Abstract
Lipids contain hydrocarbons and are the building blocks of cells. Lipids can naturally form themselves into nano-films and nano-structures, micelles, reverse micelles, and liposomes. Micelles or reverse micelles are monolayer structures, whereas liposomes are bilayer structures. Liposomes have been recognized as carriers for drug delivery. Solid lipid nanoparticles and lipoplex (liposome-polycation-DNA complex), also called lipid nanoparticles, are currently used to deliver drugs and genes to ocular tissues. A solid lipid nanoparticle (SLN) is typically spherical, and possesses a solid lipid core matrix that can solubilize lipophilic molecules. The lipid nanoparticle, called the liposome protamine/DNA lipoplex (LPD), is electrostatically assembled from cationic liposomes and an anionic protamine-DNA complex. The LPD nanoparticles contain a highly condensed DNA core surrounded by lipid bilayers. SLNs are extensively used to deliver drugs to the cornea. LPD nanoparticles are used to target the retina. Age-related macular degeneration, retinitis pigmentosa, and diabetic retinopathy are the most common retinal diseases in humans. There have also been promising results achieved recently with LPD nanoparticles to deliver functional genes and micro RNA to treat retinal diseases. Here, we review recent advances in ocular drug and gene delivery employing lipid nanoparticles.
Keywords: gene therapy, non-viral vectors, lipid nanoparticles, solid lipid nanoparticles, liposomes
1. Introduction
The eye is made up of many components, and therapeutic agents could be easily applied to the anterior part of the eye. However, it is difficult to administer these agents to the posterior part of the eye. Intravitreal or subretinal routes are the only means of targeting agents to the posterior area of the eye. The eye is one of the sensory organs of the body, and frequent administration of drugs to the eye is undesirable. Therefore, gene therapy would be an ideal way to provide sustained gene expression that could overcome these limitations. The eyes have been early targets for gene therapy because they are small—that is, they require relatively little active dose—they are self-contained, and because the tools of eye surgery have advanced enough to make these treatments possible. The eye offers an excellent target for gene therapy studies, it is easily accessible and relatively immune privileged. If we inject any drug or gene systemically, the drug or gene must then cross the blood retinal barrier (BRB). To our knowledge, most of the successful gene therapy trials use local administration of drug(s)/gene(s) into the eye.
2. Uses and Advantages of Nanoparticles in Medicine
Nanoparticles play important roles in the diagnosis of disease, delivery of drugs to target tissue, research into the organization of DNA, drug-mediated apoptosis of cancer cells, studies of the pharmacological efficiency of drugs, and tissue engineering. Their size and surface characteristics enable us to alter nanoparticle properties to allow for continuous discharge of drugs during transport and release at a defined location. Choosing the appropriate matrix is vital to drug delivery. Modifying the surface properties of nanoparticles will help to clear the drug from the patient’s body with significantly fewer side effects.
These particles are currently conjugated with either drugs or genes and administered through several avenues, including the oral, nasal, intra-ocular, and arterial routes. Researchers are exploring the use of various polymers to conjugate drugs and genes to enhance therapeutic benefits while minimizing adverse effects.
Nanoparticles for gene therapy are broadly classified into three groups, metal-based nanoparticles, lipid based-nanoparticles, and polymer-based nanoparticles. These particles are different in size, charge, shape, and structure, and have their own modes of delivering cargo into cells and assimilating the cargo into the genetic machinery for gene expression [1,2,3,4]. Compacted DNA nanoparticles formulated with polyethylene glycol-substituted polylysine have been used for gene therapy in mouse models of eye diseases [5,6,7,8]. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have been developed to improve the ocular delivery of acyclovir into excised corneal tissue [9].
A solid lipid nanoparticle is typically spherical, with an average diameter between 10 and 1000 nm, and possesses a solid lipid core matrix that can solubilize lipophilic molecules. The lipid core is stabilized by surfactants; the lipid component may be a triglyceride, diglyceride, monoglyceride, fatty acid, steroid, or wax. The lipid nanoparticle, called the liposome protamine/DNA lipoplex (LPD), is electrostatically assembled from cationic liposomes and an anionic complex of protamine and DNA. The LPD nanoparticles contain a highly condensed DNA core, surrounded by lipid bilayers with an average size of ~100 nm. Lipid nanoparticles have also been used to improve the efficiency of siRNA delivery in RPE cells and a laser-induced rat model for the treatment of choroidal neovascularization [10].
Methazolamide (MTA) is an anti-glaucoma drug; however, systemic administration produces side effects, while providing insufficient ocular therapeutic concentrations [11]. Solid lipid nanoparticles containing MTA have been shown to have higher therapeutic efficacy at low doses with more prolonged effects than those of the drug solution itself [11]. Lipid nanoparticles have also been shown to be feasible for the ocular delivery of anti-inflammatory drugs [12].
Since the 1990s, solid lipid nanoparticles have been examined as potential drug carrier systems. SLNs do not show bio-toxicity, as they are prepared from physiological lipids, and are especially useful in ocular drug delivery, as they enhance the corneal absorption of drugs and improve the ocular bioavailability of both hydrophilic and lipophilic drugs [13]. Cyclosporine is commonly prescribed for chronic dry eye, caused by inflammation, and cyclosporine A-loaded solid lipid nanoparticles have been shown to improve drug efficacy when administered to rabbit eyes [14,15].
Liquid lipid has also been incorporated into lipid nanoparticles to enhance ocular drug delivery [16]. These particles have been tested on human corneal epithelial cell lines and rabbit corneas [16]. The liquid lipid incorporation has been shown to improve the ocular retention and penetration of therapeutics [16]. Surface-modified solid lipid nanoparticles have been shown to provide an efficient way of improving the ocular bioavailability of drugs to bioengineered human corneas [17]. Solid lipid nanoparticles have also been used for retinal gene therapy and to study intracellular trafficking in RPE cells [18]. Solid lipid nanoparticles and lipid nanoparticles have been extensively reviewed and described in detailed in recently published articles [19,20].
The majority of solid lipid nanoparticles have been used to deliver drugs to the cornea [9,13,16,17,21,22]. We recently formulated a novel lipid nanoparticle, and examined its efficiency and delivery of genes and microRNA to the retina [23,24]. LPD has been used to successfully deliver the vascular endothelial growth factor gene into mesenchymal stem cells [25]. In this article, we review several important aspects of lipid nanoparticles, including their formulation, mechanism of internalization, cell-specific expression, and barriers that affect gene expression.
3. Gene Therapy and Viral Vectors
The success of gene therapy relies on the development of efficient, non-toxic gene carriers that can encapsulate and deliver foreign genetic materials into specific cell types [26]. Gene therapy carriers can be classified into two groups, viral and non-viral gene delivery systems. Although viral vectors, such as adeno-associated virus (AAV), have attractive features, particularly their high gene transduction capability, they face biosafety issues, especially innate and immune barriers [27], toxicity [28], and potential recombination of or complementation [29] to vector delivery. The size of viral vectors, which restricts the insertion of genes to <5 kb, is another limitation [30]. Table 1 lists various viral and non-viral carriers. All viral vectors have been used to deliver functional genes to the retina whereas non-viral vectors have been used to deliver both drugs (liposome nanoparticles and solid lipid nanoparticles) and genes (solid lipid nanoparticles, LPD/lipoplexes and CK30-PEG) to the retina.
Table 1.
Viral and non-viral delivery systems for ocular gene delivery.
| Vector | Carrier | Delivery | Ref. |
|---|---|---|---|
| Virus | AAV | Local/systemic | [31,32,33,34,35] |
| Adenovirus | Local | [36] | |
| Baculovirus | Local | [37,38] | |
| Lentivirus | Local | [39] | |
| Non-virus | Liposome nanoparticles | Local | [40,41,42,43] |
| Solid lipid nanoparticles | Local | [9,13,16,17,21,22] | |
| LPD/lipoplexes | Local | [23,24,44] | |
| CK30-PEG | Local | [5,6,7,8] |
Despite rapid advances in gene therapy during the last two decades, major obstacles to clinical applications for human diseases still exist. These impediments include immune response, vector toxicity, and the lack of sustained therapeutic gene expression. Therefore, new strategies are needed to achieve safe and effective gene therapy. The ideal vector should have low antigenic potential, high capacity to accommodate genetic material, high transduction efficiency, controlled and targeted transgene expression, and reasonable expense and safety for both the patients and the environment. These desired features led researchers to focus on non-viral vectors as an alternative to viral vectors.
4. Lipid-Based Nanoparticles
The main constituent of lipid nanoparticles is the liposome. A liposome is a spherical vesicle of a lamellar phase of the lipid bilayer. The liposome can be used as a transport vehicle to send nutrients and drugs into the body [45,46,47]. One can prepare these liposomes through disruption of biological membranes by sonication, a process of sending sound waves to disturb particles in a solution. Lipids can naturally form themselves into nano-films and nano-structures, called micelles, reverse micelles, and liposomes [20,48]. The monolayer structures are called micelles or reverse micelles, whereas the lipid bilayer structures are called liposomes (Figure 1A). In the lipid bilayer, phospholipids are principal lipids, which are amphiphilic molecules with hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties, sometimes described as having hydrophobic tails and hydrophilic heads. Therefore, liposomes are artificial phospholipid bilayers; as a result, liposomes have biocompatible characteristics [49,50]. This biocompatibility accounts for their most important advantages as drug carriers, (1) liposomes have almost no toxicity and low antigenicity; (2) liposomes can be biodegraded and metabolized in vivo, and (3) liposomal properties, such as membrane permeability, can be controlled to some extent [51,52,53]. Remarkably, liposomes can entrap and protect drug molecules or nucleic acids on the journey to the target site [54].
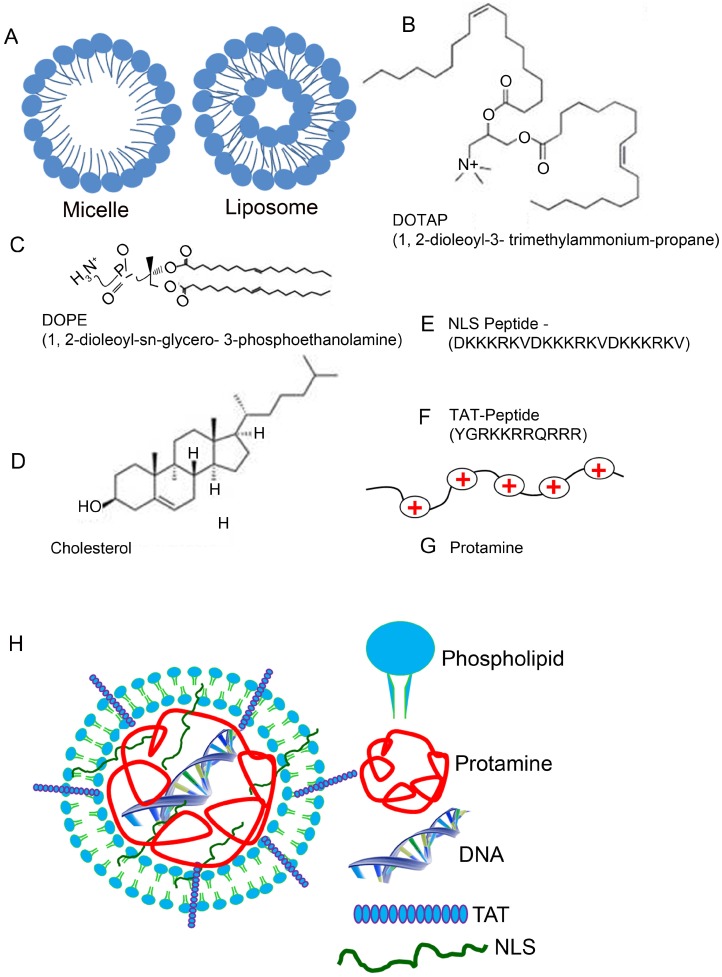
Figure 1.
Lipid, peptide, and protein components of the lipid nanoparticle. The monolayer structures are called micelles, whereas the lipid bilayer structures are called liposomes (A). Chemical structures of DOTAP (B), DOPE (C), and Cholesterol (D). NLS (E) and TAT (F) peptide sequences and protamine (small, arginine-rich, nuclear protein) (G) are also presented. DOTAP, 1, 2-dioleoyl-3-trimethylammonium-propane, DOPE, 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine; NLS, nuclear localization signal; TAT, transactivator of transcription. Formulation of a peptide-based lipid nanoparticle (H). Peptide-based nanoparticles can be formulated by mixing liposome, protamine, DNA, TAT, and NLS. TAT, transactivator of transcription; NLS, nuclear localization signal.
When nucleic acids, molecules, or drugs are enclosed in a lipid-based coating, they have a lower degradation rates than do molecules without a lipid coating. Such enclosure also increases the likelihood of endocytosis and uptake of nucleic acids or drugs into cells [4,20,55]. These desired features led researchers to focus on non-viral vectors as an alternative to viral vectors. The non-viral vectors include polymers like polyethylenimine (PEI) [56] and poly L-lysine (PLL) [57], peptides, liposomes (tiny fat-like particles) [58], and liposomes-protamine-DNA (LPD) complexes [59,60]. However, the current non-viral vectors cannot achieve tissue-specific or cell-specific sustained gene expression, nor eliminate the unwanted and harmful effects on other cells.
The use of lipid nanoparticles as part of a system delivering drugs and genes to the retina has been attempted [44]. We recently developed an artificial virus, an LPD nanoparticle in combination with nuclear localization signaling (NLS) [61] peptide and transactivator of transcription (TAT) peptide [62], to produce efficient, cell-specific gene delivery to eye tissues, with sustained gene expression. The key to our success arises from three unique features, (1) the use of biocompatible lipid molecules to pack DNA and the biocompatible protamine molecules into the nanoparticles; (2) the integration of cell-penetrating and nuclei-targeting peptides into the nanoparticles, to improve the efficiency of gene transfer and the subsequent lasting gene expression; and (3) the use of a DNA that carries the target gene, and also bears a unique promoter to achieve cell-specific gene expression.
5. Composition of Lipid Nanoparticles
Liposomes were first identified in 1965 [63], and were successfully applied as cationic liposome complexes via intravenous DNA delivery into adult mice in 1993. Since then, liposomes have been successful and widely applied in nanotechnology [64] and in various medical fields [23,24,61,65,66]. One approach for more successful nanoparticle gene therapy is the liposome protamine/DNA lipoplex (LPD), which is applied as a two-step packaging technology employing a multilayering method [61,67]. First, the DNA is packaged into a condensed core via electrostatic interactions with protamine, and various peptides (NLS and TAT) and the plasmid DNA (pDNA) are mixed at various weight ratios (Figure 1). Then, the liposomes, consisting of a cationic lipid DOTAP (1,2-dioleoyl-3-trimethylammonium-propane), a neutral “helper” lipid DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) and neutral cholesterol, are added so that the positively charged DOTAP/DOPE/Chol liposome can form a complex with the negative protamine/DNA particles, leading to the formation of LPD nanoparticles (Figure 1B–G). The negatively charged DNA is complexed with protamine, an arginine-rich, positively charged nuclear protein that replaces histone late in the haploid phase of spermatogenesis, and is essential for sperm head condensation and DNA stabilization. The advantage of adding protamine to DNA is that protamine condenses the DNA and the subsequent mixing of the protamine/DNA complex to cationic liposomes, producing a small nanoparticle. Another advantage of protamine is that the encapsulated DNA is protected from nuclease degradation [23,59]. Inclusion of protamine in solid lipid nanoparticles (SLN) has previously been shown to yield a six-fold increase in the transfection of SLN in retinal cells, due to the presence of a nuclear localization signal [68].
6. Transfer Mechanism of LPD Nanoparticles into Cells
Successful gene delivery systems have their own transfer mechanisms into the cell. Viral vectors have the advantage in cellular entry, because they bind to the cellular receptors and co-receptors, which help them to internalize and traffic to the nucleus [69,70,71,72,73]. In contrast, cationic liposomes take advantage of biocompatible characteristics and are widely used to transfect DNA into cells in culture and in vivo, since the formation of cationic lipid-DNA complexes can facilitate the association with the cell membrane and allow the complex to enter the cell through the endocytotic pathway [4,58,74]. The complex is internalized into an endosome, which will destabilize the endosome membrane and result in a flip-flop of anionic lipids that are mainly on the cytoplasmic side of the membrane. The anionic lipids will then diffuse into the complex and form charge-neutralized ion pairs with cationic lipids. This displaces the DNA from the complex and allows DNA to enter into the cytoplasm [4,74,75]. Protamine in the solid lipid nanoparticles has been reported to shift the internalization mechanism from caveolae/raft-mediated to clathrin-mediated endocytosis [68]. Some researchers also proposed that LPD nanoparticles could use two different endocytosis pathways, macropinocytosis and clathrin-mediated endocytosis [58]. In the final analysis, liposomes depend on continually improving the formulation of the nanoparticles’ coating and DNA design to increase the transfection efficiency [76,77]. The mechanisms by which peptide-modified liposome protamine/DNA lipoplex (LPD) nanoparticles improve transfer efficiency is charge-ratio-dependent and dose-dependent in vivo, and these mechanisms provide their own unique approaches to improve transfer efficiency [23,59,61].
7. Cellular Barriers in the Internalization of Lipid Nanoparticles
DNA packed into liposomes must overcome biological barriers before it can be integrated into the genome. These barriers are the cellular membrane, the nuclear membrane, and chromosomal integrity. Cell targeting and cell-internalization peptides have been extensively studied and used for efficient drug delivery and for image analysis [61]. Arginine-rich (RNA-binding, DNA-binding, and polyarginine) cell-permeable peptides have been shown to cross the cellular barrier [62]. Nuclear localization peptide of the SV40 T large antigen has been shown to promote high LPD-mediated transfection efficiency [23,24,61,78]. In designing our recently formulated lipid nanoparticle, we used a nuclear localization peptide derived from SV40 T antigen (DKKKRKVDKKKRKVDKKKRKV), and another peptide derived from human immunodeficiency virus transactivator of transcription (TAT; YGRKKRRQRRR) peptide [79,80,81,82]. The TAT-fusions have been shown to cross the blood–brain barrier [81]. A combination of these two peptides resulted in a high level of sustained gene expression in vivo (Figure 2) [23]. The TAT-peptide belongs to an arginine-rich family of peptides, which is an abundant source of membrane-permeable peptides that have potential as carriers for intracellular protein delivery [54,67]. Even with the omission of TAT-peptide, LPD nanoparticles were able to mediate gene delivery [24].
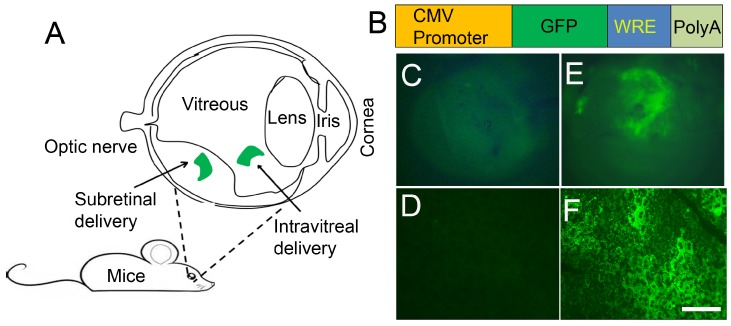
Figure 2.
LPD-mediated gene delivery into the retina. Schematic illustration of the eye and route of administration. The most commonly used and preferred mode of administration to retinal layers is subretinal (A). Generation of green fluorescent protein construct under the control of CMV promoter (B). CMV, cytomegalovirus; GFP, green fluorescent protein; WRE, posttranscriptional regulatory element from the woodchuck hepatitis virus; PolyA, polyadenylation sequence; increases the stability of the molecule. Using BalbC mice, we injected the cDNA construct subretinally into one eye. LPD was complexed with CMV-GFP-WRE-PolyA construct. The other eye was injected with LPD, with a control vector without GFP. Seventy-two hours later, eyes were removed and examined for GFP expression under inverted fluorescence microscopy. GFP expression is clearly seen in the GFP-injected eye (E), but not in the control eye (C). Whole RPE flat mounts were prepared and examined for GFP expression under inverted fluorescence microscopy. GFP expression is seen in the GFP-injected eye (F), but not in the control eye (D). Scale bar, 20 µm.
The cell-penetrating peptides (CPPs) are short peptides that facilitate cellular uptake of various molecular cargo [63,64]. In 1988, the first CPP was sequenced from an HIV-1-encoded cell-penetrating transactivator of transcription (TAT) peptide, and delivered efficiently through cell membranes; TAT has been widely applied since then [83,84,85]. The TAT mechanism of action is still poorly understood, but we do know that this TAT may possess a common internalization mechanism that is ubiquitous to arginine-rich peptides. However, the mechanism is not explained by either adsorptive-mediated endocytosis or by receptor-mediated endocytosis [62,86,87].
8. LPD Nanoparticle-Mediated Delivery of Genes to Eye Tissues
In the eye, the photochemical 11-cis-retinal allows the visual pigment rhodopsin to absorb light in the visible range. Without the photochemical, we lose the ability to see light [88]. Pre-clinical studies with viral vectors demonstrated restoration of vision upon gene transfer into retinal cells in mice and dogs [31,32,33,34]. In clinical trials, three independent groups reported vision improvements upon the viral-mediated delivery of the Rpe65 gene in patients with Rpe65-associated Leber’s congenital amaurosis (LCA) [89,90,91]. A mouse model lacking the Rpe65 gene has been commonly used for gene therapy studies [5,92,93,94].
Retinal pigment epithelium protein 65 (Rpe65) is the key enzyme in regulating the availability of photochemicals; a deficiency in this gene results in a blinding eye disease. We showed for the first time that LPD promotes efficient delivery in a cell specific-manner and long-term expression of the Rpe65 gene in mice lacking Rpe65 protein, leading to in vivo correction of blindness [23]. The efficacy of this method of restoring vision is comparable to AAV [93] and lentiviral [39] gene transfer of the Rpe65 gene to Rpe65 knockout mice. Our recently published data suggest that we successfully applied LPD to deliver miRNA-184 to the retina, to repress Wnt-mediated ischemia-induced neovascularization [24]. Thus, LPD nanoparticles could provide a promising, efficient, non-viral method of gene delivery with clinical applications in the treatment of eye disease.
9. Cell-Specific Delivery of LPD Nanoparticles
One disadvantage of nanoparticles could be cell specificity. Often, delivery and expression of genes in unwanted cells may lead to adverse or off target effects. We recently achieved specificity by cloning the genes under the control of cell-specific promoters [23]. VMD2-promoter specifically targets LPD to RPE cells, whereas rod opsin promoter specifically drives the expression into rod photoreceptor cells [23]. These studies suggest that other retinal cell specific promoters, such as cone opsin (cone), Thy1 (ganglion cell), and glial fibrillary acidic protein (Müller cells) could be used to achieve cell specificity in conjunction with LPD. The cytomegalovirus (CMV) promoter is widely used, due to its ability to induce protein expression in varied cell types [1,23]. Interestingly, our recent study suggests that CMV promoter exclusively drives expression in retinal pigment epithelial cells [23]. These features make lipid nanoparticles ideal for gene or drug delivery to ocular tissues.
10. Conclusions
Many unique genes have been associated with major retinal diseases, such as retinitis pigmentosa (RP), Leber’s congenital amaurosis (LCA), and Stargardt disease [65,69,95]. Until now, Rpe65 defection-induced LCA has been most extensively researched retinal disease. LCA-Rpe65 gene therapy is an example of successful, innovative, translational research. Further studies are needed to determine how retinal gene therapy can be improved [96,97]. The LPD is modified with cell-penetrating peptide and NLS peptide, and carries DNA capable of cell-specific gene expression. Our recent studies suggest that LPD promotes efficient and lasting gene expression in vivo without any corresponding inflammatory response [23].
The LPD system could be a promising non-viral gene delivery vector yielding long-term expression and lasting gene transfer efficiency, making it a favorable gene carrier for future applications for eye cell-based therapies. The advantage is that this system allows us to simultaneously introduce multiple biomolecules to turn on the defective signaling pathway in vivo. Thus far, non-viral vectors have traditionally been acknowledged as safer. However, non-viral vectors present their own difficulties, with low gene expression efficiency and short transient expression. Recently, the peptide-modified liposome protamine/DNA lipoplex (LPD) nanoparticle has demonstrated the potential to overcome these barriers.
Based on the successful gene therapy of Rpe65 in peptide-modified LPD nanoparticles, the optimization of liposome nanoparticle formulations is safe and efficient. Improvements in gene expression are key to the further development of liposomal nanoparticle technology for retinal gene therapy. The development of modified and safe delivery systems to optimize transfection efficiency will be a critical step toward clinical trials for human gene therapy. Thus, these new peptide-modified LPD nanoparticles open avenues to investigate and develop highly efficient liposome nanoparticles that can overcome the shortcomings of other viral vectors in the treatment of ocular diseases.
These peptide-modified LPD have many advantages for future clinical applications. First, liposome nanoparticles are able to deliver large molecular cargo. Second, the optimization of peptide-modified LPD nanoparticles allows multiple mutant genes to be simultaneously co-delivered to one vector. Third, peptide-modified LPD formulations are more biocompatible and safe. On the whole, a successful delivery formulation for gene therapy should encapsulate and protect the nucleic acid materials, escape endosomal degradation, and reach the specific target site. These new peptide-modified LPD nanoparticles offer new hope for gene therapy for ocular and other related diseases.
Acknowledgements
This study was supported by grants from the National Institutes of Health (EY016507, EY00871, and EY021725), bridge funding from the Presbyterian Health Foundation, and unrestricted departmental grants from Research to Prevent Blindness, Inc. The authors acknowledge Kathy J. Kyler, Staff Editor, University of Oklahoma Health Sciences Center, for editing this manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Adijanto J., Naash M.I. Nanoparticle-based technologies for retinal gene therapy. Eur. J. Pharm. Biopharm. 2015 doi: 10.1016/j.ejpb.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuznetsova N.R., Vodovozova E.L. Differential binding of plasma proteins by liposomes loaded with lipophilic prodrugs of methotrexate and melphalan in the bilayer. Biochemistry (Mosc.) 2014;79:797–804. doi: 10.1134/S0006297914080070. [DOI] [PubMed] [Google Scholar]
- 3.Wang F., Liu J. Liposome supported metal oxide nanoparticles, interaction mechanism, light controlled content release, and intracellular delivery. Small. 2014;10:3927–3931. doi: 10.1002/smll.201400850. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y., Szoka F.C., Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 5.Koirala A., Makkia R.S., Conley S.M., Cooper M.J., Naash M.I. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum. Mol. Genet. 2013;22:1632–1642. doi: 10.1093/hmg/ddt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Z., Conley S.M., Makkia R.S., Cooper M.J., Naash M.I. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Invest. 2012;122:3221–3226. doi: 10.1172/JCI64833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe-Rendleman C.L., Durazo S.A., Kompella U.B., Rittenhouse K.D., Di P.A., Weiner A.L., Grossniklaus H.E., Naash M.I., Lewin A.S., Horsager A., et al. Drug and gene delivery to the back of the eye, from bench to bedside. Invest. Ophthalmol. Vis. Sci. 2014;55:2714–2730. doi: 10.1167/iovs.13-13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koirala A., Conley S.M., Makkia R., Liu Z., Cooper M.J., Sparrow J.R., Naash M.I. Persistence of non-viral vector mediated RPE65 expression, case for viability as a gene transfer therapy for RPE-based diseases. J. Control. Release. 2013;172:745–752. doi: 10.1016/j.jconrel.2013.08.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyfoddin A., Al-Kassas R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev. Ind. Pharm. 2013;39:508–519. doi: 10.3109/03639045.2012.665460. [DOI] [PubMed] [Google Scholar]
- 10.Liu H.A., Liu Y.L., Ma Z.Z., Wang J.C., Zhang Q. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Invest. Ophthalmol. Vis. Sci. 2011;52:4789–4794. doi: 10.1167/iovs.10-5891. [DOI] [PubMed] [Google Scholar]
- 11.Li R., Jiang S., Liu D., Bi X., Wang F., Zhang Q., Xu Q. A potential new therapeutic system for glaucoma, solid lipid nanoparticles containing methazolamide. J. Microencapsul. 2011;28:134–141. doi: 10.3109/02652048.2010.539304. [DOI] [PubMed] [Google Scholar]
- 12.Souto E.B., Doktorovova S., Gonzalez-Mira E., Egea M.A., Garcia M.L. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr. Eye Res. 2010;35:537–552. doi: 10.3109/02713681003760168. [DOI] [PubMed] [Google Scholar]
- 13.Seyfoddin A., Shaw J., Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010;17:467–489. doi: 10.3109/10717544.2010.483257. [DOI] [PubMed] [Google Scholar]
- 14.Gokce E.H., Sandri G., Bonferoni M.C., Rossi S., Ferrari F., Guneri T., Caramella C. Cyclosporine A loaded SLNs: Evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008;364:76–86. doi: 10.1016/j.ijpharm.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Gokce E.H., Sandri G., Egrilmez S., Bonferoni M.C., Guneri T., Caramella C. Cyclosporine A-loaded solid lipid nanoparticles, ocular tolerance and in vivo drug release in rabbit eyes. Curr. Eye Res. 2009;34:996–1003. doi: 10.3109/02713680903261405. [DOI] [PubMed] [Google Scholar]
- 16.Shen J., Sun M., Ping Q., Ying Z., Liu W. Incorporation of liquid lipid in lipid nanoparticles for ocular drug delivery enhancement. Nanotechnology. 2010;21 doi: 10.1088/0957-4484/21/2/025101. [DOI] [PubMed] [Google Scholar]
- 17.Attama A.A., Reichl S., Muller-Goymann C.C. Sustained release and permeation of timolol from surface-modified solid lipid nanoparticles through bioengineered human cornea. Curr. Eye Res. 2009;34:698–705. doi: 10.1080/02713680903017500. [DOI] [PubMed] [Google Scholar]
- 18.Del Pozo-Rodriguez A., Delgado D., Solinis M.A., Gascon A.R., Pedraz J.L. Solid lipid nanoparticles for retinal gene therapy, transfection and intracellular trafficking in RPE cells. Int. J. Pharm. 2008;360:177–183. doi: 10.1016/j.ijpharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Montasser I., Shahgaldian P., Perret F., Coleman A.W. Solid lipid nanoparticle-based calix[n]arenes and calix-resorcinarenes as building blocks, synthesis, formulation and characterization. Int. J. Mol. Sci. 2013;14:21899–21942. doi: 10.3390/ijms141121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashaghi S., Jadidi T., Koenderink G., Mashaghi A. Lipid nanotechnology. Int. J. Mol. Sci. 2013;14:4242–4282. doi: 10.3390/ijms14024242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attama A.A., Reichl S., Muller-Goymann C.C. Diclofenac sodium delivery to the eye, in vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int. J. Pharm. 2008;355:307–313. doi: 10.1016/j.ijpharm.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Cavalli R., Gasco M.R., Chetoni P., Burgalassi S., Saettone M.F. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int. J. Pharm. 2002;238:241–245. doi: 10.1016/S0378-5173(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 23.Rajala A., Wang Y., Zhu Y., Ranjo-Bishop M., Ma J.X., Mao C., Rajala R.V. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano. Lett. 2014;14:5257–5263. doi: 10.1021/nl502275s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi Y., Chen Q., Rajala R.V., Ma J. Micro RNA-184 modulates cannocial Wnt signaling through regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015;589:1143–1149. doi: 10.1016/j.febslet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao B., Qiu P., Mao C. Mesoporous iron oxide nanoparticles prepared by polyacrylic acid etching and their application in gene delivery to mesenchymal stem cells. Microsc. Res. Tech. 2013;76:936–941. doi: 10.1002/jemt.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulligan R.C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 27.Herz J., Gerard R.D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci USA. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon R.H., Engelhardt J.F., Yang Y., Zepeda M., Weber-Pendleton S., Grossman M., Wilson J.M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates, toxicity study. Hum. Gene Ther. 1993;4:771–780. doi: 10.1089/hum.1993.4.6-771. [DOI] [PubMed] [Google Scholar]
- 29.Ali M., Lemoine N.R., Ring C.J. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1994;1:367–384. [PubMed] [Google Scholar]
- 30.Wu Z., Yang H., Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acland G.M., Aguirre G.D., Ray J., Zhang Q., Aleman T.S., Cideciyan A.V., Pearce-Kelling S.E., Anand V., Zeng Y., Maguire A.M., et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Li W., Dai X., Kong F., Zheng Q., Zhou X., Lu F., Chang B., Rohrer B., Hauswirth W.W., et al. Gene therapy rescues cone structure and function in the 3-month-old rd12 mouse, a model for midcourse RPE65 leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2011;52:7–15. doi: 10.1167/iovs.10-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beltran W.A., Cideciyan A.V., Lewin A.S., Iwabe S., Khanna H., Sumaroka A., Chiodo V.A., Fajardo D.S., Roman A.J., Deng W.T., et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl. Acad. Sci USA. 2012;109:2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cepko C.L. Emerging gene therapies for retinal degenerations. J. Neurosci. 2012;32:6415–6420. doi: 10.1523/JNEUROSCI.0295-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrne L.C., Dalkara D., Luna G., Fisher S.K., Clerin E., Sahel J.A., Leveillard T., Flannery J.G. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J. Clin. Invest. 2015;125:105–116. doi: 10.1172/JCI65654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park K. Cornea-targeted gene therapy using adenovirus vector. J. Control. Release. 2014;181 doi: 10.1016/j.jconrel.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 37.Kaikkonen M.U., Yla-Herttuala S., Airenne K.J. How to avoid complement attack in baculovirus-mediated gene delivery. J. Invertebr. Pathol. 2011;107(Suppl):S71–S79. doi: 10.1016/j.jip.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Luz-Madrigal A., Clapp C., Aranda J., Vaca L. In vivo transcriptional targeting into the retinal vasculature using recombinant baculovirus carrying the human flt-1 promoter. Virol. J. 2007;4 doi: 10.1186/1743-422X-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bemelmans A.P., Kostic C., Crippa S.V., Hauswirth W.W., Lem J., Munier F.L., Seeliger M.W., Wenzel A., Arsenijevic Y. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS. Med. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vadlapudi A.D., Mitra A.K. Nanomicelles, an emerging platform for drug delivery to the eye. Ther. Deliv. 2013;4:1–3. doi: 10.4155/tde.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vadlapudi A.D., Vadlapatla R.K., Earla R., Sirimulla S., Bailey J.B., Pal D., Mitra A.K. Novel biotinylated lipid prodrugs of acyclovir for the treatment of herpetic keratitis (HK), transporter recognition, tissue stability and antiviral activity. Pharm. Res. 2013;30:2063–2076. doi: 10.1007/s11095-013-1059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaishya R.D., Gokulgandhi M., Patel S., Minocha M., Mitra A.K. Novel dexamethasone-loaded nanomicelles for the intermediate and posterior segment uveitis. AAPS PharmSciTech. 2014;15:1238–1251. doi: 10.1208/s12249-014-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaudana R., Ananthula H.K., Parenky A., Mitra A.K. Ocular drug delivery. AAPS. J. 2010;12:348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Pozo-Rodriguez A., Delgado D., Gascon A.R., Solinis M.A. Lipid nanoparticles as drug/gene delivery systems to the retina. J. Ocul. Pharmacol. Ther. 2013;29:173–188. doi: 10.1089/jop.2012.0128. [DOI] [PubMed] [Google Scholar]
- 45.Paphadjopoulos D., Wilson T., Taber R. Liposomes as vehicles for cellular incorporation of biologically active macromolecules. In Vitro. 1980;16:49–54. doi: 10.1007/BF02618199. [DOI] [PubMed] [Google Scholar]
- 46.Sessa G., Weissmann G. Phospholipid spherules (liposomes) as a model for biological membranes. J. Lipid Res. 1968;9:310–318. [PubMed] [Google Scholar]
- 47.Torchilin V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Hamley I.W. Nanotechnology with soft materials. Angew. Chem Int. Ed. Engl. 2003;42:1692–1712. doi: 10.1002/anie.200200546. [DOI] [PubMed] [Google Scholar]
- 49.Jain P.K., Huang X., El-Sayed I.H., El-Sayed M.A. Noble metals on the nanoscale, optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 50.Immordino M.L., Dosio F., Cattel L. Stealth liposomes, review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 51.Honda M., Asai T., Oku N., Araki Y., Tanaka M., Ebihara N. Liposomes and nanotechnology in drug development, focus on ocular targets. Int. J. Nanomed. 2013;8:495–503. doi: 10.2147/IJN.S30725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez-Berestein G., Mehta R., Hopfer R., Mehta K., Hersh E.M., Juliano R. Effects of sterols on the therapeutic efficacy of liposomal amphotericin B in murine candidiasis. Cancer Drug Deliv. 1983;1:37–42. doi: 10.1089/cdd.1983.1.37. [DOI] [PubMed] [Google Scholar]
- 53.Van Rooijen N., van Nieuwmegen R. Liposomes in immunology, multilamellar phosphatidylcholine liposomes as a simple, biodegradable and harmless adjuvant without any immunogenic activity of its own. Immunol. Commun. 1980;9:243–256. doi: 10.3109/08820138009065997. [DOI] [PubMed] [Google Scholar]
- 54.Schnyder A., Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2:99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberle V., Bakowsky U., Zuhorn I.S., Hoekstra D. Lipoplex formation under equilibrium conditions reveals a three-step mechanism. Biophys. J. 2000;79:1447–1454. doi: 10.1016/S0006-3495(00)76396-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bragonzi A., Dina G., Villa A., Calori G., Biffi A., Bordignon C., Assael B.M., Conese M. Biodistribution and transgene expression with nonviral cationic vector/DNA complexes in the lungs. Gene Ther. 2000;7:1753–1760. doi: 10.1038/sj.gt.3301282. [DOI] [PubMed] [Google Scholar]
- 57.Kollen W.J., Mulberg A.E., Wei X., Sugita M., Raghuram V., Wang J., Foskett J.K., Glick M.C., Scanlin T.F. High-efficiency transfer of cystic fibrosis transmembrane conductance regulator cDNA into cystic fibrosis airway cells in culture using lactosylated polylysine as a vector. Hum. Gene Ther. 1999;10:615–622. doi: 10.1089/10430349950018689. [DOI] [PubMed] [Google Scholar]
- 58.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J. Control. Release. 2004;94:1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Li S., Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 60.Li S., Rizzo M.A., Bhattacharya S., Huang L. Characterization of cationic lipid-protamine-DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5:930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 61.Ma K., Wang D.D., Lin Y., Wang J., Petrenko V., Mao C. Synergetic Targeted Delivery of Sleeping-Beauty Transposon System to Mesenchymal Stem Cells Using LPD Nanoparticles Modified with a Phage-Displayed Targeting Peptide. Adv. Funct. Mater. 2013;23:1172–1181. doi: 10.1002/adfm.201102963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Futaki S., Suzuki T., Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 63.Bangham A.D., Standish M.M., Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 64.Zhu N., Liggitt D., Liu Y., Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]
- 65.Sundaram V., Moore A.T., Ali R.R., Bainbridge J.W. Retinal dystrophies and gene therapy. Eur. J. Pediatr. 2012;171:757–765. doi: 10.1007/s00431-011-1615-2. [DOI] [PubMed] [Google Scholar]
- 66.Mao Y., Triantafillou G., Hertlein E., Towns W., Stefanovski M., Mo X., Jarjoura D., Phelps M., Marcucci G., Lee L.J., et al. Milatuzumab-conjugated liposomes as targeted dexamethasone carriers for therapeutic delivery in CD74+ B-cell malignancies. Clin. Cancer Res. 2013;19:347–356. doi: 10.1158/1078-0432.CCR-12-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gandra N., Wang D.D., Zhu Y., Mao C. Virus-mimetic cytoplasm-cleavable magnetic/silica nanoclusters for enhanced gene delivery to mesenchymal stem cells. Angew. Chem Int. Ed. Engl. 2013;52:11278–11281. doi: 10.1002/anie.201301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delgado D., del Pozo-Rodriguez A., Solinis M.A., Rodriguez-Gascon A. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection, the importance of the entry pathway. Eur. J. Pharm. Biopharm. 2011;79:495–502. doi: 10.1016/j.ejpb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Han Z., Conley S.M., Naash M.I. AAV and compacted DNA nanoparticles for the treatment of retinal disorders, challenges and future prospects. Invest. Ophthalmol. Vis. Sci. 2011;52:3051–3059. doi: 10.1167/iovs.10-6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Day T.P., Byrne L.C., Schaffer D.V., Flannery J.G. Advances in AAV vector development for gene therapy in the retina. Adv. Exp. Med. Biol. 2014;801:687–693. doi: 10.1007/978-1-4614-3209-8_86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koirala A., Conley S.M., Naash M.I. A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium. Biomaterials. 2013;34:7158–7167. doi: 10.1016/j.biomaterials.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Z., Conley S.M., Makkia R., Guo J., Cooper M.J., Naash M.I. Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS. On. 2012;7:e52189. doi: 10.1371/journal.pone.0052189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powell S.K., Rivera-Soto R., Gray S.J. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov. Med. 2015;19:49–57. [PMC free article] [PubMed] [Google Scholar]
- 74.Pozzi D., Marchini C., Cardarelli F., Salomone F., Coppola S., Montani M., Zabaleta M.E., Digman M.A., Gratton E., Colapicchioni V., et al. Mechanistic evaluation of the transfection barriers involved in lipid-mediated gene delivery, interplay between nanostructure and composition. Biochim. Biophys. Acta. 2014;1838:957–967. doi: 10.1016/j.bbamem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conley S.M., Naash M.I. Nanoparticles for retinal gene therapy. Prog. Retin. Eye Res. 2010;29:376–397. doi: 10.1016/j.preteyeres.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torchilin V.P., Rammohan R., Weissig V., Levchenko T.S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Felgner P.L., Gadek T.R., Holm M., Roman R., Chan H.W., Wenz M., Northrop J.P., Ringold G.M., Danielsen M. Lipofection, a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoare M., Greiser U., Schu S., Mashayekhi K., Aydogan E., Murphy M., Barry F., Ritter T., O’Brien T. Enhanced lipoplex-mediated gene expression in mesenchymal stem cells using reiterated nuclear localization sequence peptides. J. Gene Med. 2010;12:207–218. doi: 10.1002/jgm.1426. [DOI] [PubMed] [Google Scholar]
- 79.Becker-Hapak M., McAllister S.S., Dowdy S.F. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 80.Gump J.M., Dowdy S.F. TAT transduction, the molecular mechanism and therapeutic prospects. Trends Mol. Med. 2007;13:443–448. doi: 10.1016/j.molmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Schwarze S.R., Ho A., Vocero-Akbani A., Dowdy S.F. In vivo protein transduction, delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 82.Schwarze S.R., Dowdy S.F. In vivo protein transduction, intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol. Sci. 2000;21:45–48. doi: 10.1016/S0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 83.Farkhani S.M., Valizadeh A., Karami H., Mohammadi S., Sohrabi N., Badrzadeh F. Cell penetrating peptides, efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides. 2014;57:78–94. doi: 10.1016/j.peptides.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Green M., Loewenstein P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 85.Green M., Ishino M., Loewenstein P.M. Mutational analysis of HIV-1 Tat minimal domain peptides, identification of trans-dominant mutants that suppress HIV-LTR-driven gene expression. Cell. 1989;58:215–223. doi: 10.1016/0092-8674(89)90417-0. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki T., Futaki S., Niwa M., Tanaka S., Ueda K., Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 87.Peng L.H., Niu J., Zhang C.Z., Yu W., Wu J.H., Shan Y.H., Wang X.R., Shen Y.Q., Mao Z.W., Liang W.Q., et al. TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials. 2014;35:5605–5618. doi: 10.1016/j.biomaterials.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 88.Redmond T.M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J.X., Crouch R.K., Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 89.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M., et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A., Wilson J.M., et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 92.Bemelmans A.P., Kostic C., Hornfeld D., Jaquet M., Crippa S.V., Hauswirth W.W., Lem J., Wang Z., Schorderet D.E., Munier F.L., et al. Lentiviral vectors containing a retinal pigment epithelium specific promoter for leber congenital amaurosis gene therapy. Lentiviral gene therapy for LCA. Adv. Exp. Med. Biol. 2006;572:247–253. doi: 10.1007/0-387-32442-9_35. [DOI] [PubMed] [Google Scholar]
- 93.Lai C.M., Yu M.J., Brankov M., Barnett N.L., Zhou X., Redmond T.M., Narfstrom K., Rakoczy P.E. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet. Vaccines. Ther. 2004;2 doi: 10.1186/1479-0556-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Moiseyev G., Takahashi Y., Ma J.X. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest. Ophthalmol. Vis. Sci. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 95.Ali R.R. Gene therapy for retinal dystrophies, twenty years in the making. Hum. Gene Ther. 2012;23:337–339. doi: 10.1089/hum.2012.2506. [DOI] [PubMed] [Google Scholar]
- 96.Stein L., Roy K., Lei L., Kaushal S. Clinical gene therapy for the treatment of RPE65-associated Leber congenital amaurosis. Expert. Opin. Biol. Ther. 2011;11:429–439. doi: 10.1517/14712598.2011.557358. [DOI] [PubMed] [Google Scholar]
- 97.Mowat F.M., Breuwer A.R., Bartoe J.T., Annear M.J., Zhang Z., Smith A.J., Bainbridge J.W., Petersen-Jones S.M., Ali R.R. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther. 2013;20:545–555. doi: 10.1038/gt.2012.63. [DOI] [PubMed] [Google Scholar]