Abstract
Trace elements play crucial role in the maintenance of genome stability in the cells. Many endogenous defense enzymes are containing trace elements such as superoxide dismutase and metalloproteins. These enzymes are contributing in the detoxification of reactive oxidative species (ROS) induced by ionizing radiation in the cells. Zinc, copper, manganese, and selenium are main trace elements that have protective roles against radiation-induced DNA damages. Trace elements in the free salt forms have protective effect against cell toxicity induced by oxidative stress, metal-complex are more active in the attenuation of ROS particularly through superoxide dismutase mimetic activity. Manganese-complexes in protection of normal cell against radiation without any protective effect on cancer cells are more interesting compounds in this topic. The aim of this paper to review the role of trace elements in protection cells against genotoxicity and side effects induced by ionizing radiation.
Keywords: Trace elements, DNA damage, Side effects, Radiation-protective agent, Radiation
Introduction
When ionizing radiation (IR) is passing through cell to produce free radicals and toxic substances named as reactive oxygen species (ROS). ROS elevates oxidative stress conditions in the cells. Imbalance between oxidative and endogenous antioxidant is resulting in oxidative stress damage to cell macromolecule such as DNA, RNA and proteins. Cells are equipped with defense system that neutralizes ROS and leads to cell maintenance [1]. Endogenous defense systems are mainly acted by extracellular and intracellular antioxidants, which are able to protect tissues from ROS. Various antioxidant enzymes contribute in endogenous defense system are including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). In addition, vitamin E, vitamin C, glutathione and beta carotene are non-enzymatic antioxidants, which can scavenge oxidants [2,3]. Trace elements, such as copper, manganese, zinc, and selenium, are required for the activity of antioxidant enzymes. For example, zinc is vital for the functionality of more than 300 enzymes [4]. Also, trace elements may contribute in repairing on DNA damage through regulation of proteins involved in cell signaling pathways [5,6]. Human absorb essential nutritional trace elements through supplements, and these elements can participate in protection tissues against toxicity induced by IR.
This review will focus on the role trace elements on elimination oxidative stress and cell protection against genotoxicity induced by ionizing radiation. It will concentrate on the role zinc, manganese, selenium, and copper in oxidative stress and their potential beneficial effects on deleterious side effects of IR.
Trace Elements
Trace elements are present at low concentrations (less than µg/g) in living organisms and environments. Trace elements are including zinc (Zn), selenium (Se), copper (Cu), iron (Fe), manganese (Mn), cobalt, nickel, lead, cadmium, chromium, arsenic, molybdenum, and boron. The concentrations of Zn, Se, and Cu are 33, 0.2, and 1 µg/g in human body, respectively [7]. Some of trace elements are essential for growing of organisms [8]. In biochemistry, Zn, Se, Mn, and Cu are needed at very low amount for the proper growth, development, and physiology of the organism. Deficiencies of some trace elements are associated with many diseases. Low plasma zinc levels have been reported in patients with rheumatoid arthritis [9,10]. Deficiency of selenium and vitamin E, which has been implicated in the development of Keshan (cardiomyopathy) diseases [11,12].
Biological Properties of Trace Elements
A great variety of enzymes, structural proteins, transcription factors, and ribosomal proteins are containing trace elements in their structures. Many enzymes require metals for proper functions; for example, Mn, Zn and Cu in superoxide dismutase [13,14]. Zn plays a crucial role in the repair of damaged-DNA, and regulation of cell proliferation and apoptosis through activation of various transcriptional factors and DNA or RNA polymerases [15]. Cytochrome-c oxidase sites are occupied with copper, has a crucial role in proper function of this enzyme. It is in mitochondria and contributes in electron transport chain. Cytochrome-c oxidase is an efficient energy transducer that reduces oxygen to water and converts the released chemical energy into an electrochemical membrane potential [16,17]. There a summary of role trace elements against oxidative stress with various mechanisms is discussed.
1. Antioxidant activity
Several trace elements are involvement in the catalytic activity and special conformation of antioxidant enzymes, may contribute to mitigate the oxidative stress. Enzymatic protectors include SOD, CAT, GPx, and glutathione reductase (GR). SOD and GPx are most antioxidant enzymes in the most of tissues. SOD catalyses the dismutation of superoxide (O2-) into oxygen and hydrogen peroxide (H2O2), the last is catalyzed by catalase enzyme to water and oxygen [18,19]. Proteins are containing sulfhydryl group can scavenge free radicals. Zn stabilizes sulfhydryl and increases its reactivity for neutralizing ROS [20]. GPx is belonging to peroxidase enzyme family whose main biological role is to protect the organism from oxidative stress. Most of GPx are selenium-containing proteins with a selenocysteine in the catalytic center. Selenium is necessary for proper function of this enzyme [21,22]. Zn-bound metallothioneins (MT) are thiol-rich proteins with antioxidant activities that release Zn ions for several proteins and enzymes involved in antioxidant and DNA-repair responses. They act as protective agents against deleterious effects induced by oxidative stress [23,24].
2. Anti-inflammatory effects
Adequate intakes of trace elements are required for the immune system to function efficiently. Zn is a second messenger for immune cells, and intracellular zinc participates in signaling events involved in inflammation process. Zn has a crucial role for normal development and functions of immune cells such as monocyte, macrophage, neutrophils, and natural killer cells. Zn regulates phagocytosis and pro-inflammatory cytokines production by immune cells. Zinc can inhibit nuclear factor kappa-B (NF-κB) signaling via tumor necrosis factor-alpha (TNF-α) receptor-associated factor pathways, resulting in reduction of inflammatory cytokines [25,26].
3. DNA stabilizing
Some trace elements are required for maintain genome stability. Zn and Se have been shown to affect on DNA repair responses via regulation of most cell signaling pathways and proteins involved in DNA maintenance. Severe zinc depletion caused more DNA damage in peripheral blood cells and this was normalized by zinc repletion [27]. Poly(ADP-ribose) polymerase (PARP) has a crucial role in DNA repair. PARP has Zn fingers, which plays a role in the reorganization of DNA breaks [28]. Zn-finger (Zn-f) may enable DNA ligase to rejoin chromosomal DNA strand breaks induced by oxidative stress [29]. Selenomethionine regulates p53-mediated base excision repair pathways, which corrects DNA damage caused by oxidative stress [30]. Selenium increases the activity DNA glycosylases as a repair enzyme and impact on the DNA repair process [31]. The role selenium on DNA protection was investigated in the cohort study. It was an inverse association between serum selenium level and accumulated DNA damage in human blood leukocytes [32].
Ionizing Radiation and DNA Damage
Ionizing radiation such as gamma and X-rays has sufficient energy to displace electron from atom or molecule, producing ion. IR produces ROS by ionization of water molecule. ROS are including OHo, H2O2, OHo, O2-, peroxyl radical (ROOo), alkyl hydroperoxide (ROOH), and singlet oxygen. ROS are highly chemically reactive and attack nearby molecules and lead to change their chemical structures [33]. ROS can react with many cellular biomolecules including proteins, lipids, and DNA that produce a variety of oxidative lesions. Elevated ROS level have been suggested to be involved in a variety of biological processes from cellular dysfunction to death. Radiation-induced DNA lesion is main reason for cell killing. If ROS levels increased, DNA lesions cannot be effectively repaired by endogenous systems, leading to genome instability and chromosome abnormalities [34]. Radiation-induced genome aberration has a crucial role in the mechanisms underlying radiation-induced carcinogenesis [35]. DNA as the major biomolecule is very sensitive to deleterious effect of ionizing radiation. Single strand breaks (SSB) is efficiently rejoined and repaired by base repair endogenous systems. The most deleterious damage induced by ionizing radiation is thought to be the double strand breaks (DSB), a break in the both strands of the DNA separated by disrupting about 10 base pairs or less [36,37,38]. DSB is leading to deleterious outcomes of ionizing radiation for healthy cells. Type of strand breaks is dependent to dose and type radiation, cell type and cellular environments. Presence of oxygen increases DNA damage induced by ionizing radiation, while glutathione as a thiol compound reduces radiation toxicity in cell. Persistent or misrepaired DNA damage can induce mutagenesis; genome instability is an essential step in the development of cancer. DNA damage can promote cell death responses that underlie pathologies that involve tissue dysfunction, such as bone marrow depression [39]. Ionizing radiation contributes in production of pro-inflammatory cytokines through generate ROS and reactive nitrogen species (RNS), such as superoxide, nitric oxide, hydroxyl radicals, peroxynitrite, and their derived products. There is a cross talk between ROS/RNS and some pro-inflammatory cytokines, such as TNF-α and IL-1 [40,41]. It is evidence that free radical scavengers such as N-acetyl cysteine and amifostine can cause lower pro-inflammatory cytokine expression [42,43]. TNF-α can cause DNA damages through the induction of ROS, and it is a potent mutagen. Over expression of TNF-α is related to increased levels of 8-hydroxydeoxyguanosine (8-OHdG) as a nucleotide damage marker in the liver tissue [44]. Some compounds with reduction in TNF-α level have exhibited radioprotective effects on the cells [45,46]. Ionizing radiation can activate or down-regulate multiple signaling pathways, leading to either increased cell death or increased cell survival. ROS/RNS cause or regulate apoptosis cell signaling pathways, including mitogen-activated protein kinases (MAPK), protein kinases-B/C, inhibitor-of-I-kappaB kinases. Activation of the MAPK cascades is leading to expression of target genes and resulting in a biological response, such as cell death, survival or inflammatory response, it is dependent to cell type [47,48].
Role Trace Elements on Genotoxicity Induced by IR
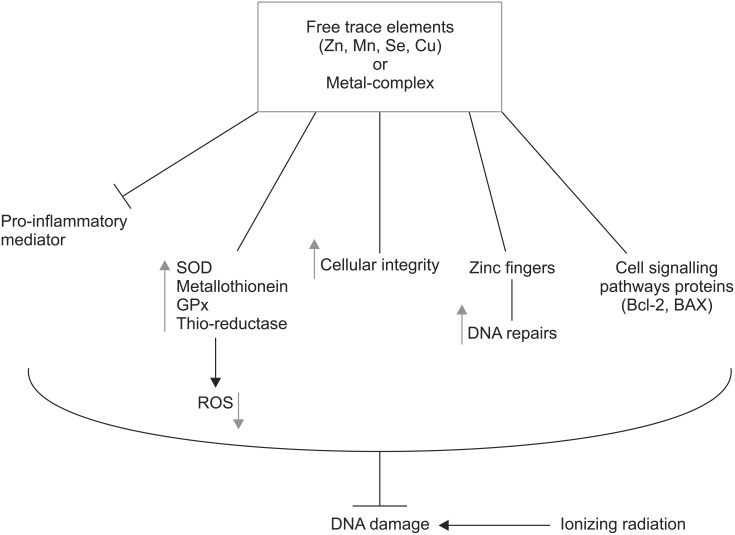
Several mechanisms are proposed for protective effects of trace elements against DNA damage induced by IR, including antioxidant, anti-inflammatory, and DNA stabilizing. Several preclinical studies reported the radioprotective effects of trace elements on the genotoxicity induced by IR. Pre-treatment with Zn both in vivo and in vitro increased metallothionein level in animal lymphocytes, and resulted in resistance to gamma radiation induced chromosome damages. Pretreatment with Cu (in vitro) did not show any protective effect on radiation-induced chromosome damage in animal lymphocytes. Induction of metallothionein synthesis by Zn is one of main factor responsible for radioprotective effect, while Cu-MT did not show any radioprotective effect [49]. Metallothionein acts as a scavenger on radiation-induced peroxides [50]. The higher protection against DNA damage induced by IR was observed for Zn-MT other than Mn-MT and Cu-MT. Metallothionein bound to Zn is high-capacity antioxidant activity to protect radiation-induced DNA damage [51]. Treatment of mice with 30 mg/kg body weight of Zn aspartate 30 minutes before exposure to radiation protected spermatogonia and tetraploid cells from radiation-induced cell killing [52,53]. Recovery bone marrow cells in groups of mice exposure to IR and fed with Zn was better than irradiated mice alone [54]. Zn can protect thyroid function against toxicity induced by 131I in rats. Zn has a role in cellular integrity of the thyroid under oxidative stress induced by ionizing radiation [55]. Zn supplementation to 131I treated rats, attenuatd the thyroid toxicity induced by 131I through the levels of MDA, GSH, SOD and catalase [56,57]. The effect of zinc on genotoxicity induced by hydrogen peroxide was evaluated in normal human lymphocytes and human myelogenous leukemia K562 cancer cell. Zn protected normal cells against DNA damage and increased this effect in cancer cell, which indicates the dual action of Zn in dependency of normal or cancer cells and can be useful in cancer therapy [58]. Zn-f motifs participate in protein-nucleic acid and protein-protein interactions in many groups of proteins, including those involved in DNA repair [59]. Zn-f may enable Lig-3 to rejoin chromosomal DNA strand breaks located at sites of clustered damage induced by ionizing radiation, resulting in maintenance of genetic integrity [29] (Fig. 1). Cu and Fe have crucial roles in Fenton reaction that transforms H2O2 into OH radical, one of the most ROS, resulting in the induction of DNA damage [60]. Normal cells treated with Cu salts to show an increased oxidative stress and DNA damage [61]. However, Cu in Cu/Zn-SOD has a protective role in detoxification of ROS [62]. Some of Cu-complexes exhibited superoxide dismutase-mimic activity and antioxidant activity [63], while other Cu-complexes showed pro-oxidant properties and generated ROS, resulting in DNA cleavage. DNA cleavage and antiproliferative effects of Cu-complexes were studied in many cancer cells for finding new anticancer agents [64,65,66].
Fig. 1. Role trace elements in mitigation DNA damage induced by ionizing radiation in cells. There are several mechanisms are involved in protection including attenuation of pro-inflammatory, increasing of super oxide dismutase (SOD), metallothionein, glutathione peroxidase (GPx), thioredoxin reductase (Thio.reductase), reduction of reactive oxygen species (ROS), and cell signaling pathways in DNA repair. Zn, zinc; Mn, manganese; Se, selenium; Cu, copper.
Selenium has several mechanisms related to cell protection against oxidative stress such as antioxidant activity by selenoenzymes, modulation of cell cycle and apoptosis and DNA repair [67]. Selenium is an essential constituent of extracellular and cellular metalloenzymes, glutathione peroxidase, thioredoxin reductase and other selenoproteins. Selenium supplementation protected healthy tissues and reduced the side effects of radiation treatment [68]. Diselenodipropionic acid (DSePA), a diselenide and a derivative of selenocystine improved survival rate in mice exposed to lethal dose IR. This selenium organic compound enhanced antioxidant enzymes and attenuated DNA damages. DSePA also inhibited radiation-induced apoptosis and reversed radiation-induced alterations in the expression of the proapoptotic BAX and the antiapoptotic Bcl-2 genes [69]. Selenoprotein GPx-1 contributes in protection DNA from oxidative stress damage [70]. Selenium, in the form of seleno-L-methionine (SeMet), induced redox-factor-1 (Ref-1) and p53 proteins in normal cells, SeMet preferentially induced the DNA repair branch of the p53 pathway. Pretreatment with SeMet protected normal fibroblasts from DNA damage, then selenium compounds are involved in DNA repair through cell signaling pathways [71]. Sodium selenite has an effective radioprotective action in the parotid gland in rats were administered before irradiation in the head and neck region with a single 15-Gy dose of gamma radiation [72]. Both the inorganic salt, sodium selenite, and the organic Se compound, selenomethionine, enhance the survival of irradiated mice when injected either before or shortly after radiation exposure. A synergistic effect of Se in combination with amifostine as a thiol compound radioprotector was observed with increasing of survival rate in irradiated mice [73,74]. Sodium selenite exhibited a radioprotective effect in the bone repair of tibia of ovariectomized rats were exposed to 10 Gy of X-ray without toxicity [75,76]. However, Se has radioprotective effect on normal cells; selenite exhibited a radiosensitizing effect in glioma cancer cell at concentration 2-3 µM [77]. These dual beneficial effects of Se are promising for patients during radiation therapy of cancer. However, Mn-complex compounds widely were investigated as radioprotector, Mn in salt form was not shown any protective effect. Manganese chloride treatment of mice 24 hours prior to irradiation did not significantly protect skin and small intestine against acute radiation injury. Mn treatment of mice did not result in increased levels of MT in the skin and small intestine [78].
Cu/Zn-SOD and Mn-SOD are conserved enzymes for scavenging superoxide radical in cells. Because of the integrity of cell membranes, exogenous SODs are not able to be incorporated into cells, which limited the application of natural SOD [79,80]. Studies on complexes of manganese and zinc have attracted considerable attention in recent time due to their various interesting properties like SOD mimetic. Kojic, chromone, 5-aminosalicylic acid, and 2-methylaminopyridine complexes with Zn, Mn and Cu exhibited radioprotective effects against mortality and genotoxicity induced by IR in animals [81,82,83,84]. Mn-containing complexes widely are evaluated as a class of radioprotective agent that acts as superoxide dismutase mimetic and other mechanisms related to mitigation radiation toxicity [85,86,87,88,89]. Mn(II) complexes with nicotinyl-L-tyrosinate and nicotinyl-L-tryptophanate schiff base ligands have radioprotective effects against genotoxicity induced by radiation in rats. Animal were administered orally with these complexes and irradiated with X-ray, DNA liver was protected by these Mn-complexes from radiation-induced damage [90] (Fig. 1). Mn-porphyrin derivatives complexes are potent, small molecular weight antioxidants that scavenge a variety of free radicals including superoxide, hydrogen peroxide, lipid peroxides and peroxynitrite. These Mn-complexes prevented radiation-induced tissue injury. MnTE-2-PyP manganese(III) Tetrakis-(N-methylpyridinium-2-yl) porphyrin reduced overall weight loss, skin damage and testicular atrophy associated with lower abdominal radiation exposure. MnTE-2-PyP reduced IR-induced DNA damage in prostate epithelial cells. The mechanism related to MnTE-2-PyP is scavenging of superoxide and hydrogen peroxide produced by IR, however, MnTE-2-PyP reduced the growth of tumors in the presence of radiation [91,92]. The radioprotective effects of MnTE-2-PyP are associated through its ability both to suppress oxidative stress and to decrease activation of key transcription factors and proangiogenic and profibrogenic cytokines [93].
Clinical Studies
Several clinical evidences indicated that trace element supplement to act as an effective radioprotector in patients during radiotherapy. In a randomized clinical study, thirty patients with head and neck cancer were randomized to receive either zinc sulfate or placebo; and oropharyngeal mucositis were evaluated in patients. Zinc sulfate was containing 50 mg zinc in a capsule that patients taken three times daily. Results indicated that the degree of mucositis in the patients in the zinc sulfate group was markedly lower than that in the placebo group. Investigators claimed this inexpensive supplement and easy to patients had no serious side effects [94]. The effect of polaprezinc (zinc L-carnosine) oral rinse was investigated on radiochemotherapy-induced oral mucositis, pain, xerostomia and taste disturbance in 16 patients with head and neck cancer, and results were compared with control azulene oral rinse. Polaprezinc oral rinse markedly reduced oral mucositis, xerostomia, taste disturbance, and the frequency of the use of analgesics in patients as compare with control group [95]. Multicenter clinical trials were performed German researchers about the beneficial effect of zinc supplement on reducing of radiation-induced diarrhea in patients. A total of 81 patients were randomized in selenium and control groups with 39 and 42 patients, respectively. Patients orally taken selenium selenite. There was a statistically significant difference between the groups towards a lower incidence of diarrhea in the selenium group compared to the control group [96]. In follow-up analysis of these patients, they demonstrated that selenium supplementation had no influence on the effectiveness of the anticancer irradiation therapy and did not negatively affect patients' long-term survival. They concluded that selenium supplement is benefit in selenium-deficient cervical and uterine cancer patients while undergoing pelvic radiation therapy [97].
Conclusion
Exposure to ionizing radiation is resulting in pathophysiological events in cells and tissues. Radiation-produced ROS and inflammations are main reasons for DNA injury in cells, which is associated to genotoxicity and cell death. Some of trace elements are involved in the protection against genome instability induced by IR through various mechanisms are including enhancement of endogenous defense enzymes and free radical scavenging proteins, medication of cell signaling pathways for DNA repair, and anti-inflammatory. However, free trace elements in the salt forms have protective effects against cell toxicity induced by oxidative stress, metal-complex are more active and interesting in attenuation of ROS particularly with super oxide dismutase mimetic activity. With regards to promising results related to Mn-complexes in protection normal cell against IR without any protective effects on cancer cells in exposure to IR, future preclinical and clinical investigations help find new medications in improvement of patients care under radiation therapy.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Hosseinimehr SJ. Flavonoids and genomic instability induced by ionizing radiation. Drug Discov Today. 2010;15:907–918. doi: 10.1016/j.drudis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Kang DH, Kang SW. Targeting cellular antioxidant enzymes for treating atherosclerotic vascular disease. Biomol Ther (Seoul) 2013;21:89–96. doi: 10.4062/biomolther.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limon-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Frassinetti S, Bronzetti G, Caltavuturo L, Cini M, Croce CD. The role of zinc in life: a review. J Environ Pathol Toxicol Oncol. 2006;25:597–610. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i3.40. [DOI] [PubMed] [Google Scholar]
- 5.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Klotz LO, Kroncke KD, Buchczyk DP, Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr. 2003;133(5 Suppl 1):1448S–1451S. doi: 10.1093/jn/133.5.1448S. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder HA, Nason AP. Trace-element analysis in clinical chemistry. Clin Chem. 1971;17:461–474. [PubMed] [Google Scholar]
- 8.He ZL, Yang XE, Stoffella PJ. Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol. 2005;19:125–140. doi: 10.1016/j.jtemb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein A. Low plasma zinc levels in active rheumatoid arthritis. J Rheumatol. 1998;25:187–188. [PubMed] [Google Scholar]
- 10.Stone J, Doube A, Dudson D, Wallace J. Inadequate calcium, folic acid, vitamin E, zinc, and selenium intake in rheumatoid arthritis patients: results of a dietary survey. Semin Arthritis Rheum. 1997;27:180–185. doi: 10.1016/s0049-0172(97)80018-2. [DOI] [PubMed] [Google Scholar]
- 11.Tong WM, Wang F. Alterations in rat pancreatic islet beta cells induced by Keshan disease pathogenic factors: protective action of selenium and vitamin E. Metabolism. 1998;47:415–419. doi: 10.1016/s0026-0495(98)90052-x. [DOI] [PubMed] [Google Scholar]
- 12.Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med. 2014;370:1756–1760. doi: 10.1056/NEJMcibr1402199. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Gladyshev VN. Comparative genomics of trace element dependence in biology. J Biol Chem. 2011;286:23623–23629. doi: 10.1074/jbc.R110.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barandier C, Tanguy S, Pucheu S, Boucher F, De Leiris J. Effect of antioxidant trace elements on the response of cardiac tissue to oxidative stress. Ann N Y Acad Sci. 1999;874:138–155. doi: 10.1111/j.1749-6632.1999.tb09232.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharif R, Thomas P, Zalewski P, Fenech M. The role of zinc in genomic stability. Mutat Res. 2012;733:111–121. doi: 10.1016/j.mrfmmm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Kim YC, Hummer G. Proton-pumping mechanism of cytochrome c oxidase: a kinetic master-equation approach. Biochim Biophys Acta. 2012;1817:526–536. doi: 10.1016/j.bbabio.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology: copper. Biomed Pharmacother. 2003;57:386–398. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988) Free Radic Biol Med. 1988;5:363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- 19.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130(5S Suppl):1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 21.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 23.Babula P, Masarik M, Adam V, et al. Mammalian metallothioneins: properties and functions. Metallomics. 2012;4:739–750. doi: 10.1039/c2mt20081c. [DOI] [PubMed] [Google Scholar]
- 24.Viarengo A, Burlando B, Ceratto N, Panfoli I. Antioxidant role of metallothioneins: a comparative overview. Cell Mol Biol (Noisy-le-grand) 2000;46:407–417. [PubMed] [Google Scholar]
- 25.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43:370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. 2009;139:1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharif R, Thomas P, Zalewski P, Fenech M. Zinc deficiency or excess within the physiological range increases genome instability and cytotoxicity, respectively, in human oral keratinocyte cells. Genes Nutr. 2012;7:139–154. doi: 10.1007/s12263-011-0248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor RM, Whitehouse CJ, Caldecott KW. The DNA ligase III zinc finger stimulates binding to DNA secondary structure and promotes end joining. Nucleic Acids Res. 2000;28:3558–3563. doi: 10.1093/nar/28.18.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung HJ, Kim HL, Kim YJ, Weon JI, Seo YR. A novel chemopreventive mechanism of selenomethionine: enhancement of APE1 enzyme activity via a Gadd45a, PCNA and APE1 protein complex that regulates p53-mediated base excision repair. Oncol Rep. 2013;30:1581–1586. doi: 10.3892/or.2013.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bera S, De Rosa V, Rachidi W, Diamond AM. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis. 2013;28:127–134. doi: 10.1093/mutage/ges064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karunasinghe N, Ryan J, Tuckey J, et al. DNA stability and serum selenium levels in a high-risk group for prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:391–397. [PubMed] [Google Scholar]
- 33.Yamamori T, Yasui H, Yamazumi M, et al. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 2012;53:260–270. doi: 10.1016/j.freeradbiomed.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704:152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. doi: 10.1093/mutage/gel048. [DOI] [PubMed] [Google Scholar]
- 36.Shikazono N, Noguchi M, Fujii K, Urushibara A, Yokoya A. The yield, processing, and biological consequences of clustered DNA damage induced by ionizing radiation. J Radiat Res. 2009;50:27–36. doi: 10.1269/jrr.08086. [DOI] [PubMed] [Google Scholar]
- 37.Lomax ME, Folkes LK, O'Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol) 2013;25:578–585. doi: 10.1016/j.clon.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Iyama T, Wilson DM., 3rd DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst) 2013;12:620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007;12:794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 42.Schaller G, Pleiner J, Mittermayer F, Posch M, Kapiotis S, Wolzt M. Effects of N-acetylcysteine against systemic and renal hemodynamic effects of endotoxin in healthy humans. Crit Care Med. 2007;35:1869–1875. doi: 10.1097/01.CCM.0000275385.45557.25. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani G, Maccio A, Madeddu C, et al. Reactive oxygen species, antioxidant mechanisms, and serum cytokine levels in cancer patients: impact of an antioxidant treatment. J Environ Pathol Toxicol Oncol. 2003;22:17–28. doi: 10.1615/jenvpathtoxoncol.v22.i1.20. [DOI] [PubMed] [Google Scholar]
- 44.Yan B, Wang H, Rabbani ZN, et al. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 45.Ozyurt H, Cevik O, Ozgen Z, et al. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic Res. 2014;48:1247–1255. doi: 10.3109/10715762.2014.945925. [DOI] [PubMed] [Google Scholar]
- 46.Ostrau C, Hulsenbeck J, Herzog M, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92:492–499. doi: 10.1016/j.radonc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Munshi A, Ramesh R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer. 2013;4:401–408. doi: 10.1177/1947601913485414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 49.Cai L, Cherian MG. Adaptive response to ionizing radiation-induced chromosome aberrations in rabbit lymphocytes: effect of pre-exposure to zinc, and copper salts. Mutat Res. 1996;369:233–241. doi: 10.1016/s0165-1218(96)90028-2. [DOI] [PubMed] [Google Scholar]
- 50.Matsubara J. Metallothionein induction: a measure of radioprotective action. Health Phys. 1988;55:433–436. doi: 10.1097/00004032-198808000-00043. [DOI] [PubMed] [Google Scholar]
- 51.Cai L, Cherian MG. Zinc-metallothionein protects from DNA damage induced by radiation better than glutathione and copper- or cadmium-metallothioneins. Toxicol Lett. 2003;136:193–198. doi: 10.1016/s0378-4274(02)00359-4. [DOI] [PubMed] [Google Scholar]
- 52.Krishnamurthy H, Jagetia GC, Jyothi P. Radioprotective effect of zinc aspartate on mouse spermatogenesis: a flow cytometric evaluation. Mutat Res. 1998;401:111–120. doi: 10.1016/s0027-5107(97)00320-5. [DOI] [PubMed] [Google Scholar]
- 53.Floersheim GL, Floersheim P. Protection against ionising radiation and synergism with thiols by zinc aspartate. Br J Radiol. 1986;59:597–602. doi: 10.1259/0007-1285-59-702-597. [DOI] [PubMed] [Google Scholar]
- 54.Huang MY, Lian SL, Wu HL, et al. Effects of zinc compound on body weight and recovery of bone marrow in mice treated with total body irradiation. Kaohsiung J Med Sci. 2007;23:453–462. doi: 10.1016/S1607-551X(08)70053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhawan D, Singh Baweja M, Dani V. Zinc sulphate following the administration of iodine-131 on the regulation of thyroid function, in rats. Hell J Nucl Med. 2007;10:167–171. [PubMed] [Google Scholar]
- 56.Dani V, Dhawan D. Zinc as an antiperoxidative agent following iodine-131 induced changes on the antioxidant system and on the morphology of red blood cells in rats. Hell J Nucl Med. 2006;9:22–26. [PubMed] [Google Scholar]
- 57.Dani V, Dhawan DK. Radioprotective role of zinc following single dose radioiodine (131I) exposure to red blood cells of rats. Indian J Med Res. 2005;122:338–342. [PubMed] [Google Scholar]
- 58.Sliwinski T, Czechowska A, Kolodziejczak M, Jajte J, Wisniewska-Jarosinska M, Blasiak J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell Biol Int. 2009;33:542–547. doi: 10.1016/j.cellbi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Witkiewicz-Kucharczyk A, Bal W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol Lett. 2006;162:29–42. doi: 10.1016/j.toxlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Romero A, Ramos E, de Los Rios C, Egea J, Del Pino J, Reiter RJ. A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res. 2014;56:343–370. doi: 10.1111/jpi.12132. [DOI] [PubMed] [Google Scholar]
- 61.Anjos VA, da Silva FM, Jr, Souza MM. Cell damage induced by copper: an explant model to study anemone cells. Toxicol In Vitro. 2014;28:365–372. doi: 10.1016/j.tiv.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Linder MC. The relationship of copper to DNA damage and damage prevention in humans. Mutat Res. 2012;733:83–91. doi: 10.1016/j.mrfmmm.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Buchtik R, Travnicek Z, Vanco J. In vitro cytotoxicity, DNA cleavage and SOD-mimic activity of copper(II) mixed-ligand quinolinonato complexes. J Inorg Biochem. 2012;116:163–171. doi: 10.1016/j.jinorgbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Liang JW, Wang Y, Du KJ, et al. Synthesis, DNA interaction and anticancer activity of copper(II) complexes with 4'-phenyl-2,2':6',2''-terpyridine derivatives. J Inorg Biochem. 2014;141:17–27. doi: 10.1016/j.jinorgbio.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Yang X, Dong S, Li X. DNA breakage induced by piceatannol and copper(II): mechanism and anticancer properties. Oncol Lett. 2012;3:1087–1094. doi: 10.3892/ol.2012.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li GY, Du KJ, Wang JQ, et al. Synthesis, crystal structure, DNA interaction and anticancer activity of tridentate copper(II) complexes. J Inorg Biochem. 2013;119:43–53. doi: 10.1016/j.jinorgbio.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 67.Brozmanova J, Manikova D, Vlckova V, Chovanec M. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010;84:919–938. doi: 10.1007/s00204-010-0595-8. [DOI] [PubMed] [Google Scholar]
- 68.Tabassum A, Bristow RG, Venkateswaran V. Ingestion of selenium and other antioxidants during prostate cancer radiotherapy: a good thing? Cancer Treat Rev. 2010;36:230–234. doi: 10.1016/j.ctrv.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Kunwar A, Bansal P, Kumar SJ, et al. In vivo radioprotection studies of 3,3'-diselenodipropionic acid, a selenocystine derivative. Free Radic Biol Med. 2010;48:399–410. doi: 10.1016/j.freeradbiomed.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Baliga MS, Diwadkar-Navsariwala V, Koh T, Fayad R, Fantuzzi G, Diamond AM. Selenoprotein deficiency enhances radiation-induced micronuclei formation. Mol Nutr Food Res. 2008;52:1300–1304. doi: 10.1002/mnfr.200800020. [DOI] [PubMed] [Google Scholar]
- 71.Fischer JL, Lancia JK, Mathur A, Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006;26(2A):899–904. [PubMed] [Google Scholar]
- 72.Tuji FM, Pontual ML, Barros SP, Almeida SM, Boscolo FN. Ultrastructural assessment of the radioprotective effects of sodium selenite on parotid glands in rats. J Oral Sci. 2010;52:369–375. doi: 10.2334/josnusd.52.369. [DOI] [PubMed] [Google Scholar]
- 73.Weiss JF, Srinivasan V, Kumar KS, Landauer MR. Radioprotection by metals: selenium. Adv Space Res. 1992;12:223–231. doi: 10.1016/0273-1177(92)90112-b. [DOI] [PubMed] [Google Scholar]
- 74.Weiss JF, Hoover RL, Kumar KS. Selenium pretreatment enhances the radioprotective effect and reduces the lethal toxicity of WR-2721. Free Radic Res Commun. 1987;3:33–38. doi: 10.3109/10715768709069767. [DOI] [PubMed] [Google Scholar]
- 75.Rocha AS, Ramos-Perez FM, Boscolo FN, Manzi FR, Cchicarelo M, Almeida SM. Effect of sodium selenite on bone repair in tibiae of irradiated rats. Braz Dent J. 2009;20:186–190. doi: 10.1590/s0103-64402009000300002. [DOI] [PubMed] [Google Scholar]
- 76.de Freitas DQ, Ramos-Perez FM, Neves EG, Marques MR, Boscolo FN, de Almeida SM. Radioprotective effect of sodium selenite on bone repair in the tibia of ovariectomized rats. Braz Dent J. 2012;23:723–728. doi: 10.1590/s0103-64402012000600017. [DOI] [PubMed] [Google Scholar]
- 77.Schueller P, Puettmann S, Micke O, Senner V, Schaefer U, Willich N. Selenium influences the radiation sensitivity of C6 rat glioma cells. Anticancer Res. 2004;24(5A):2913–2917. [PubMed] [Google Scholar]
- 78.Murata R, Nishimura Y, Hiraoka M, Abe M, Satoh M. Manganese chloride treatment does not protect against acute radiation injury of skin or crypt cells. Radiat Res. 1995;143:316–319. [PubMed] [Google Scholar]
- 79.Gu Q, Feng T, Cao H, et al. HIV-TAT mediated protein transduction of Cu/Zn-superoxide dismutase-1 (SOD1) protects skin cells from ionizing radiation. Radiat Oncol. 2013;8:253. doi: 10.1186/1748-717X-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20:1599–1617. doi: 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Emami S, Hosseinimehr SJ, Taghdisi SM, Akhlaghpoor S. Kojic acid and its manganese and zinc complexes as potential radioprotective agents. Bioorg Med Chem Lett. 2007;17:45–48. doi: 10.1016/j.bmcl.2006.09.097. [DOI] [PubMed] [Google Scholar]
- 82.Hosseinimehr SJ, Shafiee A, Mozdarani H, Akhlagpour S, Froughizadeh M. Radioprotective effects of 2-imino-3-[(chromone-2-yl)carbonyl] thiazolidines against gamma-irradiation in mice. J Radiat Res. 2002;43:293–300. doi: 10.1269/jrr.43.293. [DOI] [PubMed] [Google Scholar]
- 83.Mantena SK, Unnikrishnan MK, Chandrasekharan K. Radioprotection by copper and zinc complexes of 5-aminosalicylic acid: a preliminary study. J Environ Pathol Toxicol Oncol. 2008;27:123–134. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.50. [DOI] [PubMed] [Google Scholar]
- 84.Abou-Seif MA, El-Naggar MM, El-Far M, Ramadan M, Salah N. Prevention of biochemical changes in gamma-irradiated rats by some metal complexes. Clin Chem Lab Med. 2003;41:926–933. doi: 10.1515/CCLM.2003.141. [DOI] [PubMed] [Google Scholar]
- 85.Gridley DS, Makinde AY, Luo X, et al. Radiation and a metalloporphyrin radioprotectant in a mouse prostate tumor model. Anticancer Res. 2007;27(5A):3101–3109. [PubMed] [Google Scholar]
- 86.Li BQ, Dong X, Li N, et al. In vitro enzyme-mimic activity and in vivo therapeutic potential of HSJ-0017, a novel Mn porphyrin-based antioxidant enzyme mimic. Exp Biol Med (Maywood) 2014;239:1366–1379. doi: 10.1177/1535370214532598. [DOI] [PubMed] [Google Scholar]
- 87.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srinivasan V, Doctrow S, Singh VK, Whitnall MH. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharmacol Immunotoxicol. 2008;30:271–290. doi: 10.1080/08923970801925331. [DOI] [PubMed] [Google Scholar]
- 89.Moeller BJ, Batinic-Haberle I, Spasojevic I, et al. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 90.Karapetyan NH, Malakyan MH, Bajinyan SA, Torosyan AL, Grigoryan IE, Haroutiunian SG. Influence of amino acids Shiff bases on irradiated DNA stability in vivo. Cell Biochem Biophys. 2013;67:1137–1145. doi: 10.1007/s12013-013-9617-5. [DOI] [PubMed] [Google Scholar]
- 91.Oberley-Deegan RE, Steffan JJ, Rove KO, et al. The antioxidant, MnTE-2-PyP, prevents side-effects incurred by prostate cancer irradiation. PLoS One. 2012;7:e44178. doi: 10.1371/journal.pone.0044178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehrotra S, Pecaut MJ, Freeman TL, et al. Analysis of a metalloporphyrin antioxidant mimetic (MnTE-2-PyP) as a radiomitigator: prostate tumor and immune status. Technol Cancer Res Treat. 2012;11:447–457. doi: 10.7785/tcrt.2012.500260. [DOI] [PubMed] [Google Scholar]
- 93.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ertekin MV, Koc M, Karslioglu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58:167–174. doi: 10.1016/s0360-3016(03)01562-1. [DOI] [PubMed] [Google Scholar]
- 95.Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer. 2010;127:1984–1990. doi: 10.1002/ijc.25200. [DOI] [PubMed] [Google Scholar]
- 96.Muecke R, Schomburg L, Glatzel M, et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int J Radiat Oncol Biol Phys. 2010;78:828–835. doi: 10.1016/j.ijrobp.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 97.Muecke R, Micke O, Schomburg L, et al. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: follow-up analysis of the survival data 6 years after cessation of randomization. Integr Cancer Ther. 2014;13:463–467. doi: 10.1177/1534735414541963. [DOI] [PubMed] [Google Scholar]