Abstract
Purpose
To evaluate the treatment results in early stage non-small cell lung cancer patients who have undergone fiducial-less CyberKnife radiosurgery (CKRS).
Materials and Methods
From June 2011 to November 2013, 58 patients underwent CKRS at Asan Medical Center for stage I lung cancer. After excluding 14 patients, we retrospectively reviewed the records of the remaining 44 patients. All analyses were performed using SPSS ver. 21.
Results
The median age at diagnosis was 75 years. Most patients had inoperable primary lung cancer with a poor pulmonary function test with comorbidity or old age. The clinical stage was IA in 30 patients (68.2%), IB in 14 (31.8%). The mean tumor size was 2.6 cm (range, 1.2 to 4.8 cm), and the tumor was smaller than 2 cm in 12 patients (27.3%). The radiation dose given was 48-60 Gy in 3-4 fractions. In a median follow-up of 23.1 months, local recurrence occurred in three patients (2-year local recurrence-free survival rate, 90.4%) and distant metastasis occurred in 13 patients. All patients tolerated the radiosurgery well, only two patients developing grade 3 dyspnea. The most common complications were radiation-induced fibrosis and pneumonitis. Eight patients died due to cancer progression.
Conclusion
The results showed that fiducial-less CKRS shows comparable local tumor control and survival rates to those of LINAC-based SABR or CKRS with a fiducial marker. Thus, fiducial-less CKRS using Xsight lung tracking system can be effectively and safely performed for patients with medically inoperable stage I non-small cell lung cancer without any risk of procedure-related complication.
Keywords: Lung, NSCLC, Radiosurgery, Cyberknife, Fiducialless, Marker
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. Approximately 75% of lung cancers are non-small cell lung cancer (NSCLC). At initial diagnosis, only about 10%-15% of patients with NSCLC have localized disease (stage I) [1]. In early stage lung cancer, anatomical resection is the standard treatment. However, some patients, especially in elderly patients, cannot undergo surgery due to poor pulmonary function test (PFT) results or comorbidities, such as cardiac disease and cerebral disease. According to a survey conducted in the Netherlands, the number of untreated elderly patients with stage I NSCLC significantly reduced following the introduction of stereotactic ablative radiotherapy (SABR). In addition, this survey also showed some improvement in median survival [2,3].
SABR is a high-precision radiotherapy characterized by the use of high biological radiation doses and is delivered in 1-5 fractions as an outpatient procedure. In this treatment method, the radiation dose to normal tissue is minimized, and the dose per fraction can therefore be increased. This allows upto two-fold higher biologic doses of SABR to be delivered compared to conventional radiotherapy [4]. Initially, SABR was used as a second line therapy for patients with inoperable early stage lung cancer. Several studies have reported the results of SABR for stage I NSCLC, citing a 90%-97% of 2- to 3-year local control rate, which was competitive with that of surgical resection [5,6,7]. Nowadays the clinical use of SABR is increasing.
CyberKnife (Accuray Inc., Sunnyvale, CA, USA) is a dedicated system for radiosurgery [5,8,9,10], with a real-time tumor tracking capability via the Synchrony Respiratory Tracking System [9,10]. To detect internal tumor motion, orthogonal X-ray imaging is used. Light-emitting diode (LED) is simultaneously as a surrogate marker to monitor external respiratory motion (chest wall or abdomen). The synchrony system correlates the internal tumor motion with the external respiratory motion; hence, the tumor location can be tracked. Initially, radio-opaque fiducial markers were required to use the Synchrony system for tracking a lung tumor. However, there are some well-known demerits of marker insertion into lung lesions, such as the development of pneumothorax, migration of marker, and arrhythmia, and delays in treatment [6,11,12].
The Xsight lung tracking system (Accuray Inc.) [10] with the Synchrony Respiratory Tracking System makes it possible for direct lung tumor tracking without fiducial markers using pattern-similarity matching algorithms. As shown in Fig. 1, lung tumor location is determined by matching the orthogonal image pair of the patient from a dual X-ray in-room radiograph system with the digitally reconstructed radiographs pre-calculated from the planning computed tomography (CT) data. A series of the tumor locations obtained over various respiratory phases are then correlated with the external respiratory signal with Synchrony system for real-time tumor tracking. Over the beam delivery period, the tumor localization procedure by acquisition of the orthogonal X-ray images is repeated with the interval of 30 to 60 seconds, and used for the correction of the correlation model that can be eventually changed over time. To improve overall tracking accuracy, a spine-based alignment is performed as a pre-setup stage before the direct tumor positioning. In general, the following conditions are recommended for this system to ensure the direct tumor detectability: a tumor larger than 15 mm in all axes, located at the periphery, and not complete obstructed by spine on live X-ray projection images.
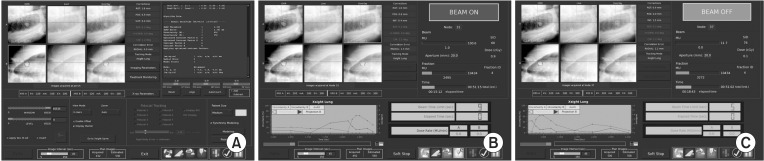
Fig. 1. Xsight lung tracking system. (A) Tracking. Before radiation delivery, Xsight lung tracking system starts to track the tumor. It will take about 5-10 minutes. (B) Beam on. If Xsight lung tracking system localized the tumor, delivery of radiation starts. (C) Beam off. If Xsight lung tracking system missed the tumor, delivery of radiation stops and the system re-tracks the location of tumor.
However, indication of fiducial-less CyberKnife radiosurgery (CKRS) had not yet been established. In addition, there were few reports on the application of this procedure.
So, to ascertain whether the indication of fiducial-less CKRS could be extended or not, we had evaluated treatment outcome of this procedure using Xsight Lung Tracking System at Asan Medical Center (AMC) and tested accuracy of this system without fiducial marker by phantom experiment [13].
In our current study, we reported the treatment outcomes of the fiducial-less CKRS using the Xsight lung tracking system for patients with stage I NSCLC, and validate the adequacy of this approach.
Materials and Methods
1. Patients
From June 2011 to November 2013, 58 patients underwent CKRS at AMC for lung cancer and lung metastases originating from other cancers. In our institute, we recommend CKRS to patient with inoperable stage I NSCLC, except some cases. Such as diameter of tumor is smaller than 1 cm or tumor cannot be tracked due to being obscured by heart or spine. In those cases, we recommend LINAC-based SABR. From 58 patients, we included patients with diagnosed to stage I NSCLC, older than 18 years, and had at least 12 month follow-up. Therefore, we retrospectively reviewed the records of 44 of these patients excluding 14 patients-advanced NSCLC (n = 6), other primary cancers diagnosed within 5 years (n = 6), and lung metastasis from other primary cancers (n = 2).
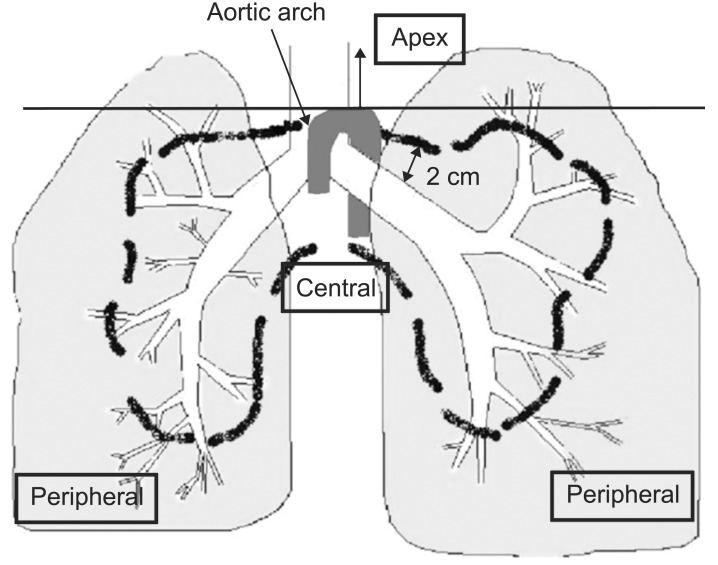
Tumor locations were classified as central, peripheral (Fig. 2), apical (superior to aortic arch) or chest wall abutted (closer than 1 cm to chest wall) according to the classification by Timmerman et al [14].
Fig. 2. Schema of central and peripheral locations. This diagram showed definition of central, peripheral and apical location of lung tumor. Tumors located at proximal bronchial tree (according to the Radiation Therapy Oncology Group) defined as centrally located tumor, tumor located above aortic arch defined as apically located tumor, and tumor located closer than 1 cm to chest wall defined as chest wall abutted. Except of these locations, others defined as peripherally located tumor.
2. Work up
Before treatment, imaging work ups such as chest-CT, fluorodeoxyglucose-positron emission tomography (FDG-PET), and PFT were conducted. Histologic confirmation was performed in 40 patients. The most common histologic subtype was squamous cell carcinoma (22 patients, 50%) followed by adenocarcinoma (16 patients, 36%).
After CKRS, a follow-up chest CT and chest X-ray were performed within 1-2 months. After the initial response evaluation, patients were followed up every 3 months with chest X-ray or CT for up to 2 years and then, every 6 months for up to 5 years.
3. Target volume delineation
For CyberKnife simulation, all patients were immobilized with a Vac-Lok and a knee pillow. Three-dimensional (3D) CT simulation was performed under end expiratory breath-holding without contrast media using a 16-slice CT (LightSpeed RT 16; GE Healthcare, Waukesha, WI, USA). Four-dimensional CT data were also acquired under free breathing with intravenous contrast to estimate the range of tumor motion.
The gross tumor volume (GTV) was delineated on a non-contrast planning 3D-CT scan. Initially, the GTV was delineated using the mediastinal window setting and modified under the lung window setting. A 5-mm margin was then added to the GTV to generate planning target volume (PTV). Using real-time tracking system, we did not necessary to add internal margin to GTV.
Both arms, the esophagus, spinal cord, trachea and bronchus, the great vessels, both lungs, the brachial plexus, and the heart were regarded as organs at risk.
4. Treatment planning and delivery
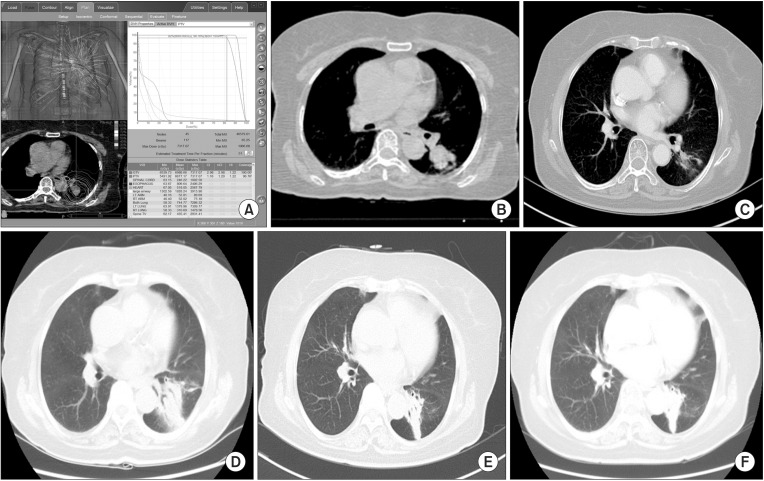
CKRS planning was performed using Multiplan ver. 4.0 (Accuray Inc.) and ray-tracing algorithm with a fixed cone (5-60 mm). In addition, CKRS was delivered to patients using CyberKnife ver. 4.0. A total 48-60 Gy was delivered in 3-4 fractions over the course of 3-11 days. Standard dose of CKRS for lung tumor in our institute is 54 Gy for 3 fractions. However, we changed this dose fractionation scheme, if tumor located near rib, skin, airway, esophagus, heart, large vessel and brachial plexus. For example, we usually prescribed 60 Gy for 4 fractions if tumor located adjacent to chest wall and 54 Gy for 4 fractions if tumor abutted to chest wall. The dose was prescribed as a 76%-85% isodose line, covering at least 95% of the PTV. Dose constraints for organs at risk were based on the previous report by Timmerman [15]. For 4 fractionated CKRS, we applied dose constraints of mid-value of 3 and 5 fractionated regimen (Table 1). Fig. 3 shows example of dose volume histogram (DVH) of centrally located tumor and CT images before and after CKRS.
Table 1. Normal tissue dose constraints for SABR in Asan Medical Center.
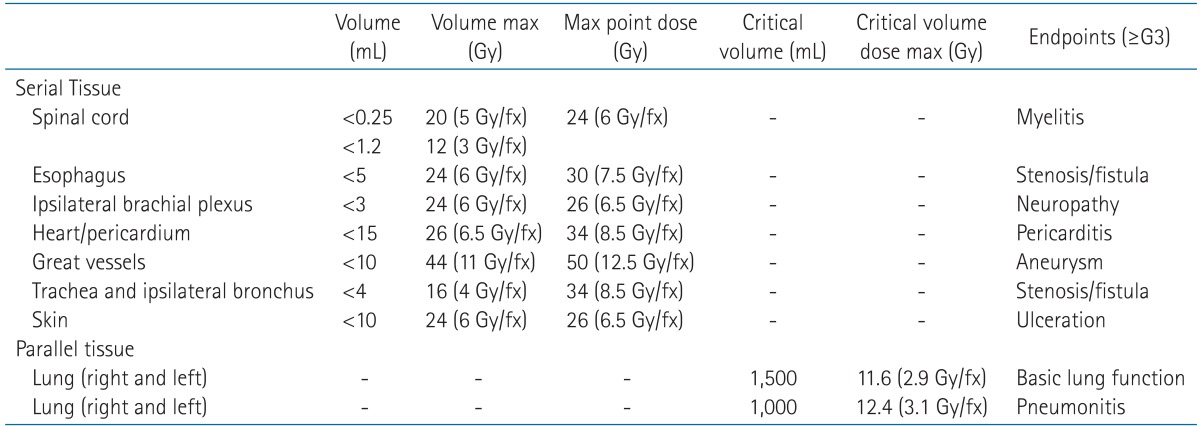
Fig. 3. DVH and example of case. (A) DVH. (B) CT image, before treatment. (C) CT image, 1 month after CKRS. After delivery of 60 Gy for 4 fractions, we checked chest CT. This figure showed partially responded tumor after CKRS. (D) CT image, 5 months after CKRS. After 5 months, asymptomatic radiation pneumonitis occurred. (E) CT image, 11 months after CKRS. After 11 months, radiation fibrosis was observed without any symptom. (F) CT image, 24 months after CKRS. Until last follow-up, tumor did not progress with radiation change. DVH, dose volume histogram; CT, computed tomography; CKRS, CyberKnife radiosurgery.
For 44 of our study patients, the Xsight lung tracking system with no insertion of a fiducial marker was used for real-time lung tumor tracking. This system can detect an internal tumor location, as long as it can recognize the tumor at least in one DRR field among candidate DRR images pre-calculated from a planning CT with varying positions and orientations. Using the Synchrony system, real-time tumor tracking was achieved. During treatment delivery, the tumor position was tracked using intermittent orthogonal X-ray imaging and a correlation model with external respiratory signal. A robot with a linear accelerator was used to maintain a precise alignment of the beam to the tumor throughout the respiratory cycle.
5. Follow-up
Local recurrence was defined as tumor recurrence or progression within the PTV, with histologic confirmation or checking change of tumor metabolism. Local enlargement defined as at least a 20% increase in the longest diameter of GTV at follow-up CT. This criteria was used previously by Timmerman et al. [7]. Regional recurrence was defined as hilar or mediastinal nodal enlargement on a chest CT or PET-CT. A distant metastasis was defined as any failure outside the ipsilateral lung. Local recurrence-free survival was defined as the period from the day of radiotherapy commencement to the day of an initially confirmed recurrence. Overall survival was defined as the period from the day of initiating radiotherapy to the day of death from any cause or to the last follow-up day.
Toxicities were evaluated using the Common Toxicity Criteria for Adverse Events ver. 4.0. Survival curves were calculated using the Kaplan-Meier method, and p-values were estimated with a log-rank test using SPSS ver. 21 (IBM SPSS, Armonk, NY, USA).
Results
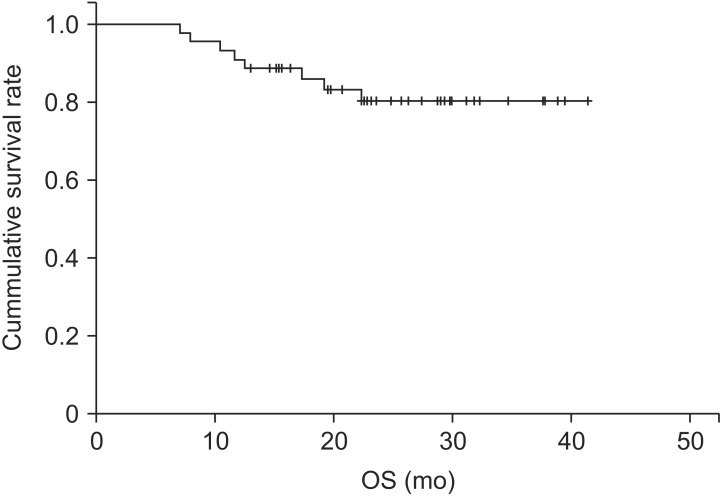
The median follow-up was 23.1 months (range, 7 to 41 months). Of the 44 patients in our study population, eight died during follow-up, due to lung cancer progression (n = 6) and other unknown causes (n = 2). The overall survival rate was 86% at 1 year and 80.3% at 2 years (Fig. 4).
Fig. 4. Overall survival (OS) outcomes in the study population.
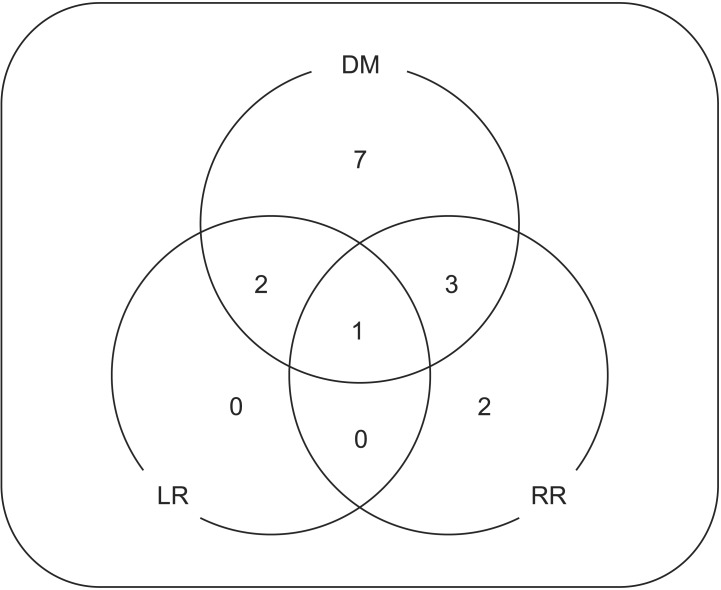
A total of 15 patients experienced disease recurrence. Local recurrence was seen in three patients (6.8%) with no isolated local recurrence, regional recurrences were seen in six patients (13.6%), and distant metastasis was evident in 13 patients (29.5%) (Fig. 5). There were no significant differences according to pathology, tumor locations and tumor size in failure patterns.
Fig. 5. Patterns of failure. DM, distant metastasis; LR, local recurrence; RR, regional recurrence.
1. Patient characteristics
Patient characteristics are listed in Table 2. The median age at diagnosis was 75 years, and there were 37 (84.1%) men. Most patients could not undergo surgery due to poor PFT-mean forced expiratory volume in one second (FEV1), 63.0% (range 24% to 138%); mean diffusing capacity of lung for carbon monoxide (DLCO), 50.8% (range 43% to 96%)-and refusal of patient. About 30% of patients were ECOG performance status 2, 3. The median tumor diameter was 2.61 cm, and tumors were most commonly located in the periphery or were abutted the chest wall.
Table 2. Patient characteristics.
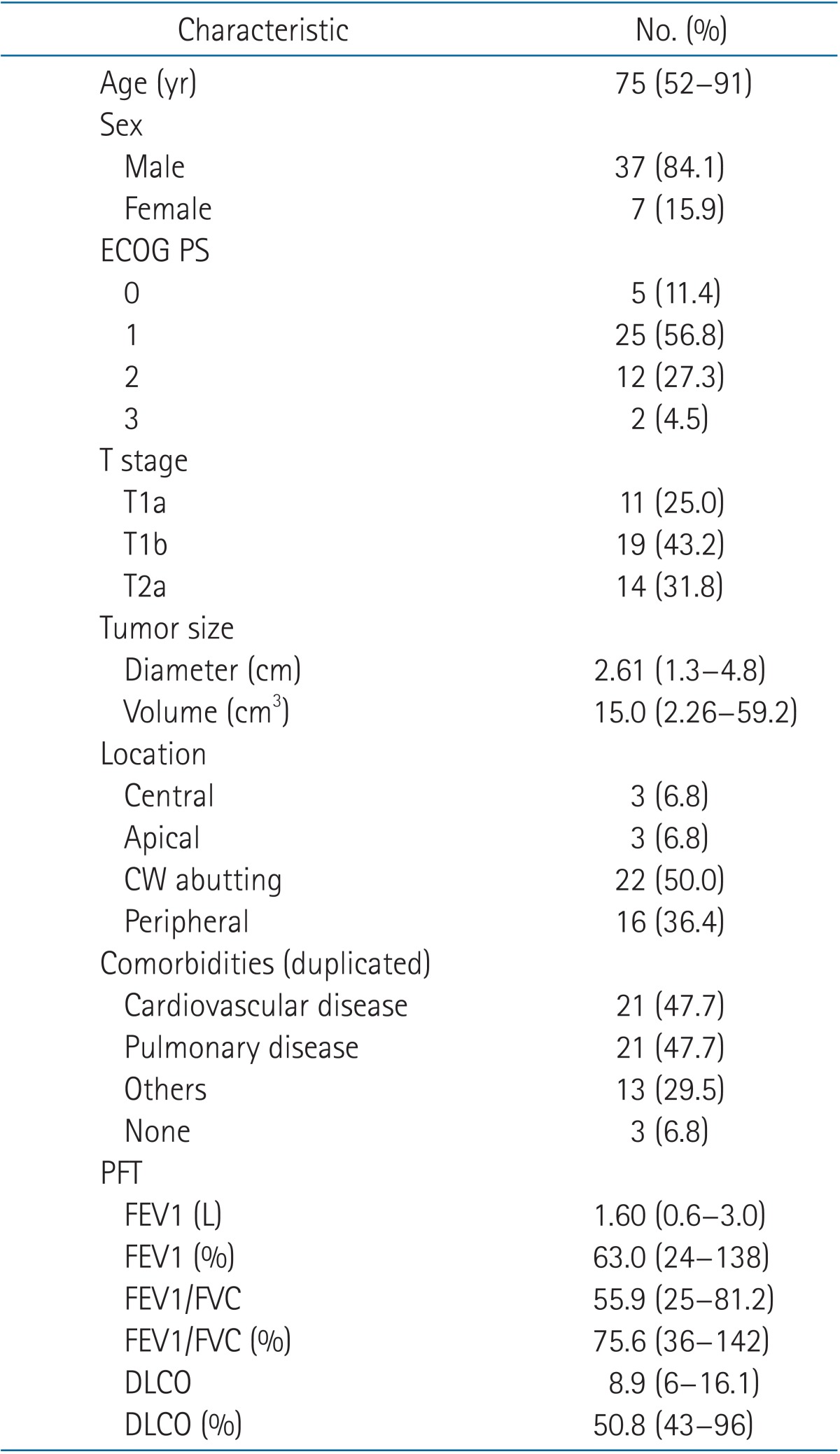
Values are presented as median (range) or number of patients (%).
ECOG PS, Eastern Cooperative Oncology Group performance statue; CW, chest wall; PFT, pulmonary function test; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; DLCO, diffusing capacity of lung for carbon monoxide.
2. Treatment characteristics
The median prescription dose was 54 Gy in 3 fractions (range, 48 to 60 Gy in 3-4 fractions) The dose was prescribed on 80% isodose line (range, 76% to 85 %). Over 100 beams were used in 24 patients (median beam number, 101; range, 31 to 170) All patients were treated within 60 minutes, except in one patient who treatment lasted for 61 minutes. After radiation, patients were evaluated by chest CT within 1-2 month.
Other treatment characteristics are described in Table 3.
Table 3. Treatment characteristics.
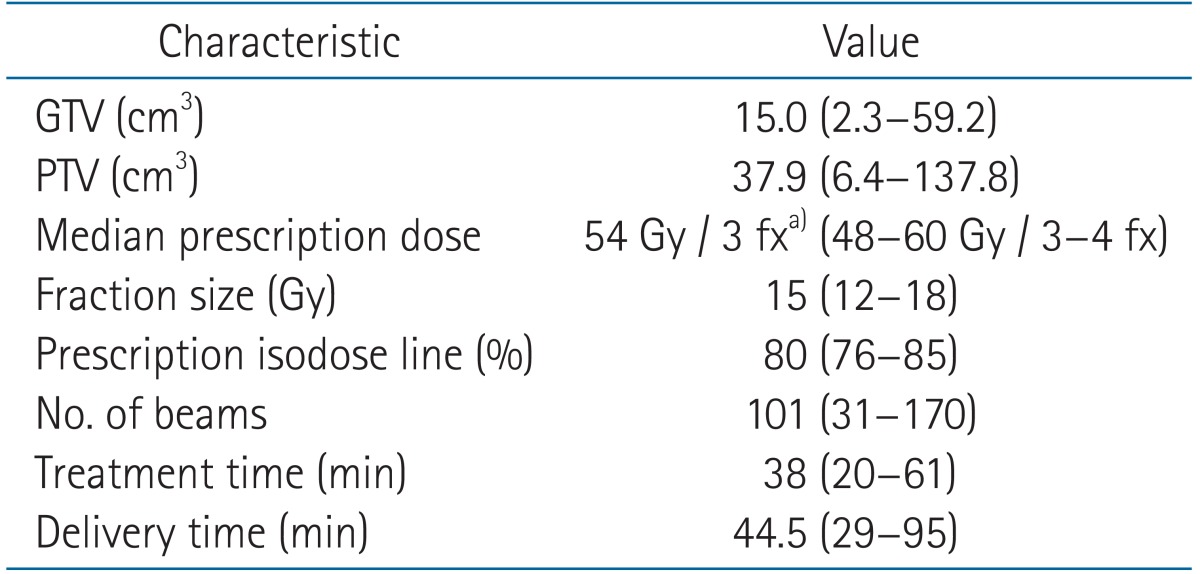
Values are presented as mean (range).
GTV, gross tumor volume; PTV, planning target volume; EQD2, equivalent dose in 2 Gy fractions. Treatment time included beam-on time and LINAC manipulating time. Delivery time = treatment time + respiratory tracking time.
a)EQD2 = 126 Gy.
3. Local control
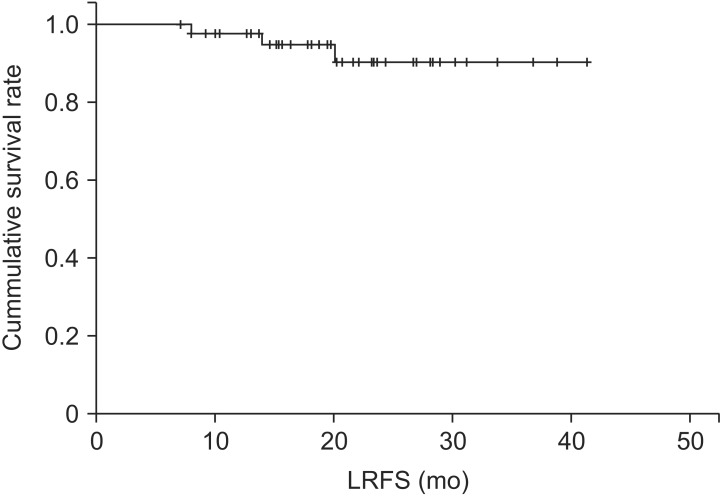
The local recurrence-free survival rate was 94.9% at 1 year and 90.4% at 2 years (Fig. 6) in our study series. There were no cases of isolated local recurrence. Local recurrence cases are described at Table 4.
Fig. 6. Local recurrence-free survival (LRFS) outcomes.
Table 4. Local recurrence cases.
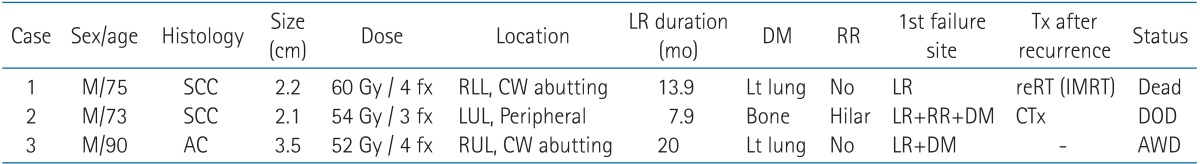
LR, local recurrence; DM, distant metastasis; RR, regional recurrence; SCC, squamous cell carcinoma; AC, adenocarcinoma; CW, chest wall; DOD, dead of disease; AWD, alive with disease; RLL, right lower lobe; LUL, left upper lobe; Tx, treatment; IMRT, intensity-modulated radiation therapy.
Local recurrence occurred in three patients, all of who had a distant metastasis, and one of who also had regional recurrence. Only one patient had local recurrence initially, while the disease recurred locally and distantly at the same time in the remaining patients. The local recurrence interval was 7-20 months. After recurrence, patients received re-irradiation or systemic chemotherapy. Eventually, two patients died and one survived among the three cases of local recurrence.
4. Toxicities
There were no grade 4 or 5 toxicities. The most common acute toxicities were G1 fibrosis and pneumonitis (Table 5). Two patients had G3 dyspnea: one had aggravated dyspnea due to aggravation of underlying interstitial lung disease (initial PFT: FVC, 2.61 L and 67%; FEV1: 1.98 L and 81%; DLCO, 44%), while the other had aggravated dyspnea due to radiation and recovered after steroid administration (initial PFT: FVC, 1.33 L and 50%; FEV1, 0.63 L and 36%; DLCO, 54%).
Table 5. Acute and chronic toxicities.
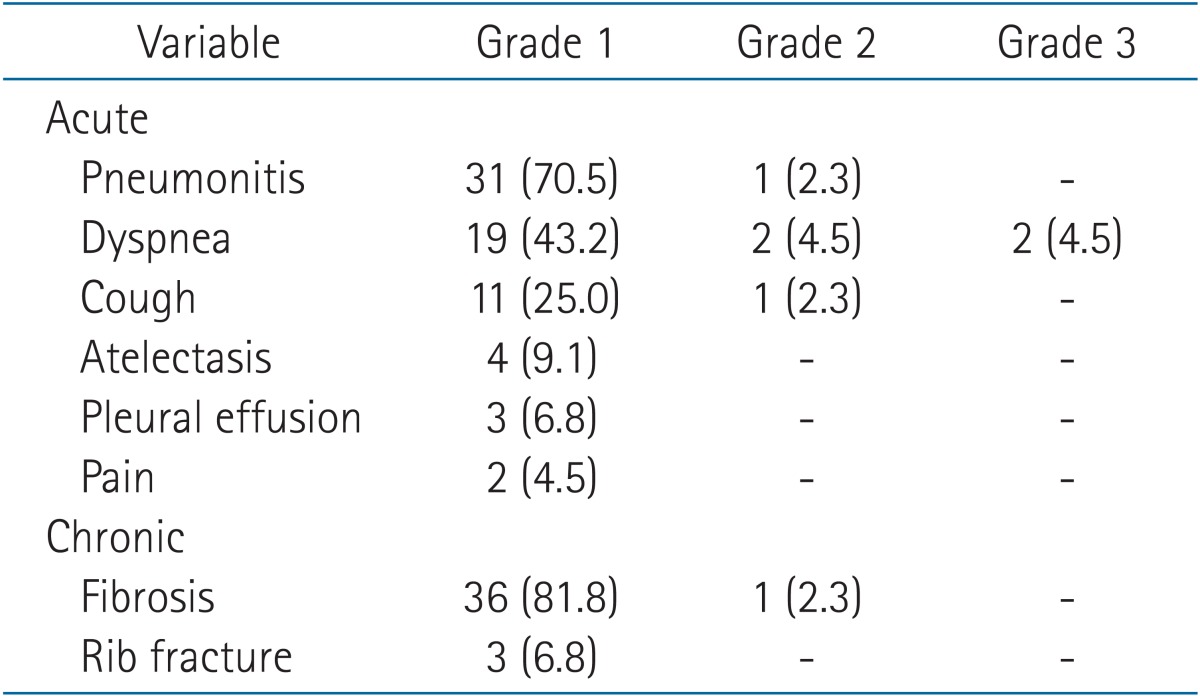
Values are presented as number (%).
Three patients experienced a grade 1 rib fracture. Only two patients experienced a grade 1 rib fracture (simple healed fracture line) among the 22 patients with a chest wall abutting lung cancer. In addition, one patient with a tumor located at the periphery experienced a simple rib fracture.
Three patients had a centrally located lung cancer and none experienced any airway obstruction.
Discussion and Conclusion
SABR is now considered the treatment standard for patients with inoperable stage I NSCLC [16] and has shown a good local control rate that is comparable to that of surgery.
In previous studies, CKRS also showed good outcomes, with a local control rate at 2 years of 86%-100% in patients with inoperable early stage NSCLC with minor complications, such as grade 1-2 radiation pneumonitis, mild chest wall discomfort [17,18,19,20,21] (Table 6). The advantages of CKRS are the ability to perform real-time tumor tracking, which makes it possible to reduce the target volume by eliminating the margin of the internal target volume. This has the potential to improve the outcomes in patients with a poor PFT.
Table 6. Studies of Cyberknife radiosurgery.
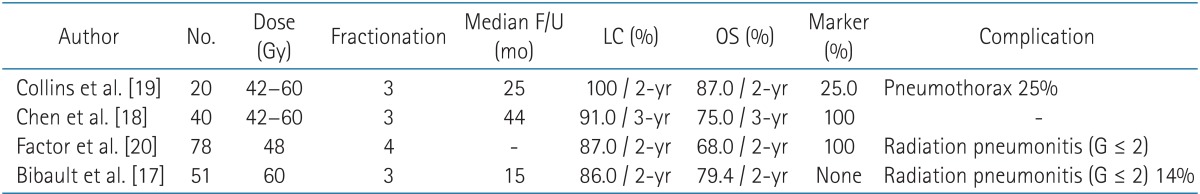
F/U, follow-up; LC, local recurrence rate; OS, overall survival rate.
Before the introduction of the Xsight lung tracking system, fiducial marker insertion was necessary for tumor tracking. However, the insertion of such markers has been associated with complications, such as pneumothorax, migration of the marker, and arrhythmia [6,11,12]. According to Collins et al. [12], after insertion of fiducial marker, pneumothorax was seen in 25% of patients. Moreover, 17% of all patients required tube thoracotomy to relive clinically significant pneumothorax. And these complications occurred after insertion of fiducial marker made radiation therapy to be delayed. Using Xsight lung tracking system, there were no more delay of radiation delivery. Moreover, comparing our data to that of published by Collins et al. [12], in this study with Xsight lung tracking system, radiation delivery time reduced to half (38 minutes [range, 20 to 61 minutes] vs. 82 minutes [range, 53 to 120 minutes]). However, sufficient tumor contrast in X-ray images was crucial for direct soft-tissue tracking, and tumors that are larger than 15 mm in all dimensions, located at the periphery were recommended for Xsight lung tracking system. But, there were no obvious indications for fiducial-less CKRS.
Hence, we conducted study of fiducial-less CKRS for medically inoperable early stage NSCLC. For theoretical background, we performed not only a clinical trial but also phantom experiments, which verified the accuracy of the Xsight lung tracking system [13]. Jung et al. [13] recently reported the results of phantom study. According to them, Xsight lung tracking system had comparable localization accuracy to the fiducial-based target tracking system using 3D lung phantom (standard deviations: Xsight lung tracking system 0.38 ± 0.54 mm, 0.13 ± 0.18 mm, 0.14 ± 0.37 mm for CC, LR, AP vs. fiducial-based target tracking system 0.36 ± 0.39 mm, 0.15 ± 0.64 mm, 0.15 ± 0.62 mm for CC, LR, AP). Volumes of included tumor were 1.87-21.45 cm3. Also, in our present study, no problems were encountered in terms of tumor tracking, despite the inclusion of tumors with a diameter of less than 15 mm (n = 4), and located at central region (n = 3). Moreover, the local control rates of our current fiducial-less CKRS study showed possibility for missing target was low (94% at 1 year and 90.6% at 2 years).
While the follow-up duration was short and the number of our study patients was relatively small, we observed good local tumor control with fiducial-less CKRS. In addition, treatment-related complications were most commonly grade 1 pneumonitis and fibrosis. There were only two cases of grade 3 dyspnea, and the symptoms were resolved after medication in both cases. There were no cases of treatment-related mortality in our present study patient (Table 5).
Despite the retrospective nature of our present study, the small study sample size, and short follow-up period, we were able to show a good local control rate of fiducial-less CKRS. Hence, CKRS may be successful even in small tumor size cases, and in more centrally located tumors, if the tumor can be clearly distinguished on an X-ray image.
Further investigations are needed to clarify the indications for fiducial-less CKRS.
In conclusion, Fiducial-less CKRS using Xsight lung tracking system can be effectively and safely performed for patients with medically inoperable stage I NSCLC without any risk of procedure-related complication. Further experience and classification of confident indication of fiducial-less system should be warranted.
Acknowledgments
This study was supported by a grant (2013-472) from the Asan Institute for Life Sciences, Seoul, Korea and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2011346).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Haasbeek CJ, Palma D, Visser O, Lagerwaard FJ, Slotman B, Senan S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23:2743–2747. doi: 10.1093/annonc/mds081. [DOI] [PubMed] [Google Scholar]
- 3.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 4.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 5.Brown WT, Wu X, Fayad F, et al. CyberKnife radiosurgery for stage I lung cancer: results at 36 months. Clin Lung Cancer. 2007;8:488–492. doi: 10.3816/CLC.2007.n.033. [DOI] [PubMed] [Google Scholar]
- 6.van der Voort van Zyp NC, Prevost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol. 2009;91:296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown WT, Wu X, Wen BC, et al. Early results of CyberKnife image-guided robotic stereotactic radiosurgery for treatment of lung tumors. Comput Aided Surg. 2007;12:253–261. doi: 10.3109/10929080701684754. [DOI] [PubMed] [Google Scholar]
- 9.Seppenwoolde Y, Berbeco RI, Nishioka S, Shirato H, Heijmen B. Accuracy of tumor motion compensation algorithm from a robotic respiratory tracking system: a simulation study. Med Phys. 2007;34:2774–2784. doi: 10.1118/1.2739811. [DOI] [PubMed] [Google Scholar]
- 10.Wong KH, Dieterich S, Tang J, Cleary K. Quantitative measurement of CyberKnife robotic arm steering. Technol Cancer Res Treat. 2007;6:589–594. doi: 10.1177/153303460700600601. [DOI] [PubMed] [Google Scholar]
- 11.Nuyttens JJ, Prevost JB, Praag J, et al. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: marker placement and early results. Acta Oncol. 2006;45:961–965. doi: 10.1080/02841860600902205. [DOI] [PubMed] [Google Scholar]
- 12.Collins BT, Erickson K, Reichner CA, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. doi: 10.1186/1748-717X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung J, Song SY, Yoon SM, et al. Verification of accuracy of CyberKnife tumor-tracking radiation therapy using patient-specific lung phantoms. Int J Radiat Oncol Biol Phys. 2015 Mar 06; doi: 10.1016/j.ijrobp.2015.02.055. [Epub] [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–222. doi: 10.1016/j.semradonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Videtic GM. The development of stereotactic body radiotherapy (SBRT) for medically inoperable early stage non-small cell lung cancer: an international phenomenon. J Radiat Oncol. 2012;1:3–10. [Google Scholar]
- 17.Bibault JE, Prevost B, Dansin E, Mirabel X, Lacornerie T, Lartigau E. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol. 2012;7:102. doi: 10.1186/1748-717X-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen VJ, Oermann E, Vahdat S, et al. CyberKnife with tumor tracking: an effective treatment for high-risk surgical patients with stage I non-small cell lung cancer. Front Oncol. 2012;2:9. doi: 10.3389/fonc.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins BT, Vahdat S, Erickson K, et al. Radical cyberknife radiosurgery with tumor tracking: an effective treatment for inoperable small peripheral stage I non-small cell lung cancer. J Hematol Oncol. 2009;2:1. doi: 10.1186/1756-8722-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Factor OB, Vu CC, Schneider JG, et al. Stereotactic body radiation therapy for stage I non-small cell lung cancer: a small academic hospital experience. Front Oncol. 2014;4:287. doi: 10.3389/fonc.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim W, Kim HJ, Park JH, Huh HD, Choi SH. Treatment results of CyberKnife radiosurgery for patients with primary or recurrent non-small cell lung cancer. J Korean Soc Ther Radiol Oncol. 2011;29:28–35. [Google Scholar]