Abstract
Voltage-dependent calcium channels (VDCCs) couple neuronal activity to diverse intracellular signals with exquisite spatiotemporal specificity. Using calcium imaging and electrophysiology, Jones and Stuart (J Neurosci 33: 19396–19405, 2013) examined the intimate relationship between distinct types of VDCCs and small-conductance calcium-activated potassium (SK) channels that contribute to the compartmentalized control of excitability in the soma and dendrites of cortical pyramidal neurons. Here we discuss the importance of calcium domains for signal specificity, explore the possible functions and mechanisms for local control of SK channels, and highlight technical considerations for the optical detection of calcium signals.
Keywords: SK channel, calcium domains, calcium buffering, neuronal excitability
calcium is an indispensible signaling molecule that controls a wide range of neuronal processes, including neurotransmitter release, gene expression, and synaptic plasticity. It is well established that calcium selectively interacts with diverse effector proteins, such as kinases, phosphatases, transmembrane sensors, and ion channels. For such interactions to be specific and reliable, calcium signals must be spatiotemporally restricted. Indeed, effector proteins commonly reside within tens to hundreds of nanometers of the calcium source in so-called nano- and microdomains where activation by a brief local calcium signal is optimal. One example of tight coupling between a calcium-sensing protein and calcium source are calcium-activated potassium (KCa) channels. The close association of depolarization-dependent calcium influx through VDCCs and activation of hyperpolarizing potassium conductances allows KCa channels to control excitability, action potential waveform, and spike patterns in many types of neurons (Faber 2010; Swensen and Bean 2003; Wolfart and Roeper 2002; Womack and Khodakhah 2003). Recent evidence also suggests that KCa channels locally attenuate excitability and regulate synaptic integration in dendritic spines of hippocampal pyramidal neurons during calcium influx through glutamate receptors and VDCCs (Bloodgood and Sabatini 2007; Cai et al. 2004; Ngo-Anh et al. 2005; Wang et al. 2014). These findings suggest that KCa channels are expressed in various neuronal compartments; however, the conditions under which they are activated, the coupling specificity to their calcium source, and their intraneuronal distribution remain incompletely understood.
Tremendous advances in the high-speed and high-resolution optical detection of calcium signals have enabled accurate estimates of their concentration and time course, even in small subcellular compartments such as synaptic terminals, dendrites, and spines (Denk et al. 1996; Higley and Sabatini 2008). These technical advances have contributed to a better understanding of local calcium-dependent processes such as transmitter release, synaptic integration, and activation of signaling cascades (Brenowitz and Regehr 2003; Neher and Taschenberger 2013; Yasuda et al. 2006). In a recent report in The Journal of Neuroscience, Jones and Stuart (2013) use a combination of confocal calcium imaging, electrophysiology, and pharmacology to provide novel insight into the differential activation of somatic and dendritic SK channels and their respective calcium sources. The authors demonstrate that SK channels in layer V cortical pyramidal neurons engage distinct calcium sources with varying coupling regimes depending on the location in the cell. This study thus provides some mechanistic detail of how activity-dependent calcium signals globally and locally control excitability and stimulates speculation on the distinct functional roles of SK channels within a neuron.
The authors build on classic work that demonstrated sodium channel-dependent backpropagation of somatically generated action potentials along the entire length of hippocampal pyramidal neuron dendrites (Magee and Johnston 1995). Subsequent studies showed that backpropagating action potentials (bAPs) provide sufficient depolarization to activate dendritic VDCCs in many neuron types, including layer V cortical pyramidal neurons (Nevian et al. 2007; Waters et al. 2005). Based on earlier findings (Bloodgood and Sabatini 2007), the authors propose that bAP-dependent calcium signals could activate dendritic SK channels to locally control excitability. To test this hypothesis, layer V pyramidal neurons were filled with the calcium indicator Oregon Green BAPTA-1 (OGB-1) via patch pipette (Fig. 1A1, Jones and Stuart 2013), allowing for the visual identification of basal dendrite branches and spines and subsequent detection of calcium transients. They then evoked somatic action potentials while performing confocal line scans through the dendritic shaft and spine heads at varying distances from the soma (Fig. 1, A2 and A3, Jones and Stuart) to record calcium-dependent increases in fluorescence (ΔF/F) upon invasion of bAPs. To investigate the effect of SK channel blockade selectively in dendrites and spines, they locally applied the SK channel antagonist apamin and found an enhancement of both spine and dendritic calcium transients, indicating that SK channels curtail calcium influx in these compartments during neuronal firing (Fig. 1, B–D, Jones and Stuart). Similarly, previous studies in hippocampal pyramidal neurons observed that SK channels limit calcium influx through NMDA receptors during synaptic activation of spines (Bloodgood and Sabatini 2007). The current report by Jones and Stuart makes the additional advance of demonstrating that bAP-dependent calcium influx triggers SK channel activation in dendrites and reveals an activation gradient along the length of the dendrite and associated spines, with the highest levels occurring at most distal locations (Fig. 1, E and F, Jones and Stuart). The most parsimonious explanation for this observation is the graded expression of SK channels themselves. However, alternate mechanisms are conceivable. For example, a somatodendritic expression gradient of VDCCs could also produce graded SK channel activation, which Jones and Stuart briefly discuss. Another mechanism could involve a tightening of coupling distance between SK channels and VDCCs along dendrites, thereby increasing efficiency of SK channel activation. Finally, a non-uniform distribution of resting conductances and a resulting membrane potential gradient along the basal dendrite could account for graded SK channel activation. At rest, Ih is a hyperpolarization-activated inward current that could depolarize distal dendrites of pyramidal cells (Biel et al. 2009). Consistent with this notion, Ih increases with distance from the soma in apical dendrites of layer V pyramidal neurons (Berger et al. 2001; Stuart and Spruston 1998) as well as CA1 pyramidal neurons (Bullis et al. 2007). Whereas SK channels are voltage independent and inhibition of Ih has little effect on bAPs in basal dendrites (Acker and Antic 2009), Ih could promote activation of distal low-threshold R-type channels by depolarizing the dendritic resting potential closer to activation threshold, even during strong attenuation of bAP amplitude, resulting in more efficient activation of SK channels. Ih is also present along basal dendrites of layer V pyramidal neurons (Branco and Hausser 2011), but a gradient has yet to be described. Two lines of evidence favor the simplest explanation: a gradient of SK channels. First, histological studies have found strongest SK immunoreactivity in distal dendrites of cortical layer V pyramidal neurons (Sailer et al. 2004), which are the same type of neuron studied by Jones and Stuart. Second, quantitative mapping using atomic force microscopy in hippocampal pyramidal neurons demonstrated the highest densities of SK channels in distal dendrites of (Maciaszek et al. 2012). However, both studies focused on apical dendrites, and only the first examined cortical layer V pyramidal neurons. It would therefore be interesting to perform similar anatomic studies in the basal dendrites of layer V pyramidal neurons, in which Jones and Stuart observed a functional SK channel gradient. Third, calcium imaging and pharmacology in hippocampal pyramidal neurons suggests relatively uniform distribution of calcium channels but increasing expression of other, voltage-gated potassium channels mediating IA with increasing somatic distance (Hoffman et al. 1997). However, channel composition varies widely between different types of neurons and dendritic locations, disallowing a definite conclusion. Therefore, further experiments are required to determine the mechanism underlying graded SK channel activation in layer V neurons. Immunohistochemistry, or for better spatial resolution, array tomography and electron microscopy combined with immunogold labeling, could be used to examine the distribution of SK channels. In the future, photocaged and fluorescently labeled inhibitors of SK channels and VDCCs may allow for spatially restricted inhibition of these ion channels and the detection of their distribution, respectively, but such elegant strategies are still limited to a small number of ion channel subtypes (Schmidt et al. 2014; Williams et al. 2012; Zayat et al. 2003).
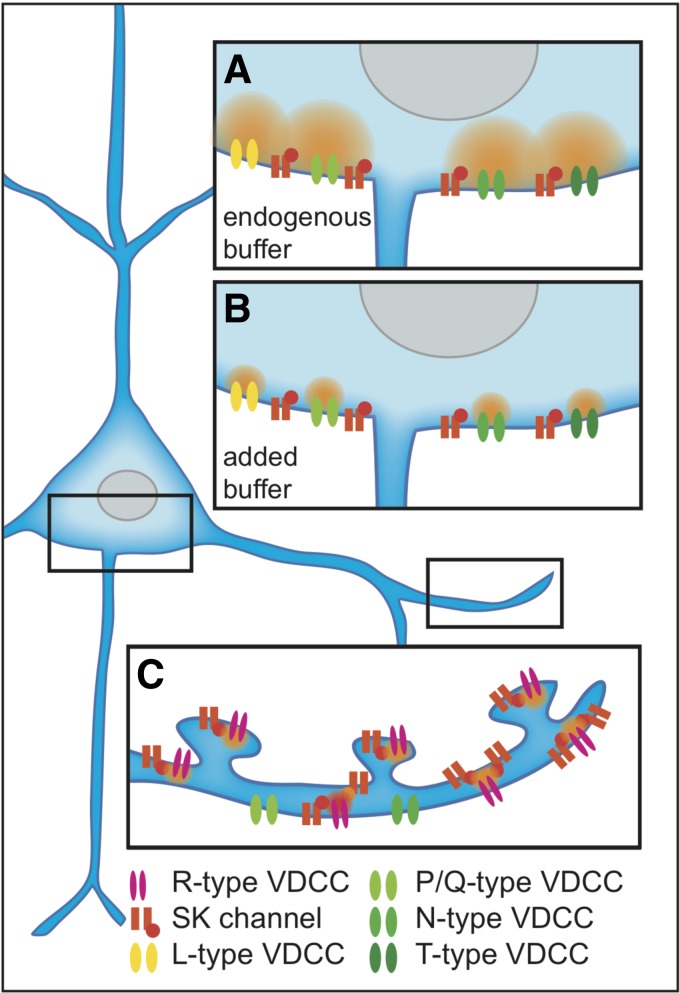
Fig. 1.
Schematic shows a layer V pyramidal neuron. Black boxes indicate somatic and dendritic compartments. A: enlarged somatic compartment containing multiple types of voltage-dependent calcium channels (VDCCs) that provide calcium (orange cloud) to small-conductance calcium-activated potassium (SK) channels in microdomain vicinity when endogenous buffer capacity is low. B: same somatic compartment but in the presence of exogenous buffer. The high-affinity buffer constrains the calcium domains, preventing SK channel activation. C: enlarged basal dendrite compartment containing R-type calcium channels in nanodomain proximity to SK channels. Because of the nanodomain relationship of R-type calcium channels and SK channels, exogenous buffer does not interfere with SK channel activation.
In the next set of experiments, the authors seek to determine the calcium source activating dendritic SK channels. They find that inhibition of R-type calcium channels occludes the effect of apamin, suggesting that they directly couple to the calcium-binding domains of SK channels (Fig. 2, Jones and Stuart). Consistent with this observation, specific coupling of SK channels to dendritic R-type calcium channels has been demonstrated elsewhere (Bloodgood and Sabatini 2007), suggesting that this coupling scheme may be used in many types of neurons, although other calcium sources for SK channels have been described as well (Marrion and Tavalin 1998; Ngo-Anh et al. 2005; Wang et al. 2014; Wolfart and Roeper 2002). The dominant role of R-type channels in activating SK channels in dendrites may be advantageous for two reasons. First, the gating properties of R-type channels allow optimal responsiveness to attenuated depolarization provided by bAPs (Nevian et al. 2007; Stuart and Sakmann 1994) and subthreshold depolarizations from synaptic input (Ngo-Anh et al. 2005). R-type channels exhibit a relatively negative activation threshold among high-voltage-activated calcium channels and deactivate more slowly (Li et al. 2007), which maximizes calcium influx even when VDCC density is low. Second, bAPs width is known to increase during propagation toward the distal dendrites, and this slower AP waveform more efficiently opens R-type calcium channels (Li et al. 2007).
The report by Jones and Stuart (2013) also provides information about the coupling distance between SK and R-type channels. The observation that the high-affinity calcium indicator OGB-1, which acts as a fast calcium buffer, did not disrupt coupling between R-type and SK channels suggests that the coupling distance is in the range of tens of nanometers (Adler et al. 1991; Eggermann et al. 2012). Although N- and P/Q-type VDCCs contribute significantly to distal calcium signals, these channels are incapable of activating SK channels. Additionally, calcium released from intracellular stores fails to activate dendritic SK channels (Fig. 4, Jones and Stuart). These results demonstrate that the general increase of the intracellular calcium concentration is insufficient to activate SK channels in spines and dendrites. Rather, exclusively R-type channels in nanodomain proximity take a privileged role in the activation of SK channels (Fig. 3, Jones and Stuart; Fig. 1C of this Neuro Forum). These results are in line with previous reports of nanodomain coupling between calcium source and SK channels in dendrites and spines (Cai et al. 2004; Ngo-Anh et al. 2005) but counter to other studies that concluded that the high-affinity calcium sensor calmodulin can mediate SK channel activation even in relatively loose microdomain coupling regimes (Fakler and Adelman 2008), similar to the loose coupling Jones and Stuart observed at the soma, where they found that both the rapid calcium buffer OGB-1 and the slow buffer EGTA (Fig. 5, A–D, Jones and Stuart) occlude the effect of SK channel inhibition on the SK channel-mediated medium afterhyperpolarization (mAHP) following the AP (Fig. 1B of this Neuro Forum). At the soma, inhibitors of L-type, P/Q-type, N-type, and T-type, but not R-type, all decreased the mAHP, and a VDCC inhibitor cocktail occluded SK-channel blockade entirely (Fig. 6, Jones and Stuart). Together, these findings suggest that promiscuous microdomain coupling of a wide range of VDCCs promotes AP-dependent activation of somatic SK channels in layer V pyramidal cells (Fig. 1A of this Neuro Forum). Whereas in dendrites extremely tight coupling of SK channels to optimally gated R-type channels may ensure reliable activation when depolarizations are small and channel densities are low, at the soma loose coupling is sufficient to control SK channels due to strong depolarizations during spiking and the abundance of calcium sources. The differential coupling of somatic and dendritic SK channels could be the consequence of different SK channel subtypes present in somatic and the dendritic compartments. Indeed, Sailer et al. (2004) demonstrated that SK2 channels concentrate in the soma of layer V pyramidal neurons, whereas SK1 primarily localizes to distal dendrites. Further experiments will have to test whether different SK channel subtypes couple to different VDCCs with varying tightness of coupling. Although technically challenging, it would also be interesting to quantify the number of VDCCs that signal to individual somatic versus dendritic SK channels, similar to what has been shown for release of synaptic vesicles (Bucurenciu et al. 2010).
Importantly, the work of Jones and Stuart (2013) demonstrates that perturbations of cellular calcium dynamics by introducing exogenous calcium binding molecules can significantly alter intrinsic properties of neurons. This is particularly evident with the use of OGB-1 in calcium imaging experiments (Fig. 5, Jones and Stuart). The authors likely chose this high-affinity calcium indicator because of its excellent signal-to-noise ratio. However, the use of high-affinity indicators can be problematic because they can significantly buffer free calcium ions, constrain calcium domains, and decouple a calcium source from its downstream targets. We estimate that adding 200 μM OGB-1 to these cells roughly quadruples their endogenous buffer capacity κ (number of bound calcium ions to free calcium ions, Higley and Sabatini 2008; equilibrium binding constant KB = 170 nM). This will dominate the neurons' relatively low endogenous calcium buffer capacity of ∼100 (Helmchen et al. 1996). Such overbuffering can slow the time course, decrease the amplitude, and increase the spatial spread of calcium (Higley and Sabatini 2008). This, together with the dye's extremely slow calcium off rate (koff ∼140 s−1, Schmidt and Eilers 2009; compare to koff of OGB-5N: ∼5,600 s−1, DiGregorio et al. 1999) may be responsible for the very slow decay time constants of calcium transients shown in this report (Figs. 1–4 and 7, Jones and Stuart), disallowing any conclusions about the true time course and amplitude of the calcium signal.
The authors carefully replicate the key experiment in their Fig. 1F with the low-affinity calcium indicator OGB-6F (KD = 3 μM) and find that the gradient of SK channel activation is unchanged. This indicates that OGB-1 does not alter SK channel activation along the dendritic shaft. Nevertheless, the study raises the more general issue that any added buffer can disrupt coupling of calcium to its effector molecules. Similar buffering effects can also occur during the long-term expression of genetically encoded calcium indicators (GECIs) that has become a ubiquitous technique to probe neuronal function in vitro and in vivo. The most prevalent GECIs, the GCaMPs, have multiple calcium binding sites and an affinity for calcium of ∼400 nM (Akerboom et al. 2012). When expressed at high concentrations, overbuffering of calcium could alter the coupling of calcium ions to molecules that are crucial for the function of SK channels and many others. Acutely, GECI overexpression could lead to increased excitability and steeper input-output functions in neurons, as is seen when OGB-1 or EGTA is included in the patch pipette in this study (Fig. 5, Jones and Stuart). Additionally, altered buffering of calcium can disrupt many more processes, including neurotransmitter release (Bucurenciu et al. 2008), synaptic plasticity (Caillard et al. 2000; Matveev et al. 2006), and gene transcription (West et al. 2001). New GECIs with fewer calcium binding sites (Thestrup et al. 2014) may allow for less alteration of intracellular calcium dynamics, and therefore preserved coupling of calcium to its effectors.
It is accepted that dendrites form distinct functional compartments that differ in many aspects and thus differentially contribute to local processing and somatic action potential firing (Branco and Hausser 2010; Spruston 2008). This functional heterogeneity is thought to underlie a complex interplay of dendrite architecture (Rall 1962), recruitment of nonlinear conductances (Branco and Hausser 2011), ion channel distribution (Lorincz et al. 2002; Mathews et al. 2010), and differences in synaptic input (Magee and Cook 2000; Petreanu et al. 2007; Somogyi et al. 1998). Jones and Stuart (2013) extend our understanding of compartmentalized regulation of neuronal excitability in layer V pyramidal neurons by introducing the graded activation of SK channels by bAPs along basal dendrites. What function could enhanced distal SK channel activation serve? It has previously been shown that bAP-mediated depolarization relieves the magnesium block of NMDA receptors, allowing incoming synaptic signals to undergo synaptic plasticity in a classic Hebbian scheme of coincidence detection (Magee and Johnston 1997). It has also been suggested that recruitment of NMDA receptors, together with other voltage-gated conductances, boosts distal dendritic excitatory postsynaptic potentials (EPSPs) in order to mitigate the effects of dendritic filtering (Branco and Hausser 2011; Nevian et al. 2007) and enhance their influence on somatic spiking. During coincident somatic activity and synaptic input, it is thus possible that bAP-activated R-type channels activating SK channels in dendrites and spines operate in a negative feedback loop that both self-limits bAP-mediated depolarization and curtails EPSP duration. SK channels would hyperpolarize the dendrite and spine and reestablish the voltage-dependent block of NMDA receptors to ultimately narrow the time window for coincidence detection. Thus strong distal SK channel activation may serve to counteract the NMDA receptor-dependent EPSP boosting observed in distal basal dendrites (Branco and Hausser 2011). It would be interesting to test whether calcium influx through NMDA receptors could synergistically act to activate dendritic SK channels, or whether there are other signaling pathways in place (Wang et al. 2014). In either case, SK channels could control the expression of synaptic plasticity, as described at other synapses (Hopf et al. 2010; Ngo-Anh et al. 2005; Ohtsuki et al. 2012), depending on the dendritic location of the input.
Additionally, the exclusive coupling between dendritic R-type and SK channels could enable specific regulation by neuromodulators, allowing synapses to adjust their sensitivity to synaptic input and increasing their dynamic range, e.g., in states of arousal. Whereas direct effects on SK channels by muscarinic M1 acetylcholine receptor and adrenergic receptor activation have been demonstrated (Buchanan et al. 2010; Faber et al. 2008; Giessel and Sabatini 2010), selective modulation of R-type channels is unknown. Future work is needed to test whether calcium influx through R-type VDCCs has other privileged roles in dendrites, such as local activation of kinases, release of retrograde messengers, or regulation of postsynaptic receptors, or whether the other many types of VDCCs present in dendrites are responsible. Distinct calcium sources for other effectors would suggest that dendrites require independent coupling regimes to allow for specialized control of separate neuronal processes. As pharmacological and optical approaches evolve, we can expect to learn much more about local calcium signaling and its consequences in the future.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R. prepared figures; S.R. and M.S.T. drafted manuscript; S.R. and M.S.T. edited and revised manuscript; S.R. and M.S.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Diasynou Fioravante for comments on our manuscript.
REFERENCES
- Acker CD, Antic SD. Quantitative assessment of the distributions of membrane conductances involved in action potential backpropagation along basal dendrites. J Neurophysiol 101: 1524–1541 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler E, Augustine G, Duffy S, Charlton M. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci 11: 1496–1507, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SSH, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci 32: 13819–13840, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher HR. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 85: 855–868, 2001. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885, 2009. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV2.3 voltage-sensitive calcium channels located in dendritic spines. Neuron 53: 249–260, 2007. [DOI] [PubMed] [Google Scholar]
- Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol 20: 494–502, 2010. [DOI] [PubMed] [Google Scholar]
- Branco T, Hausser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron 69, 885–892, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz S, Regehr W. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci 23: 6373–6384, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M1 receptors is mediated by inhibition of SK channels. Neuron 68: 948–963, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat Neurosci 13: 19–21, 2010. [DOI] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron 57: 536–545, 2008. [DOI] [PubMed] [Google Scholar]
- Bullis JB, Jones TD, Poolos NP. Reversed somatodendritic Ih gradient in a class of rat hippocampal neurons with pyramidal morphology. J Physiol 579: 431–443, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Muralidharan S, Kao JPY, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44: 351–364, 2004. [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA 97: 13372–13377, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Yuste R, Svoboda K, Tank DW. Imaging calcium dynamics in dendritic spines. Curr Opin Neurobiol 6: 372–378, 1996. [DOI] [PubMed] [Google Scholar]
- DiGregorio DA, Peskoff A, Vergara JL. Measurement of action potential-induced presynaptic calcium domains at a cultured neuromuscular junction. J Neurosci 19: 7846–7859, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 13: 7–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES. Functional interplay between NMDA receptors, SK channels and voltage-gated Ca2+ channels regulates synaptic excitability in the medial prefrontal cortex. J Physiol 588: 1281–1292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci 28: 10803–10813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler BB, Adelman JP. Control of KCa channels by calcium nano/microdomains. Neuron 59: 873–881, 2008. [DOI] [PubMed] [Google Scholar]
- Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron 68: 936–947, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J 70: 1069–1081, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron 59: 902–913, 2008. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387: 869–875, 1997. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron 65: 682–694, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Stuart GJ. Different calcium sources control somatic versus dendritic SK channel activation during action potentials. J Neurosci 33: 19396–19405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bischofberger J, Jonas P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurosci 27: 13420–13429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5: 1185–1193, 2002. [DOI] [PubMed] [Google Scholar]
- Maciaszek JL, Soh HH, Walikonis RS, Tzingounis AV, Lykotrafitis GG. Topography of native SK channels revealed by force nanoscopy in living neurons. J Neurosci 32: 11435–11440, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci 3: 895–903, 2000. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science 268: 301–304, 1995. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275: 209–213, 1997. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature 395: 900–905, 1998. [DOI] [PubMed] [Google Scholar]
- Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by Kv1 channels. Nat Neurosci 13: 601–609, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev V, Bertram R, Sherman A. Residual bound Ca2+ can account for the effects of Ca2+ buffers on synaptic facilitation. J Neurophysiol 96: 3389–3397, 2006. [DOI] [PubMed] [Google Scholar]
- Neher E, Taschenberger H. Transients in global Ca2+ concentration induced by electrical activity in a giant nerve terminal. J Physiol 591: 3189–3195, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat Neurosci 10: 206–214, 2007. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642–649, 2005. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, Piochon C, Adelman JP, Hansel C. SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron 75: 108–120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007. [DOI] [PubMed] [Google Scholar]
- Rall W. Theory of physiological properties of dendrites. Ann NY Acad Sci 96: 1071–1092, 1962. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci 26: 458–469, 2004. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Tillberg PW, Chen F, Boyden ES. A fully genetically encoded protein architecture for optical control of peptide ligand concentration. Nat Commun 5: 3019, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Eilers J. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci 27: 229–243, 2009. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26: 113–135, 1998. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9: 206–221, 2008. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci 18: 3501–3510, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367: 69–72, 1994. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci 23: 9650–9663, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thestrup T, Litzlbauer J, Bartholomaus I, Mues M, Russo L, Dana H, Kovalchuk Y, Liang Y, Kalamakis G, Laukat Y, Becker S, Witte G, Geiger A, Allen T, Rome LC, Chen TW, Kim DS, Garaschuk O, Griesinger C, Griesbeck O. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat Methods 11: 175–182, 2014. [DOI] [PubMed] [Google Scholar]
- Wang K, Lin MT, Adelman JP, Maylie J. Distinct Ca2+ sources in dendritic spines of hippocampal CA1 neurons couple to SK and Kv4 channels. Neuron 81: 379–387, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Schaefer A, Sakmann B. Backpropagating action potentials in neurones: measurement, mechanisms and potential functions. Prog Biophys Mol Biol 87: 145–170, 2005. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024–11031, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Fuchs JR, Green JT, Morielli AD. Cellular mechanisms and behavioral consequences of Kv1.2 regulation in the rat cerebellum. J Neurosci 32: 9228–9237, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci 22: 3404–3413, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci 23: 2600–2607, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Harvey CD, Zhong H, Sobczyk A, van Aelst L, Svoboda K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nat Neurosci 9: 283–291, 2006. [DOI] [PubMed] [Google Scholar]
- Zayat L, Calero C, Albores P, Baraldo L, Etchenique R. A new strategy for neurochemical photodelivery: metal-ligand heterolytic cleavage. J Am Chem Soc 125: 882–883, 2003. [DOI] [PubMed] [Google Scholar]