Nano- and molecular-scale materials and devices have demonstrated great potential for unique properties and functions that are unattainable at macroscopic scales.[1] Studies of single molecules and their interactions and reactions at the single-molecule level provide new perspectives on important fundamental issues in physics, chemistry, and biology.[2] Often it is desirable to anchor the molecules on suitable substrates to facilitate the study and application of single molecules under various conditions for physical measurement and chemical processing. Two common approaches to single-polymer immobilization are electrostatic adsorption and chemical grafting. The first method involves the deposition of a very dilute polymer solution on a substrate followed by evaporation of the solvent. A key requirement is that the polymer and the substrate possess opposite charges under the experimental conditions for the electrostatic interactions to occur.[3–5] The second method involves chemically grafting the polymer to the substrate. In this case, the polymer is derivatized with a functional group that is subsequently conjugated to the substrates. Examples include Cl-terminated poly(dimethylsiloxane) on silicon,[6] disulfide-modified polystyrene on gold,[7] and polysilanyllithium on a brominated quartz surface.[8] Relative to noncovalent and electrostatic interactions, covalent immobilization offers more stable and robust attachments that can withstand harsh experimental conditions. Herein, we report a general approach to the covalent immobilization of single polymer molecules. This method does not require chemical derivatization of the polymer and is applicable to a variety of materials, including those that do not possess reactive functional groups.
The immobilization chemistry is based on the photochemically or thermally initiated C—H/N—H insertion reactions of perfluorophenylazides (PFPAs).[9–11] The presence of fluorine atoms markedly increases the insertion reaction yield relative to their non-fluorinated counterparts.[12] In the example shown in Scheme 1, a glass or silicon wafer is first treated with PFPA–silane, thus attaching azido groups to the surface. The polymer is then coated and the azido groups activated to form covalent linkages to the polymer. We found that this immobilization method is highly reproducible and tolerant of defects. Uniform polymer films were obtained from wafers treated with PFPA–silane at concentrations of a few micromoles, or when more than 100 times of a non-photoactive silane was added,[13] which is because in principle, only one attachment point is necessary to tether the entire polymer chain to the surface. We therefore hypothesize that as the density of the surface azido groups continues to decrease, the immobilized polymer will no longer be uniform. The thickness of the polymer film will decrease and eventually the surface will be populated by isolated single polymer molecules. The density of the surface azido groups can be controlled by the concentration of PFPA–silane or by treating the surface with a mixture of PFPA–silane and a non-photoactive silane.[13]
Scheme 1.
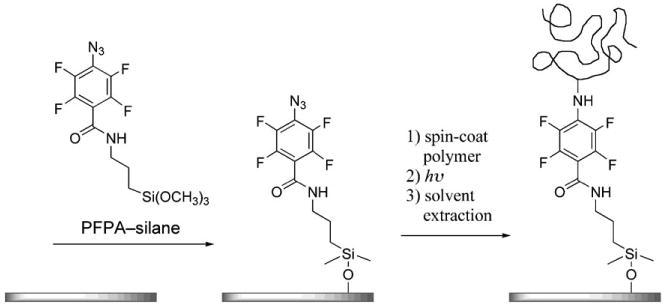
Covalent immobilization of polymers on silicon oxide using PFPA–silane as the photo-cross-linker.
Herein, we report the covalent immobilization of single polymer molecules using dilute PFPA–silane solutions. Comprehensive studies were conducted on polystyrene, a polymer that does not possess any functional groups. In the experiment, cleaned silicon wafers were treated with solutions of PFPA–silane in toluene, the concentrations of which ranged from 5 × 10−1 to 5 × 10−5 mg mL−1. The wafers were then spin-coated with polystyrene. Irradiation followed by removal of the unbound polymer with toluene yielded covalently attached polymers. The thickness and water-contact angles of the resulting films are shown in Figure 1.
Figure 1.
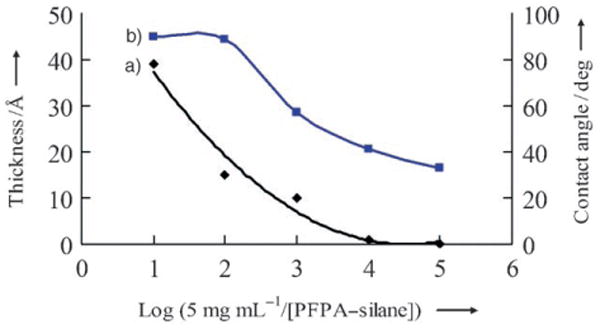
a) Thickness, measured by ellipsometry, and b) water contact angle of the immobilized polymer as a function of PFPA–silane concentration. The contact angle of the unfunctionalized silicon wafer was measured to be approximately 17°.
As the concentration of PFPA–silane decreased, the immobilized polymer became thinner (Figure 1 a). At the PFPA–silane concentration of 5 × 10−3 mg mL−1, the contact angle decreased to approximately 57°, thus indicating the collapse of the film (Figure 1 b). Further dilution resulted in lower contact angles and no film was detected by ellipsom-etry. When the immobilization was carried out on wafers treated with PFPA–silane at 5 × 10−5 mg mL−1, isolated single polystyrene molecules were observed (Figure 2 a).
Figure 2.
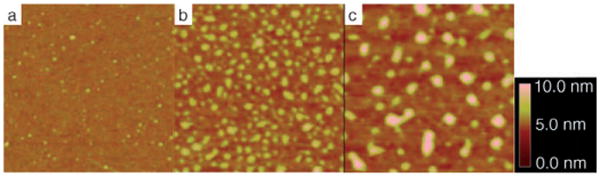
Single polystyrene molecules covalently immobilized on silicon wafers. Monodisperse polystyrene used: a) Mw = 223 200, b) 570000, and c) 1 877000 g mol−1. The wafers were treated with PFPA–silane at concentrations of a) 5×10−5, b) 1×10−5, b) 5×10−6 mg mL−1, respectively. The scan area is 1 ×1 μm2 for all three images.
To confirm that the particles were indeed polymer single molecules, polystyrene of various molecular weights was used. The size of a polymer is directly related to its molecular weight: the higher the molecular weight, the larger the radius of gyration (Rg) and thus the size of the molecule. Indeed, we observed that the size of the particles, that is, the immobilized single polymer molecules, increased with the molecular weight of the polymer (Figure 2).
We found that the higher the molecular weight, the lower the concentration of PFPA–silane was needed to obtain single-molecule immobilization (see Figure 2). This finding is consistent with the immobilization chemistry shown in Scheme 1. As the molecular weight and thus size of the polymer increases, less surface azido groups are needed to attach the polymer. The concentration of PFPA– silane was therefore lowered to observe isolated single molecules.
Atomic force microscopy (AFM) revealed that the immobilized polystyrene adopted a cone shape (Figure 3). The volume of a single polystyrene molecule V can thus be calculated according to Equation (1), where H is the height and D is the diameter of the molecule measured by AFM. Results showed that the measured values (V) are in good agreement with those calculated from the molecular weights of the polymer (Vcalcd; Table 1).[4]
Figure 3.
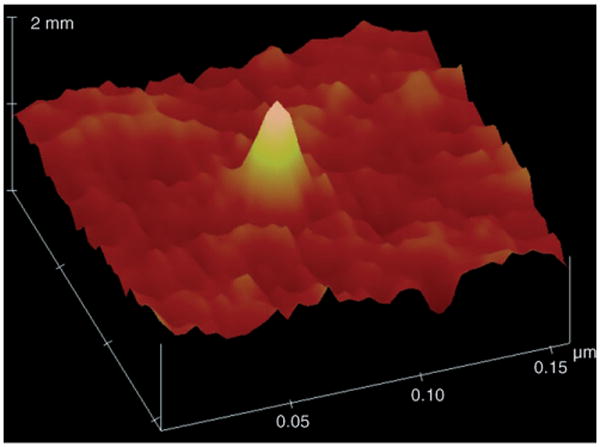
AFM topographic image enlarged from Figure 2 a.
Table 1.
Measured and calculated sizes of single molecules.
| Mw | D [nm] | H [nm] | V [nm3] | Vcalcd [nm3] |
|---|---|---|---|---|
| 223 200 | 32 ± 6[a] | 1.6 ± 0.6[a] | 429 | 370 |
| 570 000 | 50 ± 7[b] | 1.9 ± 0.5[b] | 1243 | 947 |
| 1877 000 | 69 ± 14[c] | 2.7 ± 1.2[c] | 3364 | 3118 |
The D and H values were measured and averaged from
112,
85, and
51 particles from the corresponding images in Figure 2, excluding obvious large aggregates.
| (1) |
The versatility of the method was tested with poly(2-ethyl-oxazoline) (PEOX), a hydrophilic polymer that also lacks reactive functional groups. Following a similar procedure, single molecules of PEOX were successfully immobilized (Figure 4). A much higher concentration of PFPA–silane was needed for PEOX (5 × 10−1 mg mL−1) than for polystyrene (10−5 mg mL−1, Mw = 570 000). This difference can be attributed to the difference in the immobilization efficiency of the two polymers, which is in part governed by the local concentration of the polymer in proximity to the azide-functionalized surface.[14] Evaluation of 62 particles from the AFM image yielded the average particle volume of 1764 nm3 (D = (53 ± 8) nm, H = (2.4 ± 1.1) nm), whereas the calculated molecular volume is 830 nm3 based on the average molecular weight of the sample (Mw = 500 000). Unlike polystyrene samples, which are monodisperse, the PEOX obtained from Aldrich is polydisperse (molecular weight distribution is unknown); a polydisperse polymer is more heterogeneous than its monodisperse counterpart with respect to molecular weight. When a polymer containing various sized molecules is coated on a surface with a limited amount of azido groups, larger molecules, that is, polymers of higher molecule weight, are more likely than smaller molecules to be attached. The molecular volume of the immobilized polymer is thus larger than the theoretical value that is calculated from the average molecular weight of the original sample. This preferential immobilization of larger sized polymers was also observed for polystyrene (Table 1), although the deviations were smaller than PEOX as a result of the monodisperse nature of the polystyrene samples.
Figure 4.
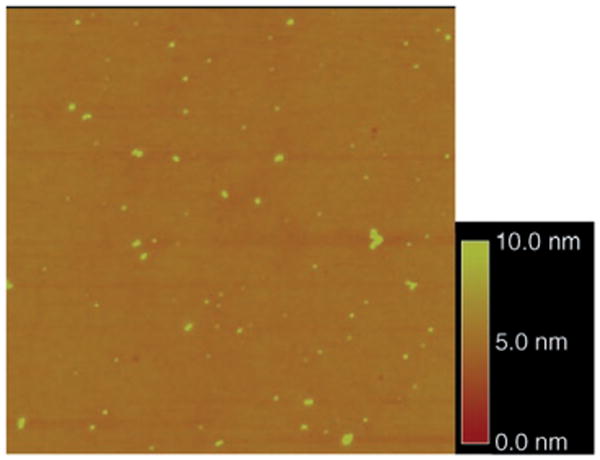
AFM topographic image of immobilized PEOX (Mw = 500000 g mol−1) on a silicon wafer treated with 5×10−1 mg mL−1 of PFPA–silane in toluene. The scan area is 3×3 μm2.
Extended single polymer chains were occasionally observed as shown in Figure 5. The curvilinear length was measured to be 430 nm. This value falls in the range of 95– 953 nm, as calculated from the molecular-weight distribution of the polystyrene sample.[15–16]
Figure 5.
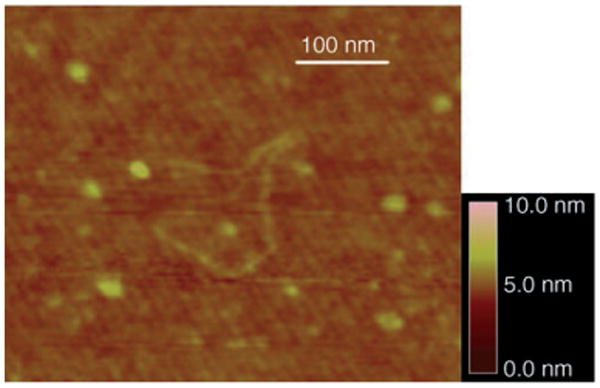
An extended polystyrene molecule (Mw = 223 200 g mol−1).
In summary, a simple and general method has been developed for the covalent immobilization of single polymer molecules on silicon wafers. Because the immobilization is based on C—H/N—H insertion reactions, it is versatile and especially suited for molecules that do not possess functional groups and are difficult to be immobilized by other means. Single-polymer immobilization was also achieved by treating the wafer surface with a mixture of PFPA–silane and a non-photoactive silane. Studies are underway to investigate the nature of the attachment, including the polymer attachment point and orientation.
Supplementary Material
Footnotes
The authors acknowledge the financial support from an NIH AREA award 1R15 GM066279-01A2.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Lee TH, Gonzalez JI, Zheng J, Dickson RM. Acc Chem Res. 2005;38:534–541. doi: 10.1021/ar040146t. [DOI] [PubMed] [Google Scholar]; b) Venkataraman L, Klare JE, Tam IW, Nuckolls C, Hybertsen MS, Steigerwald ML. Nano Lett. 2006;6:458–462. doi: 10.1021/nl052373+. [DOI] [PubMed] [Google Scholar]
- 2.a) Rosenberg SA, Quinlan ME, Forkey JN, Goldman YE. Acc Chem Res. 2005;38:583–593. doi: 10.1021/ar040137k. [DOI] [PubMed] [Google Scholar]; b) Brown FLH. Acc Chem Res. 2006;39:363–373. doi: 10.1021/ar050028l. [DOI] [PubMed] [Google Scholar]; c) Michalet X, Weiss S, Jager M. Chem Rev. 2006;106:1785–1813. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radmacher M, Fritz M, Hansma HG, Hansma PK. Science. 1994;265:1577–1579. doi: 10.1126/science.8079171. [DOI] [PubMed] [Google Scholar]
- 4.Gorodyska G, Kiriy A, Minko S, Tsitsilianis C, Stamm M. Nano Lett. 2003;3:365–368. [Google Scholar]
- 5.Bocharova V, Kiriy A, Stamm M, Stoffelbach F, Jérôme R, Detrembleur C. Small. 2006;2:910–916. doi: 10.1002/smll.200500490. [DOI] [PubMed] [Google Scholar]
- 6.Al-Maawali S, Bemis J, Akhremitchev B, Leecharoen R, Janesko B, Walker G. J Phys Chem B. 2001;105:3965–3971. [Google Scholar]
- 7.Al-Maawali S, Bemis J, Akhremitchev B, Liu H, Walker G. J Adhes. 2005;81:999–1016. [Google Scholar]
- 8.Furukawa K. Acc Chem Res. 2003;36:102–110. doi: 10.1021/ar020072q. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett M, Yan M. Adv Mater. 2001;13:1449–1451. [Google Scholar]
- 10.Yan M, Bartlett M. Nano Lett. 2002;2:275–278. [Google Scholar]
- 11.Yan M, Ren J. Chem Mater. 2004;16:1627–1632. [Google Scholar]
- 12.Borden WT, Gritsan NP, Hadad CM, Karney WL, Kemnitz CR, Platz MS. Acc Chem Res. 2000;33:765–771. doi: 10.1021/ar990030a. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Yan M. unpublished results [Google Scholar]
- 14.Yan M, Ren J. J Mater Chem. 2005;15:523–527. [Google Scholar]
- 15.Kumaki J, Nishikawa Y, Hashimoto T. J Am Chem Soc. 1996;118:3321–3322. [Google Scholar]
- 16.Kumaki J, Hashimoto T. J Am Chem Soc. 2003;125:4907–4917. doi: 10.1021/ja0290429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


