Abstract
Purpose
To evaluate the appropriateness of prophylactic inguinal nodal irradiation (PINI), we analyzed patterns of failure in anal cancer patients who were inguinal node-negative at presentation and did not receive PINI.
Materials and Methods
We retrospectively reviewed the records of 33 anal cancer patients treated by definitive concurrent chemoradiation therapy (CCRT) between 1994 and 2013. Radiotherapy consisted of a total dose of 44-45 Gy (22-25 fractions in 5 weeks) on the whole pelvis, anus, and perineum. Except inguinal lymphadenopathy was present at initial diagnosis, the entire inguinal chain was not included in the radiation field. In other words, there was no PINI.
Results
The median follow-up duration was 50 months (range, 4 to 218 months). Median survival and progression-free survival (PFS) were 57 months (range, 10 to 218 months) and 50 months (range, 4 to 218 months), respectively. Among the survival, the median follow-up duration was 51 months (range, 12 to 218 months). The 5-year overall survival and PFS rates were 93.4% and 88.8%, respectively. Although none of the patients received inguinal node irradiation for prophylactic purposes, there was no inguinal recurrence.
Conclusion
Treatment of anal cancer by omitting PINI might be considered in selected patients with clinically uninvolved inguinal nodes.
Keywords: Anus neoplasms, Prophylactic, Radiotherapy, Inguinal, Lymph nodes
Introduction
Anal cancer, a rare disease that only accounts for 1.5% of all gastrointestinal malignancies [1], has been associated with female gender, infection with human papillomavirus (HPV) and human immunodeficiency virus (HIV), lifetime number of sexual partners, and cigarette smoking [2]. In the past, abdominoperineal resection was the treatment of choice for anal cancer [3,4]. In 1974, Nigro et al. [5] observed complete tumor response in some anal cancer patients initially treated with preoperative concurrent 5-fluorouracil (5-FU), mitomycin C, and radiation therapy (RT). Later, UK Coordinating Committee on Cancer Research (UKCCCR), European Organization for Research and Treatment of Cancer (EORTC), and Radiation Therapy Oncology Group (RTOG) trials have contributed to establish concurrent chemoradiotherapy (CCRT) as the first-line treatment for anal squamous cell carcinoma [6,7,8,9]. Chie et al. [10] reported treatment results of anal cancer with regard to the 5-year overall survival (OS) between surgical and non-surgical treatment (CCRT). Although there was no statistically significant survival difference (p = 0.49) between the two groups, CCRT resulted in a high rates of anal sphincter preservation and 5-year OS compared with surgical treatment. In addition, Lee et al. [11] summarized overall clinical features, treatment modality approaches, and prognostic factors for anal cancer in 2007, showing that the CCRT group had a tendency toward better survival than the surgery group.
Anal cancer spreads mostly by direct extension and lymphatic pathways rather than hematogenous metastasis. Regional nodal involvement is present in 25% of cases at diagnosis. In addition, progression after first treatment is more common in the loco-regional area than as distant metastasis, and local progression occurs at rates up to 30%. In particular, since inguinal lymph nodes are one of the potential sites, prophylactic inguinal nodal irradiation (PINI) has been considered for treatment of anal cancer patients. Current National Comprehensive Cancer Network (NCCN) guidelines recommend that the inguinal nodes and pelvis, anus, and perineum be included in the initial radiation field. Some randomized trials suggest that PINI should be recommended for all tumors in order to reduce inguinal progression risk [8,12,13,14]. Others suggest that PINI should be omitted to reduce RT field size without compromising locoregional control for selected anal cancer patients [9,15]. Larger radiation field size leads to considerably more acute and late toxicities, including dermatitis, groin pain and leg lymphedema [14]. Acute toxicities may affect adherence to chemoradiation treatment [15]. Against this backdrop, the question of whether PINI is necessary to reduce inguinal progression remains controversial among radiation oncologists. We did not include PINI in our clinically inguinal node-negative group in order to reduce treatment-related acute and late toxicities. To evaluate the necessity for PINI, we analyzed patterns of failure in anal cancer patients who did not receive PINI.
Materials and Methods
1. Study population
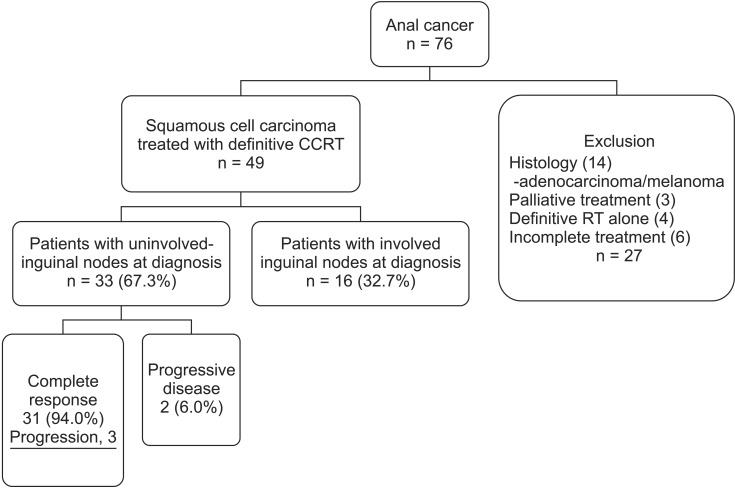
We retrospectively reviewed the records of 76 anal cancer patients who were treated between January 1, 1994 and April 8, 2013. Among these, 27 patients were excluded from the study because of biopsy-proven adenocarcinoma or melanoma (14 patients), palliative treatment (3 patients), definitive RT alone (4 patients), and incomplete treatment (6 patients). Ultimately, 49 patients with biopsy-proven squamous cell carcinoma completed definitive CCRT. Except for the patients who had inguinal lymphadenopathy at initial diagnosis (16 patients, 32.7%), 33 patients were included in this retrospective study (Fig. 1). All tumors were staged based on the seventh edition of the American Joint Committee on Cancer tumor staging criteria. Tumor assessment consisted of digital rectal examination (DRE), colonoscopy with biopsy, and abdominopelvic computed tomography (AP-CT) scan. Pelvic magnetic resonance imaging (MRI) was used starting in 2006 (14 patients, 42.4%). For metastasis work-up, chest CT) scans were conducted. In addition, positron emission tomography (PET/CT) was conducted starting in 2006 (7 patients, 21.2%). Furthermore, both clinical physical examination and abdominopelvic CT scans were carefully performed to detect inguinal nodal progression during follow-up. Revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (ver. 1.1) were used for tumor response evaluation. Tumor response was assessed eight weeks after completion of CCRT using DRE, transrectal-ultrasonography (TR-US) or sigmoidoscopy with biopsy, and AP-CT.
Fig. 1. Diagram of study population, according to inguinal involvement. CCRT, concurrent chemoradiation therapy.
2. Treatment
The primary treatment of choice for 33 patients was CCRT. RT consisted of a daily dose of 1.8-2.0 Gy up to a total dose of 44-45 Gy (22-25 fractions in 5 weeks) by external beam irradiation on the whole pelvis, anus, and perineum using a posteroanterior + paired laterals technique. An additional 9-10 Gy boost radiation was given, depending on the initial clinical stage (≥T3 or ≥N1), response, and existence of gross residual disease. The 3-dimensional conformal radiotherapy (3D-CRT) technique was introduced in 2008. There was no PINI.
Chemotherapy regimens were more heterogeneous. Nineteen patients (57.6%) received a continuous infusion of 5-FU (1,000 mg/m2 daily on days 1-4 and 29-32 of radiation therapy) and a short infusion of 10 mg/m2 mitomycin C during the first course of 5-FU, three patients (9.1%) received 5-FU plus cisplatin, and the remaining 11 patients received either capecitabine alone (18.2%) or 5-FU + leucovorin (15.1%), based on performance status.
3. Statistical analysis
OS was defined as the interval from the start date of CCRT to the date of death or final follow-up visit. Progression-free survival (PFS) was measured from the start date of CCRT to the date of first documented disease progression or final follow-up visit. Kaplan-Meier analysis was used to estimate OS and PFS.
Results
1. Patient characteristics
Overall patient, tumor, and treatment characteristics are described in Table 1. The median age of the population was 59 years (range, 32 to 79 years).
Table 1. Patient, tumor, and treatment characteristics (n = 33).
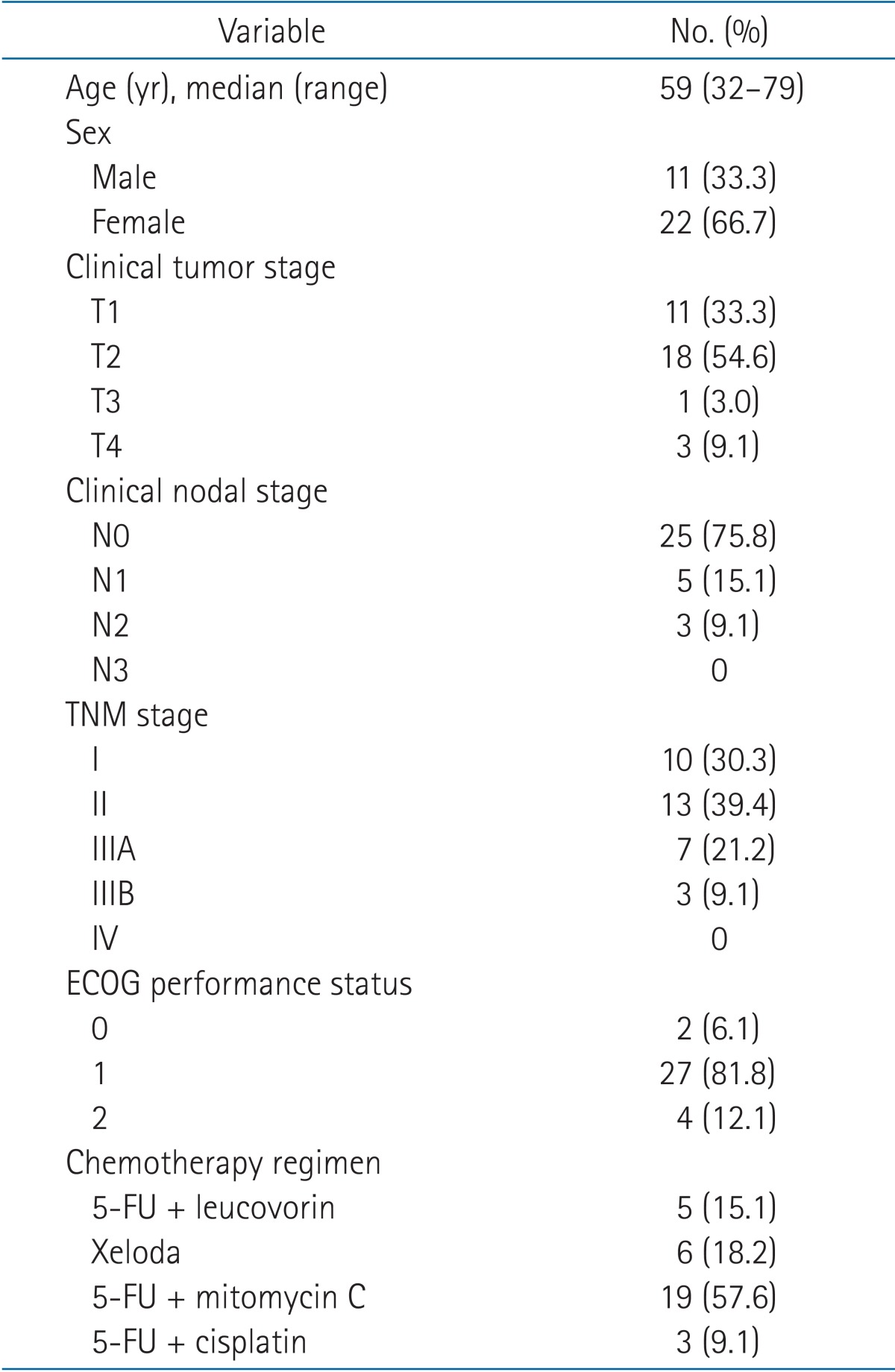
ECOG, Eastern Cooperative Oncology Group; 5-FU, 5-fluorouracil.
2. Overall treatment time and adherence to chemoradiation treatment
The median overall treatment time (OTT) was 37 days (range, 29 to 49 days). In terms of adherence to treatment, no patient dropped out because of chemoradiation-induced complications.
3. Patterns of failure
Among 33 patients, 31 patients (94.0%) and 2 patients (6.0%) experienced complete response (CR) and progressive disease (PD), respectively. During follow-up, three patients in the CR group showed disease progression. The time to progression varied from 6 to 41 months. Sites of progression varied from the local area or regional perirectal lymph nodes to distant abdominal lymph nodes or liver parenchyma. Appropriate salvage procedures were conducted depending on the site of progression (Table 2). Of note, even though none of the patients received PINI, there was no case of inguinal progression in this study.
Table 2. Characteristics of patients who progressed after initial treatment.
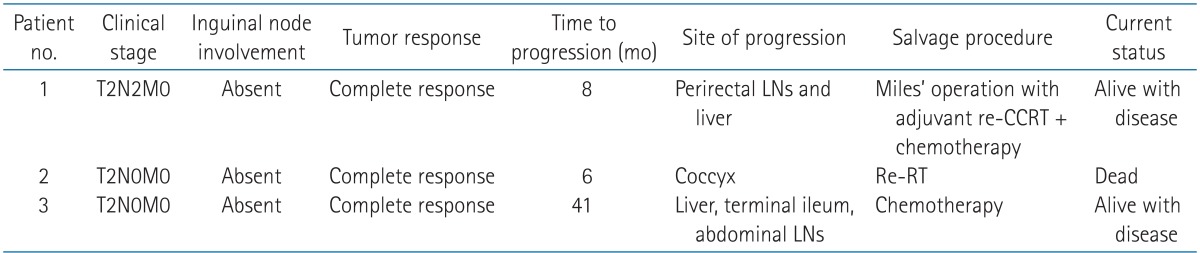
LN, lymph node; CCRT, concurrent chemoradiation therapy; RT, radiation therapy.
4. Overall survival and progression-free survival
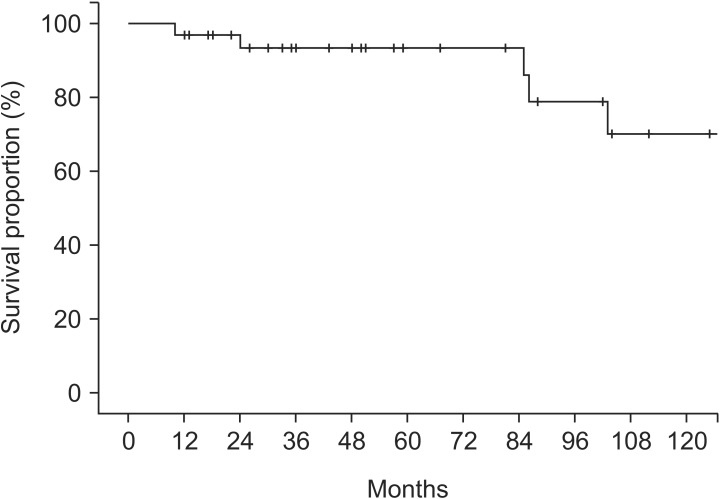
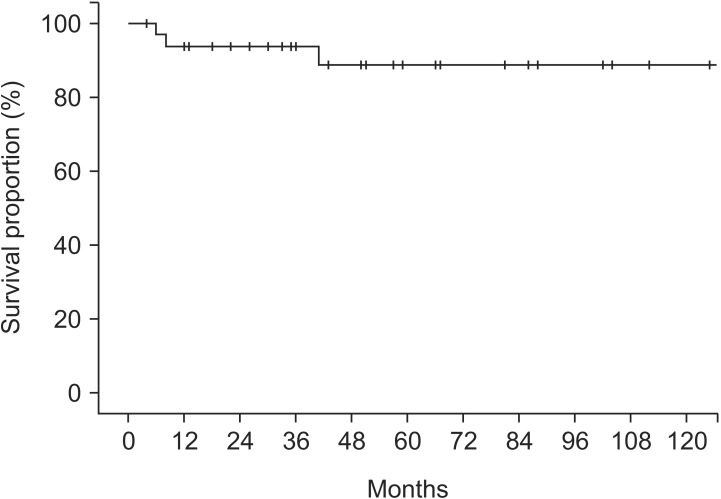
The median follow-up duration was 50 months (range, 4 to 218 months). Among the survival, a median follow-up duration was 51 months (range, 12 to 218 months). Median survival and PFS were 57 months (range, 10 to 218 months) and 50 months (range, 4 to 218 months), respectively. The 5-year OS and PFS rates were 93.4% (Fig. 2) and 88.8% (Fig. 3), respectively.
Fig. 2. Overall survival in patients with anal squamous cell carcinoma.
Fig. 3. Progression-free survival in patients with anal squamous cell carcinoma.
Discussion and Conclusion
Traditionally, most treatment for anal cancer has included inguinal node irradiation, even when no inguinal nodes were involved. Against this backdrop, we tried to analyze patterns of failure in anal cancer patients who did not receive PINI. We found three cases of progression from loco-regional (1 case), distant (1 case), and both areas (1 case). Contrary to our expectations, regional inguinal nodes were free from disease progression in this study. Also, the median OTT in the inguinal node-negative group was 37 days, which was comparable with the results of the United Kingdom National Anal Cancer Trial II (ACT II) (38 days) [13]. The median OTT was 49 days in the RTOG 8704 trial and 42 days in the RTOG 9811 trial [16,17].
As mentioned above, blanket application of PINI remains controversial among radiation oncologists. A current NCCN guideline recommends that inguinal nodes be included in the initial radiation field for prophylactic purposes. Gerard et al. [18] reported a retrospective review of 243/270 patients who were inguinal node-negative at presentation and did not receive PINI of clinically normal inguinal areas, with a resulting incidence of inguinal node progression of 6.4% for T1-T2. Das et al. [19] conducted a retrospective review of 167 patients in order to evaluate patterns of loco-regional failure and predictors of recurrence and survival in patients treated with CCRT for anal cancer. They routinely conducted PINI and reported an inguinal node failure rate of 0% for 124 cases that were inguinal node-negative at presentation. Higher T stage and N stage independently predicted a higher rate of locoregional failure. Based on these results, they advocated the importance of PINI. Recently, the CORS-03 group [14] reported a retrospective study that analyzed the outcome of 208 patients presenting with anal squamous cell carcinoma treated in four cancer centers in the south of France. Of the 181 patients with uninvolved inguinal nodes at presentation, 75 received PINI. The 5-year cumulative rate of inguinal recurrence (CRIR) was 2% and 16% in the PINI and no PINI groups, respectively (p = 0.006). In the no PINI group, the 5-year CRIR was 12% and 30% for T1-T2 and T3-T4, respectively (p = 0.02). The authors concluded that PINI should be recommended for all T3-T4 tumors and considered for early-stage tumors. However, the CORS-03 group study had some limitations. First, only 71% of the patients received concurrent chemotherapy. In addition, inguinal node involvement was assessed by AP-CT imaging and inguinal node biopsy only, without pelvis MRI or FDG-PET/CT evaluation.
In contrast to this, in a phase 3 randomized trial of the EORTC, PINI was not performed in the inguinal nodenegative group even without loco-regional compromise, and the authors concluded that clinical N staging and sex were the most important prognostic factors for local control and survival [9]. Later, Crowley et al. [15] carried out a retrospective review of 30 patients with biopsy-proven squamous cell anal cancer treated with definitive CCRT in one center and examined patterns of failure. They found only one case of inguinal progression without PINI that was inguinal nodenegative at presentation; the patient was successfully salvaged with an inguinofemoral dissection. As a result, they concluded that PINI could be omitted in selected cases of anal cancer without reducing the chance of cure and with the possibility of reducing acute and late toxicities. In addition, they emphasized two points. One was the relatively low risk of progression in the inguinal node even for higher stage tumors, in contrast to pelvic nodes. Another was increased confidence in identifying cases with uninvolved inguinal nodes through the development of imaging modalities such as MRI, PET/CT, and sentinel node biopsy.
Increasingly, MRI had become a part of routine clinical staging and is complementary to AP-CT. Recently, many trials striving to detect inguinal node involvement have more accurately used FDG-PET/CT or sentinel lymph node biopsy. Some trials suggest that FDG-PET/CT more precisely detects abnormal nodes than AP-CT and physical examination [20,21,22,23]. The root reason behind these trials was that more accurate assessment of inguinal node status subsequently guides a more reasonable RT strategy. In our cases, pelvis MRI and FDG-PET/CT were carried out as an initial staging workup procedure starting in 2006. Among patients who were diagnosed after January 2006, the proportion who received pelvis MRI and FDG-PET/CT in this study was 82.4% and 41.2%, respectively.
In conclusion, we support treatment of anal cancer without PINI in selected patients with clinically uninvolved inguinal nodes at presentation through appropriate imaging evaluation. This approach will help reduce RT field size and treatmentrelated acute and late toxicities, without compromising regional inguinal node control. Moreover, a shorter OTT will help improve patient adherence and treatment results, including loco-regional control [13].
The current study had some limitations. First, it was a retrospective study from a single institution. Second, there were small number of patients, and the median follow-up was relatively short. Third, there was lack of clinical data related to radiation-induced acute and chronic complications, and chemotherapy regimens varied among the patients. Most of all, heterogeneous methods were used for to evaluate inguinal node involvement. Further prospective studies are needed to confirm that PINI can be omitted in cases of anal cancer without inguinal node involvement.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Whiteford MH, Stevens KR, Jr, Oh S, Deveney KE. The evolving treatment of anal cancer: how are we doing? Arch Surg. 2001;136:886–891. doi: 10.1001/archsurg.136.8.886. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800. doi: 10.1056/NEJM200003163421107. [DOI] [PubMed] [Google Scholar]
- 3.Recio PM. Radical surgery in the treatment of epidermoid carcinoma of the anal canal. Philipp J Surg. 1955;10:1–8. [PubMed] [Google Scholar]
- 4.Harrison EG, Jr, Beahrs OH, Hill JR. Anal and perianal malignant neoplasms: pathology and treatment. Dis Colon Rectum. 1966;9:255–267. doi: 10.1007/BF02616920. [DOI] [PubMed] [Google Scholar]
- 5.Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–356. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 6.Cummings BJ, Keane TJ, O'Sullivan B, Wong CS, Catton CN. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys. 1991;21:1115–1125. doi: 10.1016/0360-3016(91)90265-6. [DOI] [PubMed] [Google Scholar]
- 7.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 8.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 9.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 10.Chie EK, Ha SW, Park JG, Bang YJ, Heo DS, Kim NK. Treatment results in anal cancer: non-operative treatment versus operative treatment. J Korean Soc Ther Radiol Oncol. 2002;20:62–67. [Google Scholar]
- 11.Lee WS, Chun HK, Lee WY, et al. Anal canal carcinoma: experience from a single Korean institution. Yonsei Med J. 2007;48:827–832. doi: 10.3349/ymj.2007.48.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 13.James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 14.Ortholan C, Resbeut M, Hannoun-Levi JM, et al. Anal canal cancer: management of inguinal nodes and benefit of prophylactic inguinal irradiation (CORS-03 Study) Int J Radiat Oncol Biol Phys. 2012;82:1988–1995. doi: 10.1016/j.ijrobp.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Crowley C, Winship AZ, Hawkins MA, Morris SL, Leslie MD. Size does matter: can we reduce the radiotherapy field size for selected cases of anal canal cancer undergoing chemoradiation? Clin Oncol (R Coll Radiol) 2009;21:376–379. doi: 10.1016/j.clon.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941–1948. doi: 10.1200/JCO.2011.35.4837. [DOI] [PubMed] [Google Scholar]
- 18.Gerard JP, Chapet O, Samiei F, et al. Management of inguinal lymph node metastases in patients with carcinoma of the anal canal: experience in a series of 270 patients treated in Lyon and review of the literature. Cancer. 2001;92:77–84. doi: 10.1002/1097-0142(20010701)92:1<77::aid-cncr1294>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Das P, Bhatia S, Eng C, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68:794–800. doi: 10.1016/j.ijrobp.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Cotter SE, Grigsby PW, Siegel BA, et al. FDG-PET/CT in the evaluation of anal carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:720–725. doi: 10.1016/j.ijrobp.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen BT, Joon DL, Khoo V, et al. Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol. 2008;87:376–382. doi: 10.1016/j.radonc.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Mai SK, Welzel G, Hermann B, Wenz F, Haberkorn U, Dinter DJ. Can the radiation dose to CT-enlarged but FDG-PET-negative inguinal lymph nodes in anal cancer be reduced? Strahlenther Onkol. 2009;185:254–259. doi: 10.1007/s00066-009-1944-5. [DOI] [PubMed] [Google Scholar]
- 23.Mistrangelo M, Pelosi E, Bello M, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of inguinal node metastases in patients with anal cancer. Int J Radiat Oncol Biol Phys. 2010;77:73–78. doi: 10.1016/j.ijrobp.2009.04.020. [DOI] [PubMed] [Google Scholar]