Using PD-1–deficient mice and co-adoptive transfer approaches, Odorizzi et al. demonstrate that PD-1 is not required for the induction of CD8+ T cell exhaustion (TEX) in chronic LCMV infection. The absence of PD-1 leads to more cytotoxic, but terminally differentiated TEX, with compromised long-term durability. PD-1 may well serve to protect TEX from excessive overstimulation, proliferation, and terminal differentiation.
Abstract
Programmed Death-1 (PD-1) has received considerable attention as a key regulator of CD8+ T cell exhaustion during chronic infection and cancer because blockade of this pathway partially reverses T cell dysfunction. Although the PD-1 pathway is critical in regulating established “exhausted” CD8+ T cells (TEX cells), it is unclear whether PD-1 directly causes T cell exhaustion. We show that PD-1 is not required for the induction of exhaustion in mice with chronic lymphocytic choriomeningitis virus (LCMV) infection. In fact, some aspects of exhaustion are more severe with genetic deletion of PD-1 from the onset of infection. Increased proliferation between days 8 and 14 postinfection is associated with subsequent decreased CD8+ T cell survival and disruption of a critical proliferative hierarchy necessary to maintain exhausted populations long term. Ultimately, the absence of PD-1 leads to the accumulation of more cytotoxic, but terminally differentiated, CD8+ TEX cells. These results demonstrate that CD8+ T cell exhaustion can occur in the absence of PD-1. They also highlight a novel role for PD-1 in preserving TEX cell populations from overstimulation, excessive proliferation, and terminal differentiation.
Chronic viral infections, such as HIV, HCV, and others, place a significant strain on antiviral T cell responses, forcing continued proliferation, cytokine production, and killing of infected cells for months or years (Virgin et al., 2009; Wherry, 2011). As a result, antiviral CD8+ T cell functions become suboptimal over time, a phenomenon known as T cell exhaustion (Gallimore et al., 1998; Zajac et al., 1998). Two cardinal features of exhausted CD8+ T cells (TEX cells) are the gradual loss of effector capabilities and the sustained high expression of multiple inhibitory receptors (Wherry, 2011). CD8+ TEX cells also have altered expression of key transcription factors, including Tbet, Eomesodermin (Eomes), FoxO1, and others (Shin et al., 2009; Kao et al., 2011; Paley et al., 2012; Staron et al., 2014; Martinez et al., 2015). Importantly, CD8+ T cell exhaustion contributes to failed immune control during chronic infection and cancer (Wherry, 2011; Pardoll, 2012).
The inhibitory receptor Programmed Death-1 (PD-1) is a central regulator of CD8+ T cell exhaustion. PD-1 is thought to mediate its inhibitory effects via the local and transient intracellular attenuation of positive signals from TCR/CD3 and costimulatory receptors. Upon ligation, both the ITIM and ITSM within the cytoplasmic domain of PD-1 are phosphorylated, leading to the recruitment of tyrosine phosphatases such as SHP-2 (Okazaki et al., 2001; Parry et al., 2005; Riley, 2009). SHP-2 can then dephosphorylate signaling molecules downstream of TCR/CD3 and CD28, including CD3ζ, Zap70, and PKCθ (Parry et al., 2005; Riley, 2009; Yokosuka et al., 2012). PD-1 also inhibits both the PI3K–Akt–mTOR and Ras–MEK–ERK pathways, impacting glucose metabolism and cell cycle regulation (Parry et al., 2005; Patsoukis et al., 2012). Expression of PD-1 and its primary ligand PD-L1 is highly up-regulated during chronic infection and cancer. The importance of this elevated PD-1 and PD-L1 expression has been demonstrated in several animal models where in vivo antibody-mediated blockade of the PD-1 pathway reinvigorates CD8+ TEX cell responses and decreases viral load or tumor burden (Blank et al., 2004; Iwai et al., 2005; Barber et al., 2006; Velu et al., 2009). Recent studies have extended these observations from animal models to humans, demonstrating a potent ability of PD-1 pathway blockade to revitalize antiviral immune responses (Day et al., 2006; Petrovas et al., 2006; Urbani et al., 2006; Boni et al., 2007), as well as antitumor immunity in late-stage cancer patients (Brahmer et al., 2012; Topalian et al., 2012). The observations of reversibility of exhaustion by the PD-1 pathway blockade indicate that CD8+ TEX cells, or at least a subset of the population, are not terminally dysfunctional (Blackburn et al., 2008). Furthermore, blockade of other inhibitory receptors alone and in combination with PD-1–PD-L1 blockade suggests that PD-1 is the major inhibitory receptor controlling exhaustion (Blackburn et al., 2009; Kassu et al., 2010; Sakuishi et al., 2010; Wherry, 2011). Although it is clear that PD-1–based therapies have exciting clinical potential and can dramatically improve immune responses, the precise role of PD-1 in CD8+ TEX cells remains incompletely understood.
A fundamental unresolved question is what role PD-1 signals play in initiating and/or establishing the program of T cell exhaustion. One possibility is that PD-1 directly causes the development of CD8+ T cell exhaustion. This question has previously been challenging to address because PD-1 pathway deficiency results in excessive CD8+ T cell–mediated immunopathology and altered viral pathogenesis, preventing analysis of T cell responses after the first week postinfection (p.i.; Barber et al., 2006; Frebel et al., 2012). However, the robust functionality of CD8+ T cells in the absence of PD-1 at these early time points suggests that T cell exhaustion may not develop without PD-1 signals. This outcome would implicate the PD-1 pathway as a major regulatory network inducing the development of T cell exhaustion. Alternatively, PD-1 could inhibit CD8+ T cell function during chronic infection but may not play a direct role as the initiator of the program of exhaustion. In this scenario, CD8+ T cells could still become exhausted even in settings of PD-1 deficiency. The implications of these two distinct possibilities are important for the design and implementation of therapies blocking or manipulating the PD-1 pathway in cancer, infections, and other diseases.
Here we have used a dual adoptive transfer approach that allowed us to study the development of PD-1–deficient (PD-1 KO) antiviral CD8+ T cell responses during chronic lymphocytic choriomeningitis virus (LCMV) infection in the absence of lethal immune-mediated damage and altered viral pathogenesis. Using this approach, we found that genetic deletion of PD-1 on virus-specific CD8+ T cells was insufficient to prevent the development of exhaustion. In fact, PD-1 KO virus-specific CD8+ T cells were less functional and had higher expression of other inhibitory receptors compared with WT virus-specific CD8+ T cells. Early overstimulation and robust proliferation in the absence of PD-1 led to disruption of a critical proliferative hierarchy between Tbethi and Eomeshi subsets of CD8+ TEX cells that is necessary for sustained responses during chronic infections (Paley et al., 2012). As a result, long-term proliferation and stability of TEX cell responses was compromised without PD-1. However, improved cytotoxic ability and increased localization of TEX cells to peripheral tissues without PD-1 suggests that the PD-1 pathway has previously unappreciated roles in shaping CD8+ TEX cell populations. These findings demonstrate that PD-1 signals do not instruct the development of CD8+ T cell exhaustion. However, PD-1 is required to prevent terminal differentiation of CD8+ TEX cells while simultaneously controlling important anatomical and functional aspects of these cells. These findings have important implications for understanding the long-term impact of PD-1–based therapies in humans.
RESULTS
Co-adoptive transfer strategy allows for examination of PD-1–deficient virus-specific CD8+ T cells during chronic LCMV infection
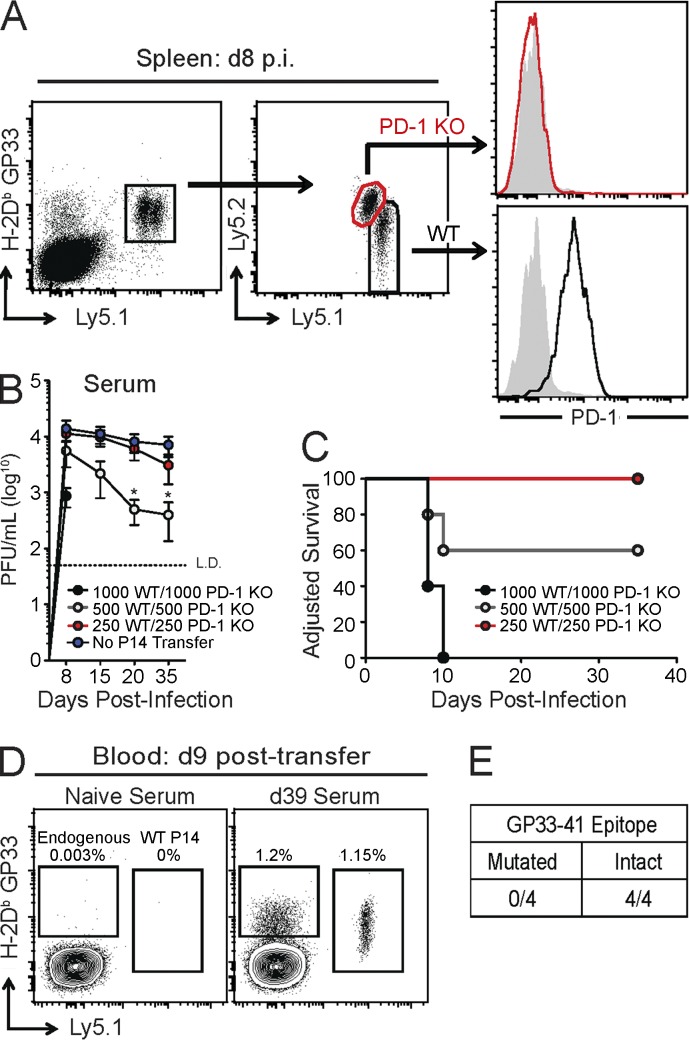
Direct infection of PD-1 or PD-L1 KO mice with LCMV clone 13 causes lethal immunopathology (Barber et al., 2006; Frebel et al., 2012). Previous experiments using adoptive transfer of LCMV-specific TCR-transgenic CD8+ T cells (P14 cells) also resulted in immunopathology when 104 PD-1 KO P14 cells were transferred (Frebel et al., 2012). We hypothesized that using lower numbers of P14 cells would avoid immune-mediated damage, allowing for analysis of PD-1 KO P14 cell exhaustion during chronic infection. Thus, a co-adoptive transfer strategy was used in which equal numbers of WT and PD-1 KO P14 cells were transferred into congenically distinct WT mice, followed by infection with LCMV clone 13 (Fig. 1 A). This strategy allowed for direct comparison of WT and PD-1 KO P14 cell responses in the same recipient mice during chronic viral infection while controlling for precursor frequency, TCR repertoire, viral load, inflammatory environment, and immunopathology. To determine whether this co-adoptive transfer strategy mitigated alterations in viral load and/or morbidity that occur as a result of transfer of high numbers of P14 cells (Blattman et al., 2009; Frebel et al., 2012), viremia and pathogenesis were assessed in mice that received varying numbers of WT and PD-1 KO P14 cells. Transfer of >1,000 total P14 cells (500 WT + 500 PD-1 KO) resulted in reduced viral load (Fig. 1 B) and significant morbidity (Fig. 1 C) compared with no P14 transfer, consistent with previous studies (Blattman et al., 2009; Frebel et al., 2012). However, transfer of 500 total P14 cells (250 WT + 250 PD-1 KO) did not significantly alter viremia or morbidity during chronic LCMV infection compared with mice that did not receive P14 cells (Fig. 1, B and C). One concern is that the presence of a monoclonal P14 T cell population might drive epitope escape (Pircher et al., 1989; Blattman et al., 2009). However, under the conditions used here, virus derived from recipients of WT and PD-1 KO P14 cells was capable of inducing a robust GP33-specific CD8+ T cell response when inoculated into new naive mice, indicating an intact GP33 sequence even in the presence of PD-1 KO P14 cells (Fig. 1, D and E). These results demonstrate that a co-adoptive transfer approach with small numbers of WT and PD-1 KO P14 cells can be used to address the development of CD8+ T cell exhaustion in the absence of cell-intrinsic PD-1 signals.
Figure 1.
Adoptive transfer of WT and PD-1 KO P14 cells as a model to study T cell exhaustion in the absence of PD-1. In brief, CD8+ T cells were isolated from peripheral blood of naive WT or PD-1 KO P14 mice. WT and PD-1 KO P14 cells were mixed at a 1:1 ratio (250 cells each), adoptively transferred into naive recipient mice, and infected with LCMV clone 13. (A) Representative FACS plots of gating scheme for WT and PD-1 KO P14 cells. P14 cells were gated from total CD8+ T cells (far left) by expression of H-2Db GP33 tetramer, congenic markers, and PD-1, as indicated (shaded gray: antibody isotype control). (B) Longitudinal analysis of viral load in serum of mice that received the indicated numbers of WT and PD-1 KO P14 cells followed by infection with LCMV clone 13 (±SEM). *, P < 0.05 (unpaired Student’s t test). (C) Survival curve of mice that received the indicated numbers of WT and PD-1 KO P14 cells after LCMV clone 13 infection. “Adjusted Survival” indicates loss of >20% of total body weight and subsequent euthanasia. (D) Representative FACS plots of endogenous GP33 and WT P14 responses in naive mice at day 9 posttransfer after inoculation with serum from a naive mouse or from a mouse that received co-adoptive transfer of WT and PD-1 KO P14 cells followed by LCMV clone 13 infection (day 39 p.i.). Values indicate frequency of endogenous GP33 and P14 responses as a percentage of CD8+ T cells. (E) Summary of the number of naive WT mice with (intact) or without (mutated) GP33-specific T cell responses after inoculation with serum from mice with WT and PD-1 KO P14 cells. All data are representative of three independent experiments with at least four mice per group (A–E).
CD8+ T cell exhaustion develops in the absence of PD-1
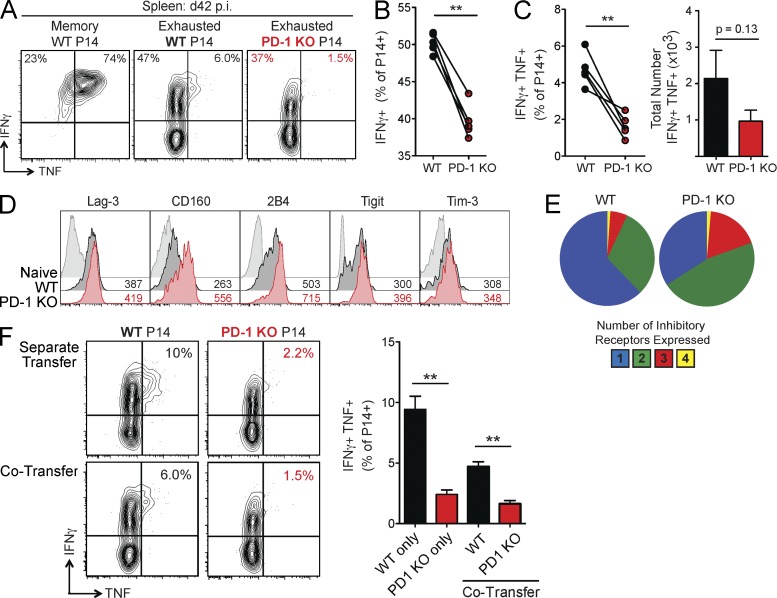
Previous studies demonstrating potent inhibition of CD8+ T cells by the PD-1 pathway suggest that PD-1 may be essential for the development of T cell exhaustion (Barber et al., 2006; Keir et al., 2008; Wherry, 2011; Frebel et al., 2012). Thus, we tested the hypothesis that PD-1 KO P14 cells would not become exhausted during chronic LCMV infection. First, we examined the ability of WT versus PD-1 KO P14 cells to produce cytokines at day 42 p.i. after ex vivo peptide restimulation. At this time point, WT P14 cells displayed characteristic features of exhaustion. Compared with control memory P14 cells generated after acute LCMV infection, exhausted WT P14 cells less efficiently produced IFNγ (mean of 86% vs. 51%) or coproduced IFNγ and TNF (mean of 76% vs. 8%; Fig. 2 A). Surprisingly, production of IFNγ was even more severely reduced in the PD-1 KO P14 population, with a mean of only 39% of cells producing IFNγ (Fig. 2, A and B). PD-1 KO P14 cells also developed a significant reduction in IFNγ production per cell, as indicated by lower mean fluorescence intensity (MFI) of IFNγ expression compared with WT P14 cells in the same mice (Fig. 2 A and not depicted). In addition, PD-1 KO P14 cells exhibited reduced poly-functionality compared with WT P14 cells, with minimal coproduction of IFNγ and TNF (Fig. 2, A and C). There was a similar trend toward decreased total numbers of PD-1 KO IFNγ+TNF+ cells compared with WT in the spleen (Fig. 2 C). In contrast, the ability to degranulate, as measured by LAMP-1/CD107a staining, was retained (not depicted). Reduced cytokine production in the absence of PD-1 also corresponded with higher expression of multiple other inhibitory receptors, a second key feature of exhaustion. PD-1 KO P14 cells expressed higher Lag-3, 2B4/CD244, CD160, and Tigit than WT P14 cells at day 42 p.i. (Fig. 2 D). A higher frequency of PD-1 KO P14 cells also simultaneously coexpressed Lag-3, 2B4, Tim-3, and CD160 compared with WT cells (Fig. 2 E). Analysis of WT and PD-1 KO P14 cells in separate mice resulted in similar findings, confirming the CD8+ T cell–intrinsic nature of this effect (Fig. 2 F and not depicted). These findings indicate that CD8+ T cell exhaustion can develop in the absence of PD-1. In fact, two defining features of CD8+ TEX cells, loss of cytokine poly-functionality, and elevated inhibitory receptor coexpression, were more severe when CD8+ T cell–intrinsic PD-1 signals were absent.
Figure 2.
CD8+ T cell exhaustion develops in the absence of PD-1. WT and PD-1 KO P14 cells were mixed at a 1:1 ratio (250 cells each), adoptively transferred into naive recipient mice, and infected with LCMV clone 13. For some experiments, 500 WT or 500 PD-1 KO P14 cells were transferred into separate naive recipient mice. P14 responses were then analyzed during the chronic phase of infection (day 42 p.i.) in the spleen. (A) Intracellular cytokine staining for IFNγ and TNF after stimulation with GP33 peptide (left). Values indicate the frequency of P14 cells producing IFNγ and/or TNF for individual mice at day 42 p.i. (B) Summary of the frequency of P14 cells producing IFNγ for multiple mice. (C) Summary of the frequency (left) and total number (right) of P14 cells coproducing IFNγ and TNF for multiple mice. (D) Protein expression of the indicated inhibitory receptors on naive CD8+ T cells, WT P14 cells, and PD-1 KO P14 cells of individual mice. Values indicate MFI of expression by FACS. (E) Boolean gating analysis of the simultaneous protein expression of multiple inhibitory receptors (Lag-3, 2B4, CD160, and Tim-3) on WT and PD-1 KO P14 cells. Pie charts display individual populations grouped according to total number of inhibitory receptors expressed. (F) Intracellular cytokine staining for IFNγ and TNF after stimulation with GP33 peptide for mice with separate transfer of WT or PD-1 KO P14 cells compared with mice with co-transferred WT and PD-1 KO P14 cells (left). Values indicate the frequency of P14 cells coproducing IFNγ and TNF for individual (left) and multiple mice (right). All error bars indicate ±SEM. **, P < 0.01 (all paired Student’s t test [A–E] except unpaired Student’s t test for separate transfer of P14 cells [F]). All data are representative of three to five independent experiments with at least five mice per group.
PD-1–deficient CD8+ T cells proliferate robustly during the acute phase of infection but develop early signs of dysfunction
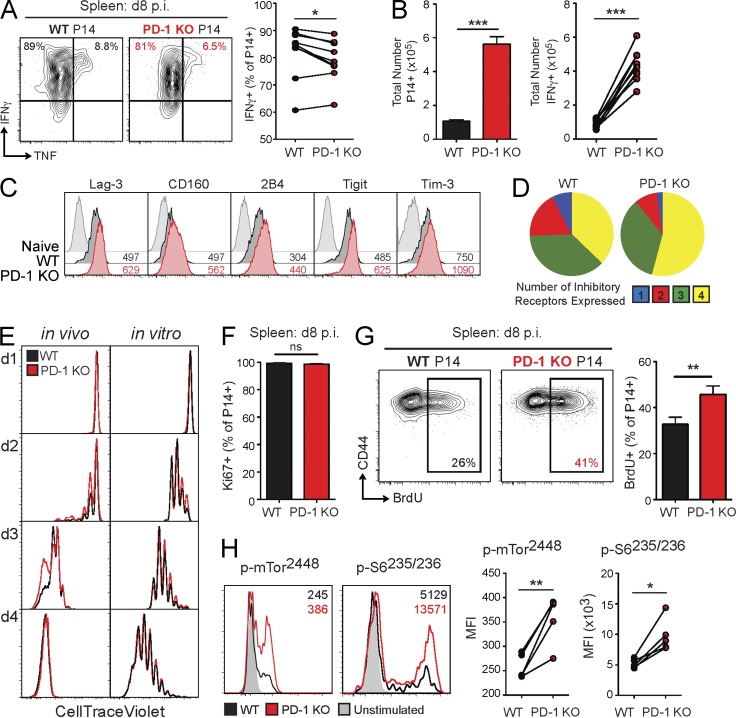
We next tested whether signs of exhaustion in the absence of PD-1 could be detected early during chronic infection. To interrogate this issue, the phenotype and function of WT and PD-1 KO P14 cells were analyzed at the peak of the effector response, day 8 p.i. Both WT and PD-1 KO P14 cells were highly capable of producing IFNγ at this time point (Fig. 3 A), consistent with previous work (Parry et al., 2005; Butte et al., 2007; Patsoukis et al., 2012; Yokosuka et al., 2012; Zinselmeyer et al., 2013). However, PD-1 KO P14 cells displayed a subtle, but significant, reduction in production of IFNγ and coproduction of IFNγ and TNF (Fig. 3 A and not depicted). PD-1 KO P14 cells also greatly outnumbered WT P14 cells in the spleen, blood, and other organs at day 8 p.i. (Fig. 3 B, left; and not depicted), consistent with the notion that PD-1 signals restrain CD8+ T cell responses. Therefore, despite the decrease in per-cell functionality indicated by a reduced frequency of IFNγ+ cells within the PD-1 KO P14 population, there was still a significant increase in the total number of PD-1 KO IFNγ-producing cells compared with WT (Fig. 3 B, right). PD-1 KO P14 cells also had elevated expression (Fig. 3 C) and coexpression (Fig. 3 D) of several other inhibitory receptors at this time point. Collectively, these data suggest that dysfunction of PD-1 KO P14 cells may begin early during LCMV clone 13 infection. However, the increase in total numbers of PD-1 KO P14 cells suggests that loss of PD-1 signals might improve the activation and proliferation of virus-specific CD8+ T cells, despite inducing slight defects in cytokine production and elevated inhibitory receptor expression.
Figure 3.
Early changes in proliferation and functionality of PD-1 KO P14 cells. For in vivo experiments, WT and PD-1 KO P14 cells were mixed at a 1:1 ratio (250 cells each), adoptively transferred into naive recipient mice, and infected with LCMV clone 13. P14 responses were then analyzed at the indicated time points. For early time points (days 1–4), a 1:1 ratio of mixed WT and PD-1 KO P14 cells (2.6 × 106 cells each) was adoptively transferred before infection. (A) Intracellular cytokine staining for IFNγ and TNF after stimulation with GP33 peptide (left). Values indicate the frequency of P14 cells producing IFNγ and TNF for individual (left) and multiple mice (right) at day 8 p.i. in the spleen. (B) Total number of WT and PD-1 KO P14 cells (left) and IFNγ-producing cells (right) at day 8 p.i. in the spleen. (C) Protein expression of the indicated inhibitory receptors by naive CD8+ T cells, WT P14 cells, and PD-1 KO P14 cells at day 8 p.i. in the spleens of individual mice. Values indicate MFI of expression by FACS. (D) Boolean gating analysis of the simultaneous protein expression of multiple inhibitory receptors (Lag-3, 2B4, CD160, and Tim-3) by WT and PD-1 KO P14 cells at day 8 p.i. in the spleen. Pie charts display individual populations grouped according to total number of inhibitory receptors expressed. (E) Expression of CTV as a measure of proliferation on WT and PD-1 KO P14 cells on the indicated days p.i. with LCMV clone 13 in the spleen (left) or after in vitro stimulation with GP33-pulsed splenocytes (right) by FACS. (F) Summary of the frequency of Ki67+ WT and PD-1 KO P14 cells for multiple mice at day 8 p.i. in the spleen. (G) Expression of CD44 versus BrdU incorporation in WT and PD-1 KO P14 cells at day 8 p.i. in the spleen after 24-h BrdU pulse for individual (left) and multiple (right) mice. Values indicate the frequency of P14 cells positive for BrdU based on FMO staining control. (H) Representative FACS histograms of protein expression of p-mTor2448 and p-S6235/236 by WT and PD-1 KO P14 cells at day 8 p.i. in the spleen after stimulation with GP33 peptide for 60 min. Values indicate MFI for phospho-proteins for individual mice (left) and multiple mice (right). All error bars indicate ±SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (paired Student’s t test) for all graphs (A–H). All data are representative of two to five independent experiments with five to eight mice per group.
To test whether the absence of PD-1 resulted in accelerated or more robust initial activation of CD8+ T cells, we examined the early activation and proliferation of WT and PD-1 KO P14 cells in the mixed chimera model. PD-1 KO P14 cells had a trend toward a proliferative advantage from days 2 to 3 p.i. in the spleen compared with WT P14 cells using CellTrace Violet (CTV) dilution to track early cell division, but there was no difference in proliferation by day 4 p.i. (Fig. 3 E, left). There was also no difference in the early proliferation of WT versus PD-1 KO P14 cells when co-cultured with GP33 peptide–pulsed splenocytes in vitro (Fig. 3 E, right). At day 8 p.i. in the spleen, nearly 100% of both WT and PD-1 KO P14 cells expressed the proliferation marker Ki67, suggesting considerable cell division by both WT and PD-1 KO P14 cells over the preceding 2–4 d (Fig. 3 F). To obtain a more precise assessment of proliferation at the later stages of effector expansion, we next used a brief BrdU pulse to examine proliferation between days 7 and 8 p.i. During this time, PD-1 KO P14 cells proliferated significantly more than WT P14 cells (Fig. 3 G). Collectively, these findings suggest that PD-1 restricts virus-specific CD8+ T cell responses during chronic infection by restraining proliferation during the effector phase.
We hypothesized that the robust proliferation of PD-1 KO P14 cells at day 8 p.i. was caused by increased TCR signaling in vivo in the absence of PD-1–mediated inhibition. Previous studies have demonstrated that loss of PD-1 signals in vitro leads to increased phosphorylation of both proximal and distal signaling molecules downstream of the TCR and costimulatory receptors (Okazaki et al., 2001; Parry et al., 2005; Yokosuka et al., 2012). Indeed, at this effector stage PD-1 KO P14 cells had augmented TCR responsiveness, as demonstrated by increased phosphorylation of Akt473 and ERK202/204 (not depicted), as well as mTOR2448 and S6235/236 (Fig. 3 H). Despite this robust TCR stimulation and proliferation, WT and PD-1 KO P14 cells had similar expression of activation and differentiation markers, such as CD69, KLRG1, CD27, CD44, and CD25, during early activation (days 1–4 p.i.) and during the effector phase (day 8 p.i.; not depicted). Thus, PD-1 controls virus-specific CD8+ T cell proliferation early during chronic infection by tempering TCR signals at the peak of infection. These data also suggest that tempering of TCR responsiveness and proliferation during the effector phase may have a benefit in preserving T cell function into the chronic phase of infection.
Decreased survival of CD8+ T cells after the effector phase in the absence of PD-1
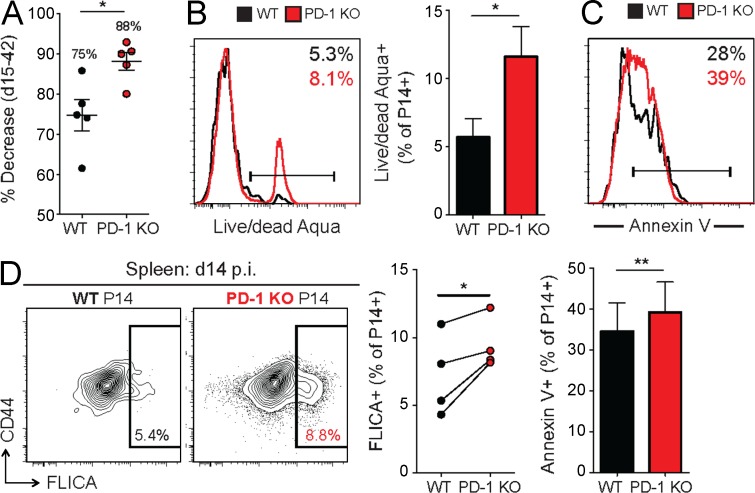
We next examined how the absence of PD-1 affected the transition to later stages of infection. After peak expansion, PD-1 KO P14 cells underwent significantly greater contraction between days 15 and 42 p.i. (88% decrease) compared with WT P14 cells (75% decrease; Fig. 4 A). We hypothesized that this more dramatic contraction was caused by decreased survival in the absence of inhibitory signals from the PD-1 pathway. To test this possibility, WT and PD-1 KO P14 cells were assessed for markers of cell death between days 14 and 20 p.i. in the spleen. A higher frequency of PD-1 KO P14 cells stained with LIVE/DEAD Aqua (Fig. 4 B) and Annexin V (Fig. 4 C), markers of compromised cell membrane integrity which are associated with apoptosis and cell death. PD-1 KO P14 cells also displayed increased staining with fluorescent caspase substrates (FLICA) at day 15 p.i. (Fig. 4 D). In addition, ex vivo survival of PD-1 KO P14 cells was decreased, as demonstrated by a significant reduction in the total frequency of live cells remaining after a 10-h in vitro culture (not depicted). Thus, in the absence of PD-1, virus-specific CD8+ T cells experience considerably more cell death than WT CD8+ T cells of the same specificity in the same inflammatory environment. These findings suggest that PD-1 prevents overstimulation of CD8+ T cells and subsequent excessive cell death during chronic viral infection.
Figure 4.
Reduced survival of PD-1 KO P14 cells during T cell contraction. WT and PD-1 KO P14 cells were mixed at a 1:1 ratio (250 cells each), adoptively transferred into naive recipient mice, and infected with LCMV clone 13. P14 responses were then analyzed at the indicated time points. (A) Percent decrease in the frequency of WT and PD-1 KO P14 cells from peak of T cell response (day 15 p.i.) to chronic phase of infection (day 42 p.i.). Values indicate the mean percent decrease for each population. (B and C) Expression of LIVE/DEAD Aqua (B) and Annexin V (C) as a measure of cell death in WT and PD-1 KO P14 cells at day 18 p.i. in the spleen for individual (histograms) and multiple mice (bar graphs). Values indicate the frequency of P14 cells positive for staining based on FMO staining controls. (D) Expression of CD44 versus FLICA in WT and PD-1 KO P14 cells at day 14 p.i. in the spleen for individual (left) and multiple mice (right). Values indicate the frequency of P14 cells positive for FLICA dye based on FMO staining control. All error bars indicate ±SEM. *, P < 0.05; **, P < 0.01 (paired Student’s t test for B–D and unpaired Student’s t test for A) for all graphs (A–D). All data are representative of three independent experiments with at least four mice per group.
Decreased proliferation and long-term durability of PD-1 KO TEX cells
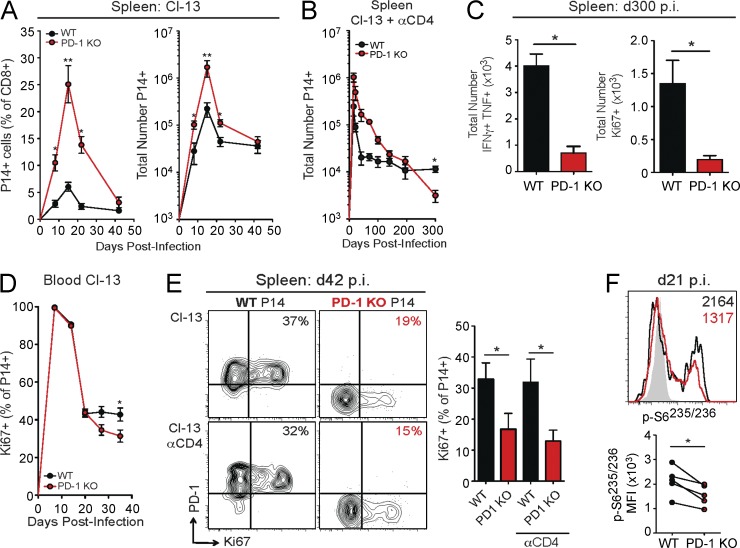
The increase in cell death of PD-1 KO P14 cells suggests that long-term durability of virus-specific CD8+ T cells may be compromised in the absence of PD-1. During LCMV clone 13 infection, the frequency and absolute number of WT P14 cells began to stabilize in the spleen and blood between days 22 and 42 p.i. (Fig. 5 A and not depicted), consistent with previous findings (Shin et al., 2007; Wherry et al., 2007; Blattman et al., 2009). In contrast, PD-1 KO P14 cells declined in both frequency and absolute number until at least day 42 p.i., suggesting that long-term durability of virus-specific CD8+ T cell responses may be compromised in the absence of PD-1 (Fig. 5 A). However, viremia during LCMV clone 13 infection of C57BL/6 mice gradually resolves by days 60–90 p.i, complicating the interpretation of changes in exhaustion at late time points (Matloubian et al., 1994; Blackburn et al., 2009). Therefore, we next used a model of chronic LCMV infection where transient depletion of CD4+ T cells before infection with LCMV clone 13 leads to persistent, life-long viremia (Matloubian et al., 1994). As observed in CD4+ T cell–sufficient clone 13 infection, WT P14 cell responses stabilized in frequency and absolute number after day 48 p.i. in CD4+ T cell–depleted mice (Fig. 5 B and not depicted). In contrast, the number of PD-1 KO P14 cells failed to stabilize and gradually declined over time. By day 300 p.i., the total number of PD-1 KO P14 cells was less than that of WT P14 cells (Fig. 5 B). This deterioration in the number of PD-1 KO P14 cells was also indicated by a significant decrease in the total numbers of IFNγ+TNF+ cells in the spleen at this time point (Fig. 5 C, left). The inability of PD-1 KO P14 cells to form a stable, long-term population of TEX cells in the spleen could be caused, at least in part, by the increased cell death observed in this population. However, ongoing antigen-driven proliferation is central to the durability of TEX cell populations (McCune et al., 2000; Shin et al., 2007). To interrogate how ongoing proliferation is impacted by PD-1 deficiency, we examined proliferation of WT and PD-1 KO P14 cells over the time course of LCMV clone 13 infection. Despite similar expression of Ki67 during the acute phase of infection, PD-1 KO P14 cells had decreased Ki67 expression beginning at day 27 p.i. in the blood (Fig. 5 D). By day 42 p.i., PD-1 KO P14 cells had dramatically reduced expression of Ki67 in the spleen of both CD4+ T cell–sufficient (clone 13) and CD4+ T cell–depleted (Cl-13 αCD4) mice (Fig. 5 E), and this defect in ongoing proliferation persisted until at least day 300 p.i. in CD4-depleted mice (Fig. 5 C, right). In addition, PD-1 KO P14 cells incorporated significantly less BrdU from days 35 to 42 p.i. than WT P14 cells (not depicted). Collectively, these findings indicate that increased cell death and loss of sustained proliferative ability contribute to the deceased stability of PD-1 KO P14 cells in chronic infection. This shift in the proliferative ability of PD-1 KO P14 cells during the chronic phase of infection corresponds with a loss of TCR responsiveness. At day 21 p.i., both WT and PD-1 KO P14 cells have decreased TCR signaling compared with day 8 p.i., as measured by phosphorylation of mTOR2448 and S6235/236 after brief ex vivo peptide restimulation (Fig. 5 F and not depicted). However, PD-1 KO P14 cells had significantly reduced phosphorylation of S6235/236 at day 21 p.i. compared with WT P14 cells (Fig. 5 F). Of note, by day 35 p.i., both WT and PD-1 KO P14 cells had minimal phosphorylation of S6235/236 and other signaling molecules (not depicted). Thus, the PD-1 KO P14 cells that survive into the chronic phase of infection are a less durable population and have reduced TCR responsiveness, as well as limited proliferative ability.
Figure 5.
Diminished long-term proliferation and stability of CD8+ T cells in the absence of PD-1. WT and PD-1 KO P14 cells were mixed at a 1:1 ratio (250 cells each), adoptively transferred into naive recipient mice, and infected with LCMV clone 13. In CD4-depleted mice, αCD4 was given at days −1 and 1 p.i. P14 responses were then analyzed at the indicated time points. (A) Longitudinal analysis of the frequency (left) and absolute numbers (right) of WT and PD-1 KO P14 cells in the spleen during LCMV clone 13 infection. (B) Longitudinal analysis of the total number of WT and PD-1 KO P14 cells in the spleen of CD4-depleted mice with LCMV clone 13 infection. (C) Summary of the total numbers of WT and PD-1 KO P14 cells coproducing IFNγ and TNF (left) or Ki67+ (right) at day 300 p.i. in the spleen. (D) Longitudinal analysis of the frequency of Ki67+ WT and PD-1 KO P14 cells in the blood during LCMV clone 13 infection. (E) Protein expression of PD-1 versus Ki67 in WT and PD-1 KO P14 cells at day 42 p.i. in the spleen during infection with LCMV clone 13 with (bottom) or without (top) CD4 depletion. Values indicate the frequency of P14 cells positive for Ki67. (F) Representative histogram of protein expression of p-S6235/236 in WT and PD-1 KO P14 cells at day 21 p.i. in the spleen after stimulation with GP33 peptide for 60 min. Values indicate the MFI of expression for phospho-proteins for individual mice (top) and multiple mice (bottom). All error bars indicate ±SEM. *, P < 0.05; **, P < 0.01 (paired Student’s t test) for all graphs (A–F). All data are representative of three independent experiments with at least five mice per group.
PD-1 controls CD8+ TEX cell proliferative dynamics by regulating Tbethi and Eomeshi subsets
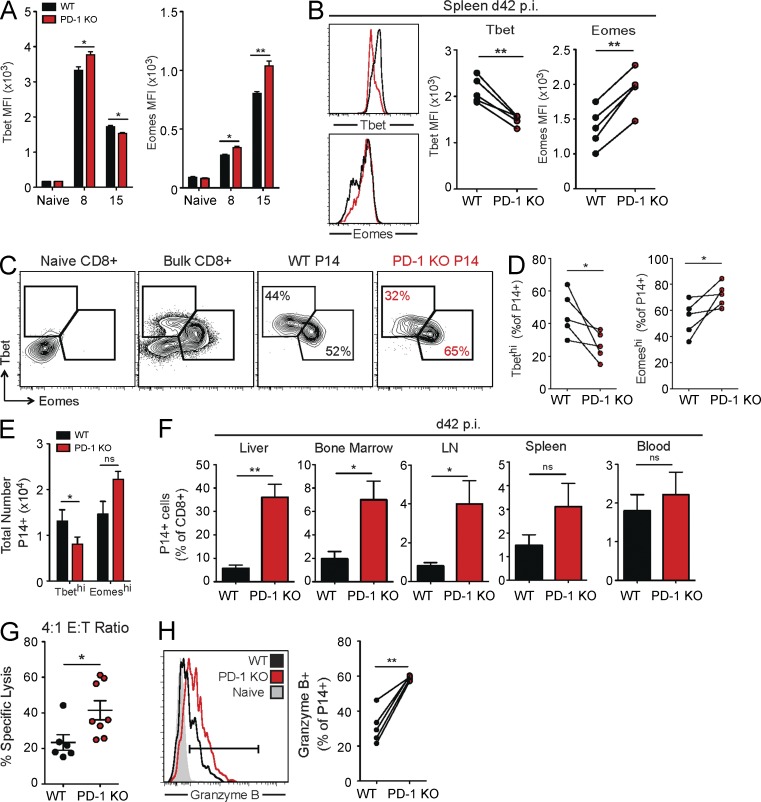
Recent work has demonstrated that two subsets of CD8+ TEX cells, Tbethi progenitors and Eomeshi progeny, exist in a proliferative hierarchy to sustain long-term CD8+ T cell responses during chronic viral infections (Paley et al., 2012). Based on these observations, we next investigated whether loss of PD-1 impacted the balance between these two subsets and whether changes in these subsets could account for the altered TCR responsiveness, proliferation, and stability of the PD-1 KO P14 population. To test this idea, we assessed expression of Tbet and Eomes at days 8, 15, and 42 p.i. in WT and PD-1 KO P14 cells. At day 8 p.i., increased expression of both Tbet and Eomes was observed in PD-1 KO P14 cells, which is consistent with the increased activation of these cells early in infection (Fig. 6 A). However, on day 15 p.i., PD-1 KO P14 cells had a significant decrease in Tbet expression (Fig. 6 A), which was followed by a substantial reduction in Tbet MFI on day 42 p.i. (Fig. 6 B). Reduced Tbet expression on a per-cell basis in PD-1 KO P14 cells was accompanied by a significant decline in the frequency and total number of Tbethi progenitor cells compared with WT P14 cells (Fig. 6, C–E). As a result, the PD-1 KO P14 cell population was skewed toward the Eomeshi subset, as demonstrated by an increase in the frequency of Eomeshi cells (Fig. 6, C and D). There was also a trend toward increased total numbers of Eomeshi cells in the PD-1 KO P14 cell population (Fig. 6 E). In addition, PD-1 KO P14 cells had increased Eomes expression on a per-cell basis at days 15 (Fig. 6 A) and 42 p.i. (Fig. 6 B).
Figure 6.
Altered dynamics of TEX cell subsets in the absence of PD-1. WT and PD-1 KO P14 cells were mixed at a 1:1 ratio (250 cells each), adoptively transferred into naive recipient mice, and infected with LCMV clone 13. P14 responses were then analyzed at the indicated time points. (A) Summary of the MFI of Tbet and Eomes in WT and PD-1 KO P14 cells in the spleen at days 0 (naive), 8, and 15 p.i. with LCMV clone 13. (B) Representative FACS histograms of Tbet and Eomes expression in WT and PD-1 KO P14 cells for individual (left) and multiple (right) mice at day 42 p.i. in the spleen. (C) Protein expression of Tbet versus Eomes in WT and PD-1 KO P14 cells at day 42 p.i. in the spleen of individual mice. Values indicate the frequency of P14 cells that are TbethiEomeslo or EomeshiTbetlo. (D and E) Total frequency (D) and numbers (E) of WT and PD-1 KO P14 TbethiEomeslo and EomeshiTbetlo cells at day 42 p.i. in the spleen. (F) Frequency of P14 cells as a percentage of total CD8+ T cells in multiple organs at day 42 p.i. with LCMV clone 13, as indicated. (G) Cytotoxicity of sorted WT and PD-1 KO P14 cells on day 22 p.i. at an E/T ratio of 4:1 after 18-d incubation. (H) Expression of Granzyme B in naive CD8+ T cells, WT P14 cells, and PD-1 KO P14 cells in individual (left) and multiple mice (right). All error bars indicate ±SEM. *, P < 0.05; **, P < 0.01 (paired Student’s t test for A–F and H and unpaired Student’s t test for G) for all graphs (A–H). All data are representative of two to five independent experiments with at least five mice per group.
We next asked whether this change in Tbet and Eomes expression observed in exhausted PD-1 KO P14 cells truly represented a shift in subset dynamics to Eomeshi progeny. To test this idea, PD-1 KO P14 cells were assessed for other cardinal features of Eomeshi cells (Paley et al., 2012). PD-1 KO P14 cells, like WT Eomeshi TEX cells, were significantly increased in peripheral tissues, including the bone marrow and liver (Fig. 6 F). Key functional properties of Eomeshi cells were also observed in PD-1 KO P14 cells. Despite decreased cytokine production (Fig. 2, A–C) and proliferation (Fig. 5, D and E), PD-1 KO P14 cells displayed enhanced cytotoxicity compared with WT P14 cells (Fig. 6 G), as well as increased expression of Granzyme B at day 42 p.i. (Fig. 6 H). These differences were also consistent with higher expression of multiple inhibitory receptors observed on PD-1 KO P14 cells, as well as the patterns of co-inhibitory receptor expression on the Eomeshi subset of WT P14 TEX cells (Fig. 2, D and E; Paley et al., 2012). Thus, PD-1 KO P14 cells accumulate mainly as terminally differentiated Eomeshi progeny. These findings suggest that PD-1 controls proliferation dynamics during chronic viral infection, at least in part, by preventing loss of Tbethi progenitor cells. In addition to changes in proliferation, loss of PD-1 signals leads to a TEX cell pool with more extensive tissue distribution and enhanced cytotoxicity, which may be an important consideration when using PD-1 blockade for chronic infections and cancer.
DISCUSSION
Expression of the inhibitory receptor PD-1 is strongly associated with CD8+ T cell exhaustion in chronic infection and cancer (Wherry, 2011; Pardoll, 2012). The ability to partially restore T cell functions by PD-1–PD-L1 pathway blockade suggests a fundamental link between this pathway and the development of CD8+ T cell exhaustion (Barber et al., 2006; Wherry, 2011). However, a key unanswered question is whether PD-1 directly causes or induces T cell exhaustion. Here, we investigated this question using approaches that control for confounding issues, such as changes in pathogenesis or viral load, in the absence of PD-1. We found that genetic absence of PD-1 on antigen-specific CD8+ T cells did not prevent exhaustion during chronic LCMV infection. Consistent with the negative regulatory role of this pathway, CD8+ T cell stimulation and proliferation were greatly enhanced by the absence of PD-1 during the acute phase of infection. However, PD-1 deficiency also led to increased apoptosis during the contraction phase. Moreover, permanent absence of PD-1 resulted in the dysregulation of a key proliferative hierarchy required to sustain CD8+ TEX cells during chronic infection (Paley et al., 2012). Without PD-1, CD8+ TEX cells accumulated as Eomeshi cells, whereas the Tbethi progenitor pool was depleted. In addition, this shift was accompanied by functional changes associated with an altered balance of these subsets. Cytokine production and proliferation declined in the absence of PD-1 as the result of loss of the slightly more functional Tbethi population, but cytotoxicity was improved because the Eomeshi subset that possesses better killing capacity was enriched. These findings reveal an unexpected dual role for PD-1 signals during chronic viral infection. Although PD-1 clearly negatively regulates CD8+ T cell responses during the chronic phase of infection (Barber et al., 2006; Wherry, 2011), these new observations indicate that this pathway also plays a critical role in protecting CD8+ TEX cells from excessive stimulation, terminal exhaustion, and erosion of the population over time. These findings have implications for prolonged clinical treatments with PD-1 pathway antagonists where the benefit of rejuvenating CD8+ TEX cells may have to be balanced with the risk of overstimulation and lack of long-term stability.
Although complete genetic absence of PD-1 is unlikely to manifest clinically, several important genetic variants in and around the PDCD1 gene have been described that are associated with autoimmunity, cancer prognosis, or chronic viral infection (James et al., 2005; Velázquez-Cruz et al., 2007; Muenst et al., 2010, 2013; Zhang et al., 2012). Although mechanistically it remains unclear how most of these genetic variants affect the expression or function of PD-1, these observations do suggest that genetic variation in the PD-1 pathway could be an important parameter affecting the development of T cell responses and PD-1 checkpoint blockade in humans. It will be interesting to determine whether there are any relationships between these human PDCD1 polymorphisms and changes in the development and/or dynamics of CD8+ TEX cells we have observed in mice. In addition to natural genetic variation, it is now possible to genetically engineer T cells for adoptive immunotherapeutic approaches (Restifo et al., 2012; Grupp et al., 2013). PD-1 might be an interesting target to consider in such therapeutically modified T cell adoptive immunotherapy approaches. However, our data suggest that such approaches to modify or genetically ablate PD-1 should be considered with caution.
One interesting aspect of the PD-1–deficient CD8+ T cell response that develops during chronic LCMV infection is the elevated expression of many other inhibitory receptors. In the absence of PD-1, we observed higher expression of Lag-3, 2B4/CD244, CD160, and Tigit. Most, if not all, of these inhibitory receptors are up-regulated after TCR signaling in acutely activated T cells, and their elevated expression may provide a mechanism to temper increased TCR stimulation in the absence of PD-1–mediated inhibition, particularly during the chronic phase of infection. In addition, the up-regulation of other inhibitory receptors in the absence of PD-1 signals could partially explain the synergy observed during co-blockade of PD-1 and other inhibitory receptors (Blackburn et al., 2009; Wherry, 2011; Pardoll, 2012) and reveal opportunities for targeting other inhibitory receptors in conjunction with PD-1. Thus, these observations support use of co-blockade of multiple inhibitory receptors in cancer patients for optimal reversal of T cell exhaustion. This issue will be interesting to examine clinically as the timing and/or sequence with which different inhibitory receptor blockades are introduced may influence the therapeutic outcome.
Our data provide a cellular mechanism to explain the population-based effects of PD-1 deficiency during chronic infection. PD-1 is critical for regulation of a proliferative hierarchy necessary to maintain CD8+ TEX cell populations. We have previously described a lineage relationship within the CD8+ TEX cell pool where Tbethi PD-1int cells give rise to Eomeshi PD-1hi cells (Paley et al., 2012). Importantly, Tbethi and Eomeshi TEX cell subsets are found not only in chronic LCMV infection of mice, but also in human HCV and HIV infections (Paley et al., 2012; Buggert et al., 2014). The transition between subsets is driven by persisting antigen and is accompanied by extensive division, down-regulation of Tbet, and up-regulation of Eomes (Paley et al., 2012). These Eomeshi progeny are more terminally differentiated and lack future proliferative capacity (Paley et al., 2012). It has recently been shown that the transcription factor FoxO1 regulates the transition between Tbethi and Eomeshi TEX cells by integrating changes in signals from the P13K–Akt–mTOR pathway. Increased FoxO1 expression leads to up-regulation of PD-1 and conversion into Eomeshi cells (Staron et al., 2014). Our current data indicate a critical, but perhaps somewhat paradoxical, role for PD-1 in this proliferative hierarchy. Without PD-1, CD8+ TEX cells accumulate as Eomeshi terminally differentiated progeny. These data support the idea that PD-1 tempers the continual activation and differentiation of the Tbethi progenitor pool and partially protects this subset from chronic overstimulation. Thus, in the steady-state, PD-1–mediated inhibition of Tbethi TEX cell stimulation may preserve these cells for future activation and generation of terminal progeny. How this PD-1–mediated effect is linked to FoxO1, TCR responsiveness, or other metabolic pathways in the TEX cell subsets remains to be determined. The ability to exploit this proliferative reserve by transiently removing PD-1 signals (e.g., via blockade) has resulted in major new clinical opportunities to treat chronic infections and cancer (Barber et al., 2006; Brahmer et al., 2012; Topalian et al., 2012). It will be important in the future to determine how transient versus permanent loss of PD-1 signaling on responding T cells affects TEX cell subset stability and lineage dynamics.
Previous work has also demonstrated that Tbet directly represses expression of the Pdcd1 gene (encoding PD-1; Kao et al., 2011). In addition, persisting antigen stimulation can lead to loss of Tbet expression (Kao et al., 2011; Paley et al., 2012). Collectively, these observations suggest a model whereby intermediate levels of PD-1 expression inhibit TCR signaling during chronic infection to help maintain Tbet expression, as well as to promote Tbethi cell survival. Tbet, in turn, partially represses Pdcd1 transcription and maintains PD-1 expression at an intermediate level (Kao et al., 2011), which may preserve cytokine production and proliferative potential in the Tbethi TEX cell subset. Interestingly, FoxO1-deficient CD8+ T cells that fail to maintain PD-1 expression also cannot be sustained during chronic LCMV infection (Staron et al., 2014), further supporting a connection between PD-1 and durability of CD8+ TEX cells. Thus, the model proposed here indicates a critical feedback loop centered on PD-1 in TEX cell subset maintenance and conversion. Our data suggest a novel dual role for PD-1 signals in negatively regulating CD8+ T cell proliferation and function during chronic infection, but also in sustaining Tbethi progenitor cells, a population critical for a proliferative hierarchy which partially controls chronic infection.
Despite the clear clinical promise and many early successes of PD-1 or PD-L1 blockade, the optimal therapeutic strategy for targeting the PD-1 pathway remains to be determined. Transient blockade of PD-1–PD-L1 can have profound effects on the reinvigoration of CD8+ TEX cells during chronic infections and cancer, as well as reduced viral or tumor burden (Wherry, 2011; Pardoll, 2012). However, we found that permanent absence of PD-1 from the onset of infection did not prevent the development of exhaustion. In fact, CD8+ T cell dysfunction was increased with genetic PD-1 deficiency, resulting in terminal exhaustion and decreased stability of the CD8+ T cell population. These findings have important implications for different therapeutic clinical approaches because long-term or “maintenance” PD-1 pathway blockade and genetically engineered T cells with altered PD-1 are being considered in human cancer patients. There are several key questions that should be addressed to determine the most effective strategies for use of PD-1 pathway inhibitors in humans. First, in the experimental model used here, PD-1 signaling is absent for CD8+ T cell priming as well as throughout chronic stimulation. It remains unclear whether PD-1 deficiency has the same effect at different stages of T cell differentiation. Conditional deletion of PD-1 signals should be used to resolve this issue in future studies. Second, the function of PD-1 may vary depending on the strength of TCR stimulation. Here, we show that robust TCR signaling in PD-1 KO P14 cells leads to increased cell death and loss of population durability. These observations are consistent with previous work demonstrating that stronger T cell stimulation leads to more severe exhaustion and clonal deletion (Wherry et al., 2003a). In LCMV clone 13 infection, CD8+ T cells responding to the NP396 epitope rapidly progress through exhaustion and are physically deleted because of overstimulation, whereas CD8+ T cells specific for GP33 and GP276 persist but become exhausted (Wherry et al., 2003a). The prevailing hypothesis is that the strength of TCR stimulation determines deletion versus exhaustion. Removal of PD-1 increases the strength of TCR signals received by GP33-specific P14 cells in the current study, which accelerates their exhaustion and leads to eventual deletion. However, deletion of PD-1 KO P14 cells is much slower than observed for the NP396-specific population in WT mice. This difference could indicate that the strength of NP396 stimulation is still greater than GP33 stimulation, even in the absence of PD-1–mediated inhibition. Alternatively, there could be other features of stimulation that are important in determining deletion versus exhaustion of different epitope-specific T cell populations (e.g., different APCs, direct versus cross-presentation, T cell precursor frequency, etc.). It will be interesting to determine the relative impact of PD-1 deficiency on CD8+ T cells of other specificities and to begin to dissect these issues. We would predict that loss of PD-1 in T cells exposed to stronger stimulation would result in accelerated and/or more severe exhaustion, as well as more rapid deletion. Third, it is unclear whether genetic absence of PD-1 is equivalent to antibody-mediated blockade. Given the broad expression of PD-1 and PD-1 ligands (Keir et al., 2008), blockade of this pathway may impact a variety of immune and nonimmune cell types. Finally, the impact of prolonged inhibition of the PD-1 pathway may be considerably different in the presence versus absence of high-level antigen stimulation. In the current setting of chronic LCMV infection, where the antigen stimulation and “stress” on the CD8+ T cell compartment is high and constant, absence of a key negative regulatory pathway may result in T cell overstimulation. In settings where antigen levels have been controlled to a low level, continued loss of PD-1 signals may be less detrimental to the stability to the T cell population and may promote CD8+ T cell functionality. Future studies will be necessary to investigate these possible outcomes.
In summary, we have identified a novel role for the PD-1 pathway in regulating CD8+ T cell exhaustion during chronic viral infection. PD-1 signals are critical for preventing the early overactivation and proliferation of CD8+ T cells in response to chronic infection, thus promoting the establishment and stability of exhausted T cell responses. Additional work will be necessary to further define the molecular pathways regulated by PD-1 in CD8+ TEX cells and to compare genetic loss of PD-1 with the more clinically relevant setting of PD-1 pathway blockade.
MATERIALS AND METHODS
Mice and infections.
PD-1–deficient (PD-1 KO) mice (Keir et al., 2007) were crossed to C57BL/6 P14 mice (Pircher et al., 1989) to generate PD-1 KO P14 mice. Mice were infected with 4 × 106 PFU of LCMV clone 13 strain by i.v. injection to generate a chronic infection. In experiments with CD4 depletion, 200 µg anti-CD4 antibody (GK1.5; Bio X Cell) was administered i.p. on days −1 and 1 of LCMV infection. Viral titers were measured as previously described (Kao et al., 2011). All animals were housed at the University of Pennsylvania (Philadelphia, PA). Experiments were performed in accordance with protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Adoptive transfer and lymphocyte isolation.
CD8+ T cells were isolated from peripheral blood or spleens of naive WT or PD-1 KO P14 mice. For spleen isolation, P14 cells were purified by CD8 negative selection (STEMCELL Technologies). The indicated number of WT or PD-1 KO cells was transferred into separate naive recipient mice or mixed at a 1:1 ratio and transferred into naive recipient mice. For proliferation assays, P14 cells were labeled with 10 mM CTV (Life Technologies) before transfer.
Flow cytometry, cell sorting, and phospho-flow.
All cells were stained with LIVE/DEAD Fixable Dead Cell Stain (Life Technologies) to discriminate live from dead cells. Surface staining was performed as described previously (Kao et al., 2011). For intracellular cytokine staining, single-cell suspensions were incubated for 5 h at 37°C in the presence of GolgiPlug (BD) and GolgiStop (BD) with or without 1 µM GP33 peptide. Cells were then stained with a Cytofix/Cytoperm kit (BD) according to the manufacturer’s instructions. For intracellular staining of transcription factors, a FoxP3 staining kit (eBioscience) was used according to the manufacturer’s instruction. For signaling experiments, cells were rested for 1–2 h at 37°C in 10% RPMI, followed by restimulation with 1 µM GP33 peptide for 60 min. Phosphorylated Akt473 (p-Akt473), p-S6235/236, p-ERK202/204, and p-mTOR2448 were detected using paraformaldehyde fixation and methanol permeabilization. Antibodies used for flow cytometry were purchased from BD (CD4, CD8, CD19, CD44, 2B4, Ki67, p-ERK202/204, and p-mTOR2448), BioLegend (PD-1 [RMP1-30 clone], Tim-3, TNF, IFNγ, and Tbet), R&D Systems (MIP-1α), eBioscience (CD8, Lag3, CD160, 2B4, CD45.1, CD45.2, CD107α, and Eomes), or Cell Signaling Technology (p-Akt473 and p-S6235/236). MHC class I peptide tetramers were made and used as described previously (Wherry et al., 2003b; Kao et al., 2011). Poly-caspase analysis was performed with Flica Vybrant FAM Poly Caspases Assay kit (Life Technologies). Data were collected on an LSRII (BD) and analyzed with FlowJo software (Tree Star). Cell sorting was performed using a FACSAria II (BD).
BrdU treatment and detection.
Animals were treated with 2 mg BrdU (Sigma-Aldrich) i.p. daily for 1–5 d before tissue harvest and analysis. BrdU incorporation was assessed by the BrdU Flow kit (BD) per the manufacturer’s instructions.
In vitro stimulation.
Naive WT and PD-1 KO P14 cells were isolated from spleens by CD8 negative selection (Stem Cell Technologies) and cultured in RPMI-1640 medium in the presence of 100 U/ml recombinant human IL-2 (R&D Systems). Naive P14 cells were stimulated for 4 d by coculturing with GP33 peptide–pulsed naive splenocytes.
Cytotoxicity assay.
Splenocytes from WT mice were labeled with two different concentrations (50 nM = “dim” or 1 µM = “bright”) of CTV. CTVdim target splenocytes were incubated with GP33 peptide, whereas CTVbright splenocytes were incubated with SIINFEKL peptide for 1 h at 37°C. 2 × 103 GP33-pulsed target cells were mixed with 2 × 103 SIINFEKL-pulsed control cells. WT and PD-1 KO P14 cells were isolated from day 29 LCMV clone 13–infected spleens by cell sorting on a FACSAria II. 8 × 103 WT or PD-1 KO P14 cells were added to wells containing target and control cells in triplicate. Unlabeled, WT CD45.1+ splenocytes were used to normalize cell concentrations in each well. After 16 h at 37°C, target cells were assessed by flow cytometry. Percent-specific lysis was calculated as 100 × [1 − (% target cells remaining/% control cells remaining)].
Statistical analysis.
Student’s t tests (paired and unpaired) were performed using Prism software (GraphPad Software).
Acknowledgments
We thank M. Ali, B. Barnett, J. Johnnidis, R. Staupe, M. Kurachi, and E. Stelekati for technical assistance in experiments and the members of the Wherry Lab for meaningful discussions and critical reading of the manuscript.
This work was funded by the T32 HIV Pathogenesis training grant (PMO), Robertson Foundation/Cancer Research Institute Irvington Fellowship (KEP), and National Institutes of Health (grants AI105343, AI112521, AI117718, AI082630, AI095608, and HHSN266200500030C) to E.J. Wherry.
E.J. Wherry has a patent licensing agreement on the PD-1 pathway. The authors declare no additional competing financial interests.
Footnotes
Abbreviations used:
- CTV
- CellTrace Violet
- LCMV
- lymphocytic choriomeningitis virus
- MFI
- mean fluorescence intensity
- p.i.
- postinfection
- TEX cell
- exhausted CD8+ T cell
References
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., and Ahmed R.. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Freeman G.J., and Wherry E.J.. 2008. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl. Acad. Sci. USA. 105:15016–15021. 10.1073/pnas.0801497105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A., Betts M.R., Freeman G.J., Vignali D.A., and Wherry E.J.. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37. 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C., Brown I., Peterson A.C., Spiotto M., Iwai Y., Honjo T., and Gajewski T.F.. 2004. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 64:1140–1145. 10.1158/0008-5472.CAN-03-3259 [DOI] [PubMed] [Google Scholar]
- Blattman J.N., Wherry E.J., Ha S.-J., van der Most R.G., and Ahmed R.. 2009. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83:4386–4394. 10.1128/JVI.02524-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni C., Fisicaro P., Valdatta C., Amadei B., Di Vincenzo P., Giuberti T., Laccabue D., Zerbini A., Cavalli A., Missale G., et al. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225. 10.1128/JVI.02844-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J.R., Tykodi S.S., Chow L.Q.M., Hwu W.-J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366:2455–2465. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggert M., Tauriainen J., Yamamoto T., Frederiksen J., Ivarsson M.A., Michaëlsson J., Lund O., Hejdeman B., Jansson M., Sönnerborg A., et al. 2014. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 10:e1004251 10.1371/journal.ppat.1004251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., and Freeman G.J.. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 27:111–122. 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- Frebel H., Nindl V., Schuepbach R.A., Braunschweiler T., Richter K., Vogel J., Wagner C.A., Loffing-Cueni D., Kurrer M., Ludewig B., and Oxenius A.. 2012. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209:2485–2499. 10.1084/jem.20121015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore A., Glithero A., Godkin A., Tissot A.C., Plückthun A., Elliott T., Hengartner H., and Zinkernagel R.. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383–1393. 10.1084/jem.187.9.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F., et al. 2013. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368:1509–1518. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Terawaki S., and Honjo T.. 2005. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 17:133–144. 10.1093/intimm/dxh194 [DOI] [PubMed] [Google Scholar]
- James E.S., Harney S., Wordsworth B.P., Cookson W.O.C.M., Davis S.J., and Moffatt M.F.. 2005. PDCD1: a tissue-specific susceptibility locus for inherited inflammatory disorders. Genes Immun. 6:430–437. 10.1038/sj.gene.6364223 [DOI] [PubMed] [Google Scholar]
- Kao C., Oestreich K.J., Paley M.A., Crawford A., Angelosanto J.M., Ali M.-A.A., Intlekofer A.M., Boss J.M., Reiner S.L., Weinmann A.S., and Wherry E.J.. 2011. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12:663–671. 10.1038/ni.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassu A., Marcus R.A., D’Souza M.B., Kelly-McKnight E.A., Golden-Mason L., Akkina R., Fontenot A.P., Wilson C.C., and Palmer B.E.. 2010. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J. Immunol. 185:3007–3018. 10.4049/jimmunol.1000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M.E., Freeman G.J., and Sharpe A.H.. 2007. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179:5064–5070. 10.4049/jimmunol.179.8.5064 [DOI] [PubMed] [Google Scholar]
- Keir M.E., Butte M.J., Freeman G.J., and Sharpe A.H.. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G.J., Pereira R.M., Äijö T., Kim E.Y., Marangoni F., Pipkin M.E., Togher S., Heissmeyer V., Zhang Y.C., Crotty S., et al. 2015. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 42:265–278. 10.1016/j.immuni.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Concepcion R.J., and Ahmed R.. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J.M., Hanley M.B., Cesar D., Halvorsen R., Hoh R., Schmidt D., Wieder E., Deeks S., Siler S., Neese R., and Hellerstein M.. 2000. Factors influencing T-cell turnover in HIV-1-seropositive patients. J. Clin. Invest. 105:R1–R8. 10.1172/JCI8647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenst S., Hoeller S., Willi N., Dirnhofera S., and Tzankov A.. 2010. Diagnostic and prognostic utility of PD-1 in B cell lymphomas. Dis. Markers. 29:47–53. 10.1155/2010/404069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenst S., Soysal S.D., Gao F., Obermann E.C., Oertli D., and Gillanders W.E.. 2013. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 139:667–676. 10.1007/s10549-013-2581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Maeda A., Nishimura H., Kurosaki T., and Honjo T.. 2001. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. USA. 98:13866–13871. 10.1073/pnas.231486598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley M.A., Kroy D.C., Odorizzi P.M., Johnnidis J.B., Dolfi D.V., Barnett B.E., Bikoff E.K., Robertson E.J., Lauer G.M., Reiner S.L., and Wherry E.J.. 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 338:1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D.M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12:252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., and Riley J.L.. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25:9543–9553. 10.1128/MCB.25.21.9543-9553.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsoukis N., Brown J., Petkova V., Liu F., Li L., and Boussiotis V.A.. 2012. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 5:ra46 10.1126/scisignal.2002796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovas C., Casazza J.P., Brenchley J.M., Price D.A., Gostick E., Adams W.C., Precopio M.L., Schacker T., Roederer M., Douek D.C., and Koup R.A.. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292. 10.1084/jem.20061496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., and Zinkernagel R.M.. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 342:559–561. 10.1038/342559a0 [DOI] [PubMed] [Google Scholar]
- Restifo N.P., Dudley M.E., and Rosenberg S.A.. 2012. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12:269–281. 10.1038/nri3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J.L. 2009. PD-1 signaling in primary T cells. Immunol. Rev. 229:114–125. 10.1111/j.1600-065X.2009.00767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., and Anderson A.C.. 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207:2187–2194. 10.1084/jem.20100643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Blackburn S.D., Blattman J.N., and Wherry E.J.. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204:941–949. 10.1084/jem.20061937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Blackburn S.D., Intlekofer A.M., Kao C., Angelosanto J.M., Reiner S.L., and Wherry E.J.. 2009. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity. 31:309–320. 10.1016/j.immuni.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron M.M., Gray S.M., Marshall H.D., Parish I.A., Chen J.H., Perry C.J., Cui G., Li M.O., and Kaech S.M.. 2014. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity. 41:802–814. 10.1016/j.immuni.2014.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S., Amadei B., Tola D., Massari M., Schivazappa S., Missale G., and Ferrari C.. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398–11403. 10.1128/JVI.01177-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Cruz R., Orozco L., Espinosa-Rosales F., Carreño-Manjarrez R., Solís-Vallejo E., López-Lara N.D., Ruiz-López I.K., Rodríguez-Lozano A.L., Estrada-Gil J.K., Jiménez-Sánchez G., and Baca V.. 2007. Association of PDCD1 polymorphisms with childhood-onset systemic lupus erythematosus. Eur. J. Hum. Genet. 15:336–341. 10.1038/sj.ejhg.5201767 [DOI] [PubMed] [Google Scholar]
- Velu V., Titanji K., Zhu B., Husain S., Pladevega A., Lai L., Vanderford T.H., Chennareddi L., Silvestri G., Freeman G.J., et al. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 458:206–210. 10.1038/nature07662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W., Wherry E.J., and Ahmed R.. 2009. Redefining chronic viral infection. Cell. 138:30–50. 10.1016/j.cell.2009.06.036 [DOI] [PubMed] [Google Scholar]
- Wherry E.J. 2011. T cell exhaustion. Nat. Immunol. 12:492–499. 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., and Ahmed R.. 2003a. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., Teichgräber V., Becker T.C., Masopust D., Kaech S.M., Antia R., von Andrian U.H., and Ahmed R.. 2003b. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. 10.1038/ni889 [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Ha S.J., Kaech S.M., Haining W.N., Sarkar S., Kalia V., Subramaniam S., Blattman J.N., Barber D.L., and Ahmed R.. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 27:670–684. 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Yokosuka T., Takamatsu M., Kobayashi-Imanishi W., Hashimoto-Tane A., Azuma M., and Saito T.. 2012. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 209:1201–1217. 10.1084/jem.20112741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., and Ahmed R.. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Han Q., Duan S., Li Z., Li N., Zhu Q., Chen J., Lv Y., Zeng X., Chen Y., and Liu Z.. 2012. PDCD1 polymorphism amplifies the predisposing effect conferred by CTLA4 polymorphism in chronic hepatitis B virus infection. Hum. Immunol. 73:421–425. 10.1016/j.humimm.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Zinselmeyer B.H., Heydari S., Sacristán C., Nayak D., Cammer M., Herz J., Cheng X., Davis S.J., Dustin M.L., and McGavern D.B.. 2013. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J. Exp. Med. 210:757–774. 10.1084/jem.20121416 [DOI] [PMC free article] [PubMed] [Google Scholar]