. 2 E). Therefore, c-Myb is essential for Ag-specific BM ASCs in response to immunization and infection.
Localization defect in the absence of c-Myb
All data presented so far support the contention that c-Myb–deficient ASCs are defective in migration to the BM. A clear intermediary in migration to the BM would be the presence of ASCs in the blood during an immune response. Detecting ASCs in blood during a primary response is technically limiting, so instead we assessed blood for migrating NP+IgG1+ ASCs after secondary immunization. Mice, immunized with NP-KLH in alum and allowed to rest for at least 4 wk, were boosted with soluble NP-KLH to assess whether secondary plasmablasts were also defective in populating the BM compartment. After secondary challenge, and reproducing our primary response data, c-Myb–deficient NP+IgG1+ ASCs were not found in the BM (Fig. 2 F). When mice were assessed 3.5–4 d after boost, we measured a 19-fold reduction in frequency of NP+IgG1+ ASCs in blood of c-Myb–deficient mice compared with controls (Fig. 2, G and H).
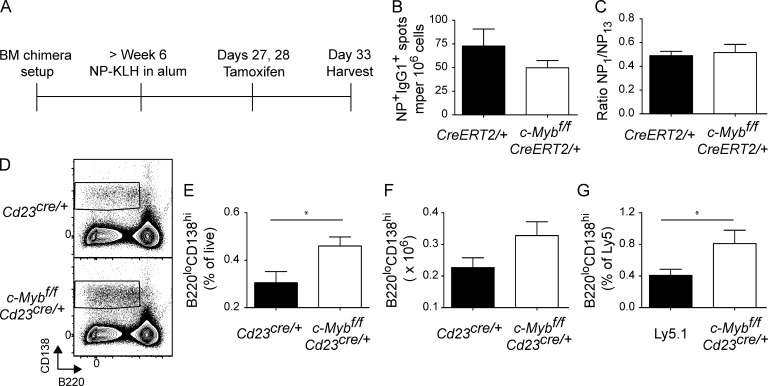
It is formally possible that c-Myb could contribute to formation of the BM ASC compartment by regulating retention or survival of ASCs once established. To investigate such a role of c-Myb, we used c-Mybfl/fl mice crossed to the tamoxifen inducible Cre system (Fig. 3). BM from μMT mice was mixed 4:1 with BM from c-Mybfl/flRosa26CreERT2/+ or Rosa26CreERT2/+ controls and used to reconstitute irradiated Ly5.1 recipients. In c-Mybfl/flRosa26CreERT2/+ BM chimeras, all B cells will delete c-Myb upon tamoxifen administration, whereas the majority of non–B cells will continue to express c-Myb. Chimeras were immunized and rested for ∼4 wk to permit the migration and formation of ASCs to the BM. Tamoxifen, delivered by oral gavage, was used to induce deletion of c-Myb in ASCs (deletion efficiency was estimated at over 95%; not depicted) and any impact on their retention was assessed (Fig. 3 A). c-Myb–deficient ASCs were present in the BM (Fig. 3 B) post-tamoxifen treatment at normal frequency and affinity (Fig. 3, B and C), demonstrating that c-Myb does not control the persistence of ASCs already occupying a bone marrow niche. Additionally, expression of the plasma cell survival factor Mcl-1 (Peperzak et al., 2013) in c-Myb–deficient splenic ASCs was comparable to controls (unpublished data). These data indicate that expression of cMyb controls emigration of ASC from secondary lymphoid organs after immunization. Consistent with this, when we assessed ASC in the spleen by flow cytometry 4 wk after primary immunization, we measured a twofold increase in B220loCD138hi cells in the absence of c-Myb at (Fig. 3, D–F), a phenomenon that was B cell intrinsic (Fig. 3 G).
Figure 3.
Survival in the BM is not affected by the absence of c-Myb. BM from μMT mice were mixed 4:1 with BM from c-Mybfl/flERT2-Cre/+ or ERT2-Cre/+ controls and used to reconstitute irradiated Ly5.1 recipients (A). Mice were rested for at least 6 wk, and then immunized with NP-KLH. After 4 wk, tamoxifen was administered 6 and 5 d before frequency (B) and affinity (C) of BM ASCs were assessed. n = 5 per genotype; results are representative of four independent experiments at multiple time points after tamoxifen treatment. (D–F) Flow cytometric representative plot (D), frequency (E), and number (F) of B220loCD138hi in the spleen 4 wk after immunization in Cd23Cre/+ (black bars) and c-Mybfl/flCd23Cre/+ (white bars) mice. n = 5 per genotype; results are representative of two independent experiments. (G) Frequency of splenic B220loCD138hi in 1:1 mixed BM chimeras as described in Fig. 2. *, P < 0.05 (Mann-Whitney nonparametric, two-tailed test; mean ± SEM).
BM ASCs present in c-Myb–deficient naive mice are mutated at a low frequency
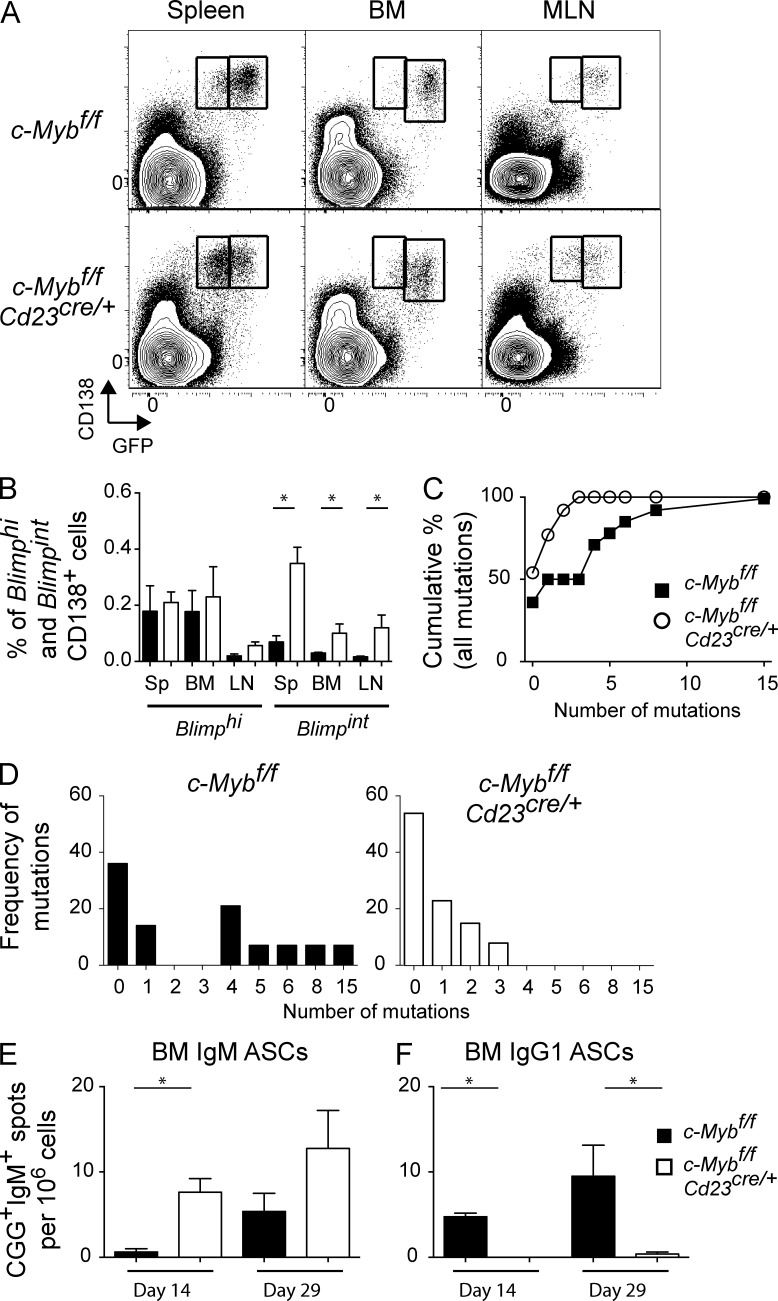
To further investigate the ASC defect, c-Mybfl/flCd23Cre/+ mice were crossed with Blimp-1gfp/+ reporter mice (Kallies et al., 2004), within which plasma cell populations can be divided into Blimp-1int and Blimp-1hi populations that have distinct characteristics (Kallies et al., 2004). Blimp-1int plasma cells are short-lived, less mature plasma cells, which is the population that retains migratory potential (Hauser et al., 2002). c-Mybfl/flCd23Cre/+ or c-Mybfl/fl littermates that were Blimp-1gfp/+ were used to track Blimp-1intCD138hi plasmablasts and Blimp-1hiCD138hi plasma cells. Whereas both populations still existed in the BM, mesenteric lymph nodes (MLNs), and spleen, there was a significant increase in Blimp-1int plasmablasts in the absence of c-Myb (Fig. 4, A and B). Furthermore, the majority of BM B220loCD138hi cells were IgM (mean ± SEM: 81 ± 3% versus 34 ± 1.7% in controls), indicating a different route of formation.
Figure 4.
Altered plasma cell populations within secondary lymphoid organs. (A and B) CD138hi GFP populations in unimmunized c-Mybfl/fl and c-Mybfl/flCd23Cre/+ mice. c-Mybfl/fl or c-Mybfl/flCd23Cre/+ mice with gfp introduced into the Blimp-1 locus on one allele were used to track Blimp-1–expressing CD138hi cells in spleen, BM, and MLN. (B) Frequency of Blimp-1hiCD138hi and Blimp-1intCD138hi cells. n ≥ 4 mice per organ, combined from two independent experiments. (C–E) CD138hi GFP cells were sort-purified from the BM of c-Mybfl/fl and c-Mybfl/flCd23Cre/+ mice (n = 3 per genotype) and pooled for sequencing. (C) Cumulative frequency of VH mutations in BM Blimp-1hiCD138hi cells and (D) number versus frequency of VH mutations in BM Blimp-1hiCD138hi cells. (E–F) Cd23Cre/+ and c-Mybfl/flCd23Cre/+ were immunized with CGG in alum and ELISPOT analysis of IgM+ (E) and IgG1+ (F) CGG-specific BM ASCs was performed at 14 and 29 d after immunization (n = 4 mice per genotype per time point). *, P < 0.05.
The apparent discordance between the lack of Ag-specific ASCs in the BM after immunization, but a resident, steady-state BM plasma cell population was investigated by sort-purification of Blimp-1hiCD138hi cells and analysis of the genes encoding the Ag receptor to determine whether these cells originated within a GC. Comparison of the DNA sequence of variable heavy-chain Igh gene segments from c-Mybfl/flCd23Cre/+ mice and wild-type controls demonstrated that the mean number of mutations per VH-IgG sequence was significantly lower in the absence of c-Myb (Cd23Cre/+, 4 mutations per sequence; c-Mybfl/flCd23Cre/+, 1.4 mutations per sequence), as was the distribution of mutations in sequences that had been mutated (Fig. 4, C and D). Given the presence of IgM+ plasma cells in the BM, we assessed the presence of IgM+ ASCs in the BM after immunization. Unimmunized mice display a significant background level of IgM+ NP-binding ASCs, but not IgM+ CGG-binding ASCs (unpublished data). We therefore immunized mice with CGG in alum and assessed BM ASCs after immunization. IgM+ (Fig. 4 E), but not IgG1+ (Fig. 4 F), ASCs were present in the BM of c-Myb–deficient mice, and at a higher frequency then control mice. The presence of IgM+ CGG-specific ASCs suggests that either c-Myb acts after isotype-switching and thus does not affect the migration of IgM+ plasma cells, that it acts primarily on GC-derived ASC, or that the IgM+ plasma cells in the bone marrow are generated in situ. Taken together, BM plasma cells that develop in the absence of c-Myb appear to be less reliant on the GC and may experience different development or selection conditions than control mice.
Differential responsiveness to chemokine signals is regulated by c-Myb
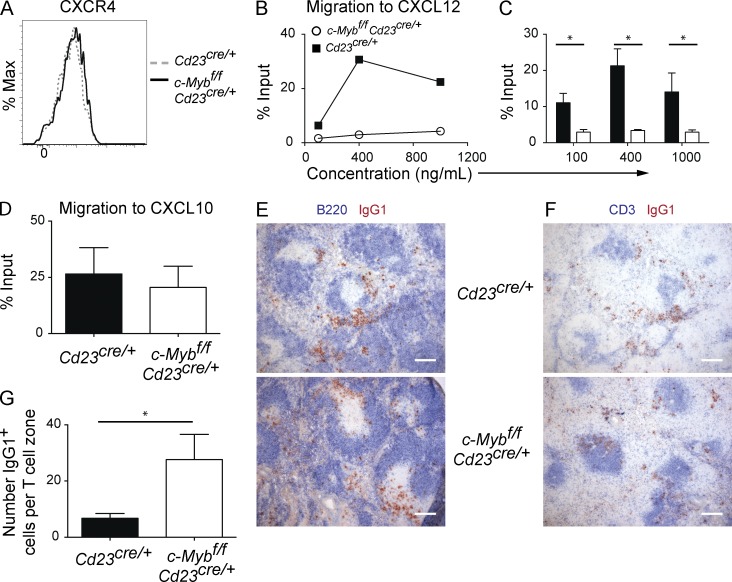
We investigated the hypothesis that dysregulated chemokine receptor expression or function may contribute to the lack of Ag-specific ASCs in the blood and BM in the absence of c-Myb. Given that Cxcr4 is regulated by c-Myb in hematopoietic progenitors (Lieu and Reddy, 2009; Quintana et al., 2011), we considered it a candidate to explain the aberrant migration of c-Myb–deficient ASCs. CXCR4 was expressed in similar amounts on c-Myb–deficient plasmablasts and controls (Fig. 5 A), and there was no difference in transcript levels of Cxcr4 (not depicted). Both c-Myb–deficient and wild-type plasmablasts responded to in vitro exposure to CXCL12 by internalization of CXCR4 as detected by equally reduced surface staining (unpublished data), demonstrating that the receptor remains coupled to downstream signaling molecules to some degree. Despite this, the chemotactic response of c-Myb–deficient plasmablasts to CXCL12 was severely impaired (Fig. 5, B and C). Other studies have also demonstrated that surface expression of CXCR4 may not indicate the ability of the cell to migrate to CXCL12 (Wehrli et al., 2001; Hauser et al., 2002; Underhill et al., 2003; Kabashima et al., 2006). c-Myb–deficient plasmablasts did not have a global deficiency in migrating to chemokines, however, as there was no difference in their migration toward CXCL10 (Fig. 5 D). Migration to S1P has been shown to be essential for plasma cells to migrate to the BM (Kabashima et al., 2006). However, the efficiency of the in vitro migration assay to S1P for splenic plasma cells is relatively low (0.5% input; Kabashima et al., 2006), and as such we could not attain a reliable assessment of c-Myb–deficient plasma cells migration to S1P. Therefore, we cannot rule out that migration to this chemokine is also defective. We have, however, assessed transcript levels of S1pr1 in c-Myb–deficient and control plasmablasts and found no significant difference (unpublished data).
Figure 5.
c-Myb regulates migration to CXCL12. (A and B) Cd23Cre/+ (dotted gray line or closed squares) and c-Mybfl/flCd23Cre/+ (black solid line or open circles) mice were immunized with NP-KLH precipitated in alum and spleens harvested at day 7 after immunization. (A) Splenic plasmablasts (B220loCD138hi) were assessed for CXCR4 expression, representative of plasmablasts assessed at multiple time points after immunization. (B–D) CD138-enriched splenic cells were assessed for the ability of plasmablasts to migrate to CXCL12 (B) representative plot, (C) combined data from three independent experiments, and (D) CXCL10, combined data from two independent experiments. *, P < 0.05 (Mann-Whitney nonparametric, two-tailed test; mean ± SEM). (E–G) Cd23Cre/+ and c-Mybfl/flCd23Cre/+ mice were immunized with NP-KLH precipitated in alum and spleens harvested at day 7 after immunization. Sections were stained with B220 (blue) and IgG1 (red; E), and CD3 (blue) and IgG1 (red; F), representative of three spleens per genotype; ASCs in T cell zones were enumerated (G; n ≥ 7 per genotype of T cell zones assessed). Bars, 200 µm. *, P < 0.05 (unpaired two-tailed t test; mean ± SEM).
Histological assessment revealed that c-Myb–deficient ASCs were mislocalized in the spleen (Fig. 5, E–G). At day 7 after immunization, control plasmablasts were detected mainly in extrafollicular foci and the splenic red pulp. In contrast, c-Myb–deficient plasmablasts had infiltrated T cell areas within the spleen (Fig. 5, E–G), suggesting an inappropriate response to localization cues had occurred, similar to CXCR4-deficient mice. It is important to note, however, that it is unclear whether CXCR4-deficient mice have an accumulation of plasma cells in T cell areas as well as the accumulation seen in the marginal zone (Kabashima et al., 2006). As c-Myb–deficient mice are not a phenocopy of CXCR4 deficiency, it is likely that other migration processes may be regulated by c-Myb. This may include migration to S1P, but as discussed above, we were limited in assessing the role of S1P. Collectively, the data presented here led us to conclude that c-Myb has an essential role in the emigration of ASCs from secondary lymphoid organs through regulation of responsiveness to CXCL12 downstream of CXCR4 expression.
Migratory programs of cell populations are reliant on chemokine receptor expression, their accompanying signal transduction pathways, and corresponding chemokine gradients within organs (Cyster, 2003). Long-lived ASCs produced in secondary lymphoid organs during an immune response rely on chemokines to migrate to the BM. In particular, ASCs expressing CXCR4 on their surface will respond to CXCL12 gradients within the spleen and LN, thus migrating into the blood and to the BM in a directed manner. Here, we demonstrate that the transcription factor c-Myb is essential for ASCs to respond to CXCL12 within the spleen, and without it, long-lived ASCs generated during a TD response do not emigrate and thus cannot establish the stable BM-resident compartment normally required for humoral immunity. This modulation of CXCL12 responsiveness occurred in the context of mislocalization of ASCs in the spleen and a failure of Ag-specific ASCs to enter the blood and migrate to the bone marrow. Collectively, these data reveal c-Myb as an essential regulator of humoral immunity.
Little is known about the transcriptional networks underlying responsiveness of ASCs to migration signals that ultimately lead to the BM. Two studies have correlated the deletion of transcription factors with a deficit or absence of Ag-specific ASCs in the BM. Germline deletion of Aiolos (Cortés and Georgopoulos, 2004) results in defects in the high-affinity BM population; however, it was unclear at what stage (production, migration, or retention) this defect was occurring. Similarly, germline deletion of KLF2 (Hart et al., 2011) demonstrated multiple defects in the formation and responses of the B cell lineage, one of which was a deficiency in Ag-specific ASCs in the BM after boost. Expression of KLF2 or Aiolos was not altered by c-Myb deficiency (unpublished data). In contrast to these studies, we pinpoint a specific role for c-Myb in the directed migration of ASCs out of organs through regulation of responsiveness to CXCL12. This phenomenon was not restricted to the spleen or immunization route, as ASCs accumulated in the organ of formation in response to influenza infection, haptenated proteins, and environmental Ags in unimmunized mice.
This study has identified a transcription factor underpinning the migration of plasma cells that is required to form the long-lived population in the BM. In sum, these experiments have revealed mechanisms underlying the formation of immunity, which will be crucial for understanding the pathogenesis of many antibody-mediated diseases.
MATERIALS AND METHODS
Mice, immunizations, and purification of cells.
Cd23-Cre (Kwon et al., 2008) were provided by M. Busslinger (The Research Institute of Molecular Pathology, Vienna, Austria) and c-Mybfl/fl mice were provided by J. Frampton (University of Birmingham, Birmingham, England, UK; Emambokus et al., 2003). Blimpgfp/+ reporter mice as previously described (Kallies et al., 2004). Ly5.1 mice were maintained at the Walter and Eliza Hall Institute. All mice are on a C57BL/6 background and are backcrossed. Animal procedures were approved by the Walter and Eliza Hall Institute Animal Ethics Committee. For primary responses, mice were injected intraperitoneally with 100 µg of NP conjugated to KLH (molar ratio between 13 and 20), precipitated on 10% alum. For analysis of CGG-specific responses, mice were injected subcutaneously with 100 µg of CGG precipitated on 10% alum. For secondary responses, 50 µg of NP-KLH in PBS injected intraperitoneally per mouse. For influenza infections, mice were inoculated with 104 PFU of HKx31 (H3N2) influenza virus as previously described (Flynn et al., 1998; Belz et al., 2000). For sort purification, cells were stained with antibodies and purified by FACSAria or Influx (BD), with purity >98%.
Flow cytometry and antibodies.
Single-cell suspensions were resuspended in PBS 2% FCS and stained for flow cytometric analysis. Cells were analyzed live (with the addition of propidium iodide) on the FACSCanto (BD) and data was analyzed with FlowJo software (Tree Star). The following antibodies were used: CD95 (JO2), IgG1 (x56), Ly5.2 (104), and CD138 (281) from BD; CD19 (ID3), CXCR4 (2B11) from eBioscience; NIP, Gr1, (8C5) and B220 (RA3-6B2) were conjugated in-house. FcγRII/III (24G2; supernatant) was used to block nonspecific binding.
BM chimeras.
For 50:50 chimeras, lethally irradiated Ly5.1 mice (2 × 5.5 Gy) were reconstituted with 50% Ly5.1 BM and 50% c-Mybfl/flCd23Cre/+ or Cd23Cre/+ BM. Mice were rested for 7–8 wk before NP-KLH/alum immunization as described above. Mice were bled before immunization to test chimerism by flow cytometry.
For µMT chimeras, lethally irradiated Ly5.1 mice (2 × 5.5 Gy) were reconstituted with 80% µMT BM and 20% c-Mybfl/flERT2-Cre/+ or ERT2-Cre/+ BM. Mice were rested for at least six weeks before NP-KLH/alum immunization as described above. 4 wk after immunization, estrogen receptor–activated deletion of loxP-flanked c-Myb alleles was triggered by oral gavage of tamoxifen (5 mg [Sigma-Aldrich] in 83 µl of a solution of 90% peanut oil (Sigma-Aldrich) and 10% ethanol) on two successive days. Deletion was assessed by PCR on sort-purified BM ASCs.
Chemotaxis.
ASCs were enriched from spleens of pooled mice per genotype using CD138 magnetic beads (Miltenyi Biotec). 105 cells were resuspended in 100 µl RPMI, supplemented with 0.5% BSA and containing PE-labeled beads to facilitate the enumeration of migrating cells. Cells were applied to the top of trans-wells containing CXCL12 (0, 0.1, 0.4, or 1 µg/ml), CXCL10 (0.1 µg/ml), or medium alone in the bottom chamber. Migrated cells were stained with antibodies to B220 and CD138, and total cell count was assessed by flow cytometry with the addition of a known number of beads to each sample. Migration to nil was also assessed and subtracted from each sample when calculating frequency of input.
Histology, ELISPOT, and ELISA.
Portions of spleens were frozen in OCT (Tissue-Tek), 7-µm sections were cut using a microtome (Leica) and stained for immunohistochemistry as detailed previously (Zotos et al., 2010). ASCs or antibody was analyzed by ELISPOT and ELISA, respectively, as previously described (Zotos et al., 2010). For influenza-specific ELISPOTs, purified HKx31 influenza virus was disrupted in a 1/10 dilution of lysis buffer (0.05 M Tris, pH 7.5, 0.5% Triton X-100, and 0.6 M KCI) in PBS, pH 7.2, and viral lysate was used to coat wells.
VH sequencing.
Blimp-1hiCD138hi cells were pre-enriched with CD138 beads and sort-purified on a FACSAria (BD). Three mice per genotype were pooled pre-enrichment. RNA was isolated using the Microkit Plus (QIAGEN) according to the manufacturer’s guidelines, cDNA was prepared using SuperScript II Reverse transcription (RT Life Technologies) and VH7183 family transcripts amplified with PFU DNA polymerase (Promega) using a degenerate VH7183-forward primer (5′-CTTAGTGMAGCCTGGAVRKTCC-3′) in combination with the IgG CH1 (5′-GGACAGGGMTCCAKAGTTCCA-3′). Gel-purified PCR products were 3′-adenylated using Taq polymerase (Applied Biosystems) and cloned into pCR2.1-TOPO (Invitrogen) according to the manufacturer’s instructions. Standard M13 primers were used to amplify cloned inserts from individual colonies and PCR products were purified over columns (QIAGEN) in preparation for sequencing with the relevant CH1 reverse primer (Australian Genome Research Facility). Germline VH7183 sequences were identified using IMGT/V-QUEST (Brochet et al., 2008).
Statistical analysis.
The Mann-Whitney nonparametric, two-tailed test or an unpaired, two-tailed Student’s t test was used for statistical analyses, using GraphPad Prism software.
Acknowledgments
We thank Ingela Vikstrom for critical reading of this manuscript, Jonathon Frampton and Meinrad Busslinger for mice, and Dana Piovesan and the Tarlinton laboratory for technical assistance.
This work is supported by a National Health and Medical Research Council (NHMRC) Australia program grant to D.M. Tarlinton, S.L. Nutt, and G.T. Belz (1054925), NHMRC project grant to K.L. Good-Jacobson (1057707), and a Leukaemia Foundation grant-in-aid to K.L. Good-Jacobson. K.L. Good-Jacobson and D.M. Tarlinton are supported by NHMRC Fellowships. S.L. Nutt and G.T. Belz are supported by Australian Research Council Future Fellowships. This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
The authors declare no competing financial interests.
Author contributions: K.L. Good-Jacobson, S.L. Nutt, and D.M. Tarlinton designed the research; K.L. Good-Jacobson and K. O’Donnell performed research; G.T. Belz provided reagents and intellectual input; and K.L. Good-Jacobson and D.M. Tarlinton wrote the manuscript.
Footnotes
Abbreviations used:
- Ag
- antigen
- ASC
- antibody-secreting plasma cells
- GC
- germinal center
- MLN
- mesenteric lymph node
- NP
- (4-Hydroxy-3-nitrophenyl)-acetyl
- TD
- T-dependent
References
- Allen C.D., Ansel K.M., Low C., Lesley R., Tamamura H., Fujii N., and Cyster J.G.. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5:943–952. 10.1038/ni1100 [DOI] [PubMed] [Google Scholar]
- Belz G.T., Xie W., Altman J.D., and Doherty P.C.. 2000. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486–3493. 10.1128/JVI.74.8.3486-3493.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet X., Lefranc M.P., and Giudicelli V.. 2008. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 36:W503-W508 10.1093/nar/gkn316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.T., and Dunnick W.A.. 1993. Germline transcripts of the murine immunoglobulin gamma 2a gene: structure and induction by IFN-gamma. Int. Immunol. 5:885–891. 10.1093/intimm/5.8.885 [DOI] [PubMed] [Google Scholar]
- Cortés M., and Georgopoulos K.. 2004. Aiolos is required for the generation of high affinity bone marrow plasma cells responsible for long-term immunity. J. Exp. Med. 199:209–219. 10.1084/jem.20031571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J.G. 2003. Homing of antibody secreting cells. Immunol. Rev. 194:48–60. 10.1034/j.1600-065X.2003.00041.x [DOI] [PubMed] [Google Scholar]
- Emambokus N., Vegiopoulos A., Harman B., Jenkinson E., Anderson G., and Frampton J.. 2003. Progression through key stages of haemopoiesis is dependent on distinct threshold levels of c-Myb. EMBO J. 22:4478–4488. 10.1093/emboj/cdg434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahl S.P., Crittenden R.B., Allman D., and Bender T.P.. 2009. c-Myb is required for pro-B cell differentiation. J. Immunol. 183:5582–5592. 10.4049/jimmunol.0901187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn K.J., Belz G.T., Altman J.D., Ahmed R., Woodland D.L., and Doherty P.C.. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 8:683–691. 10.1016/S1074-7613(00)80573-7 [DOI] [PubMed] [Google Scholar]
- Greig K.T., de Graaf C.A., Murphy J.M., Carpinelli M.R., Pang S.H., Frampton J., Kile B.T., Hilton D.J., and Nutt S.L.. 2010. Critical roles for c-Myb in lymphoid priming and early B-cell development. Blood. 115:2796–2805. 10.1182/blood-2009-08-239210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves D.C., Hyman P.L., Lu T.T., Ngo V.N., Bidgol A., Suzuki G., Zou Y.R., Littman D.R., and Cyster J.G.. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194:45–56. 10.1084/jem.194.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G.T., Wang X., Hogquist K.A., and Jameson S.C.. 2011. Krüppel-like factor 2 (KLF2) regulates B-cell reactivity, subset differentiation, and trafficking molecule expression. Proc. Natl. Acad. Sci. USA. 108:716–721. 10.1073/pnas.1013168108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A.E., Debes G.F., Arce S., Cassese G., Hamann A., Radbruch A., and Manz R.A.. 2002. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 169:1277–1282. 10.4049/jimmunol.169.3.1277 [DOI] [PubMed] [Google Scholar]
- Kabashima K., Haynes N.M., Xu Y., Nutt S.L., Allende M.L., Proia R.L., and Cyster J.G.. 2006. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 203:2683–2690. 10.1084/jem.20061289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallies A., Hasbold J., Tarlinton D.M., Dietrich W., Corcoran L.M., Hodgkin P.D., and Nutt S.L.. 2004. Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J. Exp. Med. 200:967–977. 10.1084/jem.20040973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon K., Hutter C., Sun Q., Bilic I., Cobaleda C., Malin S., and Busslinger M.. 2008. Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity. 28:751–762. 10.1016/j.immuni.2008.04.014 [DOI] [PubMed] [Google Scholar]
- Lefebvre C., Rajbhandari P., Alvarez M.J., Bandaru P., Lim W.K., Sato M., Wang K., Sumazin P., Kustagi M., Bisikirska B.C., et al. 2010. A human B-cell interactome identifies MYB and FOXM1 as master regulators of proliferation in germinal centers. Mol. Syst. Biol. 6:377 10.1038/msb.2010.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu Y.K., and Reddy E.P.. 2009. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc. Natl. Acad. Sci. USA. 106:21689–21694. 10.1073/pnas.0907623106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y., Waite J., Brewer F., Sunshine M.J., Littman D.R., and Zou Y.R.. 2004. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J. Exp. Med. 200:1145–1156. 10.1084/jem.20041185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S.L., Szabo S.J., and Glimcher L.H.. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA. 99:5545–5550. 10.1073/pnas.082114899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peperzak V., Vikström I., Walker J., Glaser S.P., LePage M., Coquery C.M., Erickson L.D., Fairfax K., Mackay F., Strasser A., et al. 2013. Mcl-1 is essential for the survival of plasma cells. Nat. Immunol. 14:290–297. 10.1038/ni.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A.M., Liu F., O’Rourke J.P., and Ness S.A.. 2011. Identification and regulation of c-Myb target genes in MCF-7 cells. BMC Cancer. 11:30 10.1186/1471-2407-11-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinson E., Fernandez C., and Stavnezer J.. 1990. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins Collectively switch recombination. Eur. J. Immunol. 20:1079–1084. 10.1002/eji.1830200520 [DOI] [PubMed] [Google Scholar]
- Tarlinton D., and Good-Jacobson K.. 2013. Diversity among memory B cells: origin, consequences, and utility. Science. 341:1205–1211. 10.1126/science.1241146 [DOI] [PubMed] [Google Scholar]
- Thomas M.D., Kremer C.S., Ravichandran K.S., Rajewsky K., and Bender T.P.. 2005. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 23:275–286. 10.1016/j.immuni.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Underhill G.H., Kolli K.P., and Kansas G.S.. 2003. Complexity within the plasma cell compartment of mice deficient in both E- and P-selectin: implications for plasma cell differentiation. Blood. 102:4076–4083. 10.1182/blood-2003-03-0947 [DOI] [PubMed] [Google Scholar]
- Victora G.D., and Nussenzweig M.C.. 2012. Germinal centers. Annu. Rev. Immunol. 30:429–457. 10.1146/annurev-immunol-020711-075032 [DOI] [PubMed] [Google Scholar]
- Wehrli N., Legler D.F., Finke D., Toellner K.M., Loetscher P., Baggiolini M., MacLennan I.C., and Acha-Orbea H.. 2001. Changing responsiveness to chemokines allows medullary plasmablasts to leave lymph nodes. Eur. J. Immunol. 31:609–616. [DOI] [PubMed] [Google Scholar]
- Zotos D., Coquet J.M., Zhang Y., Light A., D’Costa K., Kallies A., Corcoran L.M., Godfrey D.I., Toellner K.M., Smyth M.J., et al. 2010. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207:365–378. 10.1084/jem.20091777 [DOI] [PMC free article] [PubMed] [Google Scholar]